Abstract
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), is a progressive neurodegenerative disease that affects both upper and lower motor neurons, which results in loss of muscle control and eventual paralysis [1]. Currently, there are as yet unresolved challenges regarding efficient drug delivery into the central nervous system (CNS). These challenges can be attributed to multiple factors including the presence of the blood-brain barrier (BBB), blood-spinal cord barrier (BSCB), as well as the inherent characteristics of the drugs themselves (e.g. low solubility, insufficient bioavailability/bio-stability, 'off-target' effects) etc. As a result, conventional drug delivery systems may not facilitate adequate dosage of the required drugs for functional recovery in ALS patients. Nanotechnology-based strategies, however, employ engineered nanostructures that show great potential in delivering single or combined therapeutic agents to overcome the biological barriers, enhance interaction with targeted sites, improve drug bioavailability/bio-stability and achieve real-time tracking while minimizing the systemic side-effects. This review provides a concise discussion of recent advances in nanotechnology-based strategies in relation to combating specific pathophysiology relevant to ALS disease progression and investigates the future scope of using nanotechnology to develop innovative treatments for ALS patients.
Keywords: Nanotechnology, Amyotrophic lateral sclerosis (ALS), Blood-brain barrier, Neurodegenerative diseases, Central nervous system (CNS)
Graphical abstract

1. Introduction
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND) or Lou Gehrig's disease, is a rapidly progressive neurodegenerative disease that causes dysfunction of the nerves that control muscle movement [[1], [2]]. The morbidity of ALS is around one to three people per 100,000 worldwide [3]. To date, there is no effective treatment for ALS, the reported median life expectancy of ALS patients ranges from 24 to 48 months from the time of diagnosis [3]. ALS mainly affects motor neurons in the brain, brainstem, and the spinal cord (Fig. 1A) [4], leading to progressive motor neuron degradation and muscle atrophy, which ultimately result in paralysis and eventually to death due to respiratory failure [3].
Fig. 1.
(A) Schematic illustration of the human corticospinal tract. The vulnerable and resistant motor neuron (MN) groups in ALS are shown in red and blue, respectively. Reproduced with permission from Ref. [4]. (B) The proposed cellular and molecular mechanisms involved in ALS. Reproduced with permission from Refs. [6]. Schematic structure of the blood–brain barrier (BBB) (C) and the blood–spinal cord barrier (BSCB) (D) that together comprise the blood–Central Nervous System (CNS) barrier. Reproduced with permission from Ref. [13,14].
ALS can be categorized as familial (fALS) or sporadic (sALS) disease, depending on whether the patient has a family history of the disease or not. fALS is generally considered to account for 5%–20% of all cases of ALS [2]. More than 20 genes have been found associated with fALS, of which C9orf72 (40%), SOD1 (20%), FUS (1–5%), and TARDBP (1–5%) are four genes which account for most familial ALS cases [5]. The mechanisms of neuronal death mediated by these gene defects are still unclear. However, it is suggested that these converge and overlap with the same mechanisms seen in the development of sporadic ALS. Specifically, but not exhaustively, glutamate excitotoxicity, protein misfolding and aggregation, endoplasmic reticulum (ER) stress, neuroinflammation, oxidative stress, mitochondrial dysfunction, loss of trophic factors, cytoskeletal elements and defects in axonal transport. These pathophysiological defects are viewed as some of the principal events that promote ALS disease progression (Fig. 1B) [6] and many therapeutic strategies have been developed to target these mechanisms. Disappointingly, to date, the US Food and Drug Administration (FDA) has only approved two drugs that only slow ALS progression modestly: rituzole and edaravone [3]. Almost all other clinical trials have failed to show any improved clinical efficacy in the treatment of ALS over the last 20 years [7,8]. Poor understanding of mechanisms, inappropriate animal models, imperfect clinical trial design, lack of effective biomarkers, delayed diagnosis, insufficient bioavailability/biostability of drugs, and low efficiency of delivering ALS drugs to CNS are some of the potential reasons hindering significant translational progress in ALS clinical trials [7,9].
To address the above limitations in ALS treatment, new strategies are required. Encouragingly, the achievements of nanotechnology-based approaches in treating neurodegenerative diseases including Alzheimer's (AD) [10] and Parkinson's diseases (PD) [11] in the last few years offer hope that nanobased strategies may be usefully applied to improve the therapeutic efficiency of drugs in ALS clinical trials. These include, but are not limited to, improving drug bioavailability/biostability, overcoming biological barriers such as the blood-brain-barrier (BBB), reducing side-effects, attenuating off-target effect, precise targeting to disease sites and achieving real-time tracking [9,12]. Many potentially useful ALS therapies suffer from suboptimal efficacy, these may be revitalized by nanotechnology. This review outlines proposed mechanisms, current treatment, and on-going clinical trials of ALS. It further discusses the various challenges in delivering ALS drugs to CNS and how nanotechnology can be applied to address these challenges. Additionally, this review highlights the recent advances of using nanotechnology-based strategies in addressing the specific pathophysiology that is relevant to ALS disease progression.
2. Proposed mechanisms of ALS
Although the precise mechanisms of ALS are still poorly understood, it is believed that ALS is mediated by a complex interaction among cellular, molecular, and genetic pathways. The proposed principal disease mechanisms contributing to ALS are: (1) Mutations in genes that lead to impairment of normal protein function. So far, more than 20 genes have been associated with ALS, with C9orf72, SOD1, FUS, and TARDBP implicated in most familial ALS cases [15]; (2) Protein misfolding and aggregation; Essential RNA-binding proteins in ALS, such as TAR DNA binding protein of 43 kDa (TDP-43), Fused in sarcoma (FUS), ATXN2, hnRNPA1/A2, undergo cytosolic accumulation and nuclear depletion, thereby causing protein misfolding and aggregation [16,17]; The most common case is TDP-43 aggregation, which is found aggregated and mislocalized in >95% ALS patients (both sporadic and familial) [16,17]; (3) Glutamate excitotoxicity; increased synaptic glutamate mediates the rise of intracellular calcium levels, which in turn leads to excessive excitotoxicity that is thought to be one of main mechanisms resulting in neuronal death [18]; (4) Oxidative stress; when the production rate of free radicals or reactive oxygen species (ROS) is greater than the ability of endogenous radical scavenging molecules in neurons to neutralize these, excessive oxidative stress results and causes irreversible damage to cellular proteins, DNA, RNA and cell structures; indeed, most ALS patients show evidence of increased levels of oxidative damage in serum, urine samples, or cerebrospinal fluid (CSF) [19]; (5) Mitochondrial dysfunction; mitochondria are vital organelles for energy metabolism, phospholipid biogenesis, apoptosis, and calcium homeostasis; mitochondrial dysfunction has been extensively found in ALS animal models and patients and is widely thought to directly attribute to disease pathogenesis [19]; (6) Neuroinflammation; ALS is not considered an autoimmune disease as immune-system mediated acute neuroinflammation may promote motor neuron function; however, chronic neuroinflammation may lead to motor neuron degeneration, due to the excessive production of proinflammatory growth factors and cytokines which have been detected in ALS patients [20,21]; (7) Disrupted cytoskeletal and axonal transport have also been implicated in the abnormal accumulation of neurofilaments (NFs) and the mislocalization of hypophosphorylated NFs in motor neuron cell bodies or axons. These have been observed as crucial pathological hallmarks of ALS [6,22]. Current therapeutic strategies targeting the above mechanisms have shown some progress in ALS clinical trials and studies ahead of clinical usage [23]. In the next section, we will provide a concise introduction into currently approved treatments and on-going clinical trials for ALS.
3. Current treatments and clinical trials for ALS
As of now, the US Food and Drug Administration (FDA) has approved only two drugs for the treatment of ALS: riluzole and edaravone. Riluzole was first approved by the FDA in 1995, and in the ensuing decades, many other countries, including Australia, Canada, and also countries throughout Europe, also approved riluzole as the first line drug for ALS [24]. It is thought that riluzole can reduce the glutamate excitotoxicity by preventing the release of glutamate, as excessive glutamate accumulation in the brain and spinal cord is one of the main features of ALS [24]. Edaravone received FDA approval in 2017 and Health Canada approval in 2018. Edaravone treatments are also available in Japan and South Korea. Edaravone acts as an effective free radical scavenger (e.g. lipid peroxides, hydroxyl radicals) in the CNS, and excessive oxidative stress is thought to be one of the main mechanisms leading to neuronal death [25,26]. Although these two treatments have slowed down the symptoms in ALS patients, the treatment effect is still modest [26]. In recent years, more drugs and treatments are under investigation in ALS clinical trials, including drugs targeting SOD1 mutations, anti-excitotoxic agents, mitochondrial protectants, anti-apoptotic agents, anti-inflammatory agents, and neurotrophic factors etc. [26] Ongoing Phase-III interventional trials (based on US national library of medicine) are listed in Table 1 [27] and ongoing clinical trials on ALS/MND in Australia are listed in Table 2. We believe more drugs will become available to serve ALS patients in the coming years. However, given the extremely complicated causes of ALS, drugs targeting a single mechanism may not generate significant therapeutic effects, thus a combination of drugs may lead to better clinical outcomes. In addition, given the existence of biological barriers (e.g. BBB, BSCB) and the possible inherent limitations of the drug (e.g. low solubility, easy degradation, ‘off-target’ effects), there are still significant challenges in regards to effectively delivering therapeutic drugs to the CNS in order to slows the clinical progress of ALS [28]. In the next section, we will give a detailed discussion about the challenges of delivering therapeutic drugs to the CNS for the treatment of ALS.
Table 1.
Ongoing Phase-III interventional trials on ALS.
| Compound | Target/mechanism | Recruiting | Identifier |
|---|---|---|---|
| Aroursodeoxycholic acid | Anti-apoptotic | Not yet | NCT03800524 |
| Methyl cobalamin | B12 vitamin derivative | Yes | NCT03548311 |
| Masitinib | Tyrosine kinase c-Kit inhibitor | Not yet | NCT03127267 |
| CannTrust CBD Oil | Active cannabinoid | Yes | NCT03690791 |
| Arimoclomol | Stimulates repair pathways | Yes | NCT03491462 |
| Levosimendan | Calcium channel sensitizer | Yes | NCT03505021 |
| Deferiprone | Iron chelator | Not yet | NCT03293069 |
| MSC-NTF cells | Mesenchymal cell therapy | Yes | NCT03280056 |
Search query: ALS (Amyotrophic Lateral Sclerosis) https://clinicaltrials.gov/Filtered for: tatus: Recruiting – Not yet recruiting – Active, not recruiting – Enrolling by invitation; Study type: Interventional (Clinical Trial); Study Phase: Phase-III.
Table 2.
Ongoing clinical trials on ALS/MND in Australia.
| Clinical trial title | Description | Status | Recruiting |
|---|---|---|---|
| Cu(II)ATSM | A small molecule that is able to deliver copper to cells containing damaged mitochondria [29] | Phase 2/3 | Yes |
| EMERALD | Cannabis Based Medicine Extract (CannTrust CBD Oil) [30] | Phase 3 | Yes |
| Communication and Assistive Technology (CAT) | Assessment and intervention within CAT [31] | Yes | |
| Oral levosimendan (ODM-109) | Restore respiratory function in patients with ALS [32] | Phase 3 | Yes |
| Triumeq | Antiretroviral therapy [33] | Phase 3 | Not yet |
| Tecfidera | Reduce neuroinflammation and increase the levels of Regulatory T cells (Tregs) in humans [34] | Phase 2 | Completed |
| BIIB067 (Tofersen) | Antisense therapeutic specifically targeted SOD1 mutation in ALS patients [35]. | Phase 3 | Yes |
| CNM-Au8 | An oral, gold nanocrystal liquid suspension. CNM-Au8 can support bioenergetic cellular reactions and assist to remove the toxic byproducts of cellular metabolism that may induce motor neurons breakdown in ALS [36]. | Phase 2 | Yes |
| ALS-205/PMX205 | Potent non-competitive inhibitors of complement C5a receptor 1 (C5aR1) [37] | Phase 1 | Not yet |
Sources can be found from website of https://www.mndsa.org.au/Discover-our-research/Latest-research/Clinical-trials.aspx.
4. Challenges of delivering ALS therapeutic drugs to CNS
Although plenty of newly developed therapeutic agents (e.g. therapeutic proteins, neurotrophic factors, antisense oligonucleotides) have been synthesized to combat ALS [7,26], the therapeutic efficacy of these approaches have been largely disappointing due to their largely unsatisfactory in vivo properties, such as low stability in biological environments, poor BBB permeability, rapid enzymatic degradation, immune system clearance, unfavorable pharmacokinetic properties or inappropriate release profiles [7,8,38,39]. The presence of any one of these limitations may explain why a particular therapeutic agent fails to effectively target the CNS to achieve clinical efficacy. The major challenges in delivering ALS therapeutic drugs to CNS is described in the following section.
