Abstract
Point-of-care (POC) diagnostic device is an instrument that is used to acquire particular clinical information of patients in clinical as well as resource-limited settings. The conventional clinical diagnostic procedure requires high-end and costly instruments, an expert technician for operation and result interpretation, longer time, etc. that ultimately makes it exhausting and expensive. Although there are a lot of improvements in the medical facilities in the Indian healthcare system, the use of POC diagnostic devices is still in its nascent phase. This review illustrates the status of POC diagnostic devices currently used in clinical setups along with constraints in their use. The devices and technologies that are in the research and development phase across the country that has tremendous potential to elevate the clinical diagnostics scenario along with the diagnosis of ongoing COVID-19 pandemic are emphasized. The implications of using POC diagnostic devices and the future objectives for technological advancements that may eventually uplift the status of healthcare and related sectors in India are also discussed here.
Keywords: Point-of-care diagnostic devices, POC test, Resource-limited settings, Clinical diagnostics, COVID-19 pandemic, Indian healthcare system
Graphical abstract
Highlights
-
•
Need for POC devices in the Indian healthcare system.
-
•
Barriers in using POC devices.
-
•
Categorical classification of POC devices used in clinical settings.
-
•
Current Indian research and developments in POC diagnosis along with update on COVID-19.
1. Introduction
Point-of-care (POC) testing refers to obtaining particular clinical data and parameters where the patient is examined. There are numerous definitions for POC, all of them explaining a similar thing, i.e. quick acquisition of test results so that the patient can be treated in the right direction at the earliest. POC diagnostic devices are used by healthcare professionals, patients, and their families because of their user-friendly features. This ultimately results in a reduction of the turn-around time (TAT), which is the time duration between sample registrations to reporting of results. In a developing country such as India, POC tests, and devices play a pivotal role in the healthcare system. The POC testing was introduced in 1962 with the development of a method for rapid analysis of blood glucose [1], and shortly after in 1977, rapid pregnancy tests were developed that pioneered a trend for personalized diagnostics [2]. These developments introduced POC testing and devices to clinical settings and laboratories around the globe that changed the diagnostics field tremendously. Nowadays, POC diagnostic devices are used widely and available for a large variety of analytes; its utility ranges from intensive care units to outpatient clinics to personal care.
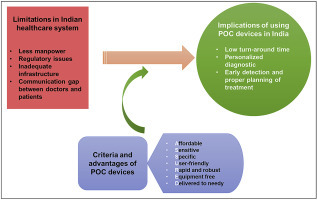
The medical facilities in India represent a very complex scenario as the country accommodates the most diverse population (about 1.38 billion) in the world [3]. Certainly, in a developing country, this huge population is one of the major reasons for the average quality of healthcare facilities along with the overall financial status. In terms of availability of healthcare personnel, it is estimated that there is only 1 doctor per 1666 people in India [4]. Because of this highly skewed ratio, healthcare professionals are under the tremendous burden that causes inefficiency and underperformance affecting the lives of millions. India is a resource-limited country in terms of healthcare and advancements, thus, the utility of POC diagnostic devices on broad-scale promises benefits and helps in uplifting the healthcare status of the large population. Fig. 1 describes limitations in the Indian healthcare system and the implications of using POC diagnostic devices in India. Despite the global advancements in laboratory-based clinical diagnostics technologies, developing countries such as India are facing problems in obtaining high-end, automated, and expensive instruments. These instruments need regular maintenance by skilled professionals, which adds up to the overall cost of diagnosis, ultimately burdening the end-user i.e. patient. This has resulted in the inaccessibility of clinicians as well as patients to advanced medical technologies [5]. Recognizing these discrepancies, World Health Organization (WHO) has laid down ‘ASSURED’ criteria for POC tests in resource-limited settings; (i) affordable (by those at risk), (ii) sensitive (few false negatives), (iii) specific (few false positive), (iv) user-friendly (simple to perform and needs minimum training), (v) rapid (producing results within few minutes) and robust (rigid storage conditions), (vi) equipment free (no complex system), (vii) delivered (to needy) [5]. Therefore, the devices fulfilling the above criteria have the potential application in the Indian healthcare system.
Fig. 1.
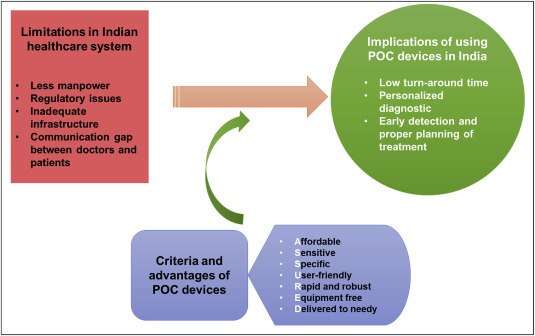
Implications of using POC devices.
This review illustrates the status of POC diagnostic devices that are currently used in Indian clinical setups along with constraints in their use. Technical details and various applications of POC diagnostic devices are discussed along with marketed products. The POC diagnostic devices and technologies that are in the development phase in India that has tremendous potential to impact the clinical diagnostics scenario are also emphasized. The importance of POC diagnostic devices in combating the current COVID-19 pandemic has also been discussed. The issues addressed in this review throw light on the status of the Indian healthcare system that may encourage various stakeholders to prioritize the focus on applications and utilization of POC diagnostic devices.
2. Prevailing status of POC tools in the Indian healthcare system
India is a growing economy and a hotspot for market investments; however, it is constantly facing challenges concerning the public healthcare system. The annual budgetary allocation towards the betterment of medical facilities lacks major support along with regulatory oversight on fulfilling the requirement of competent manpower. The country has a huge resource gap of over 4 million health workers; nearly 60% of the existing personnel work in urban areas whereas 70% of the Indian population lives in rural areas [6]. This scenario makes it even challenging to deliver and maintain the healthcare facilities in resource-limited rural areas. POC diagnostic devices have been used as a preliminary step in clinical diagnosis that can help in the reduction of burden on advanced medical ecosystem. With a rapid increase, the POC diagnostics market was estimated to advance at a compound annual growth rate (CAGR) of 9.3% from 2013 to 2018 and is expected to grow with 11.9% of CAGR during 2018–2023 worldwide [6,7]. Therefore, India provides a huge market potential for POC diagnostic devices industries to progress and fulfill the healthcare demand of the large population.
