Abstract
Background
Emerging evidence suggests that androgens and estrogens have a role in respiratory health, but it is largely unknown whether levels of these hormones can affect lung function in adults from the general population. This study investigated whether serum dehydroepiandrosterone sulfate (DHEA-S), a key precursor of both androgens and estrogens in peripheral tissues, was related to lung function in adult women participating in the European Community Respiratory Health Survey (ECRHS).
Methods
Lung function and serum DHEA-S concentrations were measured in n = 2,045 and n = 1,725 women in 1999–2002 and in 2010–2013, respectively. Cross-sectional associations of DHEA-S levels (expressed as age-adjusted z-score) with spirometric outcomes were investigated, adjusting for smoking habits, body mass index, menopausal status, and use of corticosteroids. Longitudinal associations of DHEA-S levels in 1999–2002 with incidence of restrictive pattern and airflow limitation in 2010–2013 were also assessed.
Findings
Women with low DHEA-S (z-score<-1) had lower FEV1 (% of predicted, adjusted difference: -2.2; 95%CI: -3.5 to -0.9) and FVC (-1.7; 95%CI: -2.9 to -0.5) and were at a greater risk of having airflow limitation and restrictive pattern on spirometry than women with higher DHEA-S levels. In longitudinal analyses, low DHEA-S at baseline was associated with a greater incidence of airflow limitation after an 11-years follow-up (incidence rate ratio, 3.43; 95%CI: 1.91 to 6.14).
Interpretation
Low DHEA-S levels in women were associated with impaired lung function and a greater risk of developing airflow limitation later in adult life. Our findings provide new evidence supporting a role of DHEA-S in respiratory health.
Funding
EU H2020, grant agreement no.633212
Keywords: Androgens, Sex hormones, Airflow obstruction, Cohort studies
Research in context.
Evidence before this study
We searched Pubmed for articles using the terms (“lung function” OR “pulmonary function” OR “airflow obstruction”) AND (“DHEA-S” OR “DHEA” OR “Sex steroids” OR “Sex hormones”), with no date restrictions and not limited to English-language publications. In population-based surveys, recent mendelian randomization studies indicate that changes in hormonal status (e.g. with menarche or menopause) might affect lung function. Another recent study suggested that exogenous sex steroids may reduce the lung aging in menopausal women. Low DHEA-S concentrations have been associated with inflammatory lung diseases, and greater mortality in COPD patients. DHEA-S had been associated with improved lung function in male adolescents with asthma, and nebulized DHEA-S was reported to improve asthma control. In experimental animal models, DHEA-S was found to prevent bronchospasm and relax airway smooth muscles. One cross-sectional study in men, found significant correlations but no association between DHEAs and FEV1 and FVC in multivariate analysis. We found no studies that investigated the associations between DHEA-S levels and lung function decline in women from the general population.
Added value of this study
We showed that low DHEA-S levels are associated with poor lung function, reduced FVC and FEV1/FVC ratio in a population-based sample of about 3000 women from 19 centres and 9 European countries. In longitudinal analyses, low DHEA-S levels at baseline were associated with 3-fold greater risk of developing airflow limitation (FEV1/FVC<lower limit of normal) after an 11-years follow-up.
Implications of all the available evidence
Our findings provide new evidence supporting a beneficial effect of DHEA-S in women's respiratory health. Further studies are needed to assess these relationships in men and to evaluate if restoring DHEA-S to normal levels might improve respiratory function and decrease the risk of airflow limitation.
Alt-text: Unlabelled box
1. INTRODUCTION
Sex hormones have biological effects in many non-reproductive organs. The presence of sex steroid receptors in the lungs and in the airways suggests that androgens and estrogens might play a role in the regulation of the respiratory function [1]. However, despite supporting findings from experimental studies, there is little evidence from clinical or epidemiological studies. In women, hormonal balance might be especially important, as changes of the hormonal status such as those occurring with menopause, have been associated with lung function decline [2,3]. In peripheral tissues, androgens and estrogens are produced intracellularly from dehydroepiandrosterone (DHEA) [4]. DHEA is a steroid, which is mainly produced in the zona reticularis of the adrenal glands in response to adrenocorticotropic hormone (ACTH). DHEA is mainly secreted as a sulfate ester (DHEA-S), which is the most abundant sex steroid in human serum. Its concentration peaks around the age of 20 and then steadily decreases with age [4].
There is evidence that DHEA-S can exert immunomodulatory and anti-inflammatory functions [5], [6], [7]. DHEA-S naturally antagonizes some of the immunosuppressive and catabolic effects of glucocorticoids, and its decline with age is thought to be one of the causes of immune-senescence [8], [9], [10].
Low DHEA-S levels were associated with all-cause mortality in several population-based studies [11], and were also observed in patients with cardiovascular and inflammatory diseases [12], [13], [14], [15], [16], [17], [18], [19]. No population-based study has investigated the association of DHEA-S with lung function decline, which is a significant predictor of mortality in long term follow-up studies [20]. As low DHEA-S has been associated with early death, we hypothesized that DHEA-S concentrations might be associated with lung function impairment, which may be mediating this association.
Serum DHEA-S concentrations were measured in women participating in the European Community Respiratory Health Study (ECRHS) as part of a program aimed at investigating the associations between hormonal factors and lung function decline in women [2,21], In this manuscript, we investigated if DHEA-S levels were associated with lung function and lung function decline in 10 years in women from the general population, independently from age, smoking history, and other potential confounders.
2. METHODS
2.1. Study design and population
The ECRHS (www.ecrhs.org) is an international population-based cohort study of adults born in 1945–1970 [22]. Participants underwent clinical examinations, including spirometry and standardized interviewer-led questionnaires in 1991–1993 (ECRHS-1), 1999–2002 (ECRHS-2) and 2010–2013 (ECRHS-3). At ECRHS-2 and ECRHS-3, blood samples were collected in women from centers taking part in the hormonal measurement protocol. All the women with spirometry and DHEA-S measurement in at least one of these surveys were considered for the present analysis. Overall, 2969 women from 19 centers in 9 European countries were included in this study [Table E1]. Ethical approval was obtained for each center from the appropriate ethics committee, and written consent was obtained from each participant. This manuscript adheres to the STROBE guidelines.
