Abstract
Background
Preoperative spirometry and cardiopulmonary exercise testing (CPET) may stratify risk for respiratory complications. This secondary analysis of the Measurement of Exercise Tolerance before Surgery (METS) study examined whether CPET performance (i.e., cardiopulmonary fitness) confounds associations of spirometry with outcomes.
Methods
The analysis included 1200 participants having major non-cardiac surgery at 25 hospitals in Canada, Australia, New Zealand and UK. Forced expiratory volume in 1 s (FEV1), and ratio of FEV1 to forced vital capacity (FVC) were measured during preoperative spirometry, and peak oxygen consumption and ventilatory efficiency during preoperative CPET. Outcomes were respiratory morbidity (Postoperative Morbidity Survey) and pulmonary complications (pneumonia or respiratory failure). We used multivariable logistic regression models to estimate associations of FEV1 with outcomes after adjustment for risk factors and either peak oxygen consumption or ventilatory efficiency.
Findings
128 participants (11%) developed respiratory morbidity, and 48 (4%) developed pulmonary complications. There was no strong evidence that FEV1 predicted respiratory morbidity after adjustment for peak oxygen consumption (p = 0·80) or ventilatory efficiency (p = 0·76), or FEV1 predicted pulmonary complications after adjustment for ventilatory efficiency (p = 0·37). Peak oxygen consumption (odds ratio 0·66 per 5 mL/kg/min increase; 95% CI, 0·54–0·82) was associated with respiratory morbidity. Ventilatory efficiency was associated with respiratory morbidity (p = 0·04) and pulmonary complications (p = 0·02). Peak oxygen consumption also confounded the association between FEV1 and respiratory morbidity.
Interpretation
After accounting for fitness and clinical factors, FEV1 was not strongly predictive of respiratory complications. Prior associations between FEV1 and respiratory morbidity may be explained by confounding by peak oxygen consumption.
Funding
Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research, Innovation and Science, UK National Institute of Academic Anaesthesia, UK Clinical Research Collaboration, Australian and New Zealand College of Anaesthetists, and Monash University.
Research in context.
Evidence before this study
Preoperative spirometry may stratify risk for postoperative pulmonary complications. A search (“spirometry”, “pulmonary function tests”, “postoperative pulmonary complications”, or “mortality”) of PubMed, MEDLINE, EMBASE and Cochrane Database of Systematic Reviews showed that low forced expiratory volume in 1 s (FEV1) and low ratio of FEV1 to forced vital capacity (FVC) were associated with mortality and complications after lung resection and aortocoronary bypass surgery, but these associations were inconsistent in extra-thoracic surgery. Residual unmeasured confounding by cardiopulmonary fitness may explain these prior inconsistent associations. We therefore undertook a secondary analysis of the Measurement of Exercise of Tolerance before Surgery (METS) study to determine the association of preoperative spirometry with cardiopulmonary fitness and pulmonary complications after major non-cardiac surgery.
Added value of this study
We evaluated the association of spirometry measures (FEV1 and FEV1/FVC) with cardiopulmonary exercise testing measures (peak oxygen consumption and ventilatory efficiency) and postoperative respiratory complications in 1200 adults having major elective non-cardiac surgery. Following risk adjustment, peak oxygen consumption and ventilatory efficiency – but not FEV1 – were predictive of respiratory complications. Peak oxygen consumption was a confounder in the association between FEV1 and some types of respiratory complications.
Implications of all available evidence
Inconsistent associations between spirometry and respiratory morbidity in the previous literature may be explained, in part, by residual confounding related to cardiopulmonary fitness. Unlike FEV1, cardiopulmonary fitness appears to be an important preoperative determinant of risk for postoperative pulmonary complications.
Alt-text: Unlabelled box
1. Introduction
Worldwide, about 300 million people have major surgery every year.[1], [2], [3] Following in-patient surgery, postoperative respiratory complications are common, occurring in up to 10% of patients, and important, owing to their association with increased mortality, hospital length-of-stay, and health care resource utilisation. Nonetheless, there remains a paucity of evidence on whether preoperative testing of respiratory function can help stratify risk for postoperative pulmonary complications.4,5
Spirometry is a specialised non-invasive test for measuring lung function that may be useful for identifying patients at risk of postoperative pulmonary complications.6 The prognostic value of preoperative spirometry has been reasonably well-established in lung resection and aortocoronary bypass surgery, where a reduced forced expiratory volume in 1 s (FEV1) and reduced ratio of FEV1 to forced vital capacity (FVC) are strongly associated with postoperative mortality and complications.[7], [8], [9] Following extra-thoracic surgery, however, the association of spirometry measures with outcomes have been inconsistent. While these inconsistencies have contributed to some practice guidelines recommending against preoperative spirometry for extra-thoracic surgery,[10], [11], [12], [13] the reasons underlying these inconsistent associations have been minimally explored to date.
An important possible explanation for this inconsistency in prior research is residual confounding by cardiopulmonary fitness. Previous studies examining the association of spirometry with respiratory complications included patients with varying levels of cardiopulmonary fitness. Individuals with poor spirometry results in these studies were more likely to also have poor exercise capacity and inability to perform activities of daily living.14 Thus, if preoperative fitness is measured with sufficient accuracy, spirometry may have limited incremental prognostic utility. These associations of spirometry with cardiopulmonary fitness and outcomes have not been extensively studied to-date. We therefore conducted a pre-specified secondary analysis of the Measurement of Exercise Tolerance before Surgery (METS) multicentre prospective cohort study to assess whether preoperative spirometry measures were associated with measures of cardiopulmonary fitness, and whether there was an association between preoperative spirometry and outcomes following major elective non-cardiac surgery after accounting for associated differences in cardiopulmonary fitness.15,16
2. Methods
2.1. Design
The objectives of this pre-specified secondary analysis of the METS study were to examine the associations of preoperative spirometry measures (FEV1 and FEV1/FVC ratio) with measures of cardiopulmonary fitness from cardiopulmonary exercise testing (peak oxygen consumption and ventilatory efficiency); and to examine the association of FEV1 with postoperative respiratory outcomes, while adjusting for peak oxygen consumption or ventilatory efficiency. The first objective was addressed in a nested cross-sectional analysis, while the second objective was addressed in a nested cohort analysis.
The data source was the METS study, which was a multicentre prospective cohort study of patients who had major non-cardiac surgery at 25 hospitals in Canada, New Zealand, Australia, and the United Kingdom. The METS study objectives, design, methods, and primary results have been previously reported.15,16 This secondary analysis was restricted to participants in the METS study who completed their scheduled preoperative cardiopulmonary exercise testing (including spirometry) with valid test results, had their scheduled non-cardiac surgical procedures, and successfully completed 30-day postoperative follow-up. We also excluded patients who underwent lung resection surgery because preoperative spirometry would not reflect their postoperative respiratory function.
2.2. Ethics statement
All participants in the METS study provided written informed consent, and each centre obtained research ethics board approval before commencing recruitment. Additional research ethics approval for this secondary analysis was obtained at the University Health Network (Toronto, Ontario, Canada).
