Abstract
BACKGROUND
Intrauterine adhesion (IUA) can cause serious damage to women's reproductive health, yet current treatment methods are difficult to achieve satisfactory results. In our previous studies, we demonstrated that menstrual-derived stromal stem cells (MenSCs), with high proliferative capacity and self-renewal ability, have a powerful therapeutic effect in patients with severe IUA. However, safety assessment of MenSCs transplantation is essential for its further application.
AIM
To evaluate the short-, medium-, and long-term biosafety of MenSCs via intrauterine transplantation in a rat model of IUA, with a focus on toxicity and tumorigenicity.
METHODS
MenSCs were injected into the sub-serosal layer of the uterus in an IUA rat model, for 3 d, 3 mo, and 6 mo separately, to monitor the corresponding acute, sub-chronic, and chronic effects. Healthy rats of the same age served as negative controls. Toxicity effects were evaluated by body weight, organ weight, histopathology, hematology, and biochemistry tests. Tumorigenicity of MenSCs was investigated in Balb/c-nu mice in vivo and by colony formation assays in vitro.
RESULTS
Compared with the same week-old control group, all of the IUA rats receiving MenSC transplantation demonstrated no obvious changes in body weight, main organ weight, or blood cell composition during the acute, sub-chronic, and chronic observation periods. At the same time, serum biochemical tests showed no adverse effects on metabolism or liver and kidney function. After 4 wk of subcutaneous injection of MenSCs in Balb/c-nu nude mice, no tumor formation or cell metastasis was observed. Moreover, there was no tumor colony formation of MenSCs during soft agar culture in vitro.
CONCLUSION
There is no acute, sub-chronic, or chronic poisoning, infection, tumorigenesis, or endometriosis in rats with IUA after MenSC transplantation. The above results suggest that intrauterine transplantation of MenSCs is safe for endometrial treatment.
Keywords: Menstrual blood-derived stromal cells, Endometrial treatment, Intrauterine adhesion, Stem cell transplantation, Biosafety, Toxicity
Core tip: Menstrual-derived stromal stem cells (MenSCs) with high proliferative capacity and self-renewal ability have a powerful therapeutic effect in patients with severe intrauterine adhesion. However, safety assessment of MenSC transplantation is essential for its further application. Here, we evaluated the short-, medium-, and long-term biosafety of MenSCs via intrauterine transplantation in an intrauterine adhesion rat model, with a special focus on toxicity and tumorigenicity. There was no acute, sub-chronic, or chronic poisoning, infection, tumor, or endometriosis in rats with intrauterine adhesions after MenSC transplantation, highlighting that intrauterine transplantation of MenSCs is safe for endometrial treatment.
INTRODUCTION
Intrauterine adhesion (IUA) is a traumatic disease mostly associated with intrauterine surgery[1], mainly characterized by endometrial functional disorders, including abnormal menstruation (menstrual reduction or amenorrhea), thin endometrium, pelvic pain, implantation abnormality, infertility, and abortion[2]. With the increasing frequency of intrauterine operation, the incidence of IUA is gradually increasing. Approximately 2.8%-45.5% of secondary infertility cases are related to IUA[3]. However, for patients with severe IUA, conventional treatment methods, such as surgical isolation and hormone supplementation, cannot achieve the desired therapeutic effect. Especially, it is difficult to improve the fertility in patients with IUA.
Recent studies have shown that tissue and organ damage can be repaired effectively by stem cell transplantation. Mesenchymal stem cells (MSCs) are adult stem cells that can be easily collected and cultured from tissues and organs[4]. Due to the advantages of strong proliferative ability, high genetic stability, chemotactic properties, and low immunogenetic effects, MSCs play an important role in the field of regenerative medicine[5-7]. Recently, MSCs have been used in stem cell-based infertility treatment. Bone marrow-derived MSCs (BMSCs), umbilical cord-derived MSCs (UCMSCs), and endometrial-derived MSCs were all proved to be effective in recovering damaged endometrium[8-11].
Menstrual blood-derived stromal cells (MenSCs) are shedding endometrial stem cells that are obtained from menstrual blood, and were first reported by Meng et al[12]. These cells exhibit classic MSC characteristics, such as automatic cloning, high proliferation, and pluripotency[13]. Recent studies have shown that MenSCs improved a variety of diseases, including type 1 diabetes[14], liver disease[15,16], premature ovarian failure[17], osteochondral defects[18], heart disease[19], and cartilage damage[20]. It is worth noting that MenSCs are easily obtained from abandoned menstrual blood in a non-invasive manner, which can be obtained periodically and autologously transplanted without trauma or ethical risk. Therefore, compared with BMSCs and adipose tissue-derived mesenchymal stem cells (ADSCs), MenSCs have greater clinical application potential on the premise of similar efficacy[21]. Our previous research confirmed that autologous MenSC transplantation can significantly promote endometrial morphology regeneration and functional recovery in seven patients with severe IUA, and achieved four positive pregnancies[22]. After MenSC transplantation, the endometrial pathology and uterine fertility of an IUA rat model were also improved[23]. Therefore, MenSC transplantation is a promising treatment for endometrial injury.
It is undeniable that MenSCs represent a new type of therapeutic stem cells. Many related treatment studies are still in the preclinical or phase 1 clinical trial phase. At present, the effectiveness of MenSCs in treating traumatic diseases has been confirmed. However, long-term observational data of clinical application of MenSCs is scarce, and systematic biosafety evaluation is still lacking[24].
In this study, we aimed to investigate the biosafety of intrauterine transplantation of MenSCs to treat endometrial injury over acute, sub-chronic, and chronic periods. Based on an IUA rat model, the safety of MenSC treatment was systematically evaluated for toxicity, tumorigenicity, and abnormal differentiation. Our results provide a theoretical basis for the clinical application of MenSCs in endometrial injury treatment.
MATERIALS AND METHODS
Culture and identification of MenSCs
MenSCs were cultured and identified as described previously[23]. In brief, sterile techniques were used to collect menstrual blood from three healthy volunteers, aged 25 to 30 years. After mixing with PBS, the samples of menstrual blood were lightly placed on the upper layer of an equal amount of Ficoll. The intermediate cell layer was separated and cultured in DMEM/F12 medium (1:1; HyClone, Logan, UT, United States) containing 10% fetal bovine serum (Gibco, Waltham, MA, United States) at 37 °C in a 5% atmosphere. MenSCs at passage 3 (P3) were collected and MSC surface markers were evaluated by flow cytometry (CD34, CD38, CD44, CD45, CD73, CD90, and CD105) (Supplemental Figures 1). Only well-grown and verified P3 MenSCs were used in this study.