4.1. Blood-brain barrier and blood-spinal cord barrier
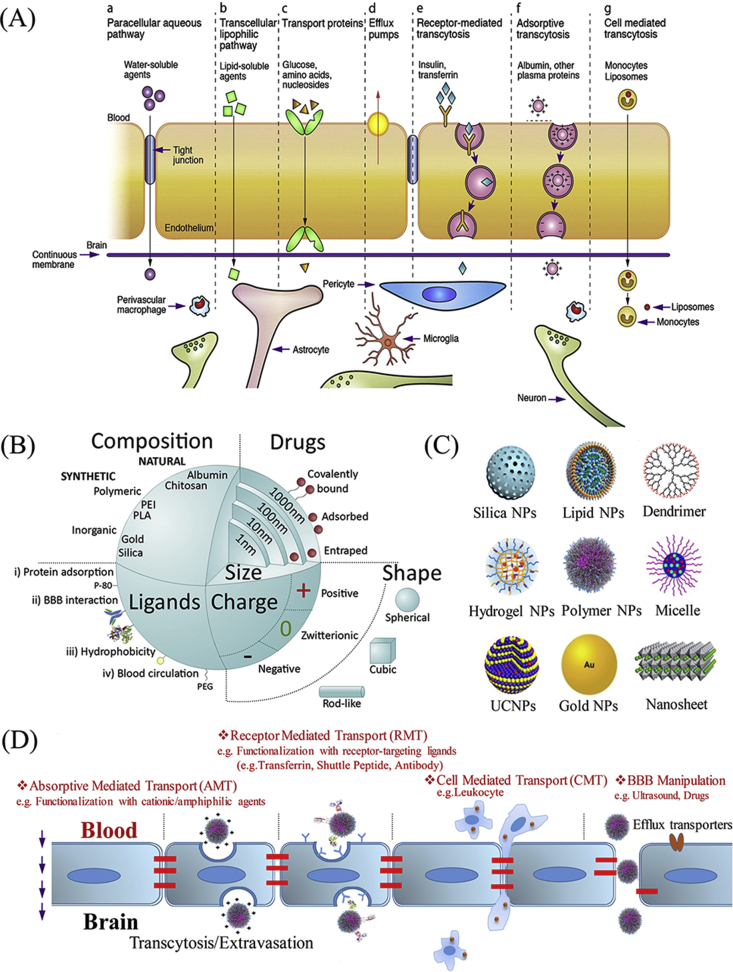
The blood-brain barrier (BBB) and the blood-spinal cord barrier (BSCB) (Fig. 1C and D) [13,14] play a crucial role in protecting the central nervous system (CNS) [40]. The BBB/BSCB serves to selectively allow substances such as nutrients and small lipid-soluble molecules to pass through the capillary endothelial membrane while limiting the entrance of pathogens or toxins (Fig. 2A) [[40], [41], [42]]. However, this protection mechanism is also a major barrier for delivering ALS therapeutic drugs to the CNS. It is believed that the BBB/BSCB hinders CNS delivery of more than 90 % of small-molecule drugs and nearly all biologics (e.g. small interfering RNA (siRNA), DNA, antisense oligonucleotides (ASOs) [38]. Moreover, the BBB/BSCB also controls brain-to-blood, spinal cord-to-blood or cerebrospinal fluid (CSF)-to-blood excretion of drugs and endogenous compounds through the action of efflux transporters [[42], [43], [44]]. The primary efflux transporter is P-glycoprotein (P-gp), which effluxes many endogenous substrates and exogenous molecules, including considerable lipid-soluble drugs. P-gp activity constitutes a major cause of the failure of many potential ALS therapeutics to reach therapeutic concentrations in the CNS [45]. Even the FDA-approved drug riluzole, only exhibited limited accumulation in the CNS due to the drug efflux mechanisms [46].Additionally, various degrading enzymes, in particular phase I and phase II enzymes, are localized at capillary endothelial cells membranes, accompanied by numbers of transporter proteins that strictly regulate the passage of therapeutic substances through the CNS. Many potential ALS drug candidates that might otherwise penetrate BBB/BSCB are blocked by these barriers [47]. However, as elaborated below, nanotechnology offers several, potentially exciting, solutions to this problem.
Fig. 2.
(A) Typical blood-brain barrier transport pathways for substances passing through the BBB. Reproduced with permission from Refs. [42]. (B) Major features of NPs which affect the permeability of NPs through the BBB. Reproduced with permission from Refs. [60]. (C) Typical nanostructures used for drug delivery or imaging. (D) Typical non-invasive nanotechnology-based CNS drug-delivery strategies to cross the BBB.
4.2. Biostability and bioavailability
Aside from poor BBB-permeability, insufficient biostability and bioavailability are additional problems that limit the effectiveness of potential ALS therapeutics. In particular, approximately half of all newly developed chemical therapeutics by the pharmaceutical industry show extremely low aqueous solubility [48]. For example, due to the cholesterol-like structure, the mitochondrial-targeted neuroprotective compound Olesoxime (TRO19622) only has limited solubility in water [49]. In another study, P7C3A20 an aminopropyl carbazole, demonstrates delayed disease progression in a G93A-SOD1 mice model, however, its solubility in water was also limited, thus further approaches were needed to improve its efficiency [50,51]. To date, most drugs used in the clinic for the treatment of CNS disease are small (<400 Da), lipid-soluble molecules (e.g. edaravone, riluzole) that can cross the BBB by active transmembrane diffusion. However, the drug absorbed by the brain capillary endothelial cells must then be delivered to the interstitial fluid of the brain tissue for therapeutic function. Consequently, a drug molecule that is too lipophilic risks sequestration by stacking at the capillary bed and, as a result, may not reach the cells behind the BBB [52]. Therefore, it is crucial to design drugs with appropriate lipid solubility to guarantee sufficient drug accumulation within the CNS [52]. Recently, new generations of biological therapeutics (e.g. nucleic acid constructs, neurotrophic factors and therapeutic proteins) have shown promising efficiency in treating ALS [26]. For instance, the use of an adeno-associated virus serotype 9 (AAV9) vehicle to deliver SOD short hairpin RNA (shRNA) led to a reduction in the synthesis of ALS-causing human Cu/Zn superoxide dismutase 1 enzyme (SOD1) mutants. This biomolecule extended survival in SOD1G93A mice by delaying both disease onset and slowing disease progression [53]. However, SOD1 shRNA is not BBB permeable and is susceptible to the action of biological enzymes, as evidenced by the extremely short half-life of SOD1 shRNA [39]. These limitations significantly hamper the effectiveness of SOD1 shRNA, and other similar biomolecules, as potential ALS therapies. Again, nanotechnology offers potential solutions to these problems.
4.3. Systemic distribution and clearance
Another major obstacle limiting drug delivery to CNS is the tendency of many lipophilic drugs to become systemically distributed, which attenuates the ‘on-target’ effect of drugs. As discussed in section 4.2, many lipophilic drugs passively diffuse into the central nervous system, but these lipophilic therapeutics are also more readily absorbed by cells in other tissues after intravenous injection [54]. Consequently, a larger drug dose must be given to achieve the required therapeutic concentration in the CNS. This non-specific systemic distribution may lead to increased systemic toxicity and adversely impact patient quality of life [55,56]. Additionally, the rapid systemic clearance of many drugs also significantly affects the concentration of drug reaching the CNS. A specific example is the oral suspension form of riluzole (Tiglutik™) which may be subject to enzymatic and non-enzymatic degradation in the gastrointestinal (GI) tract, thereby limiting systemic bioavailability [57]. While intravenous administration circumvents these problems, the major problem is that hydrophilic drug molecules typically suffer rapid renal clearance on account of poor reabsorption after glomerular filtration, whereas lipophilic drugs are often converted into hydrophilic metabolites in the liver before renal or biliary excretion [54]. Therefore, it was deduced that the suboptimal therapeutic efficacy of riluzole or edaravone might reflect the systemic distribution and fast systemic clearance [58].
5. Opportunities for nanotechnology to mediate CNS drug delivery in ALS
In the past few decades, several nanomedicines have been approved by the FDA that have shown far greater therapeutic efficacy than the parent drugs by themselves. Examples include Genexol-PM®, Abraxane®, Kadcyla® [59]. The advent of these nanomedicines suggests the strong possibility that a similar therapeutic benefit most likely will be achieved if nanotechnology is applied to develop ALS nanomedicines [59]. In particular, for CNS drug delivery, nanocarriers confer great opportunities to effectively package and protect the therapeutic agents with subsequent transport across the BBB thereby avoiding extensive systemic distribution [12].
5.1. Overcoming the BBB/BSCB
5.1.1. Non-invasive approach to cross the BBB/BSCB
Recently, great progress has been made in delivering drugs or diagnostic agents into the brain or spinal cord using nanotechnology. However, many features can affect the efficiency of NPs traversal through the BBB/BSCB. Nanoparticle type, size and surface charge, and surface functionalization are among the key features that have been extensively reviewed by Saraiva et al. [60], which are depicted in Fig. 2B [60]. The different nanostructures (e.g. Lipid NPs [61], Polymer NPs [62], Gold NPs [63], Dendrimer [64], Carbon-based nanomaterials [65]) that can be engineered to cross the BBB/BSCB are shown in Fig. 2C. The three major pathways by which nanomaterials cross the BBB/BSCB are absorptive-mediated transport (AMT), receptor-mediated transport (RMT) and cell-mediated transport (CMT) [28] as described in Fig. 2D. These approaches have been widely used to transport nanomaterials loaded with drugs or imaging substances to the CNS [28]. Nanoparticles are also usually co-engineered with various ligands that facilitate BBB/BSCB traversal. Ligands that are capable of adsorbing brain/spinal cord penetrating proteins (e.g. tween 80), ligands that are capable of interacting directly with brain/spinal cord endothelial cell receptors (e.g. transferrin, brain-specific antibody and peptides) and ligands that are able to increase endothelial cell endocytosis (e.g. cationic polymer, amphipathic peptides) are often used. Ligands that are capable of improving blood circulation (e.g. poly ethylene glycol) (PEG), cell membrane) are also widely used in coating nanomaterials [60,66]. Moreover, the capability of nanoparticles to cross the BBB/BSCB can be enhanced by utilizing a combination of additional techniques that manipulate the BBB/BSCB. For example, the BBB can be temporarily forced open with certain drugs or physical stimulation that allows better nanoparticles to penetrate the tight junction (TJ) of the brain capillary endothelial cells (BCECs) [28]. Furthermore, nanoparticle delivery of therapeutic agents can also be enhanced by coloading inhibitors of efflux pumps, especially polymeric P-gp [67]. Considering the diversity of nanostructures, we will provide a detailed illustration in section 6 to discuss the types of nanostructures that can be employed to combat specific pathophysiology relevant to ALS disease progression.
5.1.2. Invasive approach to cross the BBB/BSCB
Direct injections, such as intrathecal (IT) and intracranial injection, that can deliver large doses of therapeutic drugs directly into the cerebrospinal fluid (CSF), have received widespread attention as potential methods of ALS treatment [[68], [69], [70]]. However, direct injection of CSF in patients with ALS is still a daunting challenge in the clinic due to the serious potential risks, such as infection, edema, and neuronal damage [28,71]. In addition, poor solubility, insufficient pharmacokinetics, neurotoxicity, rapid clearance, non-specific targeting, and limited tissue diffusion of drugs are some of the vital obstacles that hinder the development of IT for clinical trials [28,71]. Given the fact that nanoscale carriers can be featured with increased drug solubility, extended SCF or SCF retention, enhanced tissue diffusion, controlled drug release, as well as facilitated cell-specific targeting, direct injection of nanomedicines can provide alternative solutions for introducing drugs into the CNS [72,73]. For example, Antonello Spinelli and colleagues, studied the kinetic distribution and stability of PEG-coated AuNPs in mice after intracisternal administration, and found that these NPs resulted in high brain penetrance, long-lasting stability, and targeting of neurons [74]. This approach might be purchased to carry ALS therapeutic agents inside the diseased sites by bypassing the BBB/BSCB. Moreover, given the poor prognosis of ALS patients and the need for actual clinical research in the near future, direct injection of nanomedicine remains a promising option for ALS treatment.
5.2. Improving bioavailability and increasing drug exposure time in the body
Many newly discovered drug molecules are insoluble in water and must be used with additional additives, such as surfactants, oils or ethanol, that normally cause inconvenience in administration or side-effects [26]. SP600125 (an anthrapyrazolone inhibitor of the c-Jun N-terminal kinase, JNK pathway), for instance, can be used to stabilize stathmin 2 (STMN2), a protein that has recently been found to stimulate axonal growth. Hence, SP600125 may be a potential therapy for ALS but as SP600125 is poorly soluble in water, dimethyl sulfoxide (DMSO) has been needed in in-vivo experiments performed ahead of clinical usage, which is a barrier for human clinical trials [75]. Nanotechnology offers the potential to encapsulate hydrophobic drugs, like SP600125, inside the NPs or conjugate hydrophobic drugs to the NP surface, resulting in increased solubility [76,77]. Another problem that hinders the application of many drugs in the CNS is short half-life due to rapid systemic excretion or enzymatic degradation. The half-life of edaravone, for example, is only 4.5–6 h while the half-life of nucleic acid constructs is even shorter (several minutes), thus higher doses and more-frequent dosing would be required for chronic lifelong treatment, leading to declined quality of life and increased risk of side-effects [78]. Drug agents encapsulated in nanocarriers have been successfully applied to various diseases, showing prolonged drug retention time in blood circulation [39]. First, as renal clearance is prevented once size <15 nm, nanoparticle encapsulation of drugs can reduce renal clearance if the particle size is increased beyond this threshold [79]. Second, nanoparticle formulation may also provide a physical barrier that protects drugs from metabolizing enzymes in the liver and other tissues [80]. Third, controlled release of drugs from nanocarriers is highly feasible making it possible to maintain an effective blood drug concentration for longer periods. Finally, by surface coating nanocarriers with extracted cell membranes (e.g. red blood cell or leukomonocyte cell membrane), natural polysaccharides (e.g. lipid) or stealth peptides (e.g. CD44) [81], the resulting nanocarriers acquire ‘stealth’ properties that can further protect drugs from clearance by the immune system, thus extending drug exposure time in the body.
5.3. Controlled drug release and targeted drug delivery
5.3.1. Controlled drug release
Therapeutic drugs absorbed, encapsulated or conjugated on/in nanocarriers allow efficient delivery of drug molecules into the CNS [82]. Most payloads in drug/nanocarrier complexes must be effectively released to exert their effort. The release mechanism can be vary depending on the drug loading manners, the inherent property of drugs, and the type of nanocarriers [83]. For example, physically adsorptive drugs can be released by molecular exchange; Encapsulated drugs can be released from nanocarriers by molecular diffusion or after the expansion or degradation of the nanostructure; And the conjugated drug can be released under certain stimulation [83]. In some cases, the drugs can still exert their function without being released. For example, in a study conducted by Bao et al. the anti-oxidative drug edaravone were absorbed on the surface of CeO2 NPs and showed very limited Edaravone release (lower than 4% within 24 h) in the phosphate buffer saline (pH = 7.4). Due to a lower density of polymer wrapped on the NPs, the absorbed edaravone was still effective at scavenging free radicals [84]. Given the fact that chemical conjugation of drugs to NPs may change their pharmacological properties, physical absorption or encapsulation is regarded a more effective approach for drug loading [85]. However, in the last few years, researchers have developed many sensitive linkers to conjugate the drug on/in nanocarriers. These linkers can be effectively cleaved under specific circumstances including changes in pH, enzymatic cleavage, and exposure to light [86,87]. Therefore, regardless of the drug loading manners, the drugs encapsulated or conjugated in/on nanocarriers can be released in an increasingly controlled manner, thus reducing the frequency of drug administration as well as systematic side-effects [83,88]. For example, in the study conducted by Bushra Nabi et al. riluzole was encapsulated in chitosan nanoparticles (CSNPs) through ionic gelation method, enabling continuous drug release for up to 24 h. This controlled drug release facilitated greater accumulation of the drug in the brain and resulted in a greater therapeutic effect [89]. Additionally, in some special designs, drugs encapsulated or conjugated on/in nanocarriers can be only effectively triggered and released under certain stimulation, such as light [90], reactive oxygen species (ROS) [91,92], ultrasound [93], these approaches allow more drugs to be released in the needed site, thereby reducing side-effects on other normal cells. Considering that excess ROS are widely observed in ALS-related neurons [94], it is thought that ROS triggering drug release is a viable method that can be used for targeting release of therapeutic agents to ALS-related cells.