2.1. Need for POC diagnostic devices
POC testing devices have enhanced the diagnostic capacity for several severe diseases and disorders. Low cost is a major advantage offered by POC diagnostic devices as the large population in India cannot afford the cost of conventional diagnostics. POC tests are very convenient for continuous monitoring of particular analyte or repetitive follow-up tests to track the progress of treatment. POC tests reduce the TAT drastically, which is necessary and very effective in managing critical cases such as heart disease. Low TAT is also favorable in resource-limited settings or for patients visiting from distant places for diagnosis because they do not have to spend extra for travel if the diagnosis is completed in a single visit. Also, the diagnosis is completed in a single visit that reduces the chance of further spread of infection to the community (if the patient goes home and comes back for the results) in cases of infectious diseases. POC testing also helps in routine analysis of millions of people in resource-limited areas that are deprived of the diagnosis since it requires traveling from remote places. High-end digital platforms employed by most of the POC devices such as in blood-pressure, glucose, oxygen monitoring devices, reduce the workloads of nurses and other first-line healthcare workers. Result interpretation is carried out by automatic multiplex analytical tools of device and does not require expert technician [8]. Integrating with appropriate software, POC test results can be stored in the medical database and shared with healthcare workers through a cloud server, which benefits the patient as well as the clinicians [9].
Blood glucose level detection and cardiological analysis are the two major sectors that have contributed to the majority of the POC diagnostic market. It has been reported that POC diagnostic devices for blood-glucose monitoring proved to be the key support for diabetes control and management [10]. The coronary heart disease (CHD) is a major threat among the Indians and has reached an epidemic proportion, which is evident from the number of cases reported at the tertiary care hospitals. Moreover, the early detection of CHD among persons with risk factors is extremely essential given its control [11]. POC diagnostic devices, such as Cobas h 232 POC System and Cholestech LDX analyzer by Roche Diagnostics India Pvt. Ltd. and Abbot India Ltd., respectively, have aided in the early detection of CHD. A mobile electrocardiogram (ECG) monitoring device ‘miBEAT’ developed by Cardea Biomedical Technologies [12] has also assisted in the diagnostic procedures for CHD. The utility of POC diagnostic devices has increased the assurance in management by rapid analysis and also stimulated the penetration of POC diagnostic devices in the Indian market as well as household. Seasonal infectious diseases such as influenza, dengue, malaria, etc. have piled on top of the already menacing diseases such as tuberculosis (TB), pneumonia, urinary tract infection, thyroid dysfunction, etc. Also, most of the cases are from rural areas, which account for the majority of the population of the country. Thus, the existing diagnostics methodologies such as the high-end lab-based systems need to be updated to POC tests, which are rapid, catered to remote places, and cost-effective.
2.2. Barriers in using POC diagnostic devices
POC diagnostic devices have been designed with perspective to have applications in the clinical settings as well as in remote places. As compared to the urban area, the literacy rate is lower in rural parts of India that affects the use of POC diagnostic devices as a basic level of understanding and interpretation ability is required for its handling. Thus, most of the POC diagnostic devices are used in clinical settings. Doctors tend to trust on the diagnostics method that gives reliable results to avoid false interpretation and consequences. Although there are numerous advantages offered by POC diagnostic devices, the sensitivity of a few POC tests is uncertain. The POC diagnostic devices may require refrigeration and other conditions to be maintained for long term storage, and it may not be possible to maintain such conditions in remote places. The scenario of clinical diagnostics in India is worrying, which gets even worse when it comes to private sector hospitals. Some of the high-profile hospitals and private practicing doctors receiving incentives for recommending diagnostic tests, prescribing unnecessary tests, negligence for good clinical practices, etc. have deteriorated the situation in the country [13]. An example and consequence of such malpractice is the situation of TB in the country. TB is a major cause of mortality worldwide and WHO has declared it a global pandemic. Poor organization, unregulated primary and private healthcare, tricky guidelines in laboratory testing, unregulated use of anti-TB drugs, and most importantly unethical administration are critical challenges towards TB management in India [14]. A root-cause analysis, which is an orderly system to identify the origin of a problem and to tackle it there itself, was carried out by Jaroslawaski et al [15]; the report mentions several causes of why serological tests that are banned by WHO still being practiced in the private healthcare sector in India. Similarly, laboratory tests in India lack proper adherence to regulations that are prescribed by the Bureau of Indian Standards [16] and are not accompanied by proper infrastructure, both in the public and private healthcare sectors. It has been observed that several spurious, unapproved, and inefficient products are available in the Indian market, which further degrades the quality of diagnosis.
In the community level healthcare system, accredited social health activist, nurses at primary health care, and auxiliary nurse midwives operate and control most of the work in primary and rural hospitals. Generally, these workforces are not well trained and not authorized to carry out POC diagnosis and prescribe drugs according to the results [13]. Although the infrastructure related issues are resolved, the coordination among care providers and between patients and care providers is crucial. The unproductive association between the stakeholders is another cause of the ineffective practice of POC diagnostic devices [17]. Fig. 2 describes various perspectives of barriers in adapting POC diagnostic devices in the Indian healthcare system.
Fig. 2.
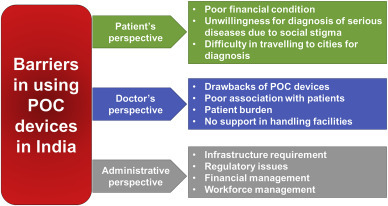
Barriers in using POC devices.
Although there are numerous challenges and barriers towards the utility of POC diagnostic devices in India, awareness about its benefits is increasing, and gradually, the healthcare system is adapting to more such valuable practices. It has been reported by Rinkoo et al [18], that going by the Tanahashi framework, which is a time verified prototype to comprehend health system efficiency, the availability of rapid tests and devices along with doctor-patient relationship have considerably improved in India. The study also reports a promising approach for developing innovative POC diagnostic devices by various stakeholders to improve the Indian healthcare scenario. The success is still not converted to substantial public health gains, suggesting impediments at different levels of the healthcare system. Thus, it is essential to position the POC diagnostic devices in the mainstream of the healthcare system to overcome the rising problems of disease management in the Indian population.
3. The utility of POC diagnostic devices in India
3.1. Types of devices and their applications
POC diagnostic devices used for monitoring ECG, oxygen saturation, blood pressure, and temperature have aided the clinical and personalized healthcare enormously [19]. Technological advancements have led to the development of several innovative POC diagnostic devices for different applications. There are a variety of devices that are used in POC settings that include disposable kits such as rapid assay dipsticks, reusable handheld devices such as glucose monitoring kit, and multifunctional benchtop devices that are mostly used in the laboratories. The POC diagnostic devices are designed with simple to complex systems and many of them are enabled with multiplex functioning. POC diagnostic devices are composed in a manner that may generate qualitative, semi-quantitative, or quantitative results depending on their application and sensitivity requirement. Some of the POC diagnostic devices are very simple and require a one-step process of a sample application to obtain the results such as rapid test pads and dipsticks, while some devices need sample processing, handling, and result interpretation steps. These devices function based on various principles and mechanisms that include colorimetry, potentiometry, fluorimetry, electrochemistry, microfluidics, etc. Fig. 3 depicts the classification of POC diagnostic devices along with examples.
Fig. 3.