Toxicology study
Establishment and treatment of a rat model of IUA: Forty-five eight-week-old female Sprague-Dawley rats were purchased from HFK Bioscience Co. (Beijing, China) and housed in a Specific-Pathogen-Free (SPF) laboratory (SYXK 2017-0004, China) at a temperature of 22 °C ± 1 °C, a relative humidity of 50% ± 1%, and a light/dark cycle of 12/12 h. Sterilized food and water were available ad libitum. All animal studies (including euthanasia procedures) were conducted in accordance with the regulations and guidelines of China Medical University institutional animal care and with the AAALAC and IACUC guidelines. A rat model of IUA was established according to the procedures outlined in our previous study. In brief, 30 female rats in estrus cycle were selected for surgery (n = 10 for each group). After anesthesia with 3% pentobarbital, the uterine horn was surgically exposed. The endometrium was then damaged mechanically using a 16 G syringe. After two estrus cycles, the abdominal wall was reopened and 5 × 105 MenSCs were injected into each uterine serosa. Five rats in each group received a placebo (PBS) and acted as controls.
Sample acquisition: The study involved three experimental groups according to observation time: Acute group (3 d), sub-chronic group (3 mo), and chronic group (6 mo). All rats were weighed prior to sacrifice and 5 mL of blood was collected from the abdominal aorta. Sodium citrate was added to 2 mL of peripheral blood to analyze the blood cell composition. The remaining peripheral blood was quickly centrifuged and the serum was separated for biochemical detection. After removing the surface adipose tissue, the brain, heart, liver, spleen, lungs, kidneys, thymus, adrenal glands, uterus, and ovaries of each rat were weighed and recorded. Subsequently, these organs were fixed in 4% paraformaldehyde, dehydrated, and then embedded in paraffin. Then, we prepared 5 μm serial sections of each tissue for staining.
Blood cell composition test and serum biochemical test: Freshly collected rat peripheral venous blood was immediately tested for blood cell composition (Procyte DX, IDEXX Laboratories, United States). Red blood cells, hematocritt, hemoglobin, average red blood cell volume, average hemoglobin concentration, red blood cell distribution width, reticulocytes, white blood cells, neutrophils, lymphocytes, monocytes, eosinophils, basophils, neutrophils, and platelet related concentrations or percentages were evaluated. Subsequently, blood glucose, urea, creatinine, total protein, albumin, globulin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and sodium, potassium, and chloride ion concentrations in the serum of each group of rats were detected (Catalyst One, IDEXX Laboratories, United States).
Histopathology
Paraffin sections of various organs were subjected to hematoxylin-eosin (HE) staining after dewaxing and dehydration. The tissue morphology of each tissue was then evaluated by light microscopy (NikonECLIPSE N80-i).
Tumorgenicity tests
In vivo: P3 MenSCs were fluorescently labeled by transfection with green fluorescence protein (GFP)-labeled lentivirus (multiplicity of infection = 20). Subcutaneous injection in Balb/c-nu mice was applied to detect tumor-forming properties of MenSCs in vivo. Eight-week-old female Balb/c-nu mice weighing 20 g, were purchased from HFK Bioscience Co. (Beijing, China), and housed in a specific-pathogen-free (SPF) laboratory (SYXK 2017-0004, China). We then injected GFP-labeled 107 MenSCs into the epidermis on the groin of Balb/c-nu mice. The intensity and range of fluorescence were then evaluated every 7 d by 470-535 nm excitation. Each mouse was placed onto the scanning stage of the in vivo MS FX Pro system (Carestream, United States). Bioluminescence imaging was carried to identify the location of GFP-MenSCs. Images were acquired and analyzed with Carestream MI SE software.
In vitro: The tumorigenicity of MenSCs was evaluated by soft-agar colony formation assays in vitro. HELA cells were used as a positive control. These cells were first suspended in complete culture medium with 0.35% low melting agarose, then the mixture was transferred onto solidified 0.6% agarose in a 6-well plate. Approximately 2. 5 × 103 and 5 × 103 cells were uniformly inoculated into the upper layer of each well and cultured in DMEM/F12 medium containing 10% fetal bovine serum at 37 °C in an atmosphere containing 5% CO2. The monoclonal formation of MenSCs and HELA cells was observed under a microscope (Nikon ECLIPSE Hi) to investigate for malignant proliferation for a total period of 2 wk.
Statistical analysis
All data in this study are presented as the mean ± standard deviation (SD), and comparisons between groups were analyzed using one-way analysis of variance (ANOVA). Bonferroni post hoc tests were used to further investigate significant differences. Statistical analyses were carried out with Prism 8 software (GraphPad, San Diego, United States) and P < 0.05 was considered to represent a statistically significant difference.
RESULTS
Toxicity
Body weights: Over the entire experimental period, no deaths or adverse response were evident in either the control group or the MenSC transplantation groups. All rats represented with normal behavior without surgical complications. As shown in Table 1, there was no difference between each experimental group and the healthy controls in terms of body weight throughout the entire experimental period (P acute = 0.207, Psub-chronic = 0.255, and P chronic = 0.696).
Table 1.
Body weight changes of mice in toxicity study
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
| Body weight (g) | 228.0 ± 18.18 | 228.8 ± 9.175 | 267.4 ± 26.16 | 265.8 ± 9.378 | 326.0 ± 22.75 | 303.7 ± 35.06 |
Measurements are given as the mean ± SD. Group 1: Control for acute group; Group 2: Acute group; Group 3: Control for subchronic group; Group 4: Subchronic group; Group 5: Control for chronic group; Group 6: Chronic group (n = 5 for each control group, n = 10 for each experimental group).
Organ weights: Next, we weighed the major organs of all rats. As shown in Table 2, there was no difference between the control groups and the treatment groups in terms of the relative weight of the brain, heart, liver, spleen, lung, kidney, thymus, adrenal glands, uterus, and ovaries, indicating that organ weights were within the normal range. No morphological change or color change was observed in any of the examined organs.
Table 2.