2.2. Respiratory outcomes
Forced vital capacity (FVC) and forced expiration volume in 1 s (FEV1) were measured according to the American Thoracic Society criteria for repeatability. FEV1, FVC, and FEV1/FVC ratio percentage of predicted and lower limit of normal (LLN) were calculated using the Global Lung Initiative equations [23]. Airflow limitation was defined as FEV1/FVC <LLN; restrictive pattern on spirometry was defined as FVC<LLN in subjects without airflow limitation [23].
2.3. DHEA-S measurements
Blood samples were drawn and stored following standardized procedures. DHEA-S serum concentrations were determined centrally using an immuno-competitive assay (Elecsys® DHEA-S electrochemiluminescence immunoassay, Roche Diagnostics, Germany) at ECHRS-2, while a high-throughput LC-MS/MS system (Aquity UPLC coupled with a Xevo TQ-S mass spectrometer, Waters Corp., Milford, MA, USA) was used at ECRHS-3.
To account for the dependency of DHEA-S on age and as the laboratory assessments differed between the two examinations, we derived age-adjusted DHEA-S z-scores separately for ECRHS-2 and ECRHS-3, using mixed-effects linear regression models on log-transformed DHEA-S concentrations with age included as independent variable and study center as a random effect. The resulting z-score represents how far (in units of the population standard deviation, SD) a measured concentration is from the mean of a same-age population. Participants with a z-score <−1 (representing 14.3% of the sample) were considered as having “low” DHEAs levels.
2.4. Statistical analysis
To investigate the cross-sectional associations of DHEA-S with subjects’ characteristics and respiratory outcomes, data from ECRHS-2 and ECRHS-3 were pooled together, and we fitted multilevel models with subject ID (as a level-2 unit, to account for repeated measures) nested in the center (level-3). Associations were modeled using mixed-effects linear or Poisson with robust error variance regressions, with DHEA-S z-score included either as a continuous variable or as a categorical variable. Body mass index, smoking history, education as proxy of socioeconomic status, physical activity, and menopausal status were considered for adjustment. Age was included as precision variable because of its strong association with spirometry outcomes. Stratified analyses were performed for median age, to investigate if a different association was observed in older women (when DHEA-S is lower), and for menopausal status, as the effects of DHEA-S might change with the hormonal context.
Longitudinal association of DHEA-S z-score at baseline (ECRHS-2) and lung function decline between ECRHS 2 and 3 was assessed using linear models. In addition, incidence of new subjects with spirometric restriction pattern and airflow limitation was evaluated at follow-up, and mixed-effects Poisson regression models were used to derive incident rate ratios (IRR) in function of baseline DHEA-S z-score.
Sensitivity analyses for the associations of DHEA-S with spirometric outcomes were performed after exclusion from the models of: 1) corticosteroids users, as corticosteroids are known to affect the adrenal function and DHEA-S secretion; 2) women with asthma, as DHEA-S has been associated in literature with asthma occurrence; 3) current smokers, to avoid potential residual confounding by smoking which can significantly alter both lung function and DHEA-S secretion; 4) women sampled in the ECHRS symptomatic sample, to ascertain that the results were not driven by unspecified respiratory conditions.
In all models, women with missing data on a covariate were removed from the models. The statistical analyses were performed using STATA v14.2 (StataCorp, College Station, TX, USA).
Additional details are provided in the online data supplement.[Text E1]
3. RESULTS
3.1. Sample characteristics and associations with DHEA-S
A total of 3770 observations with valid lung function and DHEA-S measurements (n = 2045 at ECRHS-2 and n = 1725 at ECRHS-3) were considered for the cross-sectional analyses; 801 women had DHEA-S measured at both ECRHS-2 and ECRHS-3 (repeated measures). Among the 2045 women with DHEA-S and lung function at ECRHS-2, 1216 were followed-up with spirometry and clinical interviews at ECRHS-3 (average follow-up time: 11.0 ± 1.1 years) and were included in the longitudinal analyses.[Table E1]
The distribution of the covariates by survey and their associations with DHEA-S levels are shown in Table 1. Compared to lifetime non-smokers, current smokers had significantly higher DHEA-S concentrations, while ex-heavy smokers had lower DHEA-S concentrations. DHEA-S concentrations were also higher in overweight women (Table 2). We observed no association of age-adjusted DHEA-S z-score with menopausal status, as well as with passive smoke, school education, and physical activity. Both inhaled and oral corticosteroid use in the past year were significantly associated with lower DHEA-S levels (p<0.001).
Table 1.
Participants’ characteristics by survey. Distributions are expressed as number (percentage), median [range] or mean ± standard deviation.