5.3.2. Targeting drugs to specific cells and locations
Wide systemic distribution of therapeutics can lead to insufficient accumulation in disease-active regions [38]. Hence, to improve therapeutics for ALS, targeted drug delivery is needed. For instance, nanovehicles capable of selectively targeting neurons or axons should have significant application in ALS, as these would allow specific visualization of peripheral axonal and neuronal compartments, which could be highly useful prognostically [95]. Additionally, nanocarriers functionalized with neuron-targeting ligands, such as ten-eleven translocation methylcytosine dioxygenase 1 peptide (TET1 peptide) [96], MLR2 antibody (antibody specific to the neurotrophin receptor p75) [97] and rabies virus glycoprotein peptide/rabies virus-derived peptide (RVG/RDP peptide) [98], could also deliver therapeutics directly to injury sites. Exploiting the presence of ganglioside monosialotetrahexosylganglioside (GM1), GD1a, GD1b, and GT1b (the most abundant gangliosides in the neuronal and axonal compartments of mammalian nerves) [99] may provide a means to achieve specific targeting. These moieties localize in the outer leaflet of plasma membranes. The head-groups of these gangliosides are accessible to lectins present in bacterial toxins or antibodies [100]. Thus, it may be possible that bacterial toxins or antibodies can be grafted onto the nanocarrier surface to facilitate specific delivery of therapeutic agents to peripheral neurons or axons. An alternative approach used an in vivo phage-display screen, to identify a targeted axonal import (TAxI) peptide, that delivered protein cargo directly to spinal cord motor neurons after intramuscular injection [101]. In addition to neurons, it is believed that diseased astrocytes also play an important role in the progression of ALS [102]. Synthetic nanoparticles such as antibody-modified chitosan nanoparticles [103], Glucose-Coated Gold Nanoparticles [104], peptide-modified biodegradable polymathic nanoparticle [105] can be potential candidates for astrocyte specific targeting that may be possibly utilized to target deliver drugs to these cells and subsequently slow down the progression of the ALS. These examples illustrate the opportunity for ‘arming’ nanomaterials to selectively deliver ALS therapeutics to injury sites.
5.4. Real-time tracking and imaging
In the drug development process, it is crucial to evaluate whether a drug agent has reached the desired target. However, most drugs lack an intrinsic trackable signal and attempting to conjugate a fluorophore to the drug has the potential to unfavorably change pharmacological properties. Thus, nanotechnology offers an opportunity to incorporate sensitive, selective and rapid real-time tracking and imaging functionality [[106], [107], [108]]. For example, magnetic NPs (MNPs), with intrinsic magnetic properties, are well-positioned as the most promising nanomaterial for use as contrast agents for magnetic resonance imaging (MRI) [109]. Quantum dot (QD) nanoparticles with unique optical, excitation/emission and photostable properties imbue them with several advantages over the use of chemical fluorophores in bio-imaging [110]. Carbon-based nanomaterials, such as carbon nanotubes, carbon nanodots or graphene nanoflakes, with high aqueous solubility, low cost, easily functionalized surface, ideal biocompatibility, as well as remarkable resistance to photobleaching also show great potential for in-time tracking and imaging [111]. Polymer NPs, or solid lipid NPs, can also be functionalized with a wide variety of biomolecules with unique chemistries to also facilitate drug detection and their in vitro and in vivo bio-distribution [112]. Other nanomaterials, such as intrinsically fluorescent dendrimer [112], upconversion nanoparticles (UCNPs) [113], and gold nanoparticles, with unique optical and physical properties are also showing excellent capability for real-time tracking or imaging. A practical example is the development by Linying and coworkers of a tri-responsive polymer–gold nanoparticle that can codeliver gene (siRNA) and the small-molecule drug (Curcumin) for Parkinson's disease (PD) treatment as shown in Fig. 3 [114]. In this system, siRNA is released and plays its role after endosome escape, meanwhile small molecule drugs are released and exert their effect after being stimulated by excess ROS in diseased cells. Additionally, aggregated Au clusters have been developed that specifically target diseased cells (PD dopaminergic neurons) allowing for accurate imaging of PD via enhanced computed tomography (CT) [114]. Similarly, nanotechnology could also provide the opportunity for accurate ALS imaging and precise drug delivery.
Fig. 3.
Schematic illustration of the formation of MBPC nanoparticles and their switchable assembly in vivo. a) Assembly of levodopa-quinone gold nanoparticles (GNP) and pro-drugs BPC and MPC to form MBPCs and the siRNA loading procedure. b) Switchable assembly of GNP in vivo: 1-1 drug-gene codelivery as MBPCS circulates in the blood; 1–2 MBPCS penetrate the BBB via B6 peptide-mediated transport; 1–3 Neuron targeting via Mazindol (MA); 2 ROS-mediated drug release; 2′ Aggregated Au clusters permit imaging via enhancing computed tomography (CT). Reproduced with permission from Refs. [114].
6. Designing advanced therapeutic nanomaterials specifically targeting ALS pathophysiology
The effective delivery of therapeutic drugs, trophic factors and biomacromolecules across the BBB/BSCB to CNS remains a challenge for ALS treatment [9]. As discussed in Section 3, the rapid development of nanotechnologies offers potential solutions to a variety of factors that currently limits the therapeutic efficacy of ALS treatments. In this section, various mechanisms of ALS pathophysiology are revisited and how nanomaterials may be designed and utilized to combat these are discussed, providing stimulus to transform nanomedicine from theory to ALS clinical trials.
6.1. Glutamate excitotoxicity
Glutamate is a primary excitatory neurotransmitter in the CNS with important roles in learning and memory. However, excess glutamate can lead to over-stimulation of N-methyl-d-aspartate (NMDA) receptors and subsequently induce glutamate excitotoxicity [115]. Increasing evidence suggests that glutamate excitotoxicity plays an important role in ALS progression with more than 40 % of sALS patients showing glutamate dysfunction [[115], [116], [117]]. Riluzole, the first US Food and Drug Administration (FDA)-approved drug for ALS, is a well-described as an attenuator of glutamate excitotoxicity by blocking the neuronal release of glutamate. But its low aqueous solubility, short half-life (9–15 h after repeated doses), insufficient cerebral accumulation, and side-effects at higher dosage are major limitations that hinder its clinical efficacy [118]. In the last few years, diverse nanoformulations aimed at enhancing the neuroprotective power of Riluzole have been developed. For example, Sa Bondì and coworkers produced riluzole-loaded solid lipid nanoparticles (SLNs) which increased riluzole delivery to the brain (1.3 times higher than free drug after 8 h I.V.). Moreover, the SLNs showed reduced Riluzole distribution in liver, spleen, kidneys, heart, and lungs compared to free Riluzole, which may have potential benefits in reducing side-effects in those organs [119]. In addition to using drugs to control glutamate release, re-establishing glutamate homeostasis by upregulating the expression of glutamate transporter 1 (GLT1) has demonstrated promise in animal models [117,120]. However, unfavorable pharmacokinetic properties such as poor water solubility, low oral bioavailability, as well as severe peripheral adverse effects may diminish the clinical efficacy of GLT1 regulators (e.g. ceftriaxone) [120,121]. Recent advances have demonstrated the possibility of encapsulating GLT1 upregulators in nanostructures (e.g. liposomes, polymer nanoparticles, nano-micelles) to address the above pharmacokinetic issues. For example, Sandeep Kumar et al. have encapsulated ceftriaxone (the only intravenous beta-lactam antibiotic that has been investigated in ALS clinical trial) inside the liposomes through water-in-oil-in-water (w/o/w) type double emulsification method, and showed sustained drug release for up to 24 h [122]. Given the flexibility of drug encapsulation capability of nanocarriers, other GLT1 upregulators such as neuroimmunophilin compound GPI-1046 and the glutamate transporter activator (R)-(−)-(5)-methyl-1-nicotinoyl-2-pyrazoline (MS-153) can also be loaded in these nanoformulations to exert optimal therapeutic effects [120]. Further functionalization of GLT1 upregulator-loaded nanocomplexes with brain targeting agents will facilitate their transportation across BBB to CNS (Fig. 4). GLT1-upregulator release from nanocarriers should upregulate GLT1 expression in astrocytes, thereby leading to increased astrocytic glutamate uptake and reduced glutamate excitotoxicity to neurons (Fig. 4). Other drugs that influence glutamate levels in ALS are also under active investigation (e.g. α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), Gacyclidine, Valproic acid). These drugs can also be packaged as CNS targeted nanomaterials for optimal therapeutic effect [120]. In addition, gene therapy approaches to regulate the glutamate uptake system (e.g. Glutamate-dehydrogenase 2 (GDH2) EAAT2 excitatory amino acid transporter 2 (EAAT2)) are also under extensive investigation [123]. Given the advantages in nanotechnology-based strategies for gene CNS delivery in disease therapy [39,124], using nanotechnology to transport these glutamate uptake regulatory genes to the targeted site will greatly accelerate the application of gene therapy in ALS treatments. For example, an artificial virus system (RRPHC) has shown high efficiency in delivering different kinds of gene cargoes (including siRNA, pDNA and CRISPR-Cas9) in vivo. [125] Given the flexibility of the virus biomimetic structure, it becomes possible to design a virus system which targets specific cell types. As an illustration, the engineered virus biomimetic structure modified with peptides such as T-L5 peptide [105], short gH625 peptide [126], and AS1 homing peptide [127] can be used to deliver gene therapeutics specifically towards astrocytes [128].
Fig. 4.
Schematic illustration of glutamate transporter 1 (GLT1) regulator loaded nanoparticles traversing the BBB to regulate GLT1 expression. GLT1 regulator loaded NPs can be facilitated to cross BBB through surface functionalization with brain targeting ligands. Drugs absorbed in astrocytes will regulate GLT1 expression and subsequently restore their role in regulation glutamate balance. Reproduced with permission from Refs. [117].
6.2. Oxidative stress
The excessive production of ROS combined with impaired oxidant defense mechanisms has been recognized as a major pathogenic event in ALS [116]. The second FDA approved ALS treatment edaravone (EDV) is well known for its anti-oxidant function. It protects nerve cells by clearing damaging reactive oxygen species (ROS) in the body [25]. To improve the therapeutic efficiency of EDV in ALS, several nanostructures have been developed to better deliver EDV to the brain, such as agonistic micelles and solid lipid nanoparticles [129,130]. Additionally, the combined administration of EDV and agents that transiently open the BBB have demonstrated highly efficient delivery of EDV to the brain. For example, Qu et al., encapsulated EDV into the inner hydrophobic core of the organic micelle (EDV-AM) while the outer hydrophilic shell was conjugated with an adenosine 2A receptor (A2AR) agonist. Specific delivery of EDV for the treatment of brain ischemia was facilitated by temporarily triggering tight junction (TJ) opening of the BBB by EDV-AM-A2AR signaling (Fig. 5A) [129]. After EDV release from the EDV-AM complex, a significant reduction in ROS production by diseased brain cells was noted. Importantly, irreversible BBB damage was significantly minimized with the rapid restoration of the TJs [129]. Other anti-oxidative drugs, such as Bromocriptine and Manganese Porphyrin have also been engaged in different types of nanostructures to enhance their efficiency [131]. In addition to small molecule radical scavengers, various metal oxide nanoparticles were also shown to be effective in eliminating ROS, such as magnetite nanoparticles (Fe3O4 NPs), CuFe-PB-like NPs, cerium oxide nanoparticles (CeO2 NPs) [132,133]. Their function as free radical scavengers mainly arising from non-stoichiometric crystal defects and oxygen deficiencies. CeO2 NPs, for instance, mediated significant anti-oxidant effects that were shown to ameliorate muscle strength and prolonging life in an ALS mouse model. Treatment with CeO2 NPs prolonged median survival after symptom onset in ALS mice from 22.0 ± 2.5 days (control treated vehicle) to 33.0 ± 3.7 days [134]. Although promising, these CeO2 NPs did not feature any strategy to improve BBB permeability which may have limited their accumulation in the CNS and reduced therapeutic efficacy. Functionalization of these, and similar, metal oxide nanoparticles with BBB penetrating agents (e.g. peptide, antibody, transferrin) would potentially maximize their efficacy as neuroprotective agents. Indeed, an effective stroke therapeutic has been developed by Bao and coworkers by coupling monodispersed ceria nanoparticles (CeO2 NPs) with BBB penetrating agents [84]. Specifically, the surface of CeO2 NPs were functionalized with Angiopep-2 (ANG-2) and -polyethylene glycol (PEG) to facilitate BBB penetration and system circulation, with edaravone loaded by surface adsorption as shown in Fig. 5B [84]. Selective accumulation of CeO2 NPs in brain tissues was demonstrated by receptor-mediated transport, and the elimination of ROS was achieved by the synergistic effect of the loaded edaravone and ceria nanoparticles [84]. However, optimal structures, cargoes, delivery strategies and safety of these metal nanoparticles in ALS animal models still require further investigation.
Fig. 5.
(A) Edaravone-encapsulated-agonistic micelles enhanced BBB penetration by temporarily triggering TJ opening to rescue ischemic brain tissue. Reproduced with permission from Ref. [129]. (B) Edaravone-loaded ceria nanoparticles penetrate the BBB and mediate neuroprotection in a model of stroke. Reproduced with permission from Ref. [84]. (C) Anti-inflammatory drug-loaded silica-based drug delivery system specifically targets brain injury and SCI sites. Reproduced with permission from Ref. [136].