Relative organ weights of rats in toxicity study
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
| Brain | 1.314 ± 0.220 | 1.439 ± 0.176 | 1.480 ± 0.211 | 1.477 ± 0.197 | 1.441 ± 0.047 | 1.508 ± 0.176 |
| Heart | 0.718 ± 0.048 | 0.750 ± 0.087 | 0.744 ± 0.076 | 0.805 ± 0.064 | 0.826 ± 0.039 | 0.868 ± 0.039 |
| Liver | 8.383 ± 0.764 | 8.525 ± 1.012 | 8.563 ± 0.646 | 9.205 ± 1.385 | 9.982 ± 0.282 | 10.01 ± 0.872 |
| Spleen | 0.432 ± 0.101 | 0.490 ± 0.123 | 0.515 ± 0.051 | 0.506 ± 0.067 | 0.500 ± 0.018 | 0.501 ± 0.082 |
| Lung | 1.225 ± 1.104 | 1.269 ± 0.100 | 1.375 ± 0.129 | 1.374 ± 0.088 | 1.350 ± 0.084 | 1.374 ± 0.100 |
| Kidney | 0.900 ± 0.161 | 1.016 ± 0.267 | 1.028 ± 0.032 | 1.015 ± 0.181 | 1.012 ± 0.082 | 1.009 ± 0.144 |
| Thymus | 0.298 ± 0.021 | 0.335 ± 0.051 | 0.341 ± 0.072 | 0.339 ± 0.059 | 0.352 ± 0.032 | 0.338 ± 0.052 |
| Adrenal gland | 0.027 ± 0.003 | 0.025 ± 0.005 | 0.032 ± 0.002 | 0.029 ± 0.006 | 0.031 ± 0.002 | 0.030 ± 0.006 |
| Uterus | 0.512 ± 0.025 | 0.524 ± 0.017 | 0.511 ± 0.021 | 0.514 ± 0.020 | 0.536 ± 0.031 | 0.546 ± 0.045 |
| Ovary | 0.047 ± 0.004 | 0.049 ± 0.005 | 0.049 ± 0.004 | 0.051 ± 0.003 | 0.054 ± 0.005 | 0.053 ± 0.004 |
Measurements are given as the mean ± SD. Group 1: Control for acute group; Group 2: Acute group; Group 3: Control for subchronic group; Group 4: Subchronic group; Group 5: Control for chronic group; Group 6: Chronic group.
Hematology and biochemistry: Next, blood cell composition and serum biochemical and metabolic parameters were examined in two groups of rats (Tables 3 and 4). The blood cell compositions in the MenSC transplantation groups fluctuated slightly, but were all within the range of normal values. In addition, compared with controls of the same age, there were no changes in terms of serum biochemical or metabolic parameters in the MenSC transplantation groups.
Table 3.
Selected hematology analyses of rats in toxicity study
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
| RBC (× 1012/L) | 7.196 ± 0.090 | 7.225 ± 0.340 | 7.346 ± 0.418 | 7.462 ± 0.430 | 7.276 ± 0.421 | 7.300 ± 0.347 |
| HCT (%) | 39.32 ± 0.740 | 38.98 ± 1.992 | 39.08 ± 0.958 | 38.50 ± 2.253 | 38.90 ± 2.220 | 37.05 ± 0.576 |
| MCV (fL) | 54.64 ± 0.541 | 52.28 ± 0.784 | 53.28 ± 1.767 | 53.34 ± 0.845 | 53.00 ± 0.714 | 53.04 ± 1.681 |
| MCH (pg) | 18.66 ± 0.114 | 18.01 ± 0.470 | 18.10 ± 0.765 | 18.39 ± 0.341 | 17.82 ± 1.043 | 18.51 ± 0.905 |
| MCHC (g/dL) | 34.18 ± 0.523 | 34.42 ± 0.480 | 33.96 ± 0.493 | 34.50 ± 0.245 | 34.48 ± 0.590 | 35.12 ± 0.545 |
| RDW (%) | 17.42 ± 0.444 | 17.87 ± 0.912 | 17.78 ± 0.691 | 17.29 ± 1.152 | 17.30 ± 0.570 | 17.92 ± 0.651 |
| RETIC (K/μL) | 2.86 ± 0.434 | 3.78 ± 0.630 | 2.78 ± 0.691 | 2.93 ± 0.706 | 2.48 ± 0.798 | 2.81 ± 0.802 |
| RETIC | 214.7 ± 35.25 | 270.6 ± 39.98 | 202.2 ± 42.64 | 216.6 ± 49.50 | 162.3 ± 5.118 | 178.0 ± 19.04 |
| WBC (×1012/L) | 7.644 ± 1.668 | 6.932 ± 2.50 | 7.592 ± 2.96 | 7.504 ± 2.121 | 6.440 ± 1.248 | 5.883 ± 2.897 |
| NEU (%) | 13.72 ± 4.217 | 17.50 ± 5.977 | 12.48 ± 2.104 | 15.40 ± 5.918 | 12.10 ± 2.351 | 14.66 ± 2.831 |
| LYM (%) | 75.06 ± 5.697 | 73.91 ± 6.311 | 80.36 ± 4.360 | 79.33 ± 7.194 | 78.88 ± 3.440 | 76.26 ± 2.858 |
| MONO (%) | 5.180 ± 0.832 | 6.800 ± 0.902 | 4.520 ± 1.117 | 5.540 ± 1.169 | 4.540 ± 0.963 | 6.160 ± 1.190 |
| EOS (%) | 0.60 ± 0.123 | 0.80 ± 0.340 | 0.80 ± 0.200 | 0.53 ± 0.200 | 0.62 ± 0.356 | 0.61 ± 0.166 |
| BASO (%) | 0.14 ± 0.114 | 0.25 ± 0.151 | 0.20 ± 0.141 | 0.18 ± 0.140 | 0.16 ± 0.114 | 0.19 ± 0.171 |
| NEU (× 109/L) | 1.024 ± 0.288 | 1.116 ± 0.495 | 0.976 ± 0.153 | 1.011 ± 0.468 | 1.096 ± 0.163 | 1.000 ± 0.096 |
| LYM (× 109/L) | 6.178 ± 1.593 | 4.849 ± 2.150 | 6.158 ± 2.684 | 6.002 ± 2.089 | 5.672 ± 0.854 | 5.398 ± 1.097 |
| MONO (× 109/L) | 0.386 ± 0.042 | 0.455 ± 0.161 | 0.426 ± 0.083 | 0.415 ± 0.120 | 0.406 ± 0.067 | 0.346 ± 0.111 |
| EOS (× 109/L) | 0.046 ± 0.011 | 0.05 ± 0.017 | 0.050 ± 0.029 | 0.037 ± 0.016 | 0.004 ± 0.012 | 0.034 ± 0.013 |
| BASO (× 109/L) | 0.01 ± 0.007 | 0.014 ± 0.010 | 0.001 ± 0.007 | 0.013 ± 0.07 | 0.016 ± 0.009 | 0.016 ± 0.012 |
| PLT (K/μL) | 1001 ± 193.2 | 1107 ± 178.6 | 1032 ± 142.8 | 974.4 ± 121.9 | 1169 ± 74.37 | 1075 ± 45.99 |
| MPV (fL) | 8.50 ± 0.100 | 8.43 ± 0.216 | 8.42 ± 0.148 | 8.63 ± 0.095 | 8.44 ± 0.089 | 8.47 ± 0.206 |
| PDW (fL) | 8.50 ± 0.158 | 8.62 ± 0.148 | 8.46 ± 0.422 | 8.30 ± 0.133 | 8.42 ± 0.303 | 8.71 ± 0.778 |
| PCT (%) | 1.037 ± 0.038 | 0.962 ± 0.137 | 0.870 ± 0.129 | 0.80 ± 0.151 | 0.844 ± 0.167 | 0.88 ± 0.107 |
Note: Measurements are given as the mean ± SD. RBC: Red blood cells; HCT: Hematocritt; MCV: Average red blood cell volume; MCH: Average hemoglobin concentration; RDW: Red blood cell distribution width; RETIC: Reticulocytes; WBC: White blood cells; NEU: Neutrophils; LYM: Lymphocytes; MONO: Monocytes; EOS: Eosinophils; BASO: Basophils; PLT: Platelet; MPV: Mean platelet volume; PDW: Platelet distribution width.
