The pathophysiology of SARS-CoV2 is becoming clearer with a number of recent reports in the literature after the fast viral outbreak in China and recently in other countries worldwide [1]. The major symptoms is severe acute respiratory syndrome due to virus interaction with cells expressing angiotensin-converting enzyme 2 (ACE2) and TMPRSS2, triggering important immune response with the generation of cytokines and chemokines, which attract monocytes, macrophages and T cells promoting further inflammation [2]. This may lead to further accumulation of immune cells in the lungs, with overproduction of pro-inflammatory cytokines and activation of complement and coagulation systems in lung microcirculation [3,4]. This important immune response attracts virus-specific T cells to the site of infection, where they can eliminate the infected cells. Alveolar macrophages can then recognize neutralized viruses and apoptotic cells and clear them by phagocytosis, resulting in recovery [5]. Despite these pathophysiological mechanisms are under continuous investigation, there are only a few reports on the clinical course during the recovery phase of the disease. Recent investigations [6,7] show that lung abnormalities on chest computerized tomography (CT) show the greatest severity approximately 6–11 days after the initial onset of symptoms, while in the following weeks recovery occurs consistently and in about 30 days lung tissue lesions are almost absorbed [8]. However, extended observation on COVID-19 patient during recovery are still object of investigation.
More than 2,000 patients were admitted in our hospital (Bolognini Hospital, ASST Bergamo Est) during the COVID-19 outbreak, and we recently started to follow them during recovery. We reviewed CT findings in some of these patients in which CT scans were used to investigate potential thrombotic events with and without contrast media. We report here the preliminar results of these investigations in relation to lung perfusion conditions. We did identify perfusion defects in lungs of these patients, more than one month after remission of the symptoms, that deserve attention.
1. Patient population
During the period from April 15 to 30, twenty patients (12 male and 8 female; age range 35–86, mean age 58±10) previously treated for pneumonia SARS-CoV-2, with negative swab, have been admitted to our department to assess the outcome of SARS-CoV-2 pneumonia. Chest CT was performed at an interval between 14 and 60 days after remission of the fever (average 40±13 days). Twelve patients reported an almost complete resolution of the symptoms, while 8 patients reported residual dyspnea. Of these 8 patients, 3 reported dyspnea with minimal motor activity, while in the others after prolonged effort. Control CT was performed in 5 patients with contrast media for suspected thromboembolism (including those with dyspnea with minimal motor activity, or patients with D-Dimer value over normal range). Four patients not affected by COVID-19, studied by angioCT scan for other clinical indications and with normal lung tissue, were used as a control group. Ethical approval of this retrospective evaluation was obtained by the Bergamo province Ethics Committee (Reg. Sperim. N. 80/20 on 22/04/2020).
2. Chest CT acquisitions
Conventional chest CT was performed with the patient in the supine position during end-inspiration (CT 128 slice Ingenuity, Philips, Amsterdam, The Netherlands). The chest CT protocol used is as follows: from apices to mid-renal; slice thickness 1 mm; slice increment 1 mm; pitch 0.94; Rotation time 0.5 s; Field of view 411 mm; Voltage 120 kV; mAs modulation 100−200 mA. The contrasted acquisition consisted in arterial (80 mL/Iomeron 400, injection 4 mL/s in the antecubital vein; threshold 90 HU) and venous phase acquisition (60 s from the threshold). Images were viewed using IMPAX version 6.6.1 (Agfa-Gevaert NV Septestraat 27B-2640 Mortsel - Belgium). Every chest CT examination was evaluated by two double-blind experienced radiologists. The lung perfusion evaluation was performed by using PAA (Pulmonary Artery Analysis) Software [9] using Hounsfield Units (HU)-based colormap visualization tool (color palette ranging from −749 to −983 HU). The threshold level identified for normal lung tissue perfusion in control subjects was equal to −890 HU, with lower attenuation values considered as hypoperfusion.
We quantified the volume of low perfusion lung tissue, independently by the amount of contrast media, using the following method. The previously mentioned range of HU for hypoperfused threshold (HU < −890) in the control group was on average 13.3 % of the total HU range air to contrast media in pulmonary artery. We then assumed this percentage, and calculated patient-specific threshold for hypoperfused tissue, on the basis of HU range from air to pulmonary artery. To estimate the percentage of lung volume characterized by hypoperfusion, we segmented the lung volume of the CT scans by automatic lung segmentation using the u-net (R231) convolutional network [10], a model trained on a large dataset including COVID-19 CT slices. Segmentation on individual slices allowed to extract the right and left lung mask separately, with the trachea not included in the lung segmentation. We labeled each pixel inside the lung mask as low perfusion or normal perfusion based on the patient-specific thresholds using Python implementation of SimpleITK library (https://simpleitk.org/about.html).
3. Results
Out of the 20 patients who underwent follow-up, 12 patients had complete resolution of symptoms, with complete regression of the thickening areas detected with chest CT scan in 4 patients, and persistence of fibrous stripes areas in the other 8 patients. In the remaining patients, 5 reported dyspnea after prolonged effort, with 3 of them with complete remission of interstitial pneumonia and 2 with only partial remission. The 3 other patients reported dyspnea with minimal effort but showed a completely normal CT scan and remission of parenchymal opacity. These results indicate that persistent dyspnea is not associated with signs of interstitial pneumonia.