6.3. Inflammation
Current research and clinical discoveries have shown that inflammation in the CNS plays a vital impact in causing ALS [135]. Increasing evidence suggests that the abnormal immune/inflammatory activity of non-neuronal cells, such as microglia and astrocytes, plays a crucial role in the disease onset and progression rather than an autoimmune attack of neurons [136]. An acute neuroinflammation response may benefit the survival of motor neurons while chronically activated astrocytes and microglia may be harmful to motor neurons. In ALS patients, chronic inflammation and infiltrating immune cells are suggested to be a major pathological event in ALS progress. Additionally, a large number of anti- and proinflammatory growth factors and cytokines (e.g. IFN-γ, IL-6, vascular endothelial growth factor (VEGF), tumor necrosis factor alpha (TNF-α), IL-1β, and IL-10) were found involved in pathological changes that linked to ALS [136]. Hence, targeting inflammatory mediators with anti-inflammatory therapeutic agents may thus be neuroprotective in ALS. Indeed several clinical drugs with anti-inflammatory activity like thalidomide, celecoxib, and minocycline have demonstrated efficiency in halting or delaying disease progress in ALS animal models [26,137,138]. However, clinically used anti-inflammatory drugs are often systemically administered at high doses to exert an effect and have noted side-effects due to cell toxicity. More effective and biocompatible methods for drug delivery could potentially increase the usefulness of anti-inflammatory drugs in ALS. The developments in nanotechnology noted above suggest pathways to improve the ‘on-target’ accumulation of anti-inflammatory drugs, and reduce systemic toxicity. Indeed, a practical application of nanotechnology for anti-inflammatory drug delivery has been demonstrated by the development of cluster-like mesoporous silica NPs (MSNs) loaded with the anti-inflammatory drug arctigenin (traditional Chinese herbal medicine) and surface functionalized with a peptide sequence of four amino acids, cysteine, alanine, glutamine, and lysine, CAQK peptides (a short peptide identified by in vivo phage display screening) to target brain and spinal cord injury (SCI) sites in an animal model as shown in Fig. 5C [136]. The smaller size of MSN-based drug delivery system (<100 nm) enables the nanocomplexes to pass through the BBB and BSCB. Enhanced neuronal protection and accelerated SCI recovery were demonstrated in an animal model by inhibiting the activation of astrocytes and diminishing the expression of interleukin-17 (IL-17) and other inflammatory factors [136]. In another study, Giuseppe Tripodo et al. designed a mesenchymal stromal cells (MSCs) loading curcumin-INVITE 1(Inulin-d-alfa-tocopherol succinate)-micelles platform. In this system the curcumin (a well-known anti-inflammatory drug) loaded micelles (INVITE MC) are able to release the entrapped drug while reducing the toxicity of curcumin on MSC, which has shown great potential in treating ALS [139]. Given the flexibility in designing functional nanomaterials, more engineered nanoparticles loaded with anti-inflammatory factors may become available for the potential treatment of ALS.
6.4. Transition-metal dyshomeostasis
A common pathophysiology involved in many neurodegenerative diseases such as ALS is the excessive accumulation of the transition metals like copper (Cu2+) and iron (Fe3+) in the CNS [29]. Although iron does not directly induce disease, it is nonetheless increasingly viewed as playing a crucial role in promoting disease progression by catalyzing the formation of ROS and increasing oxidative stress. Thus, iron chelators (e.g. Deferiprone, Clioquinol, Apocynin) have shown therapeutic benefit in regulating transition-metal dyshomeostasis in many neurodegenerative diseases [140,141]. However, these chelators normally have severe adverse effects and lack of tissue-specific targeting. Thus, improving the utility of metal-ion chelators with nanotechnology may improve their efficacy by promoting efficient BBB traversal and increasing their selectivity for injury sites [141]. For example, Gang Liu et al. developed an iron chelator (e.g. (2-methyl-N-(2-aminoethyl)-3-hydroxyl-4-pyridinone (MAEHP)) conjugated NPs platform and functionalized the surface of NPs with PS80 to facilitate BBB penetration. These NPs provided safer and more effective chelation treatment in neurodegenerative diseases [142]. In addition to small molecular iron chelators, several unique inorganic nanostructures also show potential metal-chelating capability that may be beneficial for reducing transition-metal dyshomeostasis, thereby sidestepping the need for drug loading and release. For example, black phosphorus (BP) nanosheets were designed as new chelating agents to capture excess redox-active Cu [143]. It was shown that these BP nanosheets were not only effective at capturing excess Cu2+ to protect neuronal cells from transition-metal induced neurotoxicity, but they also pass through the BBB under near-infrared laser irradiation via its inherent photothermal effect (Fig. 6A) [143] which is a great advantage compared to conventional chelators. Additionally, the excellent biostability and biocompatibility suggest a suitable profile. However, further studies are needed to assess therapeutic efficiency in ALS animal models.
Fig. 6.
(A) The application of Black Phosphorus Nanosheets for neurodegenerative disease therapy. BP nanosheets cross BBB via near-infrared laser irradiation and protect neurons by selectively capturing excess Cu2+. Reproduced with permission from Refs. [143]. (B) Different CeO2 nanostructures eradicate intracellular, extracellular, and mitochondria ROS, respectively. Reproduced with permission from Refs. [146]. (C) Schematic illustration of T-L5-CoQ10-NP and T-L5-(Asp)4-NP targeting mitochondria in astrocytes to facilitate neuroprotection by protecting astrocytes from mitochondrial dysfunction and oxidative damage. T-L5: TPP–(CH2)5–COOH. TPP: Tri-phenyl-phosphonium. Reproduced with permission from Ref. [105].
6.5. Mitochondrial dysfunction
Mitochondria play an important role in cellular respiration, energy production, and calcium homeostasis. Mitochondrial dysfunction in neurons significantly affects normal cellular function. Neuronal cell death caused by mitochondrial dysfunction has been proposed as a major contributing factor in ALS progression due to the abnormal generation of ROS [144]. As discussed in 4.2, various anti-oxidants and metal oxide nanoparticles were shown to be effective in combating oxidative stress [133]. Hence, selectively delivering anti-oxidants or metal oxide NPs to mitochondria might be a beneficial strategy for the therapy of neurodegenerative disease, including ALS [145]. Indeed, studies have shown that it is possible to selectively scavenge extracellular, intracellular, and mitochondrial ROS by applying different types of ceria nanoparticles (Fig. 6B) [146], Hyek Jin Kwon et al. have used these NPs for treating Parkinson disease (PD) [146,147]. In another study, Bapurao et al. developed an alternative biodegradable nanoparticle platform with high BBB penetration efficiency (~12%) and marked ability to accumulate in brain astrocytes to facilitate neuroprotection by enhanced protection of astrocytes from mitochondrial dysfunction and resultant oxidative damage (Fig. 6C) [105]. These preliminary data suggest that it should be possible to design similar nanoparticles for ALS treatment. Additionally, loss of Cu/Zn superoxide dismutase 1 enzyme (SOD1) was believed to induce accumulation of mitochondrial ROS [148], exogenous supplementation of anti-oxidant enzymes have shown therapeutic potential in eliminating the ROS-associated mitochondrial dysfunction [149]. Thus it is possible to envisage a nanotechnology-based therapeutic approach that enhances SOD1 expression as a promising regulator for mitochondrial dysfunction. For example, SOD1-loaded poly (lactic-co-glycolic acid) (PLGA) NPs have been reported to confer human neuron protection by eliminating hydrogen peroxide-induced oxidative stress [149]. However, whether such nanoparticles offer a genuine therapeutic opportunity in ALS needs verification in follow-up studies.
6.6. Protein misfolding and aggregation
Increasing evidence has demonstrated the crucial role of protein misfolding and aggregation in ALS affected neurons and glial cells, and the lack of clearance mechanisms has been recognized as one of the major factors influencing the pathogenesis of ALS [16,150]. Protein misfolding results in the formation of toxic protein aggregates and protein inclusions which affect normal neuronal function. Several proteins aggregate-prone proteins such as TDP-43, SOD1, and Ubiquilin-2, are widely found aggregated within the CNS of ALS patients [151]. In particular, protein aggregates of the RNA-binding protein TDP-43 in the brain and spinal cord neurons are found in nearly all ALS patients and rare mutations in the gene encoding TDP-43 can cause ALS [152]. ASOs therapy targeting mutant SOD-1 has been shown to substantially slow disease progression in a rat model of ALS. However, SOD1 mutations only account for 2–5% of ALS cases [152], thus therapeutic targeting of TDP-43 should, in theory, benefit the majority of ALS patients. However, downregulating the expression of TDP-43 is not feasible, given its critical cellular function [153]. Thus, more advanced strategies have emerged to combat TDP-43 aggregation. One particularly exciting strategy involves targeting ataxin-2, a gene modifier of TDP-43 aggregation. For example, Lindsay et al. developed ataxin-2 targeting ASOs and administered to the CNS in TDP-43 transgenic mice. After a single treatment pathology was largely reduced and the lifespan of mutant animals was markedly extended, which suggests that targeting ataxin-2 could represent an effective therapeutic strategy for ALS patients [154]. More recently, post-translational stabilization of stathmin 2 (STMN2) by SP600125 (an anthrapyrazolone inhibitor of c-Jun N-terminal kinase (JNK) pathway) was found to rescue neurite outgrowth and deficits in axon regeneration induced by TDP-43 depletion and significantly improved survival of mutant ALS mice [75]. As nanotechnology has been widely used for gene and drug delivery and great progress has been achieved in CNS disease, using nanovehicles to deliver these, and potentially other ASOs or inhibitor drugs to target TDP-43 aggregation may be a promising strategy for improved ALS therapeutics.
6.7. Gene defects
In ALS, around 5%–10% of cases show familial inheritance and to date, more than 25 genes have been identified that contribute to ALS, which are estimated to be involved in 70% of the familial amyotrophic lateral sclerosis (fALS) and 15% of the sporadic ALS (sALS). Among them, mutations in SOD1, TARDBP, FUS or hexanucleotide expansions in chromosome 9 open reading frame 72 (C9orf72) genes account for the majority of fALS cases (>60%) and some sALS cases. Therapeutic approaches targeting these mutations include RNA interference (RNAi) technology, antisense oligonucleotides or other small nucleic acids. Several of these have shown the ability to delay disease progression in animals [155]. However, most of these studies utilized viral vectors such as adenovirus (AAV), herpesvirus (HSV) or and lentivirus to facilitate delivery [[156], [157], [158]]. The immunogenicity and safety of these carriers is a major obstacle that hinders their further clinical application [159]. The rapid development of nanotechnology has made it possible to deliver novel DNA, antisense oligonucleotides (ASOs) and RNA for gene therapy, largely superseding viral vectors [160] with the distinct advantages of decreased immune response and design flexibility to overcome biological barriers [161]. For example, to deliver SOD1 ASOs to motor neurons, Chen et al. prepared calcium phosphate lipid-coated nanoparticles (CaP-lipid NPs) that could effectively and safely deliver SOD1 ASOs to motor neurons [162], the efficacy of these is currently being assessed in a mutant SOD1 mouse model. In another study, therapeutic siRNA targeting the β-site amyloid precursor protein-cleaving enzyme 1 (BACE1), that plays a role in the development of amyloid plaques in Alzheimer's disease (AD) was prepared through a unique self-assembly process (Fig. 7A) [163]. Using a BBB targeting peptide (CGN) and amyloid targeting ligand (QSH), the siRNA loaded nanocomplexes could actively cross BBB and accumulate in neurons near amyloid plaques. A single treatment was found to effectively reduce the expression of BACE1at the mRNA and protein levels, thereby reducing the production of Aβ production and limiting neuronal damage in an AD mouse model [163]. This practical example showcases how nanotechnology could be applied to the delivery of ALS-related therapeutic genes past the BBB to target specific injury sites. Despite these encouraging results, the reliability of nanomaterials to transport genes and ASOs and the credibility of targets still needs further studies.
Fig. 7.
(A) Delivery of small interfering RNA (siRNA) to neurons via a polymer nanoparticle platform to improve the therapeutic efficacy of amyloid-targeting ligand (QSH) in Alzheimer's disease where CGN functions as a BBB targeting peptide. Reproduced with permission from Ref. [163]. (B) Schematic illustration showing the preparation of a nanoparticle platform to deliver neurotrophic factors to the brain. The surface of NPs can be engineered with brain-specific antibodies, proteins and efflux inhibitors that facilitate NPs penetration. Reproduced with permission from Refs. [167].
6.8. Neurotrophic factors
Another potential treatment modality in ALS is increasing the expression of neurotrophic proteins or drug compounds that promote neuronal survival and regeneration. In the last few years, a large number of neurotrophic factors including brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), glial cell line-derived neurotrophic factor (GDNF), insulin-like growth factor 1 (IGF-1), fibroblast growth factor (FGF), vascular epidermal growth factor (VEGF), and Granulocyte-colony stimulating factor (G-CSF) [164] have been investigated in ALS models. These neurotrophic therapeutics have recruited large patient populations in Phase 3 trials, however, they have largely failed due to short half-life and low BBB permeability [82,83]. Encouragingly, the application of nanotechnology greatly enhanced the biological stability, brain targeting, pharmacokinetic efficiency of neurotrophic factors [165,166]. For example, nanocarriers were functionalized with an efflux inhibitor and brain-specific antibody to enable the delivery of a variety neuroprotectors across BBB by receptor-mediated transcytosis [167]. In another study by Igor and coworkers [168], poly (lactic-co-glycolic acid) (PLGA) nanoparticles were coated with poloxamer 188 (PX) to enable NPs to traverse the BBB with BDNF encapsulated in the NPs (NP-BDNF-PX). After intravenous (IV) injection in mice with traumatic brain injury (TBI), a significantly higher level of BDNF was detected in the brain compared to control and the neuroprotective effect was also markedly improved. A schematic of a practical approach to deliver neurotrophic factors to the brain is shown in Fig. 7B [167]. These studies illustrate how nanotechnology could be applied to improve the performance and efficacy of neurotrophic factors for ALS treatment.