Table 4.
Selected biochemistry analyses of rats in toxicity study
| Group 1 | Group 2 | Group3 | Group 4 | Group 5 | Group 6 | |
| Urea | 6.220 ± 0.512 | 6.260 ± 0.657 | 5.860 ± 0.493 | 6.19 ± 0.659 | 5.840 ± 0.270 | 6.380 ± 0.570 |
| CREA | 27.60 ± 5.128 | 32.30 ± 6.430 | 28.40 ± 4.722 | 26.40 ± 2.011 | 31.00 ± 6.557 | 31.10 ± 6.367 |
| BUN/CREA | 55.80 ± 11.71 | 48.90 ± 7.279 | 55.20 ± 8.899 | 58 ± 8.679 | 56.60 ± 5.771 | 57.80 ± 3.967 |
| TP | 60.8 ± 4.087 | 62.20 ± 5.673 | 61.80 ± 7.014 | 57.40 ± 2.914 | 60.20 ± 3.962 | 60.30 ± 5.165 |
| ALB | 31.80 ± 2.683 | 32.4 ± 4.060 | 36.80 ± 3.899 | 30.2 ± 3.584 | 30.00 ± 2.739 | 32.90 ± 3.695 |
| GLOB | 29.40 ± 1.817 | 29.8 ± 2.300 | 25.90 ± 3.194 | 27.4 ± 1.350 | 26.00 ± 3.674 | 27.80 ± 2.201 |
| ALT | 35.00 ± 6.042 | 34.7 ± 5.539 | 31.40 ± 8.355 | 38.30 ± 3.889 | 34.60 ± 7.162 | 36.30 ± 6.897 |
| AST | 73.40 ± 11.84 | 66.6 ± 14.010 | 70.20 ± 7.662 | 75.2 ± 15.050 | 64.20 ± 16.68 | 61.4 ± 13.16 |
| ALKP | 108 ± 25.03 | 121.7 ± 32.840 | 107.0 ± 37.36 | 100.3 ± 11.910 | 108.4 ± 23.39 | 95.20 ± 11.70 |
| Na | 142.6 ± 1.949 | 141.2 ± 2.348 | 143.2 ± 1.483 | 141.7 ± 2.627 | 142.2 ± 1.643 | 144.1 ± 2.234 |
| K | 5.180 ± 0.148 | 5.230 ± 0.457 | 4.820 ± 0.601 | 5.01 ± 0.213 | 4.700 ± 0.245 | 5.96 ± 0.390 |
| CL | 103.8 ± 2.387 | 106.2 ± 3.293 | 104.2 ± 2.387 | 106.6 ± 3.084 | 103.8 ± 2.775 | 105.4 ± 5.719 |
Note: Measurements are given as the mean ± SD. CREA: Creatinine; TP: Total protein; ALB: Albumin; GLOB: Globulin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALKP: Alkaline phosphatase; BUN: Blood urea nitrogen.
Histopathology
Compared with controls of the same week-old age, there were no changes in the main organ morphology or size in the MenSC transplantation groups. In addition, we carefully examined the abdominal cavity of every rat, and no endometriosis or tumor formation was observed at 3 d, 3 mo or 6 mo after MenSC transplantation.