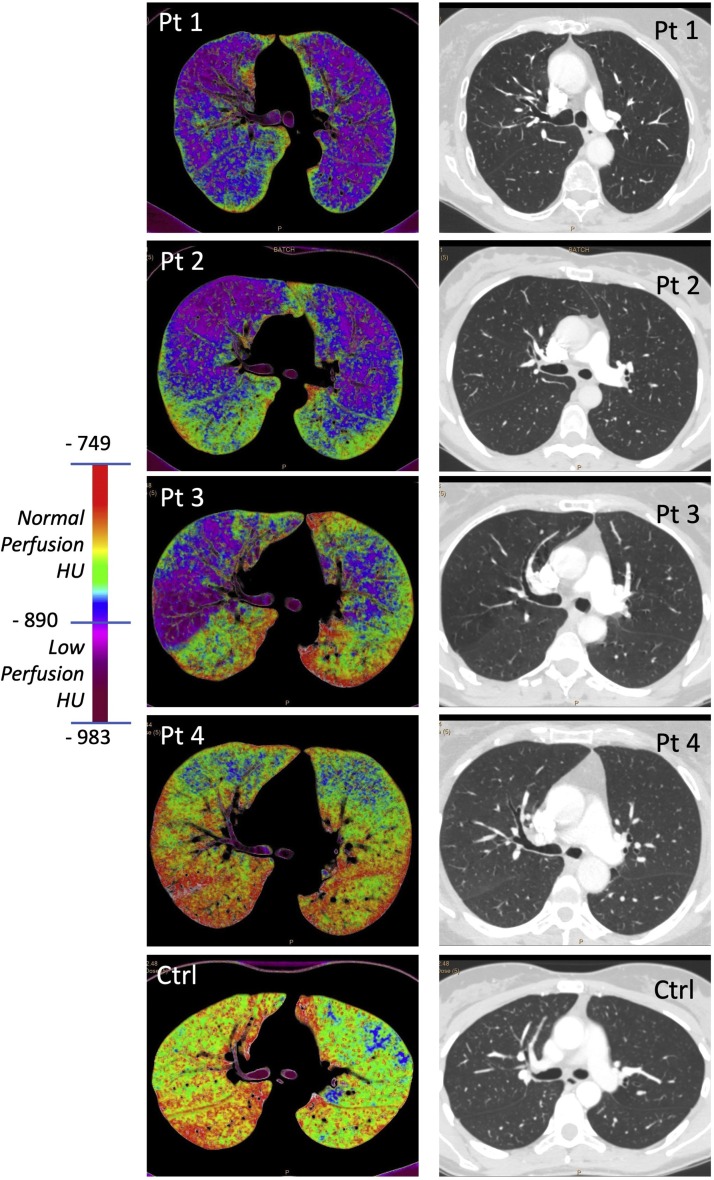
In the 5 patients studied with contrast media, 3 were those that reported dyspnea with minimal effort, while the other 2 had no such symptom. At CT evaluation, no signs of thromboembolism were present in all these patients. Of interest, in the 3 patients affected by dyspnea color map representation (see Fig. 1 ) showed diffuse hypoperfused areas (dark red/violet) not uniform between the left and right lung. Quantification of hypoperfused lung volume showed that, while in normal controls this volume was in average 8.5 %, in these patients, that reported dyspnea, the volume ranged from 21.0 % to 48.4 % (Table 1 ). Of interest, tissue lung perfusion was normal in the two patients without dyspnea (see Table 1 and Fig. 1), with hypoperfused lung volume of 7.4 % and 8.5 %, respectively. Thus, the presence of dyspnea was associated with hypoperfused lung volume rather than with abnormal lung ventilation at CT.
Fig. 1.
Contrast and non-contrast enhanced CT in COVID-19 patients and in normal control. Representative images of lung perfusion map and CT in COVID-19 patients (Pt1-4) and in a normal control (Ctrl). Perfusion maps of Pt1, 2 and 3 that were affected by dyspnea show areas of low perfusion, while Pt4 that was not affected by dyspnea shows normal perfusion map, comparable to a normal control (Ctrl). Pulmonary CT was normal in all CT scans.
Table 1.
Percentage of lung volume occupied by hypoperfused tissue in COVID-19 patients and in controls.
| Subject | Left Lung | Right Lung | Total | Time after symptoms remission (days) | Patient symptoms |
|---|---|---|---|---|---|
| Pt 1 | 19.0 % | 29.3 % | 48.4 % | 54 | Dyspnea ++ |
| Pt 2 | 15.4 % | 11.0 % | 26.4 % | 30 | Dyspnea ++ |
| Pt 3 | 13.5 % | 7.5 % | 21.0 % | 30 | Dyspnea ++ |
| Pt 4 | 4.9 % | 2.4 % | 7.4 % | 25 | – |
| Pt 5 | 3.7 % | 4.7 % | 8.5 % | 36 | – |
| Controlsa | 4.3 ± 3.2 % | 4.0 + 2.7 % | 8.5 + 5.8 % | – |
Mean + SD of 4 normal controls.