6.9. Defects in axonal transport
Axonal transport is an essential cellular process responsible for the shuttle of lipids, mitochondria, synaptic vesicles, proteins and other organelles into and out of the cell body of a neuron, and plays an important role in maintaining proper neuron function [169]. Increasing evidence from ALS patients and animal models have suggested that defects in axonal transport are largely involved in ALS process, even at a very early stage [170,171]. Genetic interference or pharmacological inhibition of histone deacetylase 6 (HDAC6) expression have been shown to effectively restore the axonal transport defects by increasing α-tubulin acetylation and endoplasmic reticulum (ER)–mitochondrial overlay [172]. However, most of HDAC6 inhibitors, such as Tubastatin A, ACY-738, are water-insoluble and inevitably hinder their clinical application. Given that HDAC6 inhibitor-encapsulated nanoparticles have been intensively investigated in cancer therapy [173,174], designing high drug loading, desirable BBB penetration, targeted neuronal delivery nanoparticles will be of great advantage in promoting those inhibitors therapeutic effect toward ALS involved axonal transport defects.
6.10. Stem cell therapy
Stem cell therapy is considered a promising alternative in ALS therapy that can potentially address many complex disease pathogenesis, and several early stage clinical trials have been initiated to verify the effectiveness of stem cells in ALS patients [175]. The transplanted stem cells could not directly replace the diseased motor neurons, they mainly differentiated into various supporting cells (e.g. astrocytes and microglia) under the stimulation of neurotrophic factors, and further formed a neuroprotective microenvironment, thus slowing down the degeneration or death of motor neurons [175]. Nanotechnology could not directly affect the effectiveness of stem cell therapy in ALS, but in terms of real time imaging or bioluminescence imaging in stem cell studies, nanotechnology can provide less invasive and higher resolution than traditional imaging methods (e.g. computed tomography and magnetic resonance imaging (MRI)) [176]. For example, with a dramatic enhancement of detection sensitivity, as few as ~10 UCNP-labeled mesenchymal stem cells (MSCs) can be detected in a mice model, which cannot be achieved with conventional exogenous agents [177]. Additionally, nanotechnology also works in improving the efficiency of stem cell transport to the brain or spinal cord, regulating cellular microenvironment, increasing the survival of the transplanted stem cells, and facilitating the stem cell differentiation efficiency, thereby promoting the effectiveness of stem cell therapy [178]. For example, in study conducted by Yung-Chih Kuo et al. heparin and nerve growth factor (NGF) loaded lipid nanoparticles can stimulate the differentiation of induced pluripotent stem cells (iPSCs) into neurons, thereby showing great potential in restoring neuron function that may beneficial to ALS patients [179].
7. Other emerging nanotechnologies with potential utility for ALS drug delivery
With the rapid development of nanotechnology, a variety of new approaches have been applied to the problem of penetrating the BBB to increase the therapeutic index of a variety of therapeutics.
7.1. Glycosylated nanocarrier
Glucose transporter-1 (GLUT1) is expressed in BCECs at a much higher level compared to many other transporters and receptors. Recently, researchers exploited this fact to enhance nanoparticle delivery across the BBB in an animal model [180]. After fasting, GLUT1 migrates from the luminal to abluminal plasma membrane. Nanocarriers functionalized to recognize and bind to GLUT1 are then delivered across the BBB following administration of glucose [180] (Fig. 8 A, B). Notably, an increase in nanocarrier accumulation in neurons was observed in mouse brain after glucose treatment in overnight fasted mice [180], demonstrating that glycosylated nanocarriers have the potential for brain drug delivery to treat neurons affected by neurodegenerative disease such as ALS.
Fig. 8.
glycemic control of GLUT1 expression increases the ability of glycosylated nanocarriers to cross the BBB into the brain. (A) Visualize the process of glycemic controlled glycosylated nanocarriers cross the brain blood vessel; After fasting for 24 h, mice were intravenously injected with 25%Gluc(6)/m(red) and intraperitoneal injected with 20% glucose 30 min later. (B) Biodistribution of Null/m and Gluc(6)/m in mice after different feeding control. Data were collected at 48 h post the injection. Reproduced with permission from Refs. [180].
7.2. Virus mimic nanomaterials
The human brain can be infected by various viruses like HIV, rabies virus and adenovirus. Nanocarriers can be designed that mimic the ability of viruses to penetrate the BBB. For example, Lee et al. have fabricated rabies virus- inspired silica- coated gold nanorods (AuNRs@SiO2) that replicate the size, shape and surface glycoprotein characteristics of the rabies virus [181]. The surface of the AuNRs@SiO2 NPs were covered with RVG29, a peptide derived from the rabies virus glycoprotein, to enable the NPs to mimic the in vivo behavior of the rabies virus in respect of penetrating the BBB and travelling into the brain through the neuronal pathway (Fig. 9A) [181]. The peptide coating also had the advantage of dramatically improving the nanosystem's biostability and biocompatibility that should ensure biosafety for potential clinical application. Therefore, nanomaterials which mimic viruses provide a new template for the delivery of therapeutic agents to the CNS.
Fig. 9.
(A) Rabies virus mimicking silica-coated gold nanorods bypass the BBB via neuronal pathways to treat brain disease. Reproduced with permission from Ref. [181]. (B) Delivery of therapeutic siRNA to the mouse brain by systemic injection of exosomes. (a) Schematic illustration of the preparation of exosomes; (b) Gene silencing efficiency by different vehicles. Reproduced with permission from Refs. [184].
7.3. Exosomes
Exosomes are intracellular membrane-based natural nanomaterials that have shown great advantages over other nanomaterials due to their non-immunogenic features and their ability to deliver a variety of cargoes [182,183]. For instance, Lydia et al. have developed exosomes can deliver siRNA into the brains of mice [184]. To reduce immunogenicity, exosomes were extracted from dendritic cells harvested from bone marrow after stimulation with interleukins. To promote brain targeting, the dendritic cells were engineered to express Lamp2b, an exosomal membrane protein, fused to the neuron-specific RVG peptide, as shown in Fig. 9B [184]. The fabricated exosomes could deliver siRNA specifically to microglia, neurons, or oligodendrocytes in the mouse brain to result in cell-specific gene knockdown [184]. In another study, exosomes were produced from human mesenchymal stem cells (MSCs) that were activated with interferon gamma (IFN-γ) (IFN-γ was used to raise the production of several important immunosuppressive cytokines in MSCs) [185]. After loading anti-inflammatory, neuroprotective RNA and protein molecules, these exosomes were intravenously administrated in an autoimmune encephalomyelitis (EAE) mouse model, showing good BBB penetration and encouragingly restoring motor skills [186]. As exosome delivery systems show good biological tolerance this should enable them to rapidly advance to clinical trials. Novel exosome nanotechnology, therefore, has the potential to deliver a wide range of therapeutics through the BBB to treat a variety of neurological diseases, including ALS [182].
8. Conclusion and perspectives
Scientists are striving to continually unravel the molecular and cellular, events that lead to ALS. To date, great progress has been made in identifying pathological mechanisms, revealing the way forward for the development of potential therapies for ALS. Indeed, a wide variety of potential therapeutic agents have been assessed in animal models of ALS. However, due to the lack of safe and effective delivery routes, efficacy is suboptimal and these promising agents are still a long way from clinical use [187]. In the last few years, significant progress has been achieved in the nanotechnology field, opening the gate for the development of nanobased therapeutic strategies in ALS. Drug properties like bioavailability, biostability, BBB penetration and ability to target neurons or astrocytes can be greatly enhanced by utilizing nanotechnology. Many therapeutic macromolecules, such as siRNA, ASOs, pDNA, their efficiency in treating ALS can also be significantly improved by using nanocarriers. Multifunctional methods of delivery combined with imaging capability will significantly benefit the field. Moreover, nanotechnology also makes it possible to transport multiple therapeutic substances simultaneously (e.g. small molecules, genes, and therapeutic proteins) to potentially facilitate more effective synergistic therapeutic outcomes [186,188].
In the last decades, nanosized biomaterials such as liposomes and polymers have been approved for clinical use [189]. However, newer classes of nanomaterials such as gold NPs, Qdots, and metallic nanoparticles are probably years away from reaching clinical trials. One of the key concerns to be addressed is that of nanomaterial biosafety, as the majority of work performed within the past decade has been focused on proof-of-principle demonstrations of nanomaterials for biomedical applications [190]. Additionally, the large-scale production of nanodrugs is another major obstacle for their clinical application [190,191]. Notwithstanding these current limitations, given the rapid growth of nanotechnologies, revolutionary new transportation and targeting strategies should emerge and increasing the likelihood that nanotechnologies will contribute to ALS treatment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work has received great support from Macquarie University Centre for Motor Neuron Disease Research (MND), ARC dementia Career Development Research Fellowship (APP 1111611), National Natural Science Foundation of China (NSFC 31640027, U1604177), National Health and Medical Research Council (NHMRC) Project grant (GNT1166024), the National Key Technologies R&D program of China (2018YFA0209800), and the International Macquarie University Research Excellence Scholarship (iMQRES).
Contributor Information
B.Y. Shi, Email: bingyang.shi@mq.edu.au.
X.J. Liang, Email: liangxj@nanoctr.cn.
References
- 1.Orsini M., Oliveira A.B., Nascimento O.J., Reis C.H.M., Leite M.A.A., de Souza J.A., Pupe C., de Souza O.G., Bastos V.H., de Freitas M.R. Amyotrophic lateral sclerosis: new perpectives and update. Neurol. Int. 2015;7(2) doi: 10.4081/ni.2015.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W., Shaw P.J., Simmons Z., Van Den Berg L.H. Amyotrophic lateral sclerosis. Nature Rev. Disease Prim. 2017;3:17071. doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 3.Oskarsson B., Gendron T.F., Staff N.P. Mayo Clinic Proceedings. Elsevier; 2018. Amyotrophic lateral sclerosis: an update for 2018; pp. 1617–1628. [DOI] [PubMed] [Google Scholar]
- 4.Ragagnin A.M., Shadfar S., Vidal M., Jamali S., Atkin J.D. Motor neuron susceptibility in ALS/FTD. Front. Neurosci. 2019;13:532. doi: 10.3389/fnins.2019.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chia R., Chiò A., Traynor B.J. Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol. 2018;17(1):94–102. doi: 10.1016/S1474-4422(17)30401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonafede R., Mariotti R. ALS pathogenesis and therapeutic approaches: the role of mesenchymal stem cells and extracellular vesicles. Front. Cell. Neurosci. 2017;11:80. doi: 10.3389/fncel.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenfeld J., Strong M.J. Challenges in the understanding and treatment of amyotrophic lateral sclerosis/motor neuron disease. Neurotherapeutics. 2015;12(2):317–325. doi: 10.1007/s13311-014-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrov D., Mansfield C., Moussy A., Hermine O. ALS clinical trials review: 20 years of failure. Are we any closer to registering a new treatment? Front. Aging Neurosci. 2017;9:68. doi: 10.3389/fnagi.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazibuko Z., Choonara Y.E., Kumar P., Du Toit L.C., Modi G., Naidoo D., Pillay V. A review of the potential role of nano-enabled drug delivery technologies in amyotrophic lateral sclerosis: lessons learned from other neurodegenerative disorders. J. Pharmacol. Sci. 2015;104(4):1213–1229. doi: 10.1002/jps.24322. [DOI] [PubMed] [Google Scholar]
- 10.Hajipour M.J., Santoso M.R., Rezaee F., Aghaverdi H., Mahmoudi M., Perry G. Advances in alzheimer's diagnosis and therapy: the implications of nanotechnology. Trends Biotechnol. 2017;35(10):937–953. doi: 10.1016/j.tibtech.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Barcia E., Boeva L., García-García L., Slowing K., Fernández-Carballido A., Casanova Y., Negro S. Nanotechnology-based drug delivery of ropinirole for Parkinson's disease. Drug Deliv. 2017;24(1):1112–1123. doi: 10.1080/10717544.2017.1359862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsou Y.H., Zhang X.Q., Zhu H., Syed S., Xu X. Drug delivery to the brain across the blood–brain barrier using nanomaterials. Small. 2017;13(43) doi: 10.1002/smll.201701921. [DOI] [PubMed] [Google Scholar]