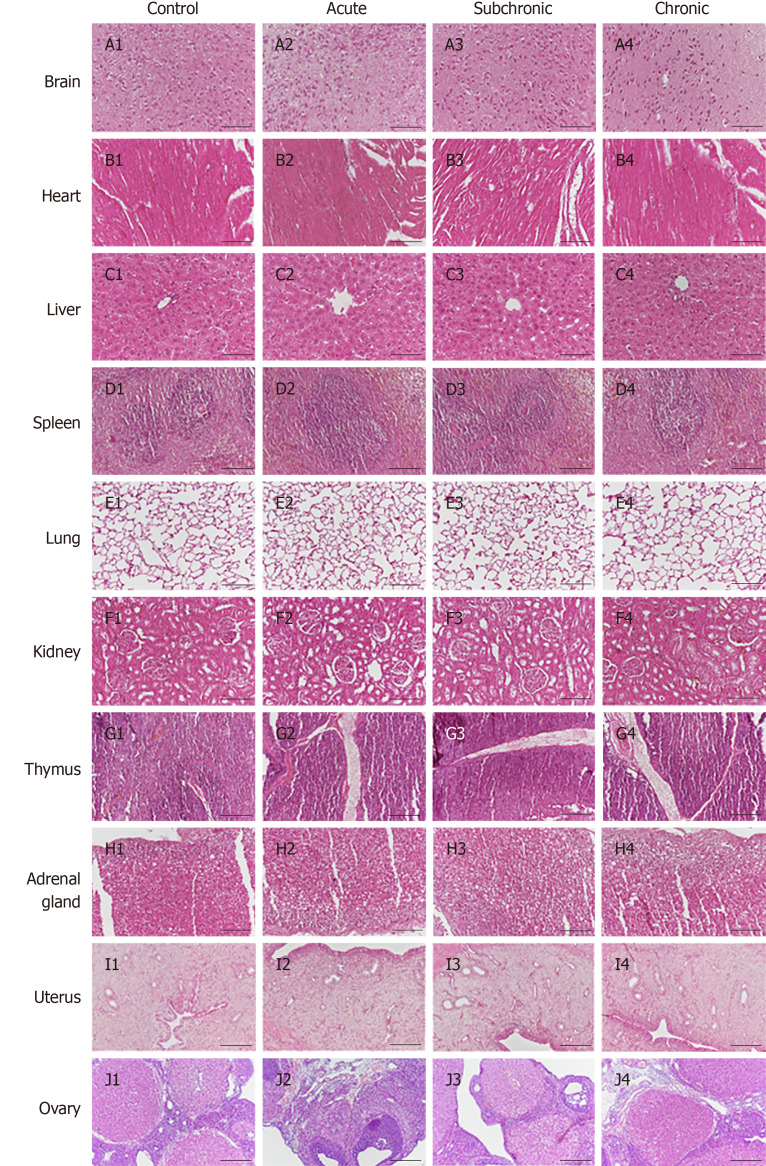
Next, histopathological examinations of the brain, heart, liver, spleen, lung, kidney thymus, thyroid, adrenal glands, uterus, and ovaries were carried out by HE staining. Representative histological images are shown in Figure 1. Compared with the control group of the same age, there were no obvious structural changes or tumor formation in the MenSC transplantation group.
Figure 1.
Histopathological analysis of intrauterine adhesion rats after menstrual-derived stromal stem cell treatment. Representative H&E staining of various organs (brain, heart, liver, spleen, lungs, kidneys, thymus, adrenal glands, uterus, and ovaries). No structural changes or injuries were detected in theses organs. Scale bar = 100 μm.
Tumorigenicity studies
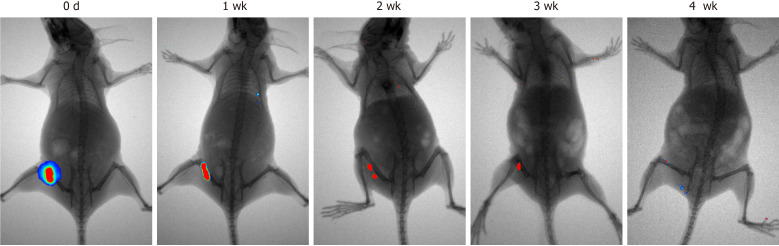
In vivo assay: The tumorigenesis of MenSCs was examined in vivo (Figure 2) via hypodermic injection into Balb/c-nu mice. Four weeks post-injection, there was no tumor formation in the nude mice injected with MenSCs and the fluorescent-labeled MenSCs gradually disappeared over time. No fluorescent signals were detected in other parts of the body. In contrast, Balb/c-nu mice with HELA cell injection showed subcutaneous tumors and had surface gangrene (Supplementary Figure 2).
Figure 2.
Tumorigenicity analysis of menstrual-derived stromal stem cells on nude mice 4 wk after subcutaneous injection. Representative photographs show the cell proliferation of menstrual-derived stromal stem cells (MenSCs) on nude mice 4 wk after subcutaneous injection. Fluorescent expression, which represented the MenSCs, gradually decreased over time. No metastatic or proliferative fluorescent signals were detected in other parts of the body.
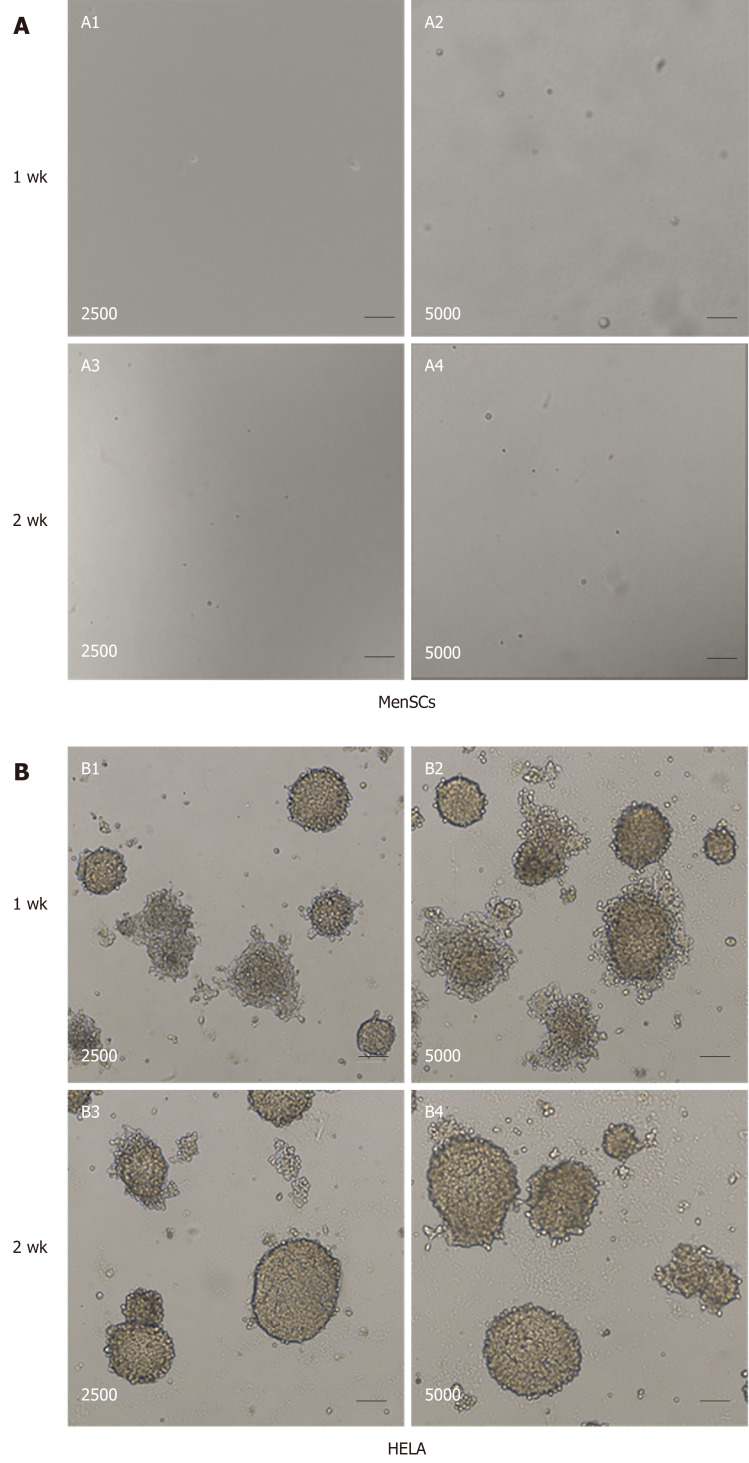
In vitro assay: The tumorigenesis of MenSCs was also investigated in vitro using soft-agar assays. The tumorigenicity was negative for MenSCs in vitro, and no cell colonies were formed during 2 wk of culture (Figure 3A). In contrast, as for Hela cells, there were numerous colonies observed in the soft agar (Figure 3B). These tumor colonies grew in stacks and spread outward with strong tumorigenicity.