4. Discussion
The lung tissue inflammation induced in COVID-19 patients by accumulation of immune cells, overproduction of pro-inflammatory cytokines and activation of complement and coagulation system in the microcirculation correlate with the initial appearance of areas of increased lung density with the “ground glass” appearance, which gradually tends to consolidate in areas preferentially located in the peripheral subpleural regions. Four stages of infection were recently proposed: early, progression, peak, and resolution [11], and in the later stage of the infection, the pattern referred to as “crazy paving” and “reversed halo sign” have been found more frequently [11]. Little is known, however, on the recovery phase of the disease. Our preliminar retrospective analysis, based on volumetric image processing, show that in symptomatic patients (dyspnea) one moths after remission from fever, despite the absence of pulmonary fibrous stripes residues, extended hypoperfused areas of lung parenchyma are still present. These findings suggest that during the recovery of COVID-19 important defects in lung blood perfusion of the microcirculation may persist even with normal lung CT.
To our knowledge, this is the first report on quantitative estimation of lung microvasculature defect, without thromboembolism, in COVID-19 patients due incomplete healing of alveolar parenchyma microcirculation that persist during recovery. Thus, while the lung tissue damage related to interstitial fluid accumulation and impairment of ventilation is almost completely recovered within one month, a persistent defect of the microcirculation may remain, likely due to residual of viral-induced inflammation, with immune cell accumulation, platelet adhesion and micro-disseminated thrombi. Whether also fibrosis may be responsible for these microcirculation perfusion defects is worth to investigate. While these complications of the disease are now well recognized during the acute phase of the infection [[12], [13], [14]], it is important to notice that this impairment of the microcirculation, we have detected, may affect lung function even in the recovery phase and may last for a long time or even not be healed with time. This evidence should be carefully considered, due to the potential clinical relevance of the problem in the large actual number of patients affected by the SARS-CoV-2 infection worldwide.
In summary, the main message from our observation is that in discharged COVID-19 patients still reporting dyspnea, chest CT should be used to quantify the presence of lung perfusion dysfunction. This is important not only for diagnosis, but also to determine the incidence of these complications, whether this damage to the lung microcirculation will resolve with time and the need for specific pharmacological interventions. Deeper knowledge of these pathological processes it is very important for this increasing in size patient population due to the ongoing SARS-CoV-2 outbreak. While extensive investigation with CT in discharged COVID19 patients is in progress by our center and by others, we believe it is urgent to draw attention to these lung complications.
Declaration of Competing Interest
Authors declare that they have no competing interests.
References
- 1.WHO . 2020. Coronavirus Disease (COVID-2019) Situation Reports. Situation Report—114. May 13, 2020.https://www.who.int/docs/default-source/coronaviruse/situation-reports published online. [Google Scholar]
- 2.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. published online April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H., Liu L., Zhang D. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dou P., Zhang S., Wang C. Serial CT features in discharged COVID-19 patients with positive RT-PCR re-test. Eur. J. Radiol. 2020;127 doi: 10.1016/j.ejrad.2020.109010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belfiore M.P., Urraro F., Grassi R. Artificial intelligence to codify lung CT in Covid-19 patients. Radiol. Med. 2020;125:500–504. doi: 10.1007/s11547-020-01195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Dong C., Hu Y. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020 doi: 10.1148/radiol.2020200843. Online ahead of print: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan F., Ye T., Sun P. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahiji K., Kligerman S., Jeudy J., White C. Improved accuracy of pulmonary embolism computer-aided detection using iterative reconstruction compared with filtered back projection. Am. J. Roentgenol. 2014;203:763–771. doi: 10.2214/AJR.13.11838. [DOI] [PubMed] [Google Scholar]
- 10.Hofmanninger J., Prayer F., Pan J., Rohrich S., Prosch H., Langs G. Automatic lung segmentation in routine imaging is a data diversity problem, not a methodology problem. arXiv. 2020 doi: 10.1186/s41747-020-00173-2. [physics, stat]; published online January 31. http://arxiv.org/abs/2001.11767 (Accessed 15 May 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zu Z.Y., Jiang M.D., Xu P.P. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020 doi: 10.1148/radiol.2020200490. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucharski A.J., Russell T.W., Diamond C. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect. Dis. 2020;20:553–558. doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry B.M., Vikse J., Benoit S., Favaloro E.J., Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin. Chim. Acta. 2020;507:167–173. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ierardi A.M., Angileri S.A., Arrichiello A., Di Meglio L., Gurgitano M., Rodà G.M., Carrafiello G. Pulmonary embolism in COVID-19: ventilation and perfusion computed tomography. IDCases. 2020;21 doi: 10.1016/j.idcr.2020.e00805. [DOI] [PMC free article] [PubMed] [Google Scholar]