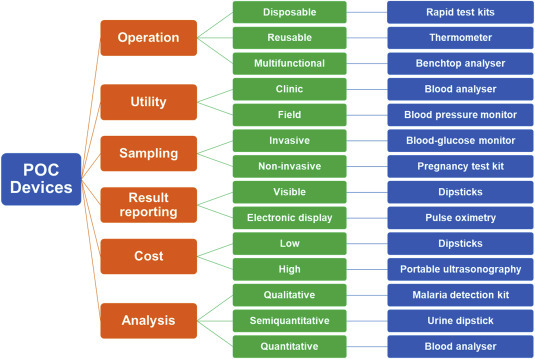
Types of POC devices.
Biosensors are the most important component in most of the POC diagnostic devices. The biosensor detects analyte with the help of a detector based on physicochemical reactions that are recognized by a biorecognition element and are transformed into a readable signal. It comprises elements such as a biorecognition element, transducer, amplifier, and a detector [20]. Nanotechnology has revolutionized the POC testing and a range of nanomaterials such as gold [21] and silver nanoparticles [22], quantum dots [23], magnetic nanoparticles [24], carbon dots [25], etc. have been used in designing the biosensors. Nanotechnology has helped the advancement of biosensing in two major ways i.e. first, by conjugation of a bio-recognition element with enhanced nanosurfaces (surface functionalization) and second, by signal amplification strategies using various nanomaterials [26].
POC diagnostic devices are categorically used in five different settings such as in homes, communities, clinics, peripheral laboratories, and hospitals [13]. Table 1 classifies the devices that are commonly used in the Indian healthcare system for a range of applications. Indian POC diagnostic devices market is majorly dominated by manufacturers such as Siemens, Roche, and Abbot. A report by Ken Research Pvt. Ltd. lists several companies that have started investing in the Indian diagnostic market [27]. TATA Elxi has developed a device that integrated the features of three distinct components such as the lancet, glucose monitoring device, and the insulin-delivery device into a single convenient accessory. It is easy-to-use, portable, and very convenient for people that need continuous blood glucose level monitoring. In 2012, Wipro has developed a wireless cardiotopograph or a fetal monitoring device for high-risk pregnancy cases. Similarly, various firms such as Yethi Medical System and Veredus Laboratories have also developed cloud-based multi-parameter diagnostic devices. In 2011, Bill and Melinda Gates Foundation donated USD 31 million for the research and development of POC diagnostic devices in developing countries [28]. This initiative was directed towards technological advances that could make diagnostic tools affordable and accessible in resource-limited areas.
Table 1.
Commonly used POC devices in the Indian healthcare system.
| Sl. No. | Application | Type of analysis | POC device used | Principle or mechanism applied in the device | Portability | Manufacturer | References |
|---|---|---|---|---|---|---|---|
| Diabetes | Glycated hemoglobin analysis (HbA1c) | DCA Vantage analyzer | HbA1c: monoclonal antibody agglutination reaction | Benchtop POC | Siemens Healthcare | [29] | |
| Glycated hemoglobin analysis (HbA1c) | Afinion HbA1c | HbA1c: monoclonal antibody agglutination reaction | Benchtop POC | Abbot India Limited | [30] | ||
| Total glucose | Cholestech LDX analyzer | Enzymatic method using glucose oxidase | Portable, lightweight | Abbot India Limited | [31] | ||
| Glucose | i-STAT Handheld using CG8+ cartridge | Sensitive biosensors on a silicon chip | Handheld | Abbot India Limited | [32] | ||
| Glucose | Cobas b 221 system | Electrochemical detection of glucose | Benchtop POC | Roche Diagnostics India Pvt. Ltd. | [33] | ||
| Glucose | HemoCue Glucose 201+ system | Micro-cuvette technology | Handheld | HemoCue India | [34] | ||
| Glycated hemoglobin analysis (HbA1c) | HemoCue HbA1c 501 | – | Benchtop POC | HemoCue India | [35] | ||
| Cardiac | Troponin analysis | Startus CS 200 Acute Care Troponin analyzer | – | Benchtop POC | Siemens Healthcare | [36] | |
| Several analytes: - Troponin T - proBNP - D-Dimer - CK-MB - M Myoglobin |
Cobas h 232 POC System | Quantitative detection of analytes | Handheld | Roche Diagnostics India Pvt. Ltd. | [37] | ||
| Troponin T | Roche cardiac troponin T sensitive test (visual) | Qualitative detection through lateral flow immunoassay | Handheld strip | Roche Diagnostics India Pvt. Ltd. | [38] | ||
| Total cholesterol, triglycerides, | Cholestech LDX analyzer | Enzymatic method and solid phase technology | Portable, lightweight | Abbot India Limited | [31] | ||
| Cardiac markers such as CK-MB, BNP | i-STAT Handheld using CG8+ cartridge | Sensitive biosensors on a silicon chip | Handheld | Abbot India Limited | [39] | ||
| Coagulation | Prothrombin time/international normalized ratio (PT/INR) | Xprecia stride coagulation analyzer | electrochemical detection using reagent test strips | Handheld | Siemens Healthcare | [40] | |
| PT/INR, activated clotting time (ACT) kaolin, ACT celite | i-STAT Handheld using CG8+ cartridge | Sensitive biosensors on a silicon chip | Handheld | Abbot India Limited | [39] | ||
| PT and activated partial prothrombin time (aPTT) | CoaguCheck Pro II | Amperometric (electrochemical) determination | Handheld | Roche Diagnostics India Pvt. Ltd. | [41] | ||
| PT/INR | CoaguCheck XS system | Amperometric (electrochemical) determination | Handheld | Roche Diagnostics India Pvt. Ltd. | [42] | ||
| Hematology | Hemoglobin and whole blood CBS | HemoCue Hb 201+ system | – | Handheld | HemoCue India | [43] | |
| Low levels of hemoglobin | HemoCue Plasma/Low Hb system | Micro-cuvette technology | Handheld | HemoCue India | [44] | ||
| Hemoglobin | i-STAT Handheld using CG8+ cartridge | Sensitive biosensors on a silicon chip | Handheld | Abbot India Limited | [39] | ||
| Oncology | Nuclear matrix protein (NMP22) in urine | Alere NMP22 Bladdercheck | Lateral flow immunochromatographic strip (colloidal gold nanoparticles) | Handheld | Abbot India Limited | [45] | |
| Head domain of nuclear mitotic apparatus protein (NuMA) | Alere NMP22 test | Enzyme immunoassay using 96 well plate | Portable (test kit) | Abbot India Limited | [46] | ||
| Urinalysis | Albumin, bilirubin, creatinine, glucose, ketone, leukocytes, nitrite, pH, protein, specific gravity, and urobilinogen | Clinitek status+ analyzer | – | Benchtop | Siemens Healthcare | [47] | |
| Leukocyte, Nitrite, Protein, Blood, Glucose, Ketone, Bilirubin, Urobilinogen, pH, Specific Gravity, Creatinine, and Protein-to-Creatinine Ratio | Clinitek Advantus Urine Chemistry Analyzer | – | Benchtop | Siemens Healthcare | [48] | ||
| Urea nitrogen/Urea, Creatinine | i-STAT Handheld using CHEM8+ cartridge | Sensitive biosensors on a silicon chip | Handheld | Abbot India Limited | [39] | ||
| Infectious diseases | Antibodies (IgG and IgM) to dengue virus in human blood | Panbio Dengue duo cassette | Immunochromatographic assay | Portable | Abbot India Limited | [49] | |
| Dengue virus Ns1 antigen and antibodies to dengue virus | SD Bioline Dengue duo | Immunochromatographic assay | Portable | Abbot India Limited | [50] | ||
| E. coli- Shiga toxin from fecal samples | Shiga Toxin Quik Chek | Immunochromatographic assay | Portable | Abbot India Limited | [51] | ||
| HIV- All the groups of HIV-1/2 | Alere determine HIV-1/2 | Lateral flow immunoassay (colloidal gold nanoparticles) | Portable | Abbot India Limited | [52] | ||