Figure 3.
Tumorigenicity analyses of menstrual-derived stromal stem cells and Hela cells in soft-agar colony formation assay. A: Representative photomicrographs show the cell malignant proliferation of 2500 and 5000 menstrual-derived stromal stem cells (MenSCs) after 1 and 2 wk of culturing. No cell colony was formed in the MenSCs group. B: Representative photomicrographs show the cell malignant proliferation of 2500 and 5000 HELA cells after 1 and 2 wk of culturing. Scale bar = 100 μm. MenSCs: Menstrual-derived stromal stem cells.
DISCUSSION
MSCs are the most commonly used stem cells in basic and clinical research. In addition to differentiation potential, MSCs also participate in the regulation of immune balance. Moreover, MSCs have the effect on microenvironment formation that is conducive to tissue regeneration[25]. Recent clinical studies have confirmed that BMSCs, ADMSCs, UCMSCs, and vascular endothelial MSCs provide therapeutic effects with regard to organ function improvement and tissue regeneration, and thus were widely used in clinical treatment research of cardiovascular disease, immune system disease, motor system injury, and digestive system disease. During the follow-up of these clinical studies, there were no tumor or serious complications associated with stem cell transplantation[26-30].
Up to now, only two clinical trials of MenSC transplantation have been reported. In 2009, Zhong’s study indicated that transplantation of allogeneic MenSCs can effectively improve multiple sclerosis. During the one-year follow-up, there were no complications related to immune response and cell transplantation observed in all four patients[31]. Similarly, in our previous clinical trial, no transplant-related complication was found in all seven severe IUA patients after receiving autologous MenSC transplantation[22].
Currently, MenSC treatment studies were mostly in the preclinical or phase 1 clinical research stage[24]. Therefore, it is essential to acquire more clinical and basic research data in order to support the further clinical application of MenSCs. To transform MenSCs from an experimental product into a clinical treatment formulation, it is necessary to consider factors directly related to clinical application, such as indications, routes of administration, and dosage. Due to the high proliferative potential and multi-pluripotency, the toxicity and tumorigenicity of stem cells are the main concerns in clinical research[32]. Meanwhile, the biological safety should be assessed in appropriate in vivo and in vitro models. In this study, we transplanted MenSCs in an IUA rat model using a dose and method consistent with the clinical application, and evaluated the safety of MenSCs for acute, sub-chronic, and chronic observations. To our knowledge, it is the first comprehensive preclinical biosafety study of MenSCs.
In our study, 30 IUA rat models received 106 MenSCs via intrauterine sub-serosa injection. During the observation periods from 3 d to 6 mo, all these rats maintained normal body weight, without death, abnormal behavior, or transplant-related diseases. At the same time, the weight, shape, and appearance of the main organs remained normal. Moreover, compared with the control group, there were no differences in blood cell composition or ratio after MenSC transplantation. Serum biochemical results showed that the liver and kidney function in these rats was normal. The above results demonstrated that MenSCs were well tolerated, without initiation of abnormal immune response or organ dysfunction.
It is worth noting that the leukocytes and lymphocytes in all experimental groups were within the normal range, indicating that the preparation and transplantation process of MenSCs was sterile. Menstrual blood is usually obtained non-invasively through the vagina, which is a unique advantage of MenSCs in regenerative medicine. However, this is also the main source of contamination risks during MenSC preparation. Therefore, the establishment of a quality control system is essential for MenSC clinical application.
Tumorigenicity is one of the most serious risk factors to be considered for the clinical application of MSCs[33], which is strictly related to genomic instability[34]. Only a few studies have investigated malignant lesions at MSC transplant sites, suggesting that the potential risk of tumor formation may still exist[35-38]. In contrast, some other studies have indicated that MSCs were not associated with tumorigenicity after intravenous or intramuscular application[39,40]. To date, only one article has reported spontaneous tumorigenic transformation due to long-term cultivation associated with genomic alterations in culture[41]. It is determined that MenSCs has no karyotype changes in long-term culture in vitro[14].
In this study, no tumor formation was observed in IUA rat models after 6 mo of intrauterine sub-serosa injection. After MenSC transplantation, no lump formed on the abdominal wall or organs. The physiological structure of all organs remained normal. Furthermore, we used Balb/c-nu mice to detect the tumorigenicity of MenSCs. The subcutaneous GFP fluorescence range gradually decreased without migration. These GFP-labled MenSCs completely disappeared at the fourth week, indicating that MenSCs are none-tumorigenic in vivo. In addition, soft agar assay demonstrated that MenSCs did not form any tumor-like cell population in vitro. These results provide good evidence that MenSCs is non-tumorigenic in clinical applications.
In conclusion, our current research confirms that intrauterine transplantation of MenSCs is safe, without toxicities or tumorigenicity. The results indicate that MenSCs are not only safe but also a promise source of cells for treating IUA and other types of endometrial damage. In addition, it is necessary to conduct longer follow-up studies on patients to fully ensure the safety of MenSC application.
ARTICLE HIGHLIGHTS
Research background
Intrauterine adhesion (IUA) can cause serious damage to women's reproductive health. In our previous studies, we demonstrated that menstrual-derived stromal stem cells (MenSCs), with high proliferative capacity and self-renewal ability, have a powerful therapeutic effect in patients with severe IUA.
Research motivation
Safety assessment of MenSCs transplantation is essential for its further application in patients with severe IUA.