2.3. Participants and study procedures
Eligible participants were aged 40 years or older, scheduled to have inpatient elective non-cardiac surgery using general and/or regional anaesthesia, and deemed to have one or more risk factors for cardiac complications or coronary artery disease. For safety reasons, participants were excluded if their preoperative FEV1 was less than 30% predicted (based on the age-, sex- and height-based reference standards used at each participating institution).
Consenting participants underwent spirometry and cardiopulmonary exercise testing within one to 90 days before their surgical procedure. Spirometry and exercise testing were performed on the same hospital visit. Both tests were protocolised, and results were masked from patients, clinicians and outcome adjudicators. For spirometry, FEV1 was defined as the volume (L) of gas exhaled during the first second of a forced expiration, starting from a position of full inspiration; FVC was defined as the total volume of gas (L) exhaled during a forced exhalation, starting from a position of full inspiration and ending at complete expiration; and the ratio of FEV1/FVC was expressed as a percentage. Both FEV1 and FVC were also expressed as percent predicted values, based on the age-, sex- and height-based reference standards used at each participating institution. Following spirometry, patients underwent cardiopulmonary exercise testing on an electromagnetically braked cycle ergometer in accordance with published guidelines,17 during which breath-by-breath measurement of minute ventilation, oxygen uptake and carbon dioxide production were performed. Patients exercised against an incremental load until they reached their limit of tolerance. The measures of interest from cardiopulmonary exercise testing were peak oxygen consumption (expressed in mL/kg/min), which was defined as the average oxygen uptake during the last 20 s of the incremental phase of exercise before attaining the limit of tolerance, and ventilatory efficiency (expressed without units), which was defined as the slope of the minute ventilation to CO2 production curves (or VE/VCO2) at the anaerobic threshold. The anaerobic threshold was determined using the modified V-slope method. Higher VE/VCO2 values represent poor ventilatory efficiency and lower values represent better ventilatory efficiency. Participants underwent routine pre-, intra-, and post-operative care at the discretion of their clinical teams. They were followed for 30 days after surgery to ascertain postoperative complications. While in hospital, participants were followed up daily by research personnel; following hospital discharge, they were contacted at home to ascertain 30-day outcomes. All outcome assessors were blinded to preoperative spirometry and cardiopulmonary exercise testing results.
The outcomes for the nested cohort analysis were postoperative respiratory morbidity and postoperative pulmonary complications. Respiratory morbidity was defined as the respiratory component of the Postoperative Morbidity Survey (POMS), which was administered on postoperative days 3 and 5. The POMS instrument assesses major organ system function postoperatively; it has construct and criterion validity.18,19 The respiratory morbidity component of the POMS instrument is defined as the de novo requirement for supplemental oxygen or other respiratory support. This definition approximates contemporary definitions of postoperative respiratory failure.4,20 Postoperative pulmonary complications were defined as unplanned mechanical ventilation within 30 days after surgery, respiratory failure (defined as any condition requiring unplanned intubation of the trachea and mechanical ventilation after completion of surgery) or pneumonia (defined as any condition with documented hypoxaemia or fever with clinical, radiologic or serologic evidence of a respiratory infection). Although previous definitions of postoperative pulmonary complications have varied and the outcome is a composite endpoint,21,22 the events comprising postoperative pulmonary complications in this present study are exclusively those of high severity.
2.4. Statistical analyses
Descriptive statistics were used to characterise the cohort. Categorical variables were described using counts and frequencies, and continuous variables were described using means with standard deviations and medians with interquartile ranges.
The nested cross-sectional analysis (first study objective) evaluated the association between FEV1 and FEV1/FVC with peak oxygen consumption and ventilatory efficiency. Initially, scatter plots were used to graphically examine the association of each of FEV1 and FEV1/FVC against each of peak oxygen consumption and ventilatory efficiency. The correlation between these variables was described using the Spearman coefficient. For this analysis, Spearman's rho of 0 to 0·2 indicated ‘very weak’; 0·2 to 0·4 indicated ‘weak’; 0·4 to 0·6 indicated ‘moderate’; 0·6 to 0·8 indicated ‘strong’, and 0·8 to 1 indicated ‘very strong’ correlation.23 To evaluate the relationship between FEV1 and FEV1/FVC with peak oxygen consumption and ventilatory efficiency, the spirometry variables of interest were expressed as a 4-knot restricted cubic spline to flexibly model possible non-linear associations while accounting for the available study sample size.
The nested cohort analysis (second study objective) evaluated the association of FEV1 with respiratory morbidity and postoperative pulmonary complications, while accounting for peak oxygen consumption or ventilatory efficiency. Unadjusted associations between demographics, comorbidities, surgical procedure, FEV1, FEV1/FVC, peak oxygen consumption, and ventilatory efficiency with each of respiratory morbidity and postoperative pulmonary complications were assessed using the Chi-square statistic for categorical variables and the Wilcoxon rank sum test for continuous variables.
A series of nested multivariable logistic regression models were then used to evaluate the adjusted association of FEV1 with respiratory morbidity, while accounting for peak oxygen consumption or ventilatory efficiency. We initially estimated a regression model where the independent variables were FEV1, age, sex, body mass index (BMI), coronary artery disease, smoking history, and surgical procedure type. These covariates were chosen a priori based on previous literature and clinical sensibility. Peak oxygen consumption and ventilatory efficiency were then included as additional covariates in two separate models. Interaction terms were also used to test for the presence of effect modification between FEV1 and either peak oxygen consumption or ventilatory efficiency. Adjusted estimates of association from these models were expressed as odds ratios (OR) with 95% confidence intervals (CIs). The underlying assumption of linear associations between continuous predictor variables and outcomes was tested by expressing spirometry (FEV1) and CPET (peak oxygen consumption or ventilatory efficiency) variables as 4-knot restricted cubic splines; if there was evidence of non-linearity, the predictor variable was expressed using the spline terms.
The number of covariates that could be included in a multivariable logistic regression model predicting postoperative pulmonary complications was limited because there were relatively few (i.e., 48) outcome events.24 Two preliminary univariable models were therefore initially developed with FEV1 and ventilatory efficiency as predictors expressed as 3-knot restricted cubic splines. These initial analyses were used to determine whether any transformations of FEV1 or ventilatory efficiency were needed to account for non-linear associations with postoperative pulmonary complications. No transformation of FEV1 was necessary; however, ventilatory efficiency had a non-linear association with the log odds of postoperative pulmonary complications and was modelled as a 3-knot restricted cubic spline. Two nested multivariable logistic regression models were then used to evaluate the adjusted association of FEV1 with postoperative pulmonary complications, while accounting for ventilatory efficiency. We initially estimated a regression model where the independent variables were FEV1, age, sex, and surgical procedure type. These covariates were chosen a priori based on previous literature and clinical sensibility. Ventilatory efficiency was then included as an additional covariate in the model.
To quantify optimism (overfitting), the multivariable logistic regression models in the nested cohort analysis were internally validated using bootstrap resampling. The optimism-corrected C index was derived from 100 bootstrap resamples. Statistical analyses were conducted using the R statistical package (Version 3.2.4).