| Rapid drug screening | Drug screening and detection in the oral fluid at workplace and vehicles | SoToxa – Mobile test system | – | Portable | Abbot India Limited | [53] |
3.2. Research and developments in POC diagnostic devices
Rising demand for rapid diagnostics in the healthcare sector has led to the research and development of numerous POC diagnostic devices and technologies. Researchers across the globe are studying and reporting novel techniques that have the potential to be translated into POC diagnostic devices. In the case of developing countries, apart from blood-glucose and cardiac biomarkers sensing there is a huge need for the POC technique for the detection of infectious diseases [54]. The pathogen can transform itself to invade or bypass the human immune system and thus, pose a major threat to human health. The development and utility of POC diagnostics for this area are extremely necessary and supportive to control and manage the infection. Globally, researchers are working on advancements in biosensors, microfluidics, bioanalytical platform, assay formats, lab-on-a-chip technologies, and huge potential is observed in the smartphone-based biosensors that can be used in POC diagnostic devices [55,56]. Similarly, in the Indian scenario, scientists and entrepreneurs have made a huge impact on the development of next-generation POC diagnostic devices, which are based on the latest technologies such as a smartphone- and cloud-based devices.
Research related to a cancer diagnosis has been in the focus as the disease load is very high in India. Nanobiosensors have found to be an excellent material for ultra-sensitive POC diagnosis of various types of cancers. It enables the development of superior and advanced diagnostic techniques as compared to the conventional methods [57]. Recently, Singh et al [58] have developed an excellent graphene-based biosensor for the detection of cancer biomarker i.e. carcinoembryonic antigen (CEA). A multiwalled-carbon-nanotubes embedded zinc oxide nanowire was used as a sensing platform for the detection of antigen-125 (ovarian cancer biomarker) [59]. In another study by Kumar et al [60], a highly sensitive paper-based biosensor was developed using a polymer and reduced graphene oxide composite for the detection of CEA, which was found to be cheaper, flexible and eco-friendly. Apart from cancer research, several research groups in India are working on the development of various POC diagnostics devices. Singh et al [61] have developed a biosensor for detection of cholesterol using enzyme immobilized on polyaniline films. In another study, same group have reported amperometric method for detection of cholesterol using biosensor developed by immobilizing cholesterol esterase and cholesterol oxidase onto polypyrrole films [62]. Chaudhari et al [63] have developed the uric acid biosensing assay using chemiluminescent alginate microspheres. This is an indirect enzymatic assay based on hydrogen peroxide production, which is correlated with the concentration of uric acid in the sample. DNA hybridization technique has been reported for the detection of dengue virus using electrospun nanofibers [64]. Malik et al [65] have reported estimation of fasting blood glucose levels using a mathematical algorithm that integrates sensing of various electrochemical parameters. An impedance spectroscopic technique-based immunosensor has been reported for the quick analysis of Salmonella typhi using gold nanoparticles [66].
Lateral flow immunoassay (LFIA)-based diagnostic tools have gained a lot of importance due to its simplicity and rapidity [67,68]. A fluorescent LFIA-based POC test was developed by Borse et al [69] for the diagnosis of orthopedic implant-associated infections. The LFIA used a double antibody sandwich technique utilizing fluorescent cadmium telluride quantum dots. In another work, various process parameters for LFIA development such as various combinations of strip component assembly, the morphology of nitrocellulose membrane, flow time and flow pattern of fluid through the strip, etc. were studied [70]. This study emphasized the optimization of different parameters for the development of LFIA with improved sensitivity. Fluorescent ‘turn-on’ biosensor has been developed for the detection of inorganic phosphate in human serum [71]. Similarly, a dual-sensing platform has been reported for analysis of glutathione and silver using carbon dots, which also have applications in mammalian cell imaging [72]. A portable fluorescence reader (PorFloRTM) and similar devices have been developed that can read the fluorescence signal on the LFIA strips [73,74]. In another work, an immunochromatographic strip was developed using colloidal gold nanoparticles to analyze 17-α-hydroxyprogesterone in serum [75]. The graphene-based nanocomposites are found to be an excellent material for surface plasmon resonance-based analysis, which have the potential for futuristic development of graphene nanosensor-based POC diagnostic devices [76]. Advancement in the area of paper-based diagnostic devices has led to the development of simple POC test platforms. These devices utilize the different forms and designs of paper to carry out multistep fluidic processes by means of wicking activity of absorbent material. Researchers have developed a POC nucleic acid amplification tests (NAATs), wherein steps such as lysis of infectious agents, purification of nucleic acids, amplification, and detection are performed on a single paper strip [77].
Electrochemical biosensors have been developed for the rapid and quantitative analysis of various entities. Recently, an electrical biosensor-based POC testing device is developed for the detection of the α-amylase enzyme in human serum [78]. An amperometric biosensor was developed by Chandra et al [79], which uses dsDNA and cardiolipin modified screen-printed electrodes in association with a microfluidic channel for the detection of anticancer drugs in urine samples. In another work reported by Akhtar et al [80], a dual probe was fabricated using conducting layers of gold nanoparticles onto which the capture antibodies were immobilized, and in the second probe, a bioconjugate of detection antibodies with gold nanoparticles was prepared. Each sensor was placed in microfluidic channels that showed excellent detection ranges for brain-derived neurotrophic factors (BDNF), which are released from cancer cells. In a study by Choudhary et al [81], an electrochemical immunosensor was developed for non-invasive detection of oral cancer marker CD 59. CD 59 antibody was immobilized on the gold electrode treated with l-cysteine and this immunosensor probe was used to detect CD 59 qualitatively in human saliva samples. Thus, from the literature reports, it is evident that many researchers in India are working on the development of POC diagnostic devices. The research reports promise a great future and in fact, there are numerous reports of these technologies being translated and commercialized.