Research objectives
The purpose of this study was to evaluate the short-, medium-, and long-term biosafety of MenSCs via intrauterine transplantation in a rat model of IUA, with a focus on toxicity and tumorigenicity.
Research methods
MenSCs were injected into the sub-serosal layer of the uterus in an IUA rat model, for 3 d, 3 mo, and 6 mo separately, to monitor the corresponding acute, sub-chronic, and chronic effects. Healthy rats of the same age served as negative controls. Toxicity effects were evaluated by body weight, organ weight, histopathology, hematology, and biochemistry tests. Tumorigenicity of MenSCs was investigated in Balb/c-nu mice in vivo and by colony formation assays in vitro.
Research results
Compared with the same week-old control group, all of the IUA rats receiving MenSC transplantation demonstrated no obvious changes in body weight, main organ weight, or blood cell composition during the acute, sub-chronic, and chronic observation periods. At the same time, serum biochemical tests showed no adverse effects on metabolism or liver and kidney function. After 4 wk of subcutaneous injection of MenSCs in Balb/c-nu nude mice, no tumor formation or cell metastasis was observed. Moreover, there was no tumor colony formation of MenSCs during soft agar culture in vitro.
Research conclusions
There was no acute, sub-chronic, or chronic poisoning, infection, tumorigenesis, or endometriosis in rats with intrauterine adhesions after MenSC transplantation. The above results suggested that intrauterine transplantation of MenSCs is safe for endometrial treatment.
Research perspectives
MenSCs are not only safe but also a promise source of cells for treating IUA and other types of endometrial damage. In addition, it is necessary to conduct longer follow-up studies on patients to fully ensure the safety of MenSC application.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of the Shengjing Hospital affiliated to China Medical University (2017PS330K).
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care of Shengjing Hospital Affiliated to China Medical University and were conducted in accordance with the AAALAC and IACUC guidelines.
Conflict-of-interest statement: The authors declare that they have no competing interests.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: February 24, 2020
First decision: March 28, 2020
Article in press: April 23, 2020
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dueland S, Haneder S, Taylor ME S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Liu MY
Contributor Information
Qi-Yuan Chang, Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China; Key Laboratory of Reproductive Dysfunction Diseases and Fertility Remodeling of Liaoning Province, Shenyang 110004, Liaoning Province, China.
Si-Wen Zhang, Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China; Key Laboratory of Reproductive Dysfunction Diseases and Fertility Remodeling of Liaoning Province, Shenyang 110004, Liaoning Province, China.
Ping-Ping Li, Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China; Key Laboratory of Reproductive Dysfunction Diseases and Fertility Remodeling of Liaoning Province, Shenyang 110004, Liaoning Province, China.
Zheng-Wei Yuan, Key Laboratory of Health Ministry for Congenital Malformation, Shengjing Hospital of China Medical University, Benxi 117004, Liaoning Province, China.
Ji-Chun Tan, Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China; Key Laboratory of Reproductive Dysfunction Diseases and Fertility Remodeling of Liaoning Province, Shenyang 110004, Liaoning Province, China. tjczjh@163.com.
Data sharing statement
No additional data are available.
References
- 1.Bosteels J, Weyers S, Mol BW, D'Hooghe T. Anti-adhesion barrier gels following operative hysteroscopy for treating female infertility: a systematic review and meta-analysis. Gynecol Surg. 2014;11:113–127. doi: 10.1007/s10397-014-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118. doi: 10.1186/1477-7827-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.March CM, Israel R, March AD. Hysteroscopic management of intrauterine adhesions. Am J Obstet Gynecol. 1978;130:653–657. doi: 10.1016/0002-9378(78)90322-8. [DOI] [PubMed] [Google Scholar]
- 4.Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010;20:121–133. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- 5.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Yu L, Sun M, Mu S, Wang C, Wang D, Yao Y. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res Int. 2013;2013:690491. doi: 10.1155/2013/690491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marofi F, Vahedi G, Biglari A, Esmaeilzadeh A, Athari SS. Mesenchymal Stromal/Stem Cells: A New Era in the Cell-Based Targeted Gene Therapy of Cancer. Front Immunol. 2017;8:1770. doi: 10.3389/fimmu.2017.01770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volarevic V, Bojic S, Nurkovic J, Volarevic A, Ljujic B, Arsenijevic N, Lako M, Stojkovic M. Stem cells as new agents for the treatment of infertility: current and future perspectives and challenges. Biomed Res Int. 2014;2014:507234. doi: 10.1155/2014/507234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N, Mohanty S, Seth T, Shankar M, Bhaskaran S, Dharmendra S. Autologous stem cell transplantation in refractory Asherman's syndrome: A novel cell based therapy. J Hum Reprod Sci. 2014;7:93–98. doi: 10.4103/0974-1208.138864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, Palmero J, Remohí J, Pellicer A, Simón C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31:1087–1096. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 11.Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, Wang J, Bai D, Wang J, Wang L, Zhou Q, Wang H, Dai C, Ding L, Xu B, Zhou Y, Hao J, Dai J, Hu Y. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9:192. doi: 10.1186/s13287-018-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, Thébaud B, Riordan NH. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossignoli F, Caselli A, Grisendi G, Piccinno S, Burns JS, Murgia A, Veronesi E, Loschi P, Masini C, Conte P, Paolucci P, Horwiz EM, Dominici M. Isolation, characterization, and transduction of endometrial decidual tissue multipotent mesenchymal stromal/stem cells from menstrual blood. Biomed Res Int. 2013;2013:901821. doi: 10.1155/2013/901821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Luo Y, Chen J, Pan R, Xiang B, Du X, Xiang L, Shao J, Xiang C. Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev. 2014;23:1245–1257. doi: 10.1089/scd.2013.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. 