2.5. Post-Hoc analyses
To address comments from an external peer-reviewer, we performed post-hoc analyses to assess the impact of age-, sex- and height-specific reference values when evaluating the prognostic significance of FEV1 and FEV1/FVC ratio with respect to predicting respiratory morbidity. Specifically, multivariable logistic regression models were used to separately estimate the adjusted association of (i) FEV1 below the lower limit of normal and (ii) FEV1/FVC ratio below the lower limit of normal with respiratory morbidity. Covariates in the models were age, sex, BMI, cardiopulmonary exercise testing performance (i.e., peak oxygen consumption and ventilatory efficiency evaluated in separate models), coronary artery disease, smoking history, and surgical procedure type.
2.6. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The first author (AS) and corresponding author (DNW) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
The study included 1200 participants, which represents about 86% of the 1401 individuals included in the primary METS study analysis.16 The sample (characteristics summarised in Table 1) was comprised of 63% males, had a median age of 65 years and a median BMI of 28 kg/m2. Common comorbidities included hypertension (54%), diabetes mellitus (18%) and coronary artery disease (11%). Frequent surgical procedures included upper abdominal and/or thoracic (45%), urologic or gynaecologic (31%), or orthopaedic (23%) procedures. With respect to preoperative spirometry, the median FEV1 was 2·66 L and median FEV1/FVC ratio was 0·76; these values were within the normal to low-normal ranges. Based on age-, sex- and height-based reference standards, 25% (n = 302) had a FEV1 below the lower limit of normal and 17% (n = 198) had a FEV1/FVC ratio below the lower limit of normal. Similarly, the median peak oxygen consumption was 18·9 mL/kg/min and median ventilatory efficiency was 31. These values were also within normal to low-normal ranges. About 11% (n = 129) of the cohort developed respiratory morbidity, while 4% (n = 48) developed postoperative pulmonary complications.
Table 1.
Sample characteristics.
| Study Cohort (n = 1200) |
|
|---|---|
| Age in years (median, IQR; mean (SD)) | 65 (57 – 72); 64 (10) |
| Male sex (%) | 757 (63%) |
| BMI in kg/m2 (median, IQR; mean (SD)) | 28 (25 – 32); 29 (6) |
| Current or recent smoker * (n,%) | 173 (14%) |
| Comorbidities | |
| Aortic stenosis (n,%) | 16 (1%) |
| Atrial fibrillation (n,%) | 45 (4%) |
| Coronary artery disease (n,%) | 135 (11%) |
| Heart failure (n,%) | 15 (1%) |
| Known obstructive lung disease (n,%) | 136 (11%) |
| Diabetes mellitus (n,%) | 215 (18%) |
| Preoperative renal insufficiency† (n,%) | 93 (8%) |
| Peripheral artery disease (n,%) | 29 (2%) |
| Hypertension (n,%) | 653 (54%) |
| Surgical procedure | |
| Upper abdominal and/or thoracic | 412 (34%) |
| Vascular | 19 (2%) |
| Urology or gynaecology | 375 (31%) |
| Orthopaedic | 280 (23%) |
| Other | 114 (10%) |
| Spirometry results | |
| FEV1 in L (median, IQR; mean (SD)) | 2.66 (2·14 – 3·16); 2·68 (0·73) |
| % FEV1 Predicted (median, IQR; mean (SD)) | 95 (82 – 108); 96 (22) |
| FEV1 below lower limit of normal‡ | 302 (25%) |
| FVC in L (median, IQR; mean (SD)) | 3·52 (2·88 – 4·24); 3·59 (0·97) |
| % FVC Predicted (median, IQR; mean (SD)) | 96 (85 – 109); 97 (19) |
| FVC below lower limit of normal‡ | 229 (19%) |