4. POC diagnostics for COVID-19
Recently, the WHO has declared public health emergency concerned with an outbreak of novel coronavirus (2019-nCoV) i.e. severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). WHO has formally termed the disease as coronavirus disease 2019 (COVID-19) [82]. COVID-19 is transmitted human-to-human and has highly non-specific symptoms such as cough, fever, dyspnea, and viral pneumonia. Upon infection, individuals with low immunity and other health complications need intensive care due to susceptibility towards respiratory distress and morbidity. COVID-19 has become a global pandemic affecting the huge population worldwide with confirmed cases of 6.04 million with 370,657 deaths globally as of 1st June 2020 [83]. The current confirmed cases in India are 190,535 with the number of deaths being 5394 as of 1st June 2020 (Source - Aarogya Setu Mobile App). Most of the countries have restricted international travel and suggested parameters such as social distancing, isolation (quarantining), personal hygiene maintenance, etc. to prevent the spread of 2019-nCoV. More than 60 days of lockdown was imposed by the Indian government to control the spread of COVID-19.
The coronavirus infection produces a large repertoire of cytokines namely IL-2, IL-7, IL-10, IP10, GSCF, MCP1, MIP1A, and TNFα [84]. These intracellular biomarkers can potentially be used for the rapid diagnosis of COVID-19 apart from conventional RNA-based real-time reverse transcription-polymerase chain reaction (RT-PCR) method [85]. A study reported a sensitive paper-based detection methodology for nucleic acid using loop-mediated isothermal amplification (LAMP), which can be further associated with smartphones for the development of a potential POC diagnostic device for personalized use [86]. The detection of the viral nucleic acids or viral antigens/antibodies (IgM and IgG) produced in response to the 2019-nCoV infection has the potential to be a significant methodology. Various techniques including LFIA, microfluidics, enzyme-linked immunosorbent assays (ELISA), etc. are in the research and development phase [87,88].
Currently, the Food and Drug Administration (FDA) approved therapeutics are unavailable, although the development of vaccines and other therapeutics is underway. Considering the huge population of India, thousands of samples are collected for analysis, which is creating a tremendous burden on inadequate lab testing. A cost-effective and rapid test that can provide results within 5–10 min is in high demand in the Indian market. Early detection of 2019-nCoV infected individuals using POC diagnostics can be extremely helpful in the implementation of control measures. The research and development of rapid test kits for COVID-19 has been actively taken up by labs all over India. Researchers at the Indian Institute of Technology Delhi have developed a real-time PCR-based detection assay for COVID-19. The assay method is validated by the Indian Council of Medical Research (ICMR) and reported to be 100% sensitive and specific [89]. Abbott India has planned to launch the antibody testing kit approved by the FDA, USA in the Indian market by the end of May 2020 [90]. For the first time in India, the National Institute of Virology, Pune has reportedly developed antibody testing kit (ELISA) that is approved by ICMR. The technology has been transferred to Zydus Cadila, a global healthcare company, partnering with ICMR, for mass production of the kits and was named as “COVID KAVACH ELISA” [91]. Bione (Bangalore-based biotech startup) has launched a rapid test kit for 2019-nCoV approved by ICMR that can be used at home [92].
5. Summary and future perspectives
The Indian healthcare system represents an exceptional case of constraints in a budget, diverse population, and multifaceted healthcare necessities. This situation demands unique solutions that are affordable, accessible, and efficient instead of pursuing insignificant technological advancements that are not related or inclined towards addressing the specific requirements of the country. POC diagnostic devices fulfill the exact demand of the situation, healthcare workers, patients as well as the rural population. Furthermore, POC tests are becoming increasingly sophisticated, whereby it has the potential to adopt lab-on-a-chip technology that can perform multitasking and increase the efficacy of patient care timely. Although there exist various barriers for use of POC diagnostic devices, the scenario is changing and encouragement is required in boosting their use to maximize the benefit to fulfill the healthcare demand of the rural population. POC diagnostic devices that are being used in clinical settings and for personalized use are categorically classified in Table 1. Indian researchers are working extensively for the development of innovative POC diagnostic devices and the translational capability and commercialization perspective are promising. Recent advancements in the field of biosensing technology, microfluidics, and paper-based diagnostics have the potential to improve the quality and efficiency of diagnostics in remote and resource-limited areas. Moreover, POC diagnostic devices may play a critical role in the containment of the COVID-19 pandemic in countries with huge populations such as India, where the cases are rising rapidly. The development of POC diagnostic devices needs to be more personalized data-centric, which can benefit the primary healthcare system and place India on the course for attaining full universal health coverage.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: NA.
Declaration of competing interest
The authors report no conflict of interest in this work.
Acknowledgment
Dr. Vivek Borse would like to acknowledge the Department of Science and Technology, Ministry of Science and Technology, Government of India for the INSPIRE Faculty Award (IFA18-ENG266, DST/INSPIRE/04/2018/000991).
References
- 1.Clark L.C., Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962;102:29–45. doi: 10.1111/j.1749-6632.1962.tb13623.x. [DOI] [PubMed] [Google Scholar]
- 2.The History of the Pregnancy Test Kit. A Timeline of Pregnancy Testing; 2014. https://history.nih.gov/exhibits/thinblueline/timeline.html accessed April 10, 2020. [Google Scholar]
- 3.Population of India | India Population 2020. StatisticsTimes.com; 2020. http://statisticstimes.com/demographics/population-of-india.php accessed April 27, 2020. [Google Scholar]
- 4.Point of Care Healthcare Technology in India - Challenges and Journey Ahead: A Clinician's Perspective. IEEE Life Sciences; 2013. https://lifesciences.ieee.org/lifesciences-newsletter/2013/january-2013/point-of-care-healthcare-technology-in-india-challenges-and-journey-ahead-a-clinician-s-perspective/ (accessed April 10, 2020) [Google Scholar]
- 5.Drain P.K., Hyle E.P., Noubary F., Freedberg K.A., Wilson D., Bishai W.R., Rodriguez W., Bassett I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014;14:239–249. doi: 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thaploo V. Essence of point-of-care diagnostic testing (POCT) in remote healthcare in India. 2017. https://www.linkedin.com/pulse/essence-point-of-care-diagnostic-testing-poct-remote-india-thaploo/ (accessed March 27, 2020)
- 7.India POCT Market Revenue is Expected to Reach over INR 4,000 Crore by FY’2023. Ken Research; 2019. https://www.prnewswire.com/in/news-releases/india-poct-market-revenue-is-expected-to-reach-over-inr-4-000-crore-by-fy-2023-ken-research-811278939.html (accessed March 29, 2020) [Google Scholar]
- 8.Impact of Point-of-Care Technologies on Healthcare. 2019. https://www.impelsys.com/blog/impact-point-care-technologies-healthcare/ (accessed April 10, 2020) [Google Scholar]
- 9.Dendukuri D.D. Health News, ET HealthWorld; 2016. Point-of-care Testing in Indian Diagnostics: Scope and Challenges.https://health.economictimes.indiatimes.com/news/diagnostics/-point-of-care-testing-in-indian-diagnostics-scope-and-challenges/53920988 (accessed March 27, 2020) [Google Scholar]
- 10.Rajendran R., Rayman G. Point-of-care blood glucose testing for diabetes care in hospitalized patients: an evidence-based review. J. Diabetes Sci. Technol. 2014;8:1081–1090. doi: 10.1177/1932296814538940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajay V.S., Prabhakaran D. Coronary heart disease in Indians: implications of the INTERHEART study. Indian J. Med. Res. 2010;132:561–566. doi: 10.4103/0971-5916.73396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardea Insight into Development. https://www.cardea-labs.com/article_licensing_final.php n.d.