2017;8:9. doi: 10.1186/s13287-016-0453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Zhang C, Chen L, Wang X, Xiang B, Wu X, Guo Y, Mou X, Yuan L, Chen B, Wang J, Xiang C. Human Menstrual Blood-Derived Stem Cells Ameliorate Liver Fibrosis in Mice by Targeting Hepatic Stellate Cells via Paracrine Mediators. Stem Cells Transl Med. 2017;6:272–284. doi: 10.5966/sctm.2015-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng P, Li P, Tan J. Human Menstrual Blood-Derived Stromal Cells Promote Recovery of Premature Ovarian Insufficiency Via Regulating the ECM-Dependent FAK/AKT Signaling. Stem Cell Rev Rep. 2019;15:241–255. doi: 10.1007/s12015-018-9867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanmohammadi M, Golshahi H, Saffarian Z, Montazeri S, Khorasani S, Kazemnejad S. Repair of Osteochondral Defects in Rabbit Knee Using Menstrual Blood Stem Cells Encapsulated in Fibrin Glue: A Good Stem Cell Candidate for the Treatment of Osteochondral Defects. Tissue Eng Regen Med. 2019;16:311–324. doi: 10.1007/s13770-019-00189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Niu R, Li W, Lin J, Stamm C, Steinhoff G, Ma N. Therapeutic potential of menstrual blood-derived endometrial stem cells in cardiac diseases. Cell Mol Life Sci. 2019;76:1681–1695. doi: 10.1007/s00018-019-03019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uzieliene I, Urbonaite G, Tachtamisevaite Z, Mobasheri A, Bernotiene E. The Potential of Menstrual Blood-Derived Mesenchymal Stem Cells for Cartilage Repair and Regeneration: Novel Aspects. Stem Cells Int. 2018;2018:5748126. doi: 10.1155/2018/5748126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv H, Hu Y, Cui Z, Jia H. Human menstrual blood: a renewable and sustainable source of stem cells for regenerative medicine. Stem Cell Res Ther. 2018;9:325. doi: 10.1186/s13287-018-1067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, Xu X, Kong L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman's syndrome. Hum Reprod. 2016;31:2723–2729. doi: 10.1093/humrep/dew235. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Li P, Yuan Z, Tan J. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res Ther. 2019;10:61. doi: 10.1186/s13287-019-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Qu J, Xiang C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. 2019;10:1. doi: 10.1186/s13287-018-1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 26.Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]) Circ Res. 2017;121:1192–1204. doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical Cord Mesenchymal Stem Cell Treatment for Crohn's Disease: A Randomized Controlled Clinical Trial. Gut Liver. 2018;12:73–78. doi: 10.5009/gnl17035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang J, Zhang H, Kong W, Deng W, Wang D, Feng X, Zhao C, Hua B, Wang H, Sun L. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: a long-term retrospective study. Stem Cell Res Ther. 2018;9:312. doi: 10.1186/s13287-018-1053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastos R, Mathias M, Andrade R, Amaral RJFC, Schott V, Balduino A, Bastos R, Miguel Oliveira J, Reis RL, Rodeo S, Espregueira-Mendes J. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: a controlled, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc. 2019:Online ahead of print. doi: 10.1007/s00167-019-05732-8. [DOI] [PubMed] [Google Scholar]
- 30.Levy ML, Crawford JR, Dib N, Verkh L, Tankovich N, Cramer SC. Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. Stroke. 2019;50:2835–2841. doi: 10.1161/STROKEAHA.119.026318. [DOI] [PubMed] [Google Scholar]
- 31.Zhong Z, Patel AN, Ichim TE, Riordan NH, Wang H, Min WP, Woods EJ, Reid M, Mansilla E, Marin GH, Drago H, Murphy MP, Minev B. Feasibility investigation of allogeneic endometrial regenerative cells. J Transl Med. 2009;7:15. doi: 10.1186/1479-5876-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldring CE, Duffy PA, Benvenisty N, Andrews PW, Ben-David U, Eakins R, French N, Hanley NA, Kelly L, Kitteringham NR, Kurth J, Ladenheim D, Laverty H, McBlane J, Narayanan G, Patel S, Reinhardt J, Rossi A, Sharpe M, Park BK. Assessing the safety of stem cell therapeutics. Cell Stem Cell. 2011;8:618–628. doi: 10.1016/j.stem.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Heslop JA, Hammond TG, Santeramo I, Tort Piella A, Hopp I, Zhou J, Baty R, Graziano EI, Proto Marco B, Caron A, Sköld P, Andrews PW, Baxter MA, Hay DC, Hamdam J, Sharpe ME, Patel S, Jones DR, Reinhardt J, Danen EH, Ben-David U, Stacey G, Björquist P, Piner J, Mills J, Rowe C, Pellegrini G, Sethu S, Antoine DJ, Cross MJ, Murray P, Williams DP, Kitteringham NR, Goldring CE, Park BK. Concise review: workshop review: understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl Med. 2015;4:389–400. doi: 10.5966/sctm.2014-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neri S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berkowitz AL, Miller MB, Mir SA, Cagney D, Chavakula V, Guleria I, Aizer A, Ligon KL, Chi JH. Glioproliferative Lesion of the Spinal Cord as a Complication of "Stem-Cell Tourism". N Engl J Med. 2016;375:196–198. doi: 10.1056/NEJMc1600188. [DOI] [PubMed] [Google Scholar]
- 36.Thirabanjasak D, Tantiwongse K, Thorner PS. Angiomyeloproliferative lesions following autologous stem cell therapy. J Am Soc Nephrol. 2010;21:1218–1222. doi: 10.1681/ASN.2009111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 38.Mohseny AB, Szuhai K, Romeo S, Buddingh EP, Briaire-de Bruijn I, de Jong D, van Pel M, Cleton-Jansen AM, Hogendoorn PC. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J Pathol. 2009;219:294–305. doi: 10.1002/path.2603. [DOI] [PubMed] [Google Scholar]
- 39.Yun JW, Ahn JH, Kwon E, Kim SH, Kim H, Jang JJ, Kim WH, Kim JH, Han SY, Kim JT, Kim JH, Kim W, Ku SY, Do BR, Kang BC. Human umbilical cord-derived mesenchymal stem cells in acute liver injury: Hepatoprotective efficacy, subchronic toxicity, tumorigenicity, and biodistribution. Regul Toxicol Pharmacol. 2016;81:437–447. doi: 10.1016/j.yrtph.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 40.Rengasamy M, Gupta PK, Kolkundkar U, Singh G, Balasubramanian S, SundarRaj S, Chullikana A, Majumdar AS. Preclinical safety & toxicity evaluation of pooled, allogeneic human bone marrow-derived mesenchymal stromal cells. Indian J Med Res. 2016;144:852–864. doi: 10.4103/ijmr.IJMR_1842_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan Q, Fouraschen SM, de Ruiter PE, Dinjens WN, Kwekkeboom J, Tilanus HW, van der Laan LJ. Detection of spontaneous tumorigenic transformation during culture expansion of human mesenchymal stromal cells. Exp Biol Med (Maywood) 2014;239:105–115. doi: 10.1177/1535370213506802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.