| ECRHS-2n = 2045 | ECRHS-3n = 1725 | |
|---|---|---|
| Age, years | 43.4 [28.0,56.8] | 53.7 [39.7,66.9] |
| Smoking habits | ||
| Non smokers | 928 (45.4%) | 774 (44.9%) |
| Past smokers with <15py | 387 (18.9%) | 472 (27.4%) |
| Past smokers with ≥15py | 119 (5.8%) | 155 (9.0%) |
| Current smokers with <15py | 249 (12.2%) | 102 (5.9%) |
| Current smokers with ≥15py | 272 (13.3%) | 132 (7.7%) |
| Smokers with unknown py | 86 (4.2%) | 89 (5.2%) |
| missing | 4 (0.2%) | 1 (0.1%) |
| Exposure to passive smoke in the last 12 months | ||
| No | 1275 (62.4%) | 1376 (79.8%) |
| Yes | 759 (37.1%) | 337 (19.5%) |
| missing | 11 (0.5%) | 12 (0.7%) |
| Body mass index, | ||
| <25 kg/m2 | 1194 (58.4%) | 736 42.7%) |
| [25–30) kg/m2 | 591 (28.9%) | 578 (33.5%) |
| ≥30 kg/m2 | 258 (12.6%) | 400 (23.2%) |
| missing | 2 (0.1%) | 11 (0.6%) |
| Age completed full time education | ||
| 16years or younger | 357 (17.5%) | 252 (14.6%) |
| 17–20 years | 653 (31.9%) | 614 (35.6%) |
| 21years or older | 1030 (50.4%) | 818 (47.4%) |
| missing | 5 (0.2%) | 41 (2.4%) |
| Vigorous physical activity | ||
| No | 1207 (59.0%) | 979 (56.8%) |
| Yes | 808 (39.5%) | 742 (43.0%) |
| missing | 30 (1.5%) | 4 (0.2%) |
| Menstrual Periods | ||
| Regular | 1199 (58.6%) | 464 (26.9%) |
| Never been regular | 120 (5.9%) | 72 (4.2%) |
| Recently irregular | 220 (10.8%) | 139 (8.1%) |
| Have stopped (>6 months) | 464 (22.7%) | 840 (48.7%) |
| missing | 42 (2.0%) | 210 (12.2%) |
| Use of corticosteroids in the last 12 months | ||
| No | 1824 (89.2%) | 1538 (89.2%) |
| Inhaled corticosteroids only | 150 (7.3%) | 68 (3.9%) |
| Oral corticosteroids | 38 (1.6%) | 20 (1.2%) |
| missing | 33 (1.6%) | 99 (5.7%) |
| FEV1, (mL) | 2959 ± 505 | 2574 ± 500 |
| FEV1,% of predicted | 98.7 ± 13.5 | 94.4 ± 14.9 |
| FVC, (mL) | 3675 ± 588 | 3392 ± 593 |
| FVC,% of predicted | 99.8 ± 12.6 | 99.0 ± 13.5 |
| FEV1/FVC ratio (%) | 80.6 ± 6.6 | 75.9 ± 6.5 |
| FEV1/FVC ratio,% of predicted | 98.5 ± 7.8 | 94.7 ± 7.9 |
| Airflow limitation (FEV1/FVC<LLN) | 129 (6.3%) | 174 (10.1%) |
| Restrictive pattern on spirometry (FVC<LLN and FEV1/FVC≥LLN) | 81 (4.2%) | 62 (4.0%) |
Table 2.
Associations of women's characteristics with DHEA-S z-score. *adjusted for age plus all the other covariates included in the table.
| Unadjusted Beta (95%CI) | p-value | Adjusted* beta (95%CI) | p-value | |
|---|---|---|---|---|
| Smoking habits | ||||
| Non smokers | reference | – | reference | – |
| Past smokers with <15py | −0.00 (−0.08,0.08) | .921 | 0.00 (−0.08,0.09) | .942 |
| Past smokers with ≥15py | −0.04 (−0.16,0.09) | .575 | −0.13 (−0.26,0.00) | .052 |
| Current smokers with <15py | 0.18 (0.07,0.29) | .001 | 0.18 (0.06,0.29) | .003 |
| Current smokers with ≥15py | 0.23 (0.13,0.33) | <0.001 | 0.21 (0.10,0.33) | <0.001 |
| Smokers with unknown py | 0.00 (−0.14,0.15) | .958 | −0.02 (−0.18,0.14) | .810 |
| Exposure to passive smoke in the last 12 months | ||||
| No | Reference | – | Reference | – |
| Yes | 0.09 (0.02,0.16) | .008 | 0.04 (−0.06,0.12) | .355 |
| Body mass index, | ||||
| <25 kg/m2 | Reference | – | Reference | – |
| [25–30) kg/m2 | 0.09 (0.02,0.16) | .014 | 0.08 (0.01,0.16) | .023 |
| ≥30 kg/m2 | 0.03 (−0.06,0.12) | .488 | 0.05 (−0.04,0.14) | .282 |
| Age completed full time education | ||||
| 16years or younger | −0.03 (−0.13,0.07) | .554 | −0.07 (−0.17,0.04) | .213 |
| 17–20 years | Reference | – | Reference | – |
| 21years or older | −0.01 (−0.08,0.06) | .705 | −0.02 (−0.09,0.05) | .569 |
| Vigorous physical activity | ||||
| No | Reference | – | Reference | – |
| Yes | −0.05 (−0.11,0.02) | .160 | −0.05 (−0.11,0.02) | .173 |
| Menstrual Periods | ||||
| Regular | reference | – | reference | – |
| Never been regular | −0.05 (−0.19,0.09) | .447 | −0.06 (−0.20,0.08) | .386 |
| Recently irregular | 0.11 (0.01,0.09) | .038 | 0.07 (−0.04,0.18) | .204 |
| Have stopped (>6 months) | −0.02 (−0.09,0.05) | .523 | −0.06 (−0.14,0.03) | .192 |
| Use of corticosteroids in the last 12 months | ||||
| No | reference | – | reference | – |
| Inhaled corticosteroids only | −0.41 (−0.54,−0.28) | <0.001 | −0.41 (−0.54,−0.27) | <0.001 |
| Oral corticosteroids | −0.82 (−1.05,−0.59) | <0.001 | −0.77 (−1.01,−0.52) | <0.001 |
3.2. Cross-sectional associations of DHEA-S levels with lung function indices
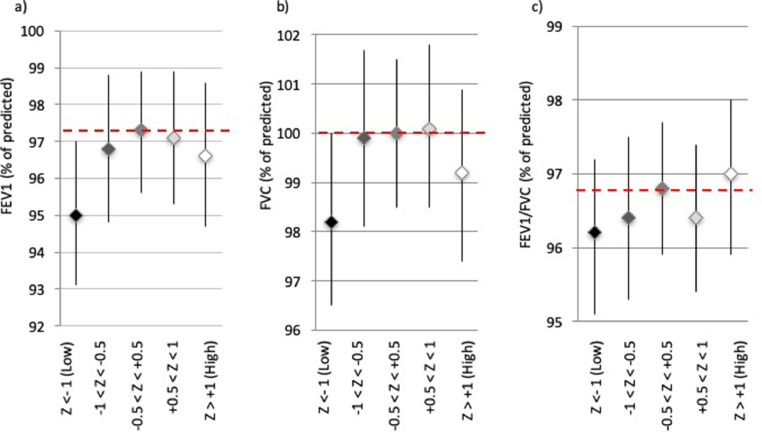
The cross-sectional associations of DHEA-S with pre-bronchodilator FEV1, FVC and FEV1/FVC ratio suggest that the association with FEV1 and FVC might not be linear [Fig. 1]. In particular, women with low DHEA-S (z-score<−1) had significantly lower FEV1 and FVC compared to women with normal levels of DHEA-S for their age (z-score between −0.5 and +0.5), while women with DHEA-S z-score above 1 did not have significantly different FEV1 or FVC. Accordingly, it was found that the proportion of women with low DHEA-S was significantly higher among women with a restrictive pattern (21.8%) than among women with normal lung function (13.5%). This difference was found also among never-smokers or ex-smokers with a cumulative history of less than 15 pack-years tobacco smoke, in whom the percentage of women with low DHEA-S reached 24.5% in women with a restrictive spirometric pattern (vs. 13.8% in women with normal lung function).