| FEV1/FVC Ratio (median, IQR; mean (SD)) | 0·76 (0·70 – 0·81); 0·75 (0·10) |
| FEV1/FVC Ratio below lower limit of normal‡ | 198 (17%) |
| CPET results | |
| Peak oxygen consumption (median, IQR; mean (SD)) | 18·9 (15·0 – 22·1); 19·5 (6·3) |
| Ventilatory efficiency (median, IQR; mean (SD)) | 31 (28 – 35); 32 (6) |
| Outcomes | |
| POMS respiratory morbidity (n,%) | 129 (11%) |
| Postoperative pulmonary complications (n,%) | 48 (4%) |
history of smoking within 1 year before surgery.
defined as an estimated glomerular filtration rate <60 mL/min/1·73 m2 based on the Chronic Kidney Disease Epidemiology Collaboration equation.
normal range based on age-, sex- and height-specific reference standards (5th percentile of a healthy non-smoking population).
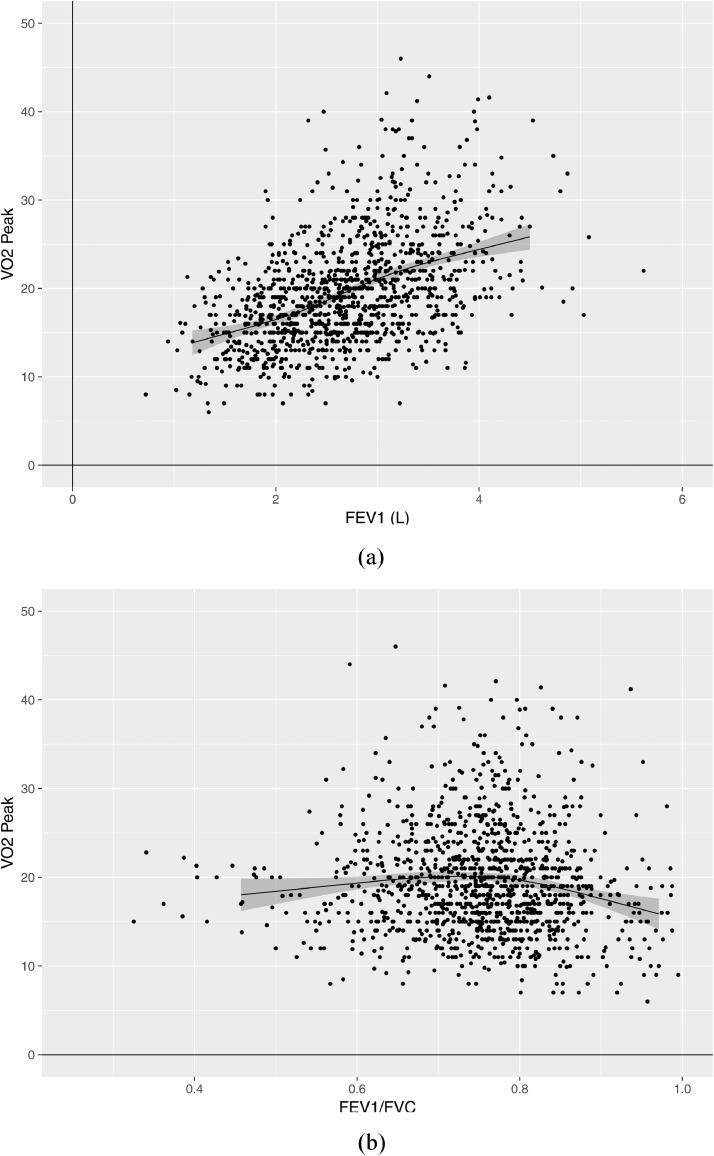
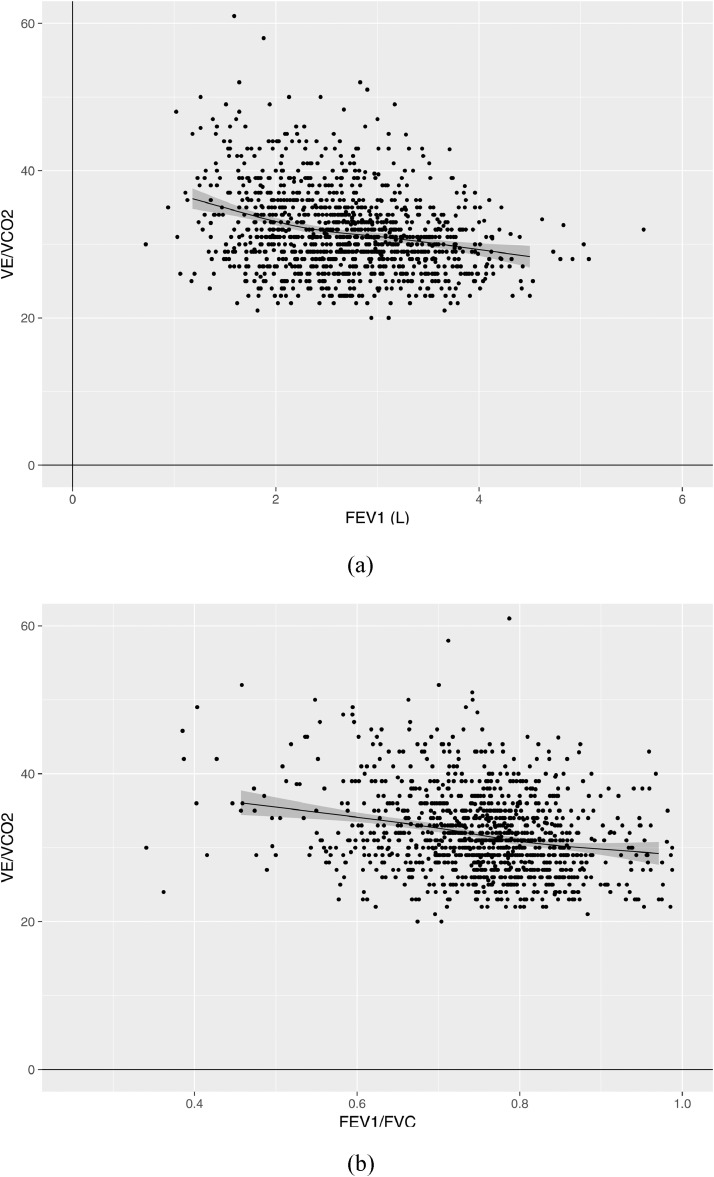
We evaluated associations between FEV1 and FEV1/FVC with peak oxygen consumption and ventilatory efficiency using scatter plots. There was a moderate positive correlation between FEV1 and peak oxygen consumption (Spearman rho 0·47; Fig. 1A), but very weak correlation between FEV1/FVC and peak oxygen consumption (Spearman rho −0·08; Fig. 1B). Scatter plots of each of FEV1 and FEV1/FVC versus ventilatory efficiency (Fig. 2) showed negative weak correlations between FEV1 and ventilatory efficiency (Spearman rho −0·25; Fig. 2A), and between FEV1/FVC and ventilatory efficiency (Spearman rho −0·23; Fig. 2B).
Fig. 1.
Correlation of FEV1 and FEV1/FVC versus peak oxygen consumption (VO2 peak). Panel A is a scatter plot presenting the association of peak oxygen consumption (VO2 peak – shown on y-axis) and forced expiratory volume in 1 s (FEV1 – shown on x-axis). The plotted line is a line of best fit (estimated using restricted cubic splines), while the grey shaded zone represents its 95% confidence limits. Panel B is a scatter plot presenting the association of peak oxygen consumption (VO2 peak – shown on y-axis) and the ratio of FEV1 to forced vital capacity (FEV1/FVC – shown on x-axis). The plotted line is a line of best fit (estimated using restricted cubic splines), while the grey shaded zone represents its 95% confidence limits.
Fig. 2.
Scatter plot of FEV1and FEV1/FVC versus ventilatory efficiency (VE/VCO2). Panel A is a scatter plot presenting the association of ventilatory efficiency (VE/VCO2 – shown on y-axis) and forced expiratory volume in 1 s (FEV1 – shown on x-axis). The plotted line is a line of best fit (estimated using restricted cubic splines), while the grey shaded zone represents its 95% confidence limits. Panel B is a scatter plot presenting the association of ventilatory efficiency (VE/VCO2 – shown on y-axis) and the ratio of FEV1 to forced vital capacity (FEV1/FVC – shown on x-axis). The plotted line is a line of best fit (estimated using restricted cubic splines), while the grey shaded zone represents its 95% confidence limits.
For the nested cohort component of this present study, the unadjusted associations of baseline characteristics with both POMS respiratory morbidity and postoperative pulmonary complications are presented in Table 2. Both FEV1 and peak oxygen consumption showed linear adjusted associations with respiratory morbidity, while the adjusted association of ventilatory efficiency with this outcome appeared to be non-linear (Appendix Figure S1).
Table 2.
Unadjusted associations with primary and secondary outcomes.