- 13.Pai N.P., Vadnais C., Denkinger C., Engel N., Pai M. Point-of-Care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandhu G. Tuberculosis: current situation, challenges and overview of its control programs in India. J. Global Infect. Dis. 2011;3:143. doi: 10.4103/0974-777X.81691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarosławski S., Pai M. Why are inaccurate tuberculosis serological tests widely used in the Indian private healthcare sector? A root-cause analysis. J. Epidemiol. Glob. Health. 2012;2:39–50. doi: 10.1016/j.jegh.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laboratory Services Overview - Bureau of Indian Standards https://bis.gov.in/index.php/laboratorys/laboratory-services-overview/ n.d.
- 17.Engel N., Ganesh G., Patil M., Yellappa V., Pai N.P., Vadnais C., Pai M. Barriers to point-of-care testing in India: results from qualitative research across different settings, users and major diseases. PloS One. 2015;10 doi: 10.1371/journal.pone.0135112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinkoo A.V., Panjiyar A.K., Sharma A., Songara D., Singh R.R., Sujit Roy D., Srivastava R.K. Getting on the bandwagon of innovative point-of-care diagnostic devices to boost primary healthcare in India: a short critique and way forward. Heal. Care Curr. Rev. 2019:1–4. doi: 10.35248/2375-4273.19.07.243. 07. [DOI] [Google Scholar]
- 19.Choudhary S., Pandey G., Mukherjee R., Joshi A. Biomed. Eng. Its Appl. Healthc. Springer Singapore; 2019. Biomedical instrumentation: focus toward point-of-care devices; pp. 297–326. [DOI] [Google Scholar]
- 20.Mahato K., Maurya P.K., Chandra P. Fundamentals and commercial aspects of nanobiosensors in point-of-care clinical diagnostics. 3 Biotech. 2018:1–14. doi: 10.1007/s13205-018-1148-8. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parab H.J., Jung C., Lee J.H., Park H.G. A gold nanorod-based optical DNA biosensor for the diagnosis of pathogens. Biosens. Bioelectron. 2010;26:667–673. doi: 10.1016/j.bios.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 22.Yun B.J., Kwon J.E., Lee K., Koh W.-G. Highly sensitive metal-enhanced fluorescence biosensor prepared on electrospun fibers decorated with silica-coated silver nanoparticles. Sensor. Actuator. B Chem. 2019;284:140–147. doi: 10.1016/j.snb.2018.12.096. [DOI] [Google Scholar]
- 23.Savin M., Mihailescu C.M., Matei I., Stan D., Moldovan C.A., Ion M., Baciu I. A quantum dot-based lateral flow immunoassay for the sensitive detection of human heart fatty acid binding protein (hFABP) in human serum. Talanta. 2018;178:910–915. doi: 10.1016/j.talanta.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 24.Huerta-Nuñez L.F.E., Gutierrez-Iglesias G., Martinez-Cuazitl A., Mata-Miranda M.M., Alvarez-Jiménez V.D., Sánchez-Monroy V., Golberg A., González-Díaz C.A. A biosensor capable of identifying low quantities of breast cancer cells by electrical impedance spectroscopy. Sci. Rep. 2019;9:6419. doi: 10.1038/s41598-019-42776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidhu J.S., Singh A., Garg N., Kaur N., Singh N. Carbon dots as analytical tools for sensing of thioredoxin reductase and screening of cancer cells. Analyst. 2018;143:1853–1861. doi: 10.1039/C7AN02040F. [DOI] [PubMed] [Google Scholar]
- 26.Mahato K., Prasad A., Maurya P.K., Chandra P. Nanobiosensors: next generation point-of-care biomedical devices for personalized diagnosis. J. Anal. Bioanal. Tech. 2016;7:1–2. doi: 10.4172/2155-9872.1000e125. [DOI] [Google Scholar]
- 27.India POCT Market Research Report, Market Future Outlook, Market Analysis, Market Major Players, India POCT Market. POCT Consumables India- Ken Research; 2019. https://www.kenresearch.com/healthcare/medical-devices/india-poct-market/209287-91.html (accessed March 29, 2020) [Google Scholar]
- 28.Point-of-care Diagnostics is Revolutionary. 2012. https://www.biospectrumasia.com/analysis/47/6203/point-of-care-diagnostics-is-revolutionary.html (accessed March 29, 2020) [Google Scholar]
- 29.DCA Vantage® Analyzer - Siemens Healthineers India https://www.siemens-healthineers.com/en-in/diabetes/diabetes/dca-vantage-analyzer n.d. (accessed March 30, 2020)
- 30.Afinion HbA1c - Alere is Now Abbott. https://www.alere.com/en/home/product-details/afinion-hba1c.html n.d. (accessed March 30, 2020)
- 31.Cholestech LDX Analyzer | Lipid Profile, Cholesterol, and Glucose Testing. - Alere is Now Abbott. https://www.alere.com/en/home/product-details/cholestech-ldx-system.html n.d.