Fig. 1.
Women's spirometric measures by DHEAS z-score group. FEV1 (a), FVC (b), and FEV1/FVC ratio (c) are expressed as percentage of predicted. P-values for the pairwise comparisons between different DHEA-S levels are shown in Table E2 in the Online Supplement.
For modeling both linear and non-linear associations, the associations of lung function outcomes with DHEA-S were estimated with z-scores included either as a continuous or dichotomous variable (Table 3). After adjusting for potential confounders, women with low DHEA-S had significantly lower FEV1 and FVC (−2.0 and −1.7% of predicted, respectively) than women with higher DHEA-S. For FEV1/FVC ratio, a linear increase of one unit in DHEA-S z-score was associated with an increase of +0.3 in FEV1/FVC% of predicted.
Table 3.
Association of DHEA-S z-scores with FEV1, FVC, and FEV1/FVC. Results from main model and sensitivity analyses.
| DHEA-S z-score (continuous) | Low* DHEA-S vs.higher | ||||
|---|---|---|---|---|---|
| Obs. | Beta (95% CI) | p-value | Beta (95% CI) | p-value | |
| FEV1 (% of predicted) | |||||
| Unadjusted | 3516 | 0.52 (0.35,1.00) | .036 | −2.18 (−3.52,−0.85) | .001 |
| Main model† | 3516 | 0.65 (0.18,1.12) | .007 | −2.02 (−3.33,−0.71) | .002 |
| w/o CS users†1 | 3245 | 0.49 (0.00,0.97) | .049 | −1.65 (−3.01,−0.28) | .018 |
| w/o asthma†2 | 3089 | 0.33 (−0.15,0.82) | .180 | −1.52 (−2.90,−0.15) | .030 |
| w/o current smokers†3 | 2633 | 0.92 (0.38,1.46) | .001 | −2.35 (−3.82,−0.87) | .002 |
| w/o symptomatic sample†4 | 2895 | 0.59 (0.06,1.12) | .029 | −1.99 (−3.44,−0.54) | .007 |
| FVC (% of predicted) | |||||
| Unadjusted | 3460 | 0.27 (−0.16,0.70) | .219 | −1.70 (−2.90,−0.49) | .006 |
| Main model† | 3460 | 0.34 (−0.08,0.77) | .114 | −1.64 (−2.83,−0.46) | .006 |
| w/o CS users†1 | 3193 | 0.20 (−0.25,0.64) | .386 | −1.40 (−2.65,−0.15) | .028 |
| w/o asthma†2 | 3037 | 0.06 (−0.40,0.52) | .800 | −1.33 (−2.62,−0.04) | .043 |
| w/o current smokers†3 | 2586 | 0.72 (0.23,1.21) | .004 | −2.15 (−3.49,−0.80) | .002 |
| w/o symptomatic sample†4 | 2848 | 0.29 (−0.20,0.78) | .241 | −1.77 (−3.11,−0.43) | .010 |
| FEV1/FVC ratio (% of predicted) | |||||
| Unadjusted | 3412 | 0.35 (0.08,0.62) | .010 | −0.60 (−1.36,0.15) | .118 |
| Main model† | 3412 | 0.31 (0.04,0.58) | .024 | −0.55 (−1.28,0.19) | .147 |
| w/o CS users†1 | 3151 | 0.29 (0.02,0.56) | .033 | −0.28 (−1.03,0.48) | .469 |
| w/o asthma†2 | 2995 | 0.28 (0.02,0.55) | .038 | −0.26 (−1.01,0.50) | .505 |
| w/o current smokers†3 | 2552 | 0.27 (−0.04,0.57) | .086 | −0.35 (−1.18,0.48) | .409 |
| w/o symptomatic sample†4 | 2812 | 0.33 (0.04,0.62) | .061 | −0.30 (−1.09,0.49) | .456 |
FEV1: Forced expiratory volume in the 1st second, FVC: Forced vital capacity, CS: corticosteroids.
*low DHEAS is defined as having Z-score < −1.
adjusted for age, BMI, smoking history, passive smoke exposure, physical activity, schooling and periods.
Sensitivity analyses were performed in (1) women who were not using corticosteroids; (2) women who did not report asthma; (3) after excluding women recruited as part of the symptomatic sample; (4) non-smokers only.
Statistically significant associations (p<0.05) are shown in bold; borderline associations (0.05≤p<0.10) are in italics.