- 13.Hu J., Yu Q., Xie L., Zhu H. Targeting the blood-spinal cord barrier: a therapeutic approach to spinal cord protection against ischemia-reperfusion injury. Life Sci. 2016;158:1–6. doi: 10.1016/j.lfs.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Abbott N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013;36(3):437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 15.Sharma A., Lyashchenko A.K., Lu L., Nasrabady S.E., Elmaleh M., Mendelsohn M., Nemes A., Tapia J.C., Mentis G.Z., Shneider N.A. ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function. Nat. Commun. 2016;7:10465. doi: 10.1038/ncomms10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soto C., Pritzkow S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 2018:1. doi: 10.1038/s41593-018-0235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou C.-C., Zhang Y., Umoh M.E., Vaughan S.W., Lorenzini I., Liu F., Sayegh M., Donlin-Asp P.G., Chen Y.H., Duong D.M. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat. Neurosci. 2018;21(2):228. doi: 10.1038/s41593-017-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howland D.S., Liu J., She Y., Goad B., Maragakis N.J., Kim B., Erickson J., Kulik J., DeVito L., Psaltis G. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc. Natl. Acad. Sci. U. S. A. 2002;99(3):1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrì M.T., Valle C., Bozzo F., Cozzolino M. Oxidative stress and mitochondrial damage: importance in non-SOD1 ALS. Front. Cell. Neurosci. 2015;9:41. doi: 10.3389/fncel.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lall D., Baloh R.H. Microglia and C9orf72 in neuroinflammation and ALS and frontotemporal dementia. J. Clin. Invest. 2017;127(9):3250–3258. doi: 10.1172/JCI90607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morello G., Spampinato A.G., Cavallaro S. Neuroinflammation and ALS: transcriptomic insights into molecular disease mechanisms and therapeutic targets. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/7070469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burk K., Pasterkamp R.J. Disrupted neuronal trafficking in amyotrophic lateral sclerosis. Acta Neuropathol. 2019:1–19. doi: 10.1007/s00401-019-01964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon M.A., Cleveland D.W. Gene therapy: gene-editing therapy for neurological disease. Nat. Rev. Neurol. 2017;13(1):7. doi: 10.1038/nrneurol.2016.190. [DOI] [PubMed] [Google Scholar]
- 24.Dharmadasa T., Kiernan M.C. Riluzole, disease stage and survival in ALS. Lancet Neurol. 2018;17(5):385–386. doi: 10.1016/S1474-4422(18)30091-7. [DOI] [PubMed] [Google Scholar]
- 25.Rothstein J.D. Edaravone: a new drug approved for ALS. Cell. 2017;171(4):725. doi: 10.1016/j.cell.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Lu H., Le W.D., Xie Y.-Y., Wang X.-P. Current therapy of drugs in amyotrophic lateral sclerosis. Curr. Neuropharmacol. 2016;14(4):314–321. doi: 10.2174/1570159X14666160120152423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cinzia V., Savina A., Mario S. Histamine beyond its effects on allergy: potential therapeutic benefits for the treatment of Amyotrophic Lateral Sclerosis (ALS) Pharmacol. Ther. October 2019;202:120–131. doi: 10.1016/j.pharmthera.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Furtado D., Björnmalm M., Ayton S., Bush A.I., Kempe K., Caruso F. Overcoming the blood–brain barrier: the role of nanomaterials in treating neurological diseases. Adv. Mater. 2018;30(46) doi: 10.1002/adma.201801362. [DOI] [PubMed] [Google Scholar]
- 29.Lovejoy D.B., Guillemin G.J. The potential for transition metal-mediated neurodegeneration in amyotrophic lateral sclerosis. Front. Aging Neurosci. 2014;6:173. doi: 10.3389/fnagi.2014.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urbi B., Broadley S., Bedlack R., Russo E., Sabet A. Study protocol for a randomised, double-blind, placebo-controlled study evaluating the Efficacy of cannabis-based Medicine Extract in slowing the disease pRogression of Amyotrophic Lateral sclerosis or motor neurone Disease: the EMERALD trial. BMJ open. 2019;9(11) doi: 10.1136/bmjopen-2019-029449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funke A., Spittel S., Grehl T., Grosskreutz J., Kettemann D., Petri S., Weyen U., Weydt P., Dorst J., Ludolph A.C. Provision of assistive technology devices among people with ALS in Germany: a platform-case management approach. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19(5–6):342–350. doi: 10.1080/21678421.2018.1431786. [DOI] [PubMed] [Google Scholar]
- 32.Al-Chalabi A., Heunks L.M., Papp Z., Pollesello P. Potential of the cardiovascular drug levosimendan in the management of amyotrophic lateral sclerosis: an overview of a working hypothesis. J. Cardiovasc. Pharmacol. 2019;74(5):389–399. doi: 10.1097/FJC.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 33.Gold J., Rowe D.B., Kiernan M.C., Vucic S., Mathers S., van Eijk R.P., Nath A., Garcia Montojo M., Norato G., Santamaria U.A. Safety and tolerability of Triumeq in amyotrophic lateral sclerosis: the Lighthouse trial. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(7–8):595–604. doi: 10.1080/21678421.2019.1632899. [DOI] [PubMed] [Google Scholar]
- 34.Vucic S., Ryder J., Mekhael L., Henderson R.D., Mathers S., Needham M., Schultz D.W., Kiernan M.C. Phase 2 randomized placebo controlled double blind study to assess the efficacy and safety of tecfidera in patients with amyotrophic lateral sclerosis (TEALS Study): study protocol clinical trial (SPIRIT Compliant) Medicine (Baltimore) 2020;99(6) doi: 10.1097/MD.0000000000018904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiò A., Mazzini L., Mora G. Disease-Modifying therapies in amyotrophic lateral sclerosis. Neuropharmacology. 2020:107986. doi: 10.1016/j.neuropharm.2020.107986. [DOI] [PubMed] [Google Scholar]
- 36.Chidambaram S.B., Ray B., Bhat A., Mahalakshmi A.M., Sunanda T., Jagadeeswari P., Gowrav M.P., Chandra R., Sakharkar M.K. Delivery of Drugs. Elsevier; 2020. Mitochondria-targeted drug delivery in neurodegenerative diseases; pp. 97–117. [Google Scholar]
- 37.Kumar V., Lee J.D., Clark R.J., Noakes P.G., Taylor S.M., Woodruff T.M. Preclinical pharmacokinetics of complement C5a receptor antagonists PMX53 and PMX205 in mice. ACS Omega. 2020;5(5):2345–2354. doi: 10.1021/acsomega.9b03735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33(9):941. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng M., Tao W., Zou Y., Farokhzad O.C., Shi B. Nanotechnology-based strategies for siRNA brain delivery for disease therapy. Trends Biotechnol. 2018;36(5):562–575. doi: 10.1016/j.tibtech.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Garbuzova-Davis S., Sanberg P.R. Blood-CNS barrier impairment in ALS patients versus an animal model. Front. Cell. Neurosci. 2014;8:21. doi: 10.3389/fncel.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14(3):133. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y., Liu L. Modern methods for delivery of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 2012;64(7):640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Löscher W., Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005;6(8):591. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 44.Jablonski M.R., Jacob D.A., Campos C., Miller D.S., Maragakis N.J., Pasinelli P., Trotti D. Selective increase of two ABC drug efflux transporters at the blood–spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol. Dis. 2012;47(2):194–200. doi: 10.1016/j.nbd.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aryal M., Fischer K., Gentile C., Gitto S., Zhang Y.-Z., McDannold N. Effects on P-glycoprotein expression after blood-brain barrier disruption using focused ultrasound and microbubbles. PloS One. 2017;12(1) doi: 10.1371/journal.pone.0166061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abrahao A., Meng Y., Llinas M., Huang Y., Hamani C., Mainprize T., Aubert I., Heyn C., Black S.E., Hynynen K. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 2019;10(1):1–9. doi: 10.1038/s41467-019-12426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grabrucker A.M., Ruozi B., Belletti D., Pederzoli F., Forni F., Vandelli M.A., Tosi G. Nanoparticle transport across the blood brain barrier. Tissue Barriers. 2016;4(1) doi: 10.1080/21688370.2016.1153568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savjani K.T., Gajjar A.K., Savjani J.K. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012 doi: 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bordet T., Berna P., Abitbol J.-L., Pruss R.M. Olesoxime (TRO19622): a novel mitochondrial-targeted neuroprotective compound. Pharmaceuticals. 2010;3(2):345–368. doi: 10.3390/ph3020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tesla R., Wolf H.P., Xu P., Drawbridge J., Estill S.J., Huntington P., McDaniel L., Knobbe W., Burket A., Tran S. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2012;109(42):17016–17021. doi: 10.1073/pnas.1213960109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Limpert A.S., Mattmann M.E., Cosford N.D. Recent progress in the discovery of small molecules for the treatment of amyotrophic lateral sclerosis (ALS) Beilstein J. Org. Chem. 2013;9(1):717–732. doi: 10.3762/bjoc.9.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banks W.A. BMC Neurol. BioMed Central; 2009. Characteristics of compounds that cross the blood-brain barrier; p. S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foust K.D., Salazar D.L., Likhite S., Ferraiuolo L., Ditsworth D., Ilieva H., Meyer K., Schmelzer L., Braun L., Cleveland D.W. Therapeutic AAV9-mediated suppression of mutant SOD1 slows disease progression and extends survival in models of inherited ALS. Mol. Ther. 2013;21(12):2148–2159. doi: 10.1038/mt.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadam R.S., Bourne D.W., Kompella U.B. Nano-advantage in enhanced drug delivery with biodegradable nanoparticles: contribution of reduced clearance. Drug Metab. Dispos. 2012;40(7):1380–1388. doi: 10.1124/dmd.112.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nau R., Sörgel F., Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 2010;23(4):858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ud Din F., Aman W., Ullah I., Qureshi O.S., Mustapha O., Shafique S., Zeb A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017;12:7291. doi: 10.2147/IJN.S146315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma M., Sharma R., Jain D.K. Nanotechnology based approaches for enhancing oral bioavailability of poorly water soluble antihypertensive drugs. Sci. Tech. Rep. 2016;2016 doi: 10.1155/2016/8525679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dash R.P., Babu R.J., Srinivas N.R. Two decades-long journey from Riluzole to Edaravone: revisiting the clinical pharmacokinetics of the only two amyotrophic lateral sclerosis therapeutics. Clin. Pharmacokinet. 2018;57(11):1385–1398. doi: 10.1007/s40262-018-0655-4. [DOI] [PubMed] [Google Scholar]
- 59.Li Z., Tan S., Li S., Shen Q., Wang K. Cancer drug delivery in the nano era: an overview and perspectives. Oncol. Rep. 2017;38(2):611–624. doi: 10.3892/or.2017.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saraiva C., Praça C., Ferreira R., Santos T., Ferreira L., Bernardino L. Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J. Contr. Release. 2016;235:34–47. doi: 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 61.Gastaldi L., Battaglia L., Peira E., Chirio D., Muntoni E., Solazzi I., Gallarate M., Dosio F. Solid lipid nanoparticles as vehicles of drugs to the brain: current state of the art. Eur. J. Pharm. Biopharm. 2014;87(3):433–444. doi: 10.1016/j.ejpb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Tosi G., Bortot B., Ruozi B., Dolcetta D., Vandelli M.A., Forni F., Severini G. Potential use of polymeric nanoparticles for drug delivery across the blood-brain barrier. Curr. Med. Chem. 2013;20(17):2212–2225. doi: 10.2174/0929867311320170006. [DOI] [PubMed] [Google Scholar]
- 63.Shilo M., Sharon A., Baranes K., Motiei M., Lellouche J.-P.M., Popovtzer R. The effect of nanoparticle size on the probability to cross the blood-brain barrier: an in-vitro endothelial cell model. J. Nanobiotechnol. 2015;13(1):19. doi: 10.1186/s12951-015-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mishra V., Kesharwani P. Dendrimer technologies for brain tumor. Drug Discov. Today. 2016;21(5):766–778. doi: 10.1016/j.drudis.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Monaco A.M., Giugliano M. Carbon-based smart nanomaterials in biomedicine and neuroengineering. Beilstein J. Nanotechnol. 2014;5(1):1849–1863. doi: 10.3762/bjnano.5.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou Y., Liu Y., Yang Z., Zhang D., Lu Y., Zheng M., Xue X., Geng J., Chung R., Shi B. Effective and targeted human orthotopic glioblastoma xenograft therapy via a multifunctional biomimetic nanomedicine. Adv. Mater. 2018;30(51):1803717. doi: 10.1002/adma.201803717. [DOI] [PubMed] [Google Scholar]
- 67.Kou L., Sun R., Bhutia Y.D., Yao Q., Chen R. Emerging advances in P-glycoprotein inhibitory nanomaterials for drug delivery. Expet Opin. Drug Deliv. 2018;15(9):869–879. doi: 10.1080/17425247.2018.1517749. [DOI] [PubMed] [Google Scholar]
- 68.Ochs G., Penn R.D., York M., Giess R., Beck M., Tonn J., Haigh J., Malta E., Traub M., Sendtner M. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2000;1(3):201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- 69.Miller T., Pestronk A., David W., Rothstein J., Simpson E., Appel S.H., Andres P.L., Mahoney K., Allred P., Alexander K. A phase I, randomised, first-in-human study of an antisense oligonucleotide directed against SOD1 delivered intrathecally in SOD1-familial ALS patients. Lancet Neurol. 2013;12(5):435. doi: 10.1016/S1474-4422(13)70061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lunn J.S., Sakowski S.A., Feldman E.L. Concise review: stem cell therapies for amyotrophic lateral sclerosis: recent advances and prospects for the future. Stem Cell. 2014;32(5):1099–1109. doi: 10.1002/stem.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang M., Gu X., Gao X. Brain Targeted Drug Delivery System. Elsevier; 2019. Nanotherapeutic strategies for the treatment of neurodegenerative diseases; pp. 321–356. [Google Scholar]
- 72.Householder K.T., Dharmaraj S., Sandberg D., Wechsler-Reya R., Sirianni R. Fate of nanoparticles in the central nervous system after intrathecal injection in healthy mice. Sci. Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-49028-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung E.P., Cotter J.D., Prakapenka A.V., Cook R.L., DiPerna D.M., Sirianni R.W. Targeting small molecule delivery to the brain and spinal cord via intranasal administration of rabies virus glycoprotein (RVG29)-Modified PLGA nanoparticles. Pharmaceutics. 2020;12(2):93. doi: 10.3390/pharmaceutics12020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spinelli A., Girelli M., Arosio D., Polito L., Podini P., Martino G., Seneci P., Muzio L., Menegon A. Intracisternal delivery of PEG-coated gold nanoparticles results in high brain penetrance and long-lasting stability. J. Nanobiotechnol. 2019;17(1):49. doi: 10.1186/s12951-019-0481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klim J.R., Williams L.A., Limone F., San Juan I.G., Davis-Dusenbery B.N., Mordes D.A., Burberry A., Steinbaugh M.J., Gamage K.K., Kirchner R. ALS-implicated protein TDP-43 sustains levels of STMN2, a mediator of motor neuron growth and repair. Nat. Neurosci. 2019;22(2):167. doi: 10.1038/s41593-018-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sahoo S.K., Misra R., Parveen S. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Cancer, Pan Stanford. 2017:73–124. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 77.Wang J., Byrne J.D., Napier M.E., DeSimone J.M. More effective nanomedicines through particle design. Small. 2011;7(14):1919–1931. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sawada H. Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis. Expet Opin. Pharmacother. 2017;18(7):735–738. doi: 10.1080/14656566.2017.1319937. [DOI] [PubMed] [Google Scholar]