- 32.CG8+ Test Cartridge | Abbott Point of Care. https://www.pointofcare.abbott/in/en/offerings/istat/istat-test-cartridges/critical-care-CG8 n.d. (accessed March 30, 2020)
- 33.cobas b 221 system https://diagnostics.roche.com/in/en_gb/products/instruments/cobas-b-221_2_roche-omni-s2-system.html n.d. (accessed March 30, 2020)
- 34.Glucose Test - HemoCue® Glucose 201+ System - HemoCue. https://www.hemocue.in/en-in/solutions/diabetes/hemocue-glucose-201plus-system n.d. (accessed March 30, 2020)
- 35.HbA1c Test - HemoCue® HbA1c 501 System - HemoCue India. https://www.hemocue.in/en-in/solutions/diabetes/hemocue-hba1c-501-system n.d. (accessed March 30, 2020)
- 36.Stratus CS Acute Care Siemens healthineers India. https://www.siemens-healthineers.com/en-in/cardiac/cardiac-systems/stratus-cs-acute-care n.d. (accessed March 30, 2020)
- 37.Cobas h 232 POC system. https://diagnostics.roche.com/in/en_gb/products/instruments/cobas-h-232.html n.d. (accessed March 31, 2020)
- 38.Roche CARDIAC Trop T Sensitive Test (Visual) https://diagnostics.roche.com/in/en_gb/products/params/roche-cardiac-trop-t-sensitive-test-visual.html n.d. (accessed March 31, 2020)
- 39.i-STAT Test Cartridges | Abbott Point of Care. https://www.pointofcare.abbott/in/en/offerings/istat/istat-test-cartridges n.d. (accessed March 31, 2020)
- 40.Xprecia stride coagulation analyzer - Siemens healthineers India. https://www.siemens-healthineers.com/en-in/coagulation/coagulation/xprecia-stride-analyzer n.d. (accessed March 31, 2020)
- 41.CoaguChek® Pro II. https://diagnostics.roche.com/in/en_gb/products/instruments/coaguchek-pro-ii.html#productInfo n.d. (accessed March 31, 2020)
- 42.CoaguChek® XS system https://diagnostics.roche.com/in/en_gb/products/instruments/coaguchek-xs.html#productInfo n.d. (accessed March 31, 2020)
- 43.Hemoglobin Test - HemoCue Hb 201+ System - HemoCue India. https://www.hemocue.in/en-in/solutions/hematology/hemocue-hb-201plus-system n.d. (accessed March 31, 2020)
- 44.Hemolysis - HemoCue® Plasma/Low Hb System - HemoCue. https://www.hemocue.in/en-in/solutions/hematology/hemocue-plasma-low-hb-system n.d. (accessed April 1, 2020)
- 45.NMP22 BladderChek - Alere is Now Abbott. https://www.alere.com/en/home/product-details/nmp22-bladderchek.html n.d. (accessed April 1, 2020)
- 46.NMP22 Test - Alere is Now Abbott. https://www.alere.com/en/home/product-details/nmp22-test.html n.d. (accessed April 1, 2020)
- 47.CLINITEK Status Plus - Siemens Healthineers India. https://www.siemens-healthineers.com/en-in/urinalysis-products/urinalysis-systems/clinitek-status-analyzer n.d. (accessed April 1, 2020)
- 48.CLINITEK Advantus Analyzer - Siemens Healthineers India https://www.siemens-healthineers.com/en-in/urinalysis-products/urinalysis-systems/clinitek-advantus-urine-chemistry-analyzer n.d. (accessed April 1, 2020)
- 49.Panbio Dengue Duo Cassette - Alere is Now Abbott. https://www.alere.com/en/home/product-details/panbio-dengue-duo-cassette.html n.d. (accessed April 1, 2020)
- 50.SD BIOLINE Dengue Duo NS1 Ag + Ab Combo - Alere is Now Abbott. https://www.alere.com/en/home/product-details/sd-bioline-dengue-duo-ns1-ag---ab-combo.html n.d. (accessed April 1, 2020)
- 51.SHIGA TOXIN QUIK CHEK - Alere is Now Abbott. https://www.alere.com/en/home/product-details/shiga-toxin-quik-chek-test.html n.d. (accessed April 1, 2020)
- 52.Alere Determine HIV-1/2 - Alere is Now Abbott. https://www.alere.com/en/home/product-details/determine-hiv-1-2.html n.d. (accessed April 1, 2020)
- 53.SoToxa Mobile Test System - Alere is Now Abbott. https://www.alere.com/en/home/product-details/sotoxa-mobile-test-system.html n.d. (accessed April 1, 2020)
- 54.St John A., Price C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. 2014;35:155–167. http://www.ncbi.nlm.nih.gov/pubmed/25336761 [PMC free article] [PubMed] [Google Scholar]
- 55.Vashist S.K. Point-of-care diagnostics: recent advances and trends. Biosensors. 2017;7 doi: 10.3390/bios7040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu X., Akay A., Wei H., Wang S., Pingguan-Murphy B., Erlandsson B.-E., Li X., Lee W., Hu J., Wang L., Xu F. Advances in smartphone-based point-of-care diagnostics. Proc. IEEE. 2015;103:236–247. doi: 10.1109/JPROC.2014.2378776. [DOI] [Google Scholar]
- 57.Chandra P., Tan Y.N., Singh S.P. Springer Singapore; Singapore: 2017. Next Generation Point-of-care Biomedical Sensors Technologies for Cancer Diagnosis. [DOI] [Google Scholar]
- 58.Singh V.K., Kumar S., Pandey S.K., Srivastava S., Mishra M., Gupta G., Malhotra B.D., Tiwari R.S., Srivastava A. Fabrication of sensitive bioelectrode based on atomically thin CVD grown graphene for cancer biomarker detection. Biosens. Bioelectron. 2018;105:173–181. doi: 10.1016/j.bios.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Paul K.B., Singh V., Vanjari S.R.K., Singh S.G. One step biofunctionalized electrospun multiwalled carbon nanotubes embedded zinc oxide nanowire interface for highly sensitive detection of carcinoma antigen-125. Biosens. Bioelectron. 2017;88:144–152. doi: 10.1016/j.bios.2016.07.114. [DOI] [PubMed] [Google Scholar]
- 60.Kumar S., Kumar S., Srivastava S., Yadav B.K., Lee S.H., Sharma J.G., Doval D.C., Malhotra B.D. Reduced graphene oxide modified smart conducting paper for cancer biosensor. Biosens. Bioelectron. 2015;73:114–122. doi: 10.1016/j.bios.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 61.Singh S., Solanki P.R., Pandey M.K., Malhotra B.D. Cholesterol biosensor based on cholesterol esterase, cholesterol oxidase and peroxidase immobilized onto conducting polyaniline films. Sensor. Actuator. B Chem. 2006;115:534–541. doi: 10.1016/j.snb.2005.10.025. [DOI] [Google Scholar]
- 62.Singh S., Chaubey A., Malhotra B.D. Amperometric cholesterol biosensor based on immobilized cholesterol esterase and cholesterol oxidase on conducting polypyrrole films. Anal. Chim. Acta. 2004;502:229–234. doi: 10.1016/j.aca.2003.09.064. [DOI] [Google Scholar]
- 63.Chaudhari R.D., Joshi A.B., Srivastava R. Uric acid biosensor based on chemiluminescence detection using a nano-micro hybrid matrix. Sensor. Actuator. B Chem. 2012;173:882–889. doi: 10.1016/j.snb.2012.08.001. [DOI] [Google Scholar]