| Respiratory Morbidity |
Pulmonary Complications |
|||||
|---|---|---|---|---|---|---|
| Present | Absent | p-value | Present | Absent | p-value | |
| (n = 129) | (n = 1071) | (n = 48) | (n = 1152) | |||
| Age in years (median, IQR) | 63 (56 – 70) | 65 (57 – 72) | 0·1 | 65 (59 – 72) | 65 (56 – 72) | 0·92 |
| Male sex (%) | 81 (63%) | 676 (63%) | 1 | 36 (75%) | 721 (63%) | 0·11 |
| BMI in kg/m2 (median, IQR) | 28 (24 – 31) | 28 (25 – 32) | 0·45 | 27 (24 – 31) | 28 (25 – 32) | 0·28 |
| Current or recent smoker† (n,%) | 20 (16%) | 153 (14%) | 0·81 | 6 (13%) | 167 (15%) | 0·84 |
| Comorbidities | ||||||
| Aortic stenosis (n,%) | 1 (1%) | 15 (1%) | 1 | 0 (0%) | 16 (1%) | 0·99 |
| Atrial fibrillation (n,%) | 7 (5%) | 38 (4%) | 0·32 | 4 (8%) | 41 (4%) | 0·1 |
| Coronary artery disease (n,%) | 11 (9%) | 124 (12%) | 0·37 | 6 (13%) | 129 (11%) | 0·81 |
| Heart failure (n,%) | 2 (2%) | 13 (1%) | 0·67 | 0 (0%) | 15 (1%) | 0·99 |
| Cerebrovascular disease (n,%) | 7 (5%) | 38 (4%) | 0·32 | 3 (6%) | 42 (4%) | 0·42 |
| Diabetes mellitus (n,%) | 18 (14%) | 197 (18%) | 0·26 | 9 (19%) | 206 (18%) | 0·99 |
| Renal insufficiency‡ (n,%) | 10 (8%) | 83 (8%) | 1 | 2 (4%) | 91 (8%) | 0·58 |
| Peripheral artery disease (n,%) | 2 (2%) | 27 (3%) | 0·76 | 1 (2%) | 28 (2%) | 0·99 |
| Hypertension (n,%) | 59 (46%) | 594 (56%) | 0·05 | 21 (44%) | 632 (55%) | 0·17 |
| Surgical Procedure | ||||||
| Upper abdominal and/or thoracic | 86 (67%) | 326 (30%) | <0·001 | 28 (58%) | 384 (33%) | <0·001 |
| Vascular | 4 (3%) | 15 (1%) | 2 (4%) | 17 (2%) | ||
| Urology or gynaecology | 25 (19%) | 350 (33%) | 11 (23%) | 364 (32%) | ||
| Orthopaedic | 5 (4%) | 275 (26%) | 3 (6%) | 277 (24%) | ||
| Other | 9 (7%) | 105 (10%) | 4 (8%) | 110 (10%) | ||
| Spirometry Results | ||||||
| FEV1 in L (median, IQR) | 2·67 (2·13 – 3·11) | 2·66 (2·14 – 3·16) | 0·77 | 2·77 (2·31 – 3·05) | 2·66 (2·13 – 3·16) | 0·72 |
| % FEV1 Predicted (median, IQR) | 96 (84 – 106) | 95 (82 – 108) | 0·68 | 96 (82 – 105) | 95 (82 – 108) | 0·61 |
| FEV1 below lower limit of normal‡ | 30 (23%) | 272 (25%) | 0·59 | 13 (27%) | 289 (25%) | 0·76 |
| FVC in L (median, IQR) | 3·52 (2·85 – 3·99) | 3·52 (2·88 – 4·27) | 0·28 | 3·62 (3·38 – 4·18) | 3·51 (2·87 – 4·24) | 0·29 |
| % FVC Predicted (median, IQR) | 94 (83 – 108) | 96 (85 – 109) | 0·3 | 95 (90 – 105) | 96 (85 – 110) | 0·94 |
| FVC below lower limit of normal‡ | 22 (17%) | 207 (19%) | 0·54 | 5 (10%) | 224 (19%) | 0·12 |
| FEV1/FVC Ratio (median, IQR) | 0·78 (0·71 – 0·84) | 0·76 (0·70 – 0·81) | 0·03 | 0·76 (0·69 – 0·79) | 0·76 (0·70 – 0·81) | 0·24 |
| FEV1/FVC Ratio below lower limit of normal‡ | 24 (19%) | 174 (16%) | 0·50 | 10 (21%) | 188 (16%) | 0·41 |
| CPET Results | ||||||
| Peak oxygen consumption (median, IQR) | 17·0 (14·0 – 21·0) | 19·0 (15·0 – 22·9) | 0·001 | 18·8 (15·1 – 21·0) | 18·9 (15·0 – 22·3) | 0·63 |
| Ventilatory efficiency (median, IQR) | 32 (29 – 35) | 31 (28 – 35) | 0·03 | 32 (30 – 35) | 31 (28 – 35) | 0·04 |
*history of smoking within 1 year before surgery.
defined as an estimated glomerular filtration rate <60 mL/min/1·73 m2 based on the Chronic Kidney Disease Epidemiology Collaboration equation.
normal range based on age-, sex- and height-specific reference standards (5th percentile of a healthy non-smoking population).
The unadjusted association of FEV1 with respiratory morbidity was an OR of 0·97 (95% CI, 0·75 to 1·25; p = 0·81) per 1 L increase. After adjustment for demographics and clinical factors (including surgery type), the OR was 0·85 (95% CI, 0·61 to 1·18; p = 0·33) per 1 L increase. Following further adjustment for either peak oxygen consumption or ventilatory efficiency, there was no strong statistical evidence of an association between FEV1 and respiratory morbidity (Table 3). The association of FEV1 with respiratory morbidity changed substantially with further adjustment for peak oxygen consumption (i.e., change in adjusted OR from 0.85 to 1.05 – consistent with a 23% relative change in the OR), while it was minimally changed after further adjustment for ventilatory efficiency (i.e., change in adjusted OR from 0.85 to 0.88 – consistent with a 4% relative change in the OR). There was also stronger evidence for both peak oxygen consumption (OR 0·66 per 5 mL/kg/min increase; 95% CI, 0·54 to 0·82; p<0·001) and ventilatory efficiency (non-linear association, p = 0·04; OR 2·06 for increase from 25 to 30; CI, 1·15 to 3·71, Appendix Table S1) being associated with respiratory morbidity. There was little evidence for an interaction of FEV1 with either peak oxygen consumption (p = 0·42) or ventilatory efficiency (p = 0·44).
Table 3.
Adjusted association of respiratory morbidity with FEV1, clinical factors and peak oxygen consumption or ventilatory efficiency (separate models).
| A. Models* predicting respiratory morbidity using FEV1, clinical factors, and peak oxygen consumption. | |||
|---|---|---|---|
| Factor | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (no CPET) (95% CI) | Adjusted Odds Ratio (with CPET) (95% CI) |
| FEV1 (per 1 L increase) | 0·97 (0·75 - 1.25) | 0·85 (0·61 - 1·18) | 1·05 (0·74 - 1·47; p = 0·8) |
| Peak oxygen consumption (per 5 mL/kg/min increase) | 0·74 (0·63 - 0·88) | N.A. | 0·66 (0·54 - 0·82; p = 0·0001) |
| Age (per 10-year increase) | 0·89 (0·75 - 1·06) | 0·93 (0·75 - 1·15) | 0·88 (0·71 – 1·09; p = 0·23) |
| Female sex | 1·01 (0·69 - 1·48) | 0·91 (0·57 - 1·47) | 0·77 (0·47 – 1·25; p = 0·29) |
| BMI (per 5-unit increase, in kg/m2) | 0·92 (0·79 - 1·09) | 0·93 (0·79 - 1·10) | 0·84 (0·71 – 1·01; p = 0·06) |
| Coronary artery disease | 0·71 (0·37 - 1·36) | 0·77 (0·39 - 1·51) | 0·69 (0·35 – 1·37; p = 0·28) |
| Current or recent smoker | 1·10 (0·66 - 1·83) | 1·05 (0·61 - 1·81) | 1·02 (0·59 – 1·76; p = 0·94) |
| Upper abdominal or thoracic surgery | 4·57 (3·10 - 6·74) | 4·52 (3·05 - 6·69) | 4·25 (2·86 – 6·32; p<0.0001) |
| B. Models† predicting respiratory morbidity using FEV1, clinical factors, and ventilatory efficiency | |||
|---|---|---|---|
| Factor | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (no CPET) (95% CI) | Adjusted Odds Ratio (with CPET) (95% CI) |
| FEV1 (per 1 L increase) | 0·97 (0·75 - 1·25) | 0·85 (0·61 - 1·18) | 0·88 (0·52 - 1·49; p = 0·76) |
| Ventilatory efficiency | Non-linear | N.A. | Non-linear (p = 0·02) |
| Age (per 10-year increase) | 0·89 (0·75 - 1·06) | 0·93 (0·75 - 1·15) | 0·87 (0·70 – 1·08; p = 0·21) |
| Female sex | 1·01 (0·69 - 1·48) | 0·91 (0·57 - 1·47) | 0·88 (0·54 – 1·43; p = 0·6) |
| BMI (per 5-unit increase, in kg/m2) | 0·92 (0·79 - 1·09) | 0·93 (0·79 - 1·10) | 0·97 (0·81 – 1·15; p = 0·7) |
| Coronary artery disease | 0·71 (0·37 - 1·36) | 0·77 (0·39 - 1·51) | 0·75 (0·37 – 1·49; p = 0·4) |
| Current or recent smoker | 1·10 (0·66 - 1·83) | 1·05 (0·61 - 1·81) | 1·04 (0·60 – 1·80; p = 0·89) |
| Upper abdominal or thoracic surgery | 4·57 (3·10 - 6·74) | 4·52 (3·05 - 6·69) | 4·46 (3·00 – 6·62; p<0.0001) |
Abbreviations: BMI, body mass index; CPET, cardiopulmonary exercise test.