- 79.Choi H.S., Liu W., Misra P., Tanaka E., Zimmer J.P., Ipe B.I., Bawendi M.G., Frangioni J.V. Renal clearance of nanoparticles. Nat. Biotechnol. 2007;25(10):1165. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agrawal U., Sharma R., Gupta M., Vyas S.P. Is nanotechnology a boon for oral drug delivery? Drug Discov. Today. 2014;19(10):1530–1546. doi: 10.1016/j.drudis.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 81.Bourquin J., Milosevic A., Hauser D., Lehner R., Blank F., Petri-Fink A., Rothen-Rutishauser B. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv. Mater. 2018;30(19):1704307. doi: 10.1002/adma.201704307. [DOI] [PubMed] [Google Scholar]
- 82.Zhou Y., Peng Z., Seven E.S., Leblanc R.M. Crossing the blood-brain barrier with nanoparticles. J. Contr. Release. 2018;270:290–303. doi: 10.1016/j.jconrel.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 83.Kamaly N., Yameen B., Wu J., Farokhzad O.C. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bao Q., Hu P., Xu Y., Cheng T., Wei C., Pan L., Shi J. Simultaneous blood–brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano. 2018;12(7):6794–6805. doi: 10.1021/acsnano.8b01994. [DOI] [PubMed] [Google Scholar]
- 85.Morachis J.M., Mahmoud E.A., Almutairi A. Physical and chemical strategies for therapeutic delivery by using polymeric nanoparticles. Pharmacol. Rev. 2012;64(3):505–519. doi: 10.1124/pr.111.005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leriche G., Chisholm L., Wagner A. Cleavable linkers in chemical biology. Bioorg. Med. Chem. 2012;20(2):571–582. doi: 10.1016/j.bmc.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 87.Chang M., Zhang F., Wei T., Zuo T., Guan Y., Lin G., Shao W. Smart linkers in polymer–drug conjugates for tumor-targeted delivery. J. Drug Target. 2016;24(6):475–491. doi: 10.3109/1061186X.2015.1108324. [DOI] [PubMed] [Google Scholar]
- 88.Zhang A., Jung K., Li A., Liu J., Boyer C. Recent advances in stimuli-responsive polymer systems for remotely controlled drug release. Prog. Polym. Sci. 2019:101164. [Google Scholar]
- 89.Nabi B., Rehman S., Fazil M., Khan S., Baboota S., Ali J. Riluzole loaded nanoparticles to alleviate the symptoms of neurological disorders by attenuating oxidative stress. Drug Dev. Ind. Pharm. 2020:1–42. doi: 10.1080/03639045.2020.1730396. (just-accepted) [DOI] [PubMed] [Google Scholar]
- 90.Ma Y., Liang X., Tong S., Bao G., Ren Q., Dai Z. Gold nanoshell nanomicelles for potential magnetic resonance imaging, light-triggered drug release, and photothermal therapy. Adv. Funct. Mater. 2013;23(7):815–822. [Google Scholar]
- 91.Zheng M., Liu Y., Wang Y., Zhang D., Zou Y., Ruan W., Yin J., Tao W., Park J.B., Shi B. ROS-Responsive polymeric siRNA nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy. Adv. Mater. 2019;31(37):1903277. doi: 10.1002/adma.201903277. [DOI] [PubMed] [Google Scholar]
- 92.Liu L., Li Y., Liu R., Shen Q., Li Y., Shi Z., Shen J., Ji W., Zhang X. Switchable nanoparticle for programmed gene-chem delivery with enhanced neuronal recovery and CT imaging for neurodegenerative disease treatment. Mater. Horiz. 2019;6(9):1923–1929. [Google Scholar]
- 93.Sirsi S.R., Borden M.A. State-of-the-art materials for ultrasound-triggered drug delivery. Adv. Drug Deliv. Rev. 2014;72:3–14. doi: 10.1016/j.addr.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barber S.C., Shaw P.J. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic. Biol. Med. 2010;48(5):629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 95.Silva Adaya D., Aguirre-Cruz L., Guevara J., Ortiz-Islas E. Nanobiomaterials' applications in neurodegenerative diseases. J. Biomater. Appl. 2017;31(7):953–984. doi: 10.1177/0885328216659032. [DOI] [PubMed] [Google Scholar]
- 96.Mathew A., Fukuda T., Nagaoka Y., Hasumura T., Morimoto H., Yoshida Y., Maekawa T., Venugopal K., Kumar D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer's disease. PloS One. 2012;7(3) doi: 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rogers M.-L., Smith K.S., Matusica D., Fenech M., Hoffman L., Rush R.A., Voelcker N.H. Non-viral gene therapy that targets motor neurons in vivo. Front. Mol. Neurosci. 2014;7:80. doi: 10.3389/fnmol.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwon E.J., Skalak M., Lo Bu R., Bhatia S.N. Neuron-targeted nanoparticle for siRNA delivery to traumatic brain injuries. ACS Nano. 2016;10(8):7926–7933. doi: 10.1021/acsnano.6b03858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Posse de Chaves E., Sipione S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010;584(9):1748–1759. doi: 10.1016/j.febslet.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 100.Sheikh K.A., Zhang G. Antibody-based neuronal and axonal delivery vectors for targeted ligand delivery. Neural Regen. Res. 2016;11(5):712. doi: 10.4103/1673-5374.182685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sellers D.L., Bergen J.M., Johnson R.N., Back H., Ravits J.M., Horner P.J., Pun S.H. Targeted axonal import (TAxI) peptide delivers functional proteins into spinal cord motor neurons after peripheral administration. Proc. Natl. Acad. Sci. U. S. A. 2016;113(9):2514–2519. doi: 10.1073/pnas.1515526113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamanaka K., Komine O. The multi-dimensional roles of astrocytes in ALS. Neurosci. Res. 2018;126:31–38. doi: 10.1016/j.neures.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 103.Gu J., Al-Bayati K., Ho E.A. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv. Transl. Res. 2017;7(4):497–506. doi: 10.1007/s13346-017-0368-5. [DOI] [PubMed] [Google Scholar]
- 104.Gromnicova R., Davies H.A., Sreekanthreddy P., Romero I.A., Lund T., Roitt I.M., Phillips J.B., Male D.K. Glucose-coated gold nanoparticles transfer across human brain endothelium and enter astrocytes in vitro. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0081043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Surnar B., Basu U., Banik B., Ahmad A., Marples B., Kolishetti N., Dhar S. Nanotechnology-mediated crossing of two impermeable membranes to modulate the stars of the neurovascular unit for neuroprotection. Proc. Natl. Acad. Sci. U. S. A. 2018;115(52):E12333–E12342. doi: 10.1073/pnas.1816429115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shimoni O., Shi B., Adlard P.A., Bush A.I. Delivery of fluorescent nanoparticles to the brain. J. Mol. Neurosci. 2016;60(3):405–409. doi: 10.1007/s12031-016-0833-5. [DOI] [PubMed] [Google Scholar]
- 107.Koffie R.M., Farrar C.T., Saidi L.-J., William C.M., Hyman B.T., Spires-Jones T.L. Nanoparticles enhance brain delivery of blood–brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc. Natl. Acad. Sci. U. S. A. 2011;108(46):18837–18842. doi: 10.1073/pnas.1111405108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi B., Du X., Chen J., Fu L., Morsch M., Lee A., Liu Y., Cole N., Chung R. Multifunctional hybrid nanoparticles for traceable drug delivery and intracellular microenvironment-controlled multistage drug-release in neurons. Small. 2017;13(20):1603966. doi: 10.1002/smll.201603966. [DOI] [PubMed] [Google Scholar]
- 109.Wu H.C., Wang T.W., Bohn M.C., Lin F.H., Spector M. Novel magnetic hydroxyapatite nanoparticles as non-viral vectors for the glial cell line-derived neurotrophic factor gene. Adv. Funct. Mater. 2010;20(1):67–77. [Google Scholar]
- 110.Minami S.S., Sun B., Popat K., Kauppinen T., Pleiss M., Zhou Y., Ward M.E., Floreancig P., Mucke L., Desai T. Selective targeting of microglia by quantum dots. J. Neuroinflammation. 2012;9(1):22. doi: 10.1186/1742-2094-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin J., Huang Y., Huang P. Biomed. Appl. Funct. Nanomater. Elsevier; 2018. Graphene-based nanomaterials in bioimaging; pp. 247–287. [Google Scholar]
- 112.Nicolas J., Mura S., Brambilla D., Mackiewicz N., Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013;42(3):1147–1235. doi: 10.1039/c2cs35265f. [DOI] [PubMed] [Google Scholar]
- 113.Fu L., Morsch M., Shi B., Wang G., Lee A., Radford R., Lu Y., Jin D., Chung R. A versatile upconversion surface evaluation platform for bio–nano surface selection for the nervous system. Nanoscale. 2017;9(36):13683–13692. doi: 10.1039/c7nr03557h. [DOI] [PubMed] [Google Scholar]
- 114.Liu L., Li Y., Liu R., Shen Q., Li Y., Shi Z., Shen J., Ji W., Zhang X. Switchable nanoparticle for programmed gene-chem delivery with enhanced neuronal recovery and CT imaging for neurodegenerative disease treatment. Mater. Horiz. 2019;6:1923–1929. [Google Scholar]
- 115.Blasco H., Mavel S., Corcia P., Gordon P. The glutamate hypothesis in ALS: pathophysiology and drug development. Curr. Med. Chem. 2014;21(31):3551–3575. doi: 10.2174/0929867321666140916120118. [DOI] [PubMed] [Google Scholar]
- 116.Zarei S., Carr K., Reiley L., Diaz K., Guerra O., Altamirano P.F., Pagani W., Lodin D., Orozco G., Chinea A. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 2015;6 doi: 10.4103/2152-7806.169561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rizzo F., Riboldi G., Salani S., Nizzardo M., Simone C., Corti S., Hedlund E. Cellular therapy to target neuroinflammation in amyotrophic lateral sclerosis. Cell. Mol. Life Sci. 2014;71(6):999–1015. doi: 10.1007/s00018-013-1480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Verma S.K., Arora I., Javed K., Akhtar M., Samim M. Enhancement in the neuroprotective power of riluzole against cerebral ischemia using a brain targeted drug delivery vehicle. ACS Appl. Mater. Interfaces. 2016;8(30):19716–19723. doi: 10.1021/acsami.6b01776. [DOI] [PubMed] [Google Scholar]
- 119.Bondì M.L., Craparo E.F., Giammona G., Drago F. Brain-targeted solid lipid nanoparticles containing riluzole: preparation, characterization and biodistribution. Nanomedicine. 2010;5(1):25–32. doi: 10.2217/nnm.09.67. [DOI] [PubMed] [Google Scholar]
- 120.Rao P., Yallapu M.M., Sari Y., Fisher P.B., Kumar S. Designing novel nanoformulations targeting glutamate transporter excitatory amino acid transporter 2: implications in treating drug addiction. J. Personalized Med. 2015;1(1):3. [PMC free article] [PubMed] [Google Scholar]
- 121.File T.M., Jr., Wilcox M.H., Stein G.E. Summary of ceftaroline fosamil clinical trial studies and clinical safety. Clin. Infect. Dis. 2012;55(suppl_3):S173–S180. doi: 10.1093/cid/cis559. [DOI] [PubMed] [Google Scholar]
- 122.Kumar S., Bhanjana G., Kumar A., Taneja K., Dilbaghi N., Kim K.-H. Synthesis and optimization of ceftriaxone-loaded solid lipid nanocarriers. Chem. Phys. Lipids. 2016;200:126–132. doi: 10.1016/j.chemphyslip.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 123.Benkler C., Barhum Y., Ben-Zur T., Offen D. Multifactorial gene therapy enhancing the glutamate uptake system and reducing oxidative stress delays symptom onset and prolongs survival in the SOD1-G93A ALS mouse model. J. Mol. Neurosci. 2016;58(1):46–58. doi: 10.1007/s12031-015-0695-2. [DOI] [PubMed] [Google Scholar]
- 124.Wong J.K., Mohseni R., Hamidieh A.A., MacLaren R.E., Habib N., Seifalian A.M. Will nanotechnology bring new hope for gene delivery? Trends Biotechnol. 2017;35(5):434–451. doi: 10.1016/j.tibtech.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 125.Li L., Song L., Liu X., Yang X., Li X., He T., Wang N., Yang S., Yu C., Yin T. Artificial virus delivers CRISPR-Cas9 system for genome editing of cells in mice. ACS Nano. 2017;11(1):95–111. doi: 10.1021/acsnano.6b04261. [DOI] [PubMed] [Google Scholar]
- 126.Valiante S., Falanga A., Cigliano L., Iachetta G., Busiello R.A., La Marca V., Galdiero M., Lombardi A., Galdiero S. Peptide gh625 enters into neuron and astrocyte cell lines and crosses the blood–brain barrier in rats. Int. J. Nanomed. 2015;10:1885. doi: 10.2147/IJN.S77734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Terashima T., Ogawa N., Nakae Y., Sato T., Katagi M., Okano J., Maegawa H., Kojima H. Gene therapy for neuropathic pain through siRNA-IRF5 gene delivery with homing peptides to microglia. Mol. Ther. Nucleic Acids. 2018;11:203–215. doi: 10.1016/j.omtn.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Valori C.F., Guidotti G., Brambilla L., Rossi D. Astrocytes: emerging therapeutic targets in neurological disorders. Trends Mol. Med. September 01, 2019;25(9):750–759. doi: 10.1016/j.molmed.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 129.Jin Q., Cai Y., Li S., Liu H., Zhou X., Lu C., Gao X., Qian J., Zhang J., Ju S. Edaravone-encapsulated agonistic micelles rescue ischemic brain tissue by tuning blood-brain barrier permeability. Theranostics. 2017;7(4):884. doi: 10.7150/thno.18219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang T., Ferrill L., Gallant L., McGillicuddy S., Fernandes T., Schields N., Bai S. Verapamil and riluzole cocktail liposomes overcome pharmacoresistance by inhibiting P-glycoprotein in brain endothelial and astrocyte cells: a potent approach to treat amyotrophic lateral sclerosis. Eur. J. Pharmaceut. Sci. 2018;120:30–39. doi: 10.1016/j.ejps.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 131.Moujalled D., White A.R. Advances in the development of disease-modifying treatments for amyotrophic lateral sclerosis. CNS Drugs. 2016;30(3):227–243. doi: 10.1007/s40263-016-0317-8. [DOI] [PubMed] [Google Scholar]
- 132.Wei H., Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 2013;42(14):6060–6093. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 133.Wu J., Wang X., Wang Q., Lou Z., Li S., Zhu Y., Qin L., Wei H. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II) Chem. Soc. Rev. 2019;48(4):1004–1076. doi: 10.1039/c8cs00457a. [DOI] [PubMed] [Google Scholar]