- 64.Tripathy S., Krishna Vanjari S.R., Singh V., Swaminathan S., Singh S.G. Electrospun manganese (III) oxide nanofiber based electrochemical DNA-nanobiosensor for zeptomolar detection of dengue consensus primer. Biosens. Bioelectron. 2017;90:378–387. doi: 10.1016/j.bios.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Malik S., Parikh H., Shah N., Anand S., Gupta S. Non-invasive platform to estimate fasting blood glucose levels from salivary electrochemical parameters. Healthc. Technol. Lett. 2019;6:87–91. doi: 10.1049/htl.2018.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pal N., Sharma S., Gupta S. Sensitive and rapid detection of pathogenic bacteria in small volumes using impedance spectroscopy technique. Biosens. Bioelectron. 2016;77:270–276. doi: 10.1016/j.bios.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 67.Borse V., Srivastava R. Nanobiomaterial Eng. Springer Singapore; Singapore: 2020. Nanobiotechnology advancements in lateral flow immunodiagnostics; pp. 181–204. [DOI] [Google Scholar]
- 68.Borse V.B., Konwar A.N., Jayant R.D., Patil P.O. Perspectives of characterization and bioconjugation of gold nanoparticles and their application in lateral flow immunosensing. Drug Deliv. Transl. Res. 2020 doi: 10.1007/s13346-020-00771-y. [DOI] [PubMed] [Google Scholar]
- 69.Borse V., Srivastava R. Fluorescence lateral flow immunoassay based point-of-care nanodiagnostics for orthopedic implant-associated infection. Sensor. Actuator. B Chem. 2019;280:24–33. doi: 10.1016/j.snb.2018.10.034. [DOI] [Google Scholar]
- 70.Borse V., Srivastava R. Process parameter optimization for lateral flow immunosensing. Mater. Sci. Energy Technol. 2019;2:434–441. doi: 10.1016/j.mset.2019.04.003. [DOI] [Google Scholar]
- 71.Borse V., Jain P., Sadawana M., Srivastava R. “Turn-on” fluorescence assay for inorganic phosphate sensing. Sensor. Actuator. B Chem. 2016;225:340–347. doi: 10.1016/j.snb.2015.11.054. [DOI] [Google Scholar]
- 72.Borse V., Thakur M., Sengupta S., Srivastava R. N-doped multi-fluorescent carbon dots for ‘turn off-on’ silver-biothiol dual sensing and mammalian cell imaging application. Sensor. Actuator. B Chem. 2017;248:481–492. doi: 10.1016/j.snb.2017.03.158. [DOI] [Google Scholar]
- 73.Borse V., Patil A.S., Srivastava R. 2017 9th Int. Conf. Commun. Syst. Networks. IEEE; 2017. Development and testing of portable fluorescence reader (PorFloRTM) pp. 498–501. [DOI] [Google Scholar]
- 74.Makkar R.L., Syeda Aliya S., Borse V., Srivastava R. In: Opt. Sens. Detect. V. Berghmans F., Mignani A.G., editors. SPIE; 2018. Design and development of portable fluorescence reader using silicon photo multiplier (SiPM) sensor; p. 12. [DOI] [Google Scholar]
- 75.Tripathi V., Nara S., Singh K., Singh H., Shrivastav T.G. A competitive immunochromatographic strip assay for 17-α-hydroxy progesterone using colloidal gold nanoparticles. Clin. Chim. Acta. 2012;413:262–268. doi: 10.1016/j.cca.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 76.Patil P.O., Pandey G.R., Patil A.G., Borse V.B., Deshmukh P.K., Patil D.R., Tade R.S., Nangare S.N., Khan Z.G., Patil A.M., More M.P., Veerapandian M., Bari S.B. Graphene-based nanocomposites for sensitivity enhancement of surface plasmon resonance sensor for biological and chemical sensing: a review. Biosens. Bioelectron. 2019;139:111324. doi: 10.1016/j.bios.2019.111324. [DOI] [PubMed] [Google Scholar]
- 77.Kaur N., Toley B.J. Paper-based nucleic acid amplification tests for point-of-care diagnostics. Analyst. 2018;143:2213–2234. doi: 10.1039/C7AN01943B. [DOI] [PubMed] [Google Scholar]
- 78.Mandal N., Bhattacharjee M., Chattopadhyay A., Bandyopadhyay D. Point-of-care-testing of α-amylase activity in human blood serum. Biosens. Bioelectron. 2019;124–125:75–81. doi: 10.1016/j.bios.2018.09.097. [DOI] [PubMed] [Google Scholar]
- 79.Chandra P., Zaidi S.A., Noh H.-B., Shim Y.-B. Separation and simultaneous detection of anticancer drugs in a microfluidic device with an amperometric biosensor. Biosens. Bioelectron. 2011;28:326–332. doi: 10.1016/j.bios.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 80.Akhtar M.H., Hussain K.K., Gurudatt N.G., Chandra P., Shim Y.-B. Ultrasensitive dual probe immunosensor for the monitoring of nicotine induced-brain derived neurotrophic factor released from cancer cells. Biosens. Bioelectron. 2018;116:108–115. doi: 10.1016/j.bios.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 81.Choudhary M., Yadav P., Singh A., Kaur S., Ramirez-Vick J., Chandra P., Arora K., Singh S.P. CD59 targeted ultrasensitive electrochemical immunosensor for fast and noninvasive diagnosis of oral cancer. Electroanalysis. 2016;28:2565–2574. doi: 10.1002/elan.201600238. [DOI] [Google Scholar]
- 82.Coronavirus Disease 2019 (COVID-19) Situation Report-23. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200212-sitrep-23-ncov.pdf?sfvrsn=41e9fb78_4 [Google Scholar]
- 83.Coronavirus Disease 2019. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Google Scholar]
- 84.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang T., Wang Y.-C., Shen C.-F., Cheng C.-M. Point-of-Care RNA-based diagnostic device for COVID-19. Diagnostics. 2020;10:165. doi: 10.3390/diagnostics10030165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H., Mubareka S., Gubbay J.B., Chan W.C.W. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 88.Xiang J., Yan M., Li H., Liu T., Lin C., Huang S., Shen C. Evaluation of enzyme-linked immunoassay and colloidal gold- immunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (COVID-19) MedRxiv. 2020 doi: 10.1101/2020.02.27.20028787. 2020.02.27.20028787. [DOI] [Google Scholar]
- 89.News . Indian Institute of Technology Delhi; 2020. https://home.iitd.ac.in/covide-probe.php [Google Scholar]
- 90.Abbott's COVID-19 Laboratory-Based Antibody Tests to Be Available in India by May-End, Health News, ET HealthWorld. 2020. https://health.economictimes.indiatimes.com/news/diagnostics/abbotts-covid-19-laboratory-based-antibody-tests-to-be-available-in-india-by-may-end/75535891 (accessed May 28, 2020) [Google Scholar]
- 91.Combating COVID-19: NIV-Pune Develops India's First Antibody Testing Kit -ELISA. Health News, ET HealthWorld; 2020. https://health.economictimes.indiatimes.com/news/diagnostics/combating-covid-19-niv-pune-develops-indias-first-antibody-testing-kit-elisa/75666103 (accessed May 28, 2020) [Google Scholar]
- 92.Rapid and Cheap: These New Testing Kits Could Change the Course of COVID-19 in India - Firstpost. 2020. https://www.firstpost.com/health/rapid-and-cheap-these-new-testing-kits-could-change-the-course-of-covid-19-in-india-8224721.html (accessed May 28, 2020) [Google Scholar]