Although different methods were used at ECRHS 2 and ECRHS 3 to measure DHEA-S, the associations of z-score DHEA-S with FEV1 and FVC were similar at the two surveys (Table E3). No significant interaction was found between DHEA-S and age or between DHEA-S and menopausal status (Table E4).
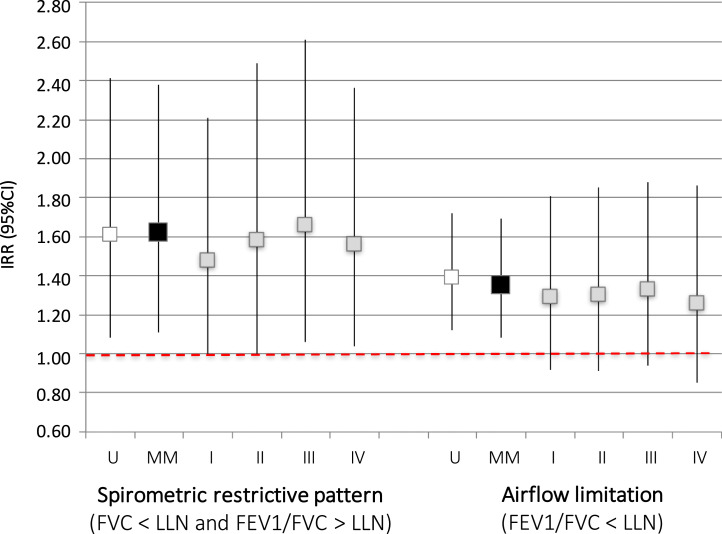
When analyzing spirometric outcomes as binary variables, we found that women with low DHEA-S had a 62% greater risk of having restrictive pattern (RR: 1.62, 95%CI: 1.11–2.38; p = 0.013) and 35% greater risk of having pre-bronchodilator airflow limitation (RR: 1.35, 95%CI: 1.08–1.69; p = 0.008) than women with higher DHEA-S [Fig. 2]. When we repeated the cross-sectional analysis using only data at ECRHS-3, where post-bronchodilator measures were available, we found that low DHEA-S was also associated with a 66% significantly greater risk of post-bronchodilator airflow limitation (Table E3).
Fig. 2.
Cross sectional associations of DHEA-S z-scores (low DHEA-S vs. higher) with the risk of airflow limitation (FEV1/FVC<LLN) and of restrictive pattern on spirometry (FVC<LLN and FEV1/FVC≥LLN). Results from main model (MM) and sensitivity analyses. Models: U: unadjusted; MM: adjusted for age, BMI, smoking history, passive smoke exposure, physical activity, schooling and periods (main model); I: MM with exclusion of corticosteroids users, II: MM with exclusion of women with asthma, III: MM with exclusion of current smokers, IV: MM with exclusion of women recruited in the ECRHS symptomatic sample.
3.3. Longitudinal associations of DHEA-S levels with lung function decline and incidence of spirometric restriction and airflow limitation
The characteristics of women who did and did not participate in both surveys and were included in the longitudinal analyses are shown in the online supplement.[Table E5] A faster decline in pre-bronchodilator FEV1/FVC ratio (−0.13% of predicted/year, p = 0.003) was observed in women with low DHEA-S compared to those who had higher DHEA-S levels at baseline.[Table E6] DHEA-S at baseline was not associated with FVC decline.
After excluding women with prevalent airflow limitation at ECRHS-2, women with low DHEA-S at baseline had a 3-fold greater risk to develop pre-bronchodilator airflow limitation at ECRHS-3 (IRR = 3.43, p<0.001) (Table 4). This association showed a significant interaction with age (p for interaction=0.013), and low DHEA-S was prospectively associated with a greater risk of developing airflow limitation in women older than the median age (i.e.age≥43 years at baseline, IRR=9.59; 95%CI:4.03–22.8), but not in younger women (IRR=1.32;95%CI:0.48–3.68). Low DHEA-S at baseline was also associated with a higher risk of post-bronchodilator airflow limitation at ECRHS-3 (IRR = 2.81, p = 0.040) (Table 4). No association between DHEA-S at baseline and having restrictive pattern at follow-up was observed, but the number of incident cases was very low.
Table 4.
Longitudinal associations of DHEAS z-scores with incidence of spirometric restrictive pattern (FVC < LLN and FEV1/FVC ≥ LLN) and pre- and post-bronchodilator airway limitation (FEV1/FVC < LLN).
| Person-years | Incident cases | Rates x1000/year | Unadjusted IRR (95% CI) | p | Adjusted^ IRR (95% CI) | p | |
|---|---|---|---|---|---|---|---|
| Spirometric restritive pattern | |||||||
| Low DHEA-S* | 1559 | 3 | 1.9 | 1.07 (0.31,3.71) | .916 | 1.40 (0.39,5.04) | .611 |
| Higher DHEA-S | 10,129 | 17 | 1.7 | reference | reference | ||
| Air flow limitation (pre-bronchodilator) | |||||||
| Low DHEA-S* | 1699 | 18 | 10.6 | 3.38 (1.92,5.93) | <0.001 | 3.43 (1.91,6.14) | <0.001 |
| Higher DHEA-S | 11,792 | 42 | 4.0 | reference | reference | ||
| Air flow limitation (post-bronchodilator) | |||||||
| Low DHEA-S* | 1665 | 6 | 3.6 | 3.15 (1.21,8.21) | .019 | 2.81 (1.05,7.53) | .040 |
| Higher DHEA-S | 10,277 | 16 | 1.6 | reference | reference |
IRR: incidence rate ratio. ^IRR are adjusted for age and BMI at baseline, change in BMI, history of smoking between the two examinations. *z-score <−1.