- 134.DeCoteau W., Heckman K.L., Estevez A.Y., Reed K.J., Costanzo W., Sandford D., Studlack P., Clauss J., Nichols E., Lipps J. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomed. Nanotechnol. Biol. Med. 2016;12(8):2311–2320. doi: 10.1016/j.nano.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 135.Zhao W., Beers D.R., Appel S.H. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J. Neuroimmune Pharmacol. 2013;8(4):888–899. doi: 10.1007/s11481-013-9489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun G., Zeng S., Liu X., Shi H., Zhang R., Wang B., Zhou C., Yu T. Synthesis and characterization of a silica-based drug delivery system for spinal cord injury therapy. Nano-Micro Lett. 2019;11(1):23. doi: 10.1007/s40820-019-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khalid S.I., Ampie L., Kelly R., Ladha S.S., Dardis C. Immune modulation in the treatment of amyotrophic lateral sclerosis: a review of clinical trials. Front. Neurol. 2017;8:486. doi: 10.3389/fneur.2017.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kiaei M., Petri S., Kipiani K., Gardian G., Choi D.-K., Chen J., Calingasan N.Y., Schafer P., Muller G.W., Stewart C. Thalidomide and lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2006;26(9):2467–2473. doi: 10.1523/JNEUROSCI.5253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tripodo G., Chlapanidas T., Perteghella S., Vigani B., Mandracchia D., Trapani A., Galuzzi M., Tosca M.C., Antonioli B., Gaetani P. Mesenchymal stromal cells loading curcumin-INVITE-micelles: a drug delivery system for neurodegenerative diseases. Colloids Surf., B. 2015;125:300–308. doi: 10.1016/j.colsurfb.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 140.Ward R.J., Dexter D.T., Crichton R.R. Neurodegenerative diseases and therapeutic strategies using iron chelators. J. Trace Elem. Med. Biol. 2015;31:267–273. doi: 10.1016/j.jtemb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 141.Bonda D.J., Liu G., Men P., Perry G., Smith M.A., Zhu X. Nanoparticle delivery of transition-metal chelators to the brain: oxidative stress will never see it coming! CNS Neurol. Disord. - Drug Targets. 2012;11(1):81–85. doi: 10.2174/187152712799960709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu G., Men P., Harris P.L., Rolston R.K., Perry G., Smith M.A. Nanoparticle iron chelators: a new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci. Lett. 2006;406(3):189–193. doi: 10.1016/j.neulet.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 143.Chen W., Ouyang J., Yi X., Xu Y., Niu C., Zhang W., Wang L., Sheng J., Deng L., Liu Y.N. Black phosphorus nanosheets as a neuroprotective nanomedicine for neurodegenerative disorder therapy. Adv. Mater. 2018;30(3):1703458. doi: 10.1002/adma.201703458. [DOI] [PubMed] [Google Scholar]
- 144.Di Carlo M., Giacomazza D., Picone P., Nuzzo D., San Biagio P.L. Are oxidative stress and mitochondrial dysfunction the key players in the neurodegenerative diseases? Free Radic. Res. 2012;46(11):1327–1338. doi: 10.3109/10715762.2012.714466. [DOI] [PubMed] [Google Scholar]
- 145.Wen R., Banik B., Pathak R.K., Kumar A., Kolishetti N., Dhar S. Nanotechnology inspired tools for mitochondrial dysfunction related diseases. Adv. Drug Deliv. Rev. 2016;99:52–69. doi: 10.1016/j.addr.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kwon H.J., Kim D., Seo K., Kim Y.G., Han S.I., Kang T., Soh M., Hyeon T. Ceria nanoparticle systems for selective scavenging of mitochondrial, intracellular, and extracellular reactive oxygen species in Parkinson's disease. Angew. Chem. Int. Ed. 2018;57(30):9408–9412. doi: 10.1002/anie.201805052. [DOI] [PubMed] [Google Scholar]
- 147.Kwon H.J., Cha M.-Y., Kim D., Kim D.K., Soh M., Shin K., Hyeon T., Mook-Jung I. Mitochondria-targeting ceria nanoparticles as antioxidants for Alzheimer's disease. ACS Nano. 2016;10(2):2860–2870. doi: 10.1021/acsnano.5b08045. [DOI] [PubMed] [Google Scholar]
- 148.Fischer L.R., Igoudjil A., Magrane J., Li Y., Hansen J.M., Manfredi G., Glass J.D. SOD1 targeted to the mitochondrial intermembrane space prevents motor neuropathy in the Sod1 knockout mouse. Brain. 2010;134(1):196–209. doi: 10.1093/brain/awq314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reddy M.K., Wu L., Kou W., Ghorpade A., Labhasetwar V. Superoxide dismutase-loaded PLGA nanoparticles protect cultured human neurons under oxidative stress. Appl. Biochem. Biotechnol. 2008;151(2–3):565. doi: 10.1007/s12010-008-8232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee S., Kim H.-J. Prion-like mechanism in amyotrophic lateral sclerosis: are protein aggregates the key? Exp. Neurobiol. 2015;24(1):1–7. doi: 10.5607/en.2015.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Taylor J.P., Brown R.H., Jr., Cleveland D.W. Decoding ALS: from genes to mechanism. Nature. 2016;539(7628):197. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Scotter E.L., Chen H.-J., Shaw C.E. TDP-43 proteinopathy and ALS: insights into disease mechanisms and therapeutic targets. Neurotherapeutics. 2015;12(2):352–363. doi: 10.1007/s13311-015-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ratti A., Buratti E. Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J. Neurochem. 2016;138:95–111. doi: 10.1111/jnc.13625. [DOI] [PubMed] [Google Scholar]
- 154.Becker L.A., Huang B., Bieri G., Ma R., Knowles D.A., Jafar-Nejad P., Messing J., Kim H.J., Soriano A., Auburger G. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature. 2017;544(7650):367. doi: 10.1038/nature22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.O'Connor D.M., Boulis N.M. Gene therapy for neurodegenerative diseases. Trends Mol. Med. 2015;21(8):504–512. doi: 10.1016/j.molmed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 156.Manabe Y., Nagano I., Gazi M., Murakami T., Shiote M., Shoji M., Kitagawa H., Setoguchi Y., Abe K. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents motor neuron loss of transgenic model mice for amyotrophic lateral sclerosis. Apoptosis. 2002;7(4):329–334. doi: 10.1023/a:1016123413038. [DOI] [PubMed] [Google Scholar]
- 157.Suzuki M., Svendsen C.N. Gene Therapy for Neurological Disorders. Springer; 2016. Ex vivo gene therapy using human mesenchymal stem cells to deliver growth factors in the skeletal muscle of a familial ALS rat model; pp. 325–336. [DOI] [PubMed] [Google Scholar]
- 158.Reddy L.V., Miller T.M. RNA-targeted therapeutics for ALS. Neurotherapeutics. 2015;12(2):424–427. doi: 10.1007/s13311-015-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rezaee M., Oskuee R.K., Nassirli H., Malaekeh-Nikouei B. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J. Contr. Release. 2016;236:1–14. doi: 10.1016/j.jconrel.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 160.Scarrott J.M., Herranz-Martín S., Alrafiah A.R., Shaw P.J., Azzouz M. Current developments in gene therapy for amyotrophic lateral sclerosis. Expet Opin. Biol. Ther. 2015;15(7):935–947. doi: 10.1517/14712598.2015.1044894. [DOI] [PubMed] [Google Scholar]
- 161.Chen J., Guo Z., Tian H., Chen X. Production and clinical development of nanoparticles for gene delivery. Mol. Ther. Methods Clin. Dev. 2016;3:16023. doi: 10.1038/mtm.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen L., Watson C., Morsch M., Cole N.J., Chung R.S., Saunders D.N., Yerbury J.J., Vine K.L. Improving the delivery of SOD1 antisense oligonucleotides to motor neurons using calcium phosphate-lipid nanoparticles. Front. Neurosci. 2017;11:476. doi: 10.3389/fnins.2017.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guo Q., Zheng X., Yang P., Pang X., Qian K., Wang P., Xu S., Sheng D., Wang L., Cao J. Small interfering RNA delivery to the neurons near the amyloid plaques for improved treatment of Alzheimer׳ s disease. Acta Pharm. Sin. B. 2019;9(3):590–603. doi: 10.1016/j.apsb.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shruthi S., Sumitha R., Varghese A.M., Ashok S., Sagar B.C., Sathyaprabha T., Nalini A., Kramer B.W., Raju T.R., Vijayalakshmi K. Brain-derived neurotrophic factor facilitates functional recovery from ALS-cerebral spinal fluid-induced neurodegenerative changes in the NSC-34 motor neuron cell line. Neurodegener. Dis. 2017;17(1):44–58. doi: 10.1159/000447559. [DOI] [PubMed] [Google Scholar]
- 165.Tan J., Wang Y., Yip X., Glynn F., Shepherd R.K., Caruso F. Nanoporous peptide particles for encapsulating and releasing neurotrophic factors in an animal model of neurodegeneration. Adv. Mater. 2012;24(25):3362–3366. doi: 10.1002/adma.201200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chung C.-Y., Lin M., Lee I., Lee T.-H., Lee M.-H., Yang J.-T. Brain-derived neurotrophic factor loaded PS80 PBCA nanocarrier for in vitro neural differentiation of mouse induced pluripotent stem cells. Int. J. Mol. Sci. 2017;18(3):663. doi: 10.3390/ijms18030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kanwar J.R., Sriramoju B., Kanwar R.K. Neurological disorders and therapeutics targeted to surmount the blood–brain barrier. Int. J. Nanomed. 2012;7:3259. doi: 10.2147/IJN.S30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Khalin I., Alyautdin R., Wong T.W., Gnanou J., Kocherga G., Kreuter J. Brain-derived neurotrophic factor delivered to the brain using poly (lactide-co-glycolide) nanoparticles improves neurological and cognitive outcome in mice with traumatic brain injury. Drug Deliv. 2016;23(9):3520–3528. doi: 10.1080/10717544.2016.1199609. [DOI] [PubMed] [Google Scholar]
- 169.Millecamps S., Julien J.-P. Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 2013;14(3):161–176. doi: 10.1038/nrn3380. [DOI] [PubMed] [Google Scholar]
- 170.Marinković P., Reuter M.S., Brill M.S., Godinho L., Kerschensteiner M., Misgeld T. Axonal transport deficits and degeneration can evolve independently in mouse models of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2012;109(11):4296–4301. doi: 10.1073/pnas.1200658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fischer L.R., Glass J.D. Axonal degeneration in motor neuron disease. Neurodegener. Dis. 2007;4(6):431–442. doi: 10.1159/000107704. [DOI] [PubMed] [Google Scholar]
- 172.Guo W., Naujock M., Fumagalli L., Vandoorne T., Baatsen P., Boon R., Ordovás L., Patel A., Welters M., Vanwelden T. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat. Commun. 2017;8(1):861. doi: 10.1038/s41467-017-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yuan Y.-g., Peng Q.-l., Gurunathan S. Combination of palladium nanoparticles and tubastatin-A potentiates apoptosis in human breast cancer cells: a novel therapeutic approach for cancer. Int. J. Nanomed. 2017;12:6503. doi: 10.2147/IJN.S136142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.El Bahhaj F., Denis I., Pichavant L., Delatouche R., Collette F., Linot C., Pouliquen D., Grégoire M., Héroguez V., Blanquart C. Histone deacetylase inhibitors delivery using nanoparticles with intrinsic passive tumor targeting properties for tumor therapy. Theranostics. 2016;6(6):795. doi: 10.7150/thno.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Goutman S.A., Savelieff M.G., Sakowski S.A., Feldman E.L. Stem cell treatments for amyotrophic lateral sclerosis: a critical overview of early phase trials. Expet Opin. Invest. Drugs. 2019;28(6):525–543. doi: 10.1080/13543784.2019.1627324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Deb K.D., Griffith M., Muinck E., Rafat M. Nanotechnology in stem cells research: advances and applications. Front. Biosci. 2012;17:1747–1760. doi: 10.2741/4016. [DOI] [PubMed] [Google Scholar]
- 177.Wang C., Cheng L., Xu H., Liu Z. Towards whole-body imaging at the single cell level using ultra-sensitive stem cell labeling with oligo-arginine modified upconversion nanoparticles. Biomaterials. 2012;33(19):4872–4881. doi: 10.1016/j.biomaterials.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 178.Vissers C., Ming G.-l., Song H. Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders. Adv. Drug Deliv. Rev. 2019;148:239–251. doi: 10.1016/j.addr.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kuo Y.-C., Rajesh R. Nerve growth factor-loaded heparinized cationic solid lipid nanoparticles for regulating membrane charge of induced pluripotent stem cells during differentiation. Mater. Sci. Eng. C. 2017;77:680–689. doi: 10.1016/j.msec.2017.03.303. [DOI] [PubMed] [Google Scholar]
- 180.Anraku Y., Kuwahara H., Fukusato Y., Mizoguchi A., Ishii T., Nitta K., Matsumoto Y., Toh K., Miyata K., Uchida S. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat. Commun. 2017;8(1):1001. doi: 10.1038/s41467-017-00952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee C., Hwang H.S., Lee S., Kim B., Kim J.O., Oh K.T., Lee E.S., Choi H.G., Youn Y.S. Rabies virus-inspired silica-coated gold nanorods as a photothermal therapeutic platform for treating brain tumors. Adv. Mater. 2017;29(13):1605563. doi: 10.1002/adma.201605563. [DOI] [PubMed] [Google Scholar]
- 182.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A.V. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J. Contr. Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Andaloussi S.E., Lakhal S., Mäger I., Wood M.J. Exosomes for targeted siRNA delivery across biological barriers. Adv. Drug Deliv. Rev. 2013;65(3):391–397. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 184.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29(4):341. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 185.Kim D.S., Jang I.K., Lee M.W., Ko Y.J., Lee D.-H., Lee J.W., Sung K.W., Koo H.H., Yoo K.H. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-γ. EBioMedicine. 2018;28:261–273. doi: 10.1016/j.ebiom.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Riazifar M., Mohammadi M.R., Pone E.J., Yeri A., Lässer C., Segaliny A.I., McIntyre L.L., Shelke G.V., Hutchins E., Hamamoto A. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13(6):6670–6688. doi: 10.1021/acsnano.9b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., del Pilar Rodriguez-Torres M., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu X., Ho W., Zhang X., Bertrand N., Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends Mol. Med. 2015;21(4):223–232. doi: 10.1016/j.molmed.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016;1(1):10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Su H., Wang Y., Gu Y., Bowman L., Zhao J., Ding M. Potential applications and human biosafety of nanomaterials used in nanomedicine. J. Appl. Toxicol. 2018;38(1):3–24. doi: 10.1002/jat.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Muthu M.S., Wilson B. Challenges posed by the scale-up of nanomedicines. Nanomedicine. 2012;7(3):307–309. doi: 10.2217/nnm.12.3. [DOI] [PubMed] [Google Scholar]