Regression model had an optimism-corrected c-index of 0·71 (1198 observations with 129 outcome events).
Regression model had an optimism-corrected c-index of 0·69 (1149 observations with 128 outcome events).
In post-hoc analyses, there was little statistical evidence that a preoperative FEV1 below the lower limit of normal was predictive of respiratory morbidity after adjustment for either peak oxygen consumption (Appendix Table S3: p = 0·10) or ventilatory efficiency (Appendix Table S4; p = 0·60). Similarly, we found little evidence that a preoperative FEV1/FVC ratio below the lower limit of normal was associated with respiratory morbidity after adjustment for either peak oxygen consumption (Appendix Table S5; p = 0·39) or ventilatory efficiency (Appendix Table S6; p = 0·80).
The unadjusted association of FEV1 with postoperative pulmonary complications was an OR of 1.00 (95% CI, 0·67 to 1·50; p = 0·98) per 1 L increase. After adjustment for demographics and clinical factors (including surgery type), the adjusted association was an OR of 0·78 (95% CI, 0·48 to 1·28; p = 0·33) per 1 L increase. Following further adjustment for ventilatory efficiency, there was no strong statistical evidence of an association between FEV1 and postoperative pulmonary complications (Table 3). The association between FEV1 and postoperative pulmonary complications remained essentially unchanged with further adjustment for ventilatory efficiency (i.e., change in adjusted OR from 0.78 to 0.79 – consistent with a 1% relative change in the OR). Conversely, ventilatory efficiency showed a stronger non-linear adjusted association with postoperative pulmonary complications (p = 0·02; OR 2·51 for increase from 25 to 30; CI, 1·11 to 5·69, Appendix Table S2).
4. Discussion
An examination of the relationship between commonly used spirometry measures (i.e., FEV1, FEV1/FVC) with measures from cardiopulmonary exercise testing (peak oxygen consumption, ventilatory efficiency) helps inform their utility in clinical practice. In this secondary analysis of an international multicentre cohort study of patients having major elective non-cardiac surgery, the measures of spirometry and cardiopulmonary fitness in the sample were predominantly in the low-normal to normal range. There was moderate correlation between FEV1 and peak oxygen consumption, very weak correlation of FEV1/FVC with peak oxygen consumption, and weak correlation of both FEV1 and FEV1/FVC with ventilatory efficiency. We found no strong evidence that preoperative FEV1 predicted postoperative respiratory complications; however, there was stronger evidence that diminished preoperative cardiopulmonary fitness (as characterized by peak oxygen consumption and ventilatory efficiency) predicted postoperative complications. Our findings also suggest that previously observed associations of spirometry results (specifically FEV1) with respiratory morbidity was explained, in part, by residual confounding related to peak oxygen consumption.
The moderate correlation between FEV1 and peak oxygen consumption highlights the possible overlap in the underlying constructs measured by these tests. Some overlap is to be expected as they are both effort-dependant, and respiratory function measured by FEV1 should contribute to cardiopulmonary fitness.6,17 Reduction in FEV1 is predictive of incident cardiovascular disease (including ischaemic heart disease, stroke, and heart failure) in the general population, further highlighting potential overlap with cardiovascular fitness measured by peak oxygen consumption.25,26 However, the moderate magnitude of this correlation highlights important differences between the measures. Peak oxygen consumption reflects the cardiovascular and pulmonary systems’ integrated physiological response to incremental exercise stress. In contrast, FEV1 is a measure of lung function that may be influenced by non-pulmonary conditions such as frailty.27 Another factor contributing to the weak-to-moderate correlation between spirometry and cardiopulmonary testing measures in the METS study cohort might its relatively low prevalence of pre-existing respiratory disease (i.e., 11% prevalence of known obstructive pulmonary disease). This correlation has been higher in older patients with respiratory disease such as chronic obstructive pulmonary disease,[28], [29], [30] suggesting that respiratory limitations are a relatively larger contributor to impaired cardiopulmonary fitness in these higher risk patients.
The weak correlations of FEV1/FVC with peak oxygen consumption, and both FEV1 and FEV1/FVC with ventilatory efficiency were unexpected. Several factors may explain these weak associations. First, FEV1 and FEV1/FVC assess lung function obtained during rest, while ventilatory efficiency is a measure of the respiratory system's dynamic ability to adapt to increasing exercise requirements.6,17 Second, FEV1 and FEV1/FVC are effort-dependant, while ventilatory efficiency is calculated at the anaerobic threshold, which is effort-independent. Third, determination of the anaerobic threshold is subject to inter-rater variability. In the pragmatic design of the METS study, anaerobic threshold was determined by trained investigators at each study site; therefore, these results need to be replicated following central determination of anaerobic thresholds, and by extension, ventilatory efficiency. Notably, limitations in ventilatory efficiency have been observed in patients with symptomatic chronic obstructive pulmonary disease who have ‘out-of-proportion breathlessness’ despite preserved FEV1, suggesting that unlike FEV1, ventilatory efficiency is influenced by cardiocirculatory factors such as heart failure and pulmonary hypertension.[31], [32], [33]
Our observation that spirometry measures were not predictive of postoperative respiratory complications after accounting for cardiopulmonary fitness is a notable addition to the literature, where previously reported associations of spirometry measures with outcomes following extra-thoracic surgery have been inconsistent.[10], [11], [12], [13] A prior small single-centre study of colorectal surgery patients showed that patients with cardiopulmonary complications had lower anaerobic threshold values but no differences in FEV1 or FEV1/FVC; however, this analysis did not include any risk adjustment.34 Our results suggest that the extent to which spirometry (specifically FEV1) predicted outcomes in prior studies may be related, in part, to its association with cardiopulmonary fitness (i.e., maximal exercise capacity as characterized by peak oxygen consumption). These findings have important implications for clinical practice, as pulmonary complications are important contributors to postoperative morbidity, length-of-stay and resource utilisation. Improved preoperative risk stratification for these complications will better inform risk discussions surrounding surgery, allow for initiating interventions to decrease risk, and facilitate targeted use of postoperative monitored beds. Our findings suggest that risk stratification for pulmonary complications must consider an assessment of patients’ cardiopulmonary fitness, which was measured objectively using CPET in this study. This association is plausible since fitness impacts the ability to cope with perioperative stress, which may play an important role in postoperative recovery including propensity to develop complications.35 Though an assessment of functional capacity has been emphasised in prior guidelines pertaining to postoperative cardiac complications,36 the results of our present study suggest that cardiopulmonary fitness is an important element of risk stratification for respiratory complications. These findings also have implications for existing risk calculators for postoperative pulmonary complications, since these tools include clinical factors such as age and anaemia, but no measures of fitness.20,21 The addition of measures of fitness may enhance assessment of risk for respiratory complications, and future prospective study addressing this topic is warranted.