3.4. Sensitivity analyses
We found similar results when data from ECRHS 2 and ECRHS 3 were analyzed separately for cross-sectional associations (Table E4). The cross-sectional associations of DHEA-S with lung function outcomes were less strong, but still generally statistically significant, when women who used corticosteroids or had asthma were excluded from the analyses (Table 3, Fig. 2, Table E7). The sensitivity analyses performed considering only women sampled in the ECRHS “random sample” to exclude potential bias related to other respiratory conditions, as well as in “non-smokers only” to avoid potential residual confounding by smoking, confirmed the main results (Table 3, Fig. 2, Table E7). The longitudinal association between low DHEA-S and higher risk of developing airflow limitation remained significant in the sensitivity analyses (Table E8).
4. DISCUSSION
To the best of our knowledge, this is the first epidemiological study investigating the association between DHEA-S levels and lung function in adult women from the general population, and the first longitudinal study investigating the associations of DHEA-S with lung function decline. We found that women with low DHEA-S levels were significantly more likely to have airflow limitation and restrictive pattern on spirometry than women with higher DHEA-S levels. Moreover, in longitudinal analyses, having low DHEA-S was associated with a greater risk of developing airflow limitation after 11-years of follow-up.
These associations were independent from potential confounders, such as smoking habits and use of corticosteroids, which were found to affect the concentration of DHEA-S. In fact, DHEA-S levels were severely reduced in women treated with oral corticosteroids, but also in women who used only inhaled corticosteroids, reflecting that even inhaled corticosteroids may have systemic effects and cause adrenal suppression. Nicotine has also been reported to affect the HPA axis [24]. In our study, current smokers had higher DHEA-S concentrations than non-smokers, confirming previous reports [25].
Women who had DHEA-S concentrations lower than one standard deviation from the average for their age had significantly lower FEV1 and FVC than women with higher DHEA-S, resulting in a greater risk of both airflow limitation and restrictive pattern on spirometry. There are no other studies in women to be compared to ours. We only found similar investigation in two cohorts of community-dwelling men: the Tromsø and the Busselton studies [26,27]. In these cross-sectional studies, including respectively 2197 Norwegian and 1768 Australian adult men, high serum levels of testosterone and dehydrotestosterone, but not estrogens, were significantly associated with better lung function [26,27]. In the Tromsø study, DHEA-S concentrations were also measured, but no significant linear association was found between FEV1 and FVC and DHEA-S after adjustments [26]. However, our findings suggest that the associations between DHEA-S and these lung function outcomes might not be linear. Moreover, as the Tromsø cohort was composed exclusively of men, the difference with our findings might be due to the lower concentrations of DHEA-S in women as compared to men, to different distribution of unmeasured confounders, or indicate that DHEA-S has a different impact on respiratory function in men and women.
Considering that DHEA-S is almost the exclusive source of androgens in peripheral tissues in women [4], these data together suggests that DHEA-S, directly or indirectly through its androgenic metabolites, might contribute in sustaining the respiratory function. Since androgens have known anabolic effects, one might speculate that DHEA-S deficiency contributes to respiratory muscle weakness [9], with a simultaneous restrictive lung function impairment. In our study, low DHEA-S was associated with restrictive pattern on spirometry only in the cross-sectional analysis, while it was not associated with FVC decline in the longitudinal analysis. If the association was causal, this might point toward a short-term, maybe reversible, effect of DHEA-S deficiency on pulmonary vital capacity. Noteworthy, among never-smokers or light smokers, one out of four women with a restrictive pattern was found to have low DHEA-S levels.
As regards airflow limitation, we found significant relationships in the cross-sectional analyses and in the longitudinal analysis. Low DHEA-S at baseline was prospectively associated with faster FEV1/FVC ratio decline and with about 3-fold greater risk of developing airflow limitation at follow-up, either when assessed pre- or post-bronchodilator.
Our findings on airflow limitation are consistent with previous studies that reported an association between low DHEA-S and asthma, of which airway obstruction is one of the principal features [16], [17], [18], [19]. Although the cross-sectional association of DHEA-S with airflow limitation was not significant after excluding women with asthma, the magnitude of the association in women without asthma was similar as in the total sample. Furthermore, the longitudinal association of baseline DHEA-S with airflow limitation incidence remained significant in women without asthma. DHEA-S might impact on airflow limitation through its immunomodulatory and anti-inflammatory properties. In vitro studies have shown that DHEA-S has direct effects on immune cells migration and inhibits the secretion of pro-inflammatory cytokines (namely IL-6, IL-2, IL-10, and TNF) from several cell types in humans [5], [6], [7], [8]. The hypothetic mechanisms of action might be both via direct inhibition of NF-kB and indirectly via downstream testosterone and estrogens [5], [6], [7], [8]. In addition, in vivo studies have shown that DHEA-S and testosterone can act as bronchoactive steroids, regulating airway contractility by relaxing the airway smooth muscles and preventing bronchospasm [28]. Together, these data suggest that DHEA-S may attenuate airway inflammation and bronchospasm and, vice-versa, low DHEA-S may increase the hyper-responsiveness of smooth muscle surrounding the airways and inflammation-driven loss of elastic recoil of lungs and large airways [29], contributing to the development of airflow limitation.
As low DHEA-S was prospectively associated also with incident fixed airflow limitation at follow-up, we hypothesize that low DHEA-S might be a risk factor for the development of COPD. Of note, a recent 6-year follow-up study reported that low DHEA-S levels were associated with greater mortality in COPD patients with acute exacerbation [30], suggesting that DHEA-S might be linked to both disease development and progression.
Previous reports showed that patients with asthma or using corticosteroids have low DHEA-S [[16], [17], [18], [19],31]. In our study, however, we found that the associations of DHEA-S with airflow limitation at follow-up were not limited to women having a respiratory disease or taking corticosteroids at baseline.
The strengths of our study include the large sample size, longitudinal design, and the use of a standardized protocol across centers, with spirometry and serum biomarkers collected in women from the general population.