While the focus of this present study was patients’ performance on spirometry and cardiopulmonary exercise testing, it is noteworthy that the dominant predictor of respiratory morbidity and postoperative pulmonary complications in the study cohort was the surgical procedure. Upper abdominal or thoracic procedures plausibly influence the risk of pulmonary complications because these procedures significantly impact the mechanical properties of respiratory muscles after surgery. In the study cohort, about 60% to 70% of respiratory morbidity events and postoperative pulmonary complications (Table 2) occurred in patients having upper abdominal or thoracic surgery. Even after multivariable risk adjustment, these procedures were associated with a three to four times higher adjusted odds of respiratory complications (Tables 3 and 4). This finding, which is consistent with prior research,20,21 suggests that the surgical procedure – as opposed to patient characteristics such as spirometry performance – is the major predictor of pulmonary complications.
Table 4.
Adjusted association of postoperative pulmonary complications with FEV1, clinical factors and ventilatory efficiency*.
| Factor | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (no CPET) (95% CI) | Adjusted Odds Ratio (with CPET) (95% CI) |
|---|---|---|---|
| FEV1 (per 1 L increase) | 1·00 (0·68 - 1·49) | 0·78 (0·48 - 1·32) | 0·79 (0·47 - 1·32; p = 0·37) |
| Ventilatory efficiency | Non-linear | N.A. | Non-linear (p = 0·02) |
| Age (per 10-year increase) | 1·04 (0·78 - 1·37) | 1·01 (0·74 - 1·38) | 0·93 (0·68 - 1·29; p = 0·68) |
| Female sex | 0·55 (0·29 - 1·08) | 0·48 (0·22 - 1·03) | 0·47 (0·21 - 1·01; p = 0·05) |
| Upper abdominal or thoracic surgery | 2·80 (1·56 - 5·03) | 2·84 (1·57 - 5·14) | 2·96 (1·62 - 5·40; p = 0·0004) |
Abbreviations: CPET, cardiopulmonary exercise test.
Regression model had an optimism-corrected c-index of 0·66 (1149 observations with 47 outcome events).
This study has several strengths. The METS study had a robust design, including standardised inclusion criteria to select elective inpatient non-cardiac surgical patients at risk for complications, a large sample in a multi-centre design, preoperative testing that was conducted in standardised fashion, blinding of treating clinicians and outcome assessors to results of preoperative investigations, minimal loss to follow-up, and statistical risk adjustment for confounding variables.15 However, this study also has certain limitations. First, the requirement that participants undergo strenuous preoperative exercise testing purely for research purposes was likely associated with self-selection bias, whereby more fit patients were more likely to participate. Consistent with this possibility, the consent rate amongst eligible patients approached for participation in the METS study was 27%.16 The exclusion of patients with FEV1 values less than 30% predicted, while instituted in the interest of patient safety, likely compounded this bias. The study cohort therefore had spirometry results with skewed distributions towards normal values. As such, the generalisability of these findings to the sicker spectrum of surgical patients requires further study. For instance, examining the comparative utility of these measures in predicting postoperative complications amongst surgical patients with respiratory comorbidities such as COPD requires further study. Second, the relatively low number of postoperative pulmonary complications events precluded it from being the primary outcome for the nested cohort analysis component of this present study. Instead, the primary outcome was respiratory morbidity, which was defined based on POMS as the de novo oxygen requirement following surgery.18 While this primary outcome has limitations in that oxygen may be prescribed based on clinical judgement for different reasons, the endpoint still has similarities to the contemporary definition of postoperative respiratory failure.20 Finally, the comparisons of FEV1, FEV1/FVC, peak oxygen consumption, ventilatory efficiency, respiratory morbidity and postoperative pulmonary complications necessitated multiple comparisons. No statistical correction was employed, as these comparisons are not independent and were planned a priori. Furthermore, the consistent associations observed regardless of how these parameters were expressed provide support to study findings.
Overall, our findings highlight limitations in using a single investigation such as spirometry in predicting complications following major surgery. What is warranted is a comprehensive approach that considers the patient's fitness, specialised investigations and especially the planned surgical procedure to generate complication-specific risk profiles. Further work is needed to translate these risk profiles to tailored perioperative management that ultimately improves outcomes amongst surgical patients.
Author contribution statement
All authors contributed to the design of the study. AS, KET, and DNW analysed the data set. All authors contributed to the interpretation of the data. AS and DNW wrote the first draft of the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. DNW obtained funding for the study. All authors have read and agreed to the final version of the manuscript.
Declaration of Competing Interest
Dr. Granton has received support, through his hospital's charitable foundation, from unrestricted grants from Bayer and Jennsen Pharmaceuticals to support research, quality improvement projects, and development of educational tools. He has also received payment for participation in steering and adjudication committees of clinical trials sponsored by United Therapeutics and Bellerophon. The other authors have nothing to disclose.
Acknowledgments
Acknowledgement
The authors also thank all the participating patients and staff across the 25 study sites.
Funding statement
The METS study was supported by grants from the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, United Kingdom (UK) National Institute of Academic Anaesthesia, UK Clinical Research Collaboration, Australian and New Zealand College of Anaesthetists, and Monash University (Melbourne, Victoria, Australia). These sponsors had no role in the design and conduct of this substudy; collection, management, analysis and interpretation of the data; preparation, review or approval of this paper; and decision to submit this manuscript for publication.
Dr. Wijeysundera and Dr. Gershon are supported in part by New Investigator Awards from the Canadian Institutes of Health Research. Dr. Wijeysundera is supported in part by a Merit Award from the Department of Anesthesiology and Pain Medicineology anbd at the University of Toronto, and the Endowed Chair in Translational Anesthesiology Research at St. Michael's Hospital and University of Toronto.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100396.