We recognize that measurements of DHEA-S using two different methods is a potential limitation of our study. However through transformation of DHEA-S concentrations in z-scores we were able to harmonize and combine the measurements obtained by immunoassay and by mass spectrometry. Although the two methods have different sensitivity and specificity (with LC-MS/MS considered the gold standard for DHEA-S measurement), the associations found in the main analyses were overall confirmed when performing separate analyses for the measurements obtained by immunoassay and mass-spectrometry, supporting the validity of our findings.[Table E3] Our observation that DHEA-S seemed associated with incident airflow limitation especially at older ages suggests that there might be a threshold effect, which needs further investigation.
As only women were sampled, the results of this study may not be generalized to men. Furthermore, as our sample was selected from the general population and relatively young at baseline, the number of incident cases of airflow limitation and restrictive pattern on spirometry was limited. Although we do acknowledge these limits, this is the first study with lung function in women and the only study with longitudinal data on lung function decline. Furthermore, we found significant associations with the incidence of airflow limitation. Our study - with repeated measures of DHEA-S and spirometry data on such a large sample – provides valuable evidence on the association between DHEA-S and lung function decline.
Independently from smoking habits and use of corticosteroids, low DHEA-S was associated with impaired lung function in women and predicted airflow limitation. Our findings provide new evidence supporting a protective role of DHEA-S in respiratory health. It might be particularly relevant to investigate DHEA-S deficiency in non-smoking women with unexplained airflow restriction. Further studies are needed to assess these relationships in men and to evaluate if restoring DHEA-S to normal levels might improve respiratory function and decrease the risk of developing airflow limitation.
Acknowledgments
Acknowledgements
The present analyses are part of the ALEC Study (www.alecstudy.org). The ALEC project leader is Prof Deborah Jarvis. The manuscript was done with ALEC Workpackage 4 led by Dr Garcia-Aymerich.
Authors’ contributions
Dr Pesce and Dr Leynaert conceived and designed the study. Dr Pesce planned and performed the statistical analyses, and wrote the first draft of the manuscript. All authors contributed in the collection of data in/from original studies, discussion of the statistical analysis plan and interpretation of study results. All authors critically reviewed and approved the final version of the manuscript.
Funding sources
The current study is part of the Ageing for Lungs in European Cohorts (ALEC) study (www.alecstudy.org), ALEC has received funding from the European Union's Horizon 2020 research and innovation program [grant agreement No. 633212]. The coordination of the ECRHS was supported by the European Commission [grant agreement no. QLK4-CT-1999–01237] and the Medical Research Council [grant agreement no. 92091]. The hormones measures at ECRHS III were funded by the Norwegian Research Council [grant agreement no. 228174]. Hormones measures at ECRHS II were funded by the local budget of the ECRHS Paris team, INSERM U700, Epidemiology, with further support from the Comité National contre les Maladies Respiratoires (CNMR), the centre d'Investigation Clinique (CIC), Bichat Hospital, and the French Agence Nationale de la Recherche (ANR). Bodies funding the local studies are listed in the Online Supplement. The funding sources had no role in the writing of the manuscript or the decision to submit it for publication. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
Dr Demoly reports presentation honoraria from Stallergène Greer, ALK, Mylan, Astra Zeneca, Bausch&Lomb and personal fees as board advisor from Chiesi, Sanofi, Thermo Fisher Scientific, outside the submitted work. Dr Jogi reports personal grants from the Estonian Research Council [personal research grant no.562], and reports travel grants and presentation honoraria from GSK, Novartis, Boehringer, outside the submitted work. Dr Pin reports travel grants and presentation honoraria from GSK, Novartis, Astra Zeneca, Zambon Pharma, outside the submitted work. All the authors declare no competing interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100389.
Contributor Information
Giancarlo Pesce, Email: giancarlo.pesce@inserm.fr.
Bénédicte Leynaert, Email: benedicte.leynaert@inserm.fr.
Appendix. Supplementary materials
References
- 1.Townsend E.A., Miller V.M., Prakash Y.S. Sex differences and sex steroids in lung health and disease. Endocrine Rev. 2012;33(1):1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Triebner K., Matulonga B., Johannessen A. Menopause is associated with accelerated lung function decline. Am J Rrespir Crit Care Med. 2017;195(8):1058–1065. doi: 10.1164/rccm.201605-0968OC. [DOI] [PubMed] [Google Scholar]
- 3.van der Plaat D.A., Pereira M., Pesce G. Age at menopause and lung function: a Mendelian Randomization study. Eur Respir J. 2019; Oct 17;54(4) doi: 10.1183/13,993,003.02421–2018. pii: 1,802,421. [DOI] [PubMed] [Google Scholar]
- 4.Labrie F., Martel C., Belanger A., Pelletier G. Androgens in women are essentially made from DHEA in each peripheral tissue according to intracrinology. J Steroid Biochem Mol Biol. 2017;168:9–18. doi: 10.1016/j.jsbmb.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Hazeldine J., Arlt W., Lord J.M. Dehydroepiandrosterone as a regulator of immune cell function. J Steroid Biochem Mol Biol. 2010;120:127–136. doi: 10.1016/j.jsbmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Choi I.S., Cui Y., Koh Y.A., Hyun-Chul L., CHo Y.B., Won Y.H. Effects of dehydroepiandrosterone on Th2 cytokine production in peripheral blood mononuclear cells from asthmatics. Kor J Internal Med. 2008;23:176–181. doi: 10.3904/kjim.2008.23.4.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koziol-White DHEA-S inhibits human neutrophil and human airway smooth muscle migration. Biochim Biophys Acta. 2012;1822(10):1638–1642. doi: 10.1016/j.bbadis.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straub R.H. Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits IL-6 secretion from mononuclear cells in man in vitro: possible link between endocrinosenescence and immunosenescence. K Clin Endocrinol Metab. 1998;83:2012–2017. doi: 10.1210/jcem.83.6.4876. [DOI] [PubMed] [Google Scholar]
- 9.Robinzon B., Cutolo M. Should dehydroepiandrosterone replacement therapy be provided with glucocorticoids? Rheumatol. 1999;38 doi: 10.1093/rheumatology/38.6.488. 848–495. [DOI] [PubMed] [Google Scholar]
- 10.Straub R.H., Schoelmerich J., Zietz B. Replacement therapy with DHEA plus corticosteroids in patients with chronic inflammatory diseases – substitutes of adrenal and sex hormones. Z Rheumatol. 2000;59(Suppl2):108–118. [PubMed] [Google Scholar]
- 11.Ohlsson C., Vandenput L., Tivesten A. DHEA and mortality: what is the nature of the association? J Steroid Biochem Mol Biol. 2015;145:248–253. doi: 10.1016/j.jsbmb.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Shufelt C., Bretsky P., Almeida C.M. DHEA-S Levels and Cardiovascular Disease Mortality in Postmenopausal Women: Results from the National Institutes of Health—National Heart, Lung, and Blood Institute (NHLBI)-Sponsored Women's Ischemia Syndrome Evaluation (WISE) J Clin Endocrinol Metab. 2010;95(11):4985–4992. doi: 10.1210/jc.2010-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimenez M.C., Sun Q., Schurks M. Low dehydroepiandrosterone sulfate is associated with increased risk of ischemic stroke among women. Stroke. 2013;44:1784–1789. doi: 10.1161/STROKEAHA.111.000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedman M., Nilsson E., de la Torre B. Low blood and synovial fluid levels of sulpho-conjugated steroids in rheumatoid arthritis. Clin Exp Rheumatol. 1992;10(1):25–30. [PubMed] [Google Scholar]
- 15.Suzuki T., Suzuki N., Engleman E.G., Mizushima Y., Sakane T. Low serum levels of dehydroepiandrosterone may cause deficient IL-2 production by lymphocites in patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1995;99(2):251–255. doi: 10.1111/j.1365-2249.1995.tb05541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn P.J., Mahood C.B., Speed J.F., Jury D.R. Dehydroepiandrosterone sulfate concentrations in asthmatic patients: pilot study. N Z Med J. 1984;97:805–808. [PubMed] [Google Scholar]
- 17.Weinstein R.E., Lobocki C.A., Gravett S. Decreased adrenal sex steroid levels in the absence of glucocorticoid suppression in postmenopausal asthmatic women. J Allergy Clin Immunol. 1996;97(1):1–8. doi: 10.1016/s0091-6749(96)70276-2. [DOI] [PubMed] [Google Scholar]
- 18.Reinke S.N., Gallart-Ayala H., Gomez C. Metabolomics analysis identifies different metabotypes of asthma severity. Eur Respir J. 2017;49 doi: 10.1183/13993003.01740-2016. 1,601,740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBoer M.D., Phillips B.R., Mauger D.T. Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulmon Med. 2018;18:58. doi: 10.1186/s12890-018-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannino D.M., Buist A.S., Petty T.L., Enright P.L., Redd S.C. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58:388–393. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macsali F., Real F.G., Plana E. Early age at menarche, lung function, and adult asthma. Am J Respir Crit Care Med. 2011;183(1) doi: 10.1164/rccm.200912-1886OC. 8.14. [DOI] [PubMed] [Google Scholar]
- 22.European Community Respiratory Health Survey II Steering Committee The European Community Respiratory Health Survey II. Eur Respir J. 2002;20(5):1071–1079. doi: 10.1183/09031936.02.00046802. [DOI] [PubMed] [Google Scholar]
- 23.Quanjer p.H., Hall G.L., Stanojevic S., Cole T.J., Stocks J., Global Lungs Initiative Age- and height-based prediction bias in spirometry reference equations. Eur Respir J. 2012;40(1):190–197. doi: 10.1183/09031936.00161011. [DOI] [PubMed] [Google Scholar]
- 24.Rohleder N., Kirschbaum C. The hypothalamic-pituatary-adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 2006;59(3):236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Nagaya T., Kondo Y., Okinaka T. Serum dehydroepiandrosterone reflects age better than health status, and may increase with cigarette smoking and alcohol drinking in middle-aged men. Aging Clin Exp Res. 2012;24(2):134–138. doi: 10.1007/BF03325159. [DOI] [PubMed] [Google Scholar]
- 26.Svartberg J., Schirmer H., Medbø A., Melbye H., Aasebø U. Reduced pulmonary function is associated with lower levels of endogenous total and free testosterone. The Tromsø Study. Eur J Epidemiol. 2007;22:107–112. doi: 10.1007/s10654-006-9095-9. [DOI] [PubMed] [Google Scholar]
- 27.Mohan S.S., Knuiman M.W., Divitini M.L. Higher serum testosterone and dihyrdrotestosterone, but not oestradiol, are independently associated with favourable indices of lung function in community-dwelling men. Clin Endocrinol. 2015;83:268–276. doi: 10.1111/cen.12738. [DOI] [PubMed] [Google Scholar]
- 28.Montaño L.M., Espinoza K., Flores-Soto E., Chavez J., Perusquia M. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. J Endocrinol. 2014;222:1–13. doi: 10.1530/JOE-14-0074. [DOI] [PubMed] [Google Scholar]
- 29.Tonga K.O., Chapman D.G., Farah C.S. Reduced lung elastic recoil and fixed airflow obstruction in asthma. Respirol. 2019 doi: 10.1111/resp.13688. [DOI] [PubMed] [Google Scholar]
- 30.Zurfluh S., Nickler M., Ottiger M. Association of adrenal hormone metabolites and mortality over a 6-year follow-up in COPD patients with acute exacerbation. Clin Chem Lab Med. 2018;56(4):669–680. doi: 10.1515/cclm-2017-0873. [DOI] [PubMed] [Google Scholar]
- 31.Cavkaytar O., Vuralli D., Arik Ylmaz E. Evidence of hypothalamic-pituitary-adrenal axis suppression during moderate-to-high-dose inhaled corticosteroid use. Eur J Pediatr. 2015;174(11):1421–1431. doi: 10.1007/s00431-015-2610-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.