Appendix. Supplementary materials
References
- 1.Fleischmann K.E., Goldman L., Young B., Lee T.H. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med. 2003;115:515–520. doi: 10.1016/s0002-9343(03)00474-1. [DOI] [PubMed] [Google Scholar]
- 2.International Surgical Outcomes Study (ISOS) group. Prospective observational cohort study on grading the severity of postoperative complications in global surgery research. Br J Surg. 2019;106:e73–e80. doi: 10.1002/bjs.11025. [DOI] [PubMed] [Google Scholar]
- 3.Weiser T.G., Haynes A.B., Molina G. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385:S11. doi: 10.1016/S0140-6736(15)60806-6. [DOI] [PubMed] [Google Scholar]
- 4.Canet J., Gallart L... Postoperative respiratory failure: pathogenesis, prediction, and prevention. Curr Opin Crit Care. 2014;20:56–62. doi: 10.1097/MCC.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 5.Ferreyra G., Long Y., Ranieri V.M. Respiratory complications after major surgery. Curr Opin Crit Care. 2009;15:342–348. doi: 10.1097/MCC.0b013e32832e0669. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrino R., Viegi G., Brusasco V. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 7.Kallianos A., Rapti A., Tsimpoukis S. Cardiopulmonary exercise testing (CPET) as preoperative test before lung resection. In Vivo (Brooklyn) 2014;28:1013–1020. [PubMed] [Google Scholar]
- 8.McAllister D.A., Wild S.H., MacLay J.D. Forced expiratory volume in one second predicts length of stay and in-hospital mortality in patients undergoing cardiac surgery: a retrospective cohort study. PLoS ONE. 2013;8:e64565. doi: 10.1371/journal.pone.0064565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saleh H.Z., Mohan K., Shaw M. Impact of chronic obstructive pulmonary disease severity on surgical outcomes in patients undergoing non-emergent coronary artery bypass grafting. Eur J Cardiothorac Surg. 2012;42:108–113. doi: 10.1093/ejcts/ezr271. [DOI] [PubMed] [Google Scholar]
- 10.De Hert S., Imberger G., Carlisle J. Preoperative evaluation of the adult patient undergoing non-cardiac surgery: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:684–722. doi: 10.1097/EJA.0b013e3283499e3b. [DOI] [PubMed] [Google Scholar]
- 11.De Hert S., Staender S., Fritsch G. Pre-operative evaluation of adults undergoing elective noncardiac surgery: updated guideline from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2018;35:407–465. doi: 10.1097/EJA.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 12.Qaseem A., Snow V., Fitterman N. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144:575–580. doi: 10.7326/0003-4819-144-8-200604180-00008. [DOI] [PubMed] [Google Scholar]
- 13.Smetana G.W., Lawrence V.A., Cornell J.E. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581–595. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y.J., Macera C.A., Addy C.L., Sy F.S., Wieland D., Blair S.N. Effects of physical activity on exercise tests and respiratory function. Br J Sports Med. 2003;37:521–528. doi: 10.1136/bjsm.37.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijeysundera D.N., Pearse R.M., Shulman M.A. Measurement of Exercise Tolerance before Surgery (METS) study: a protocol for an international multicentre prospective cohort study of cardiopulmonary exercise testing prior to major non-cardiac surgery. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijeysundera D.N., Pearse R.M., Shulman M.A. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet. 2018;391:2631–2640. doi: 10.1016/S0140-6736(18)31131-0. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society and American College of Chest Physicians ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 18.Grocott M.P., Browne J.P., Van der Meulen J. The Postoperative Morbidity Survey was validated and used to describe morbidity after major surgery. J Clin Epidemiol. 2007;60:919–928. doi: 10.1016/j.jclinepi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Davies S.J., Francis J., Dilley J., Wilson R.J., Howell S.J., Allgar V. Measuring outcomes after major abdominal surgery during hospitalization: reliability and validity of the Postoperative Morbidity Survey. Perioper Med (Lond) 2013;2:1. doi: 10.1186/2047-0525-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canet J., Sabaté S., Mazo V. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: a prospective, observational study. Eur J Anaesthesiol. 2015;32:458–470. doi: 10.1097/EJA.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 21.Canet J., Gallart L., Gomar C. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 22.Tomlinson G., Detsky A.S... Composite end points in randomized trials: there is no free lunch. JAMA. 2010;303:267–268. doi: 10.1001/jama.2009.2017. [DOI] [PubMed] [Google Scholar]
- 23.Streiner D.L., Norman G.R., Cairney J. 5th Edition. Oxford University Press; Oxford, UK: 2014. Health measurement scales: a practical guide to their development and use. [Google Scholar]
- 24.Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 25.Duong M., Islam S., Rangarajan S. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): an international, community-based cohort study. Lancet Glob Health. 2019;7:e613–e623. doi: 10.1016/S2214-109X(19)30070-1. [DOI] [PubMed] [Google Scholar]
- 26.Wannamethee S.G., Shaper A.G., Papacosta O., Lennon L., Welsh P., Whincup P.H. Lung function and airway obstruction: associations with circulating markers of cardiac function and incident heart failure in older men-the British Regional Heart Study. Thorax. 2016;71:526–534. doi: 10.1136/thoraxjnl-2014-206724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaz Fragoso C.A., Enright P.L., McAvay G., Van Ness P.H., Gill T.M. Frailty and respiratory impairment in older persons. Am J Med. 2012;125:79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassel E., Stensvold D., Halvorsen T., Wisløff U., Langhammer A., Steinshamn S. Association between pulmonary function and peak oxygen uptake in elderly: the Generation 100 study. Respir Res. 2015;16:156. doi: 10.1186/s12931-015-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pastré J., Prévotat A., Tardif C., Langlois C., Duhamel A., Wallaert B. Determinants of exercise capacity in cystic fibrosis patients with mild-to-moderate lung disease. BMC Pulm Med. 2014;14:74. doi: 10.1186/1471-2466-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pianosi P., LeBlanc J., Almudevar A. Relationship between FEV1 and peak oxygen uptake in children with cystic fibrosis. Pediatr Pulmonol. 2005;40:324–329. doi: 10.1002/ppul.20277. [DOI] [PubMed] [Google Scholar]
- 31.Apostolo A., Laveneziana P., Palange P. Impact of chronic obstructive pulmonary disease on exercise ventilatory efficiency in heart failure. Int J Cardiol. 2015;189:134–140. doi: 10.1016/j.ijcard.2015.03.422. [DOI] [PubMed] [Google Scholar]
- 32.Schwaiblmair M., Faul C., von Scheidt W., Berghaus T.M. Ventilatory efficiency testing as prognostic value in patients with pulmonary hypertension. BMC Pulm Med. 2012;12:23. doi: 10.1186/1471-2466-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eapen M.S., Grover R., Ahuja K., Williams A., Sohal S.S. Ventilatory efficiency slope as a predictor of suitability for surgery in chronic obstructive pulmonary disease patients with lung cancer. Ann Transl Med. 2016;4:296. doi: 10.21037/atm.2016.07.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikolopoulos I., Ellwood M., George M., Carapeti E., Williams A. Cardiopulmonary exercise testing versus spirometry as predictors of cardiopulmonary complications after colorectal surgery. European Surgery. 2015;47:324–330. [Google Scholar]
- 35.Dronkers J.J., Chorus A.M., van Meeteren N.L., Hopman-Rock M. The association of pre-operative physical fitness and physical activity with outcome after scheduled major abdominal surgery. Anaesthesia. 2013;68:67–73. doi: 10.1111/anae.12066. [DOI] [PubMed] [Google Scholar]
- 36.Fleisher L.A., Fleischmann K.E., Auerbach A.D. ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;130:e278–e333. doi: 10.1161/CIR.0000000000000106. 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.