NOCEBO DEFINITIONS AND PATHOPHYSIOLOGIC MECHANISMS
The management of patients with rheumatic diseases in routine practice is guided by established measures of response to treatment with a target of low disease activity or remission. In addition to objective clinical data, subjective patient-reported outcomes (such as the number of tender joints or the patient’s perception of their general health status) are taken into account when assessing efficacy and tolerability of disease-modifying anti-rheumatic drugs (DMARDs), including bio-originator and biosimilar biologic DMARDs. A growing body of evidence suggests that the impact of DMARDs on subjective symptoms in rheumatic diseases is significantly affected by the patients’ expectations of treatment effects, either favorably when pre-existing expectations are positive (placebo effect), or detrimentally when pre-existing expectations are negative (nocebo effect).1–4
The word nocebo (from Latin noceo, meaning “to harm”) denotes a medical intervention (a medication, inert substance, procedure, or patient-physician encounter) that causes noxious changes in a patient’s symptoms or physiologic condition because of negative expectations.5,6 These nocebo effects may manifest as new adverse symptoms, or as a recurrence of unpleasant symptoms previously experienced in the setting of a chronic disease. Nocebo effects often mimic the side effects described in clinical trial consent forms or patient information leaflets, but may also be nonspecific subjective complains, such as dizziness, fatigue, or pain. Neurobiological research based on functional MRI imaging has shown that nocebos activate specific areas in the modulatory pain network, which may result in exacerbation of the patient’s pain or even generate the perception of a pain signal in the patient’s brain. These areas include parts of the limbic system linked with anxiety and emotional response to stress (hippocampus, amygdala), centers for decision-making, reward, and survival behavior in response to threat (insula, nucleus accumbens, anterior cingulate cortex), and their interconnections with the periaqueductal grey and rostral ventral medulla, which modulate ascending pain signals.7–11 By facilitating nocebo effects and materializing negative preconceptions, these neural circuits may promote risk avoidance and learning from other people’s adverse experience.12 Additionally, relevant studies show that nocebo circuits manifest neuronal plasticity and increased activity as a result of prior conditioning with adverse drug effects, stressful situations and co-existing anxiety disorders.10,13
NOCEBO RISK FACTORS
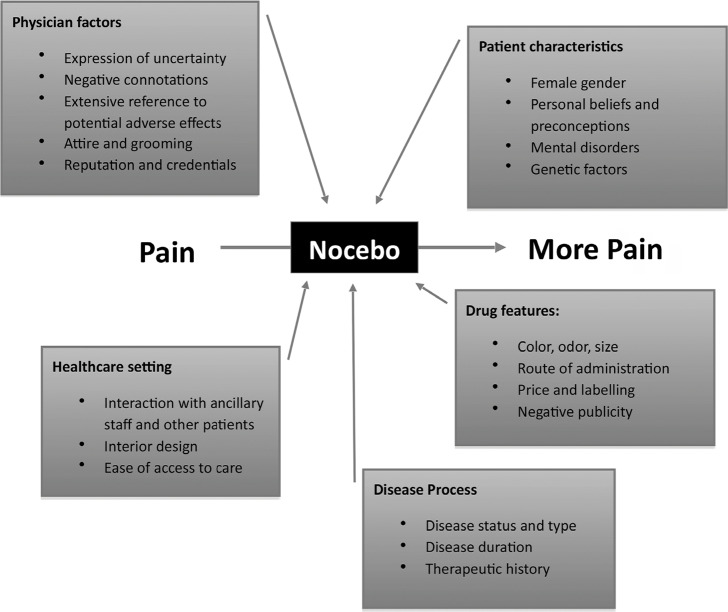
Experimental and translational research has identified several risk factors for the development of nocebo effects, most of which are very prevalent among patients with rheumatic disease. These risk factors can be classified as physician attributes, patient characteristics, drug-related factors, features of the healthcare setting, and factors related to the disease process (Figure 1).5 Among these, the interaction between patient and physician at the time of the medical encounter has the most catalytic impact on the development of nocebo effects. Nocebos affect patients of all ages, but a recent meta-analysis suggests women are more susceptible than men.14 Patients with somatisation, depression and anxiety disorders, chronic pain and pain catastrophizing, cognitive impairment, or language barriers all predispose patients to nocebo effects.15–18 History of adverse effects to prior treatment, and a recurrently flaring disease course with failure to multiple lines of therapy results in negative conditioning, also leading to nocebo effects.3 Refusal of interventions, such as preventive vaccination, and/or extensive online researching may be indications of deep apprehension and negative bias toward medications.
Figure 1.
Nocebo risk factors in clinical practice.
On behalf of the physician, expressing uncertainty or anxiety about the proposed intervention, using negative connotations and framing while informing patients, or overtly focusing on potential adverse effects using lengthy and vivid descriptions, has been linked to a higher rate of nocebo effects.19–21 A dismissive communication style (including language, posture, and grimace), as well as unbefitting comments, attire, and grooming, will affect patient expectations, both consciously and subconsciously. Finally, other features of the healthcare setting may predispose patients to nocebo effects, such as architecture, interior design, technology, accessibility and affordability of care, and behaviour of non-physician staff. Patients who spend a lot of time in waiting rooms and infusion suites may be exposed to negative suggestions and groupthink effects as a result of discussions with fellow patients.
NOCEBO EFFECTS IN PATIENTS WITH RHEUMATIC DISEASE
It is known that patients with rheumatic disease and chronic pain have altered pain transmission and central sensitization even after the inflammatory aspect of their pain has resolved.22 It is estimated that up to 30% of rheumatoid arthritis (RA) patients achieving low disease activity or remission targets with biologic DMARDs experience residual non-inflammatory pain, which can be attributed to impaired pain modulation.2 Patients with fibromyalgia syndrome suffer primarily due to impaired pain signal inhibition.23 Functional brain imaging evidence suggests that in osteoarthritis, changes in the peripheral and central processing of ascending pain signals may be largely responsible for the difficult-to-treat chronic pain cases.24 Due to its subjective nature and prevalence as a cardinal symptom of arthritis, pain increase is an expected nocebo-related adverse outcome in patients with rheumatic diseases.
Although nocebo may occur commonly in routine rheumatology practice, it can be hard to discern whether an unfavourable response to therapy is a pharmacological adverse event, a result of spontaneous fluctuations in disease activity, or a nocebo effect. In the randomized clinical trial (RCT) context, nocebo effects are measured as adverse events occurring in the inert substance arm and classified as “drug-related” by the blinded investigators. Meta-analyses of inert substance arm dropouts from RCTs for rheumatic diseases have shown that dropouts due to nocebo effects are more prevalent in patients with fibromyalgia syndrome (∼10% in trials of analgesic therapies), as compared to patients with RA (∼3% in trials of bio-originator biologic DMARDs and relevant meta-analyses of analgesic trials).5,25,26 On the other hand, trial participants with RA, have higher rates of inert substance discontinuations due to lack of efficacy (7% in a recent meta-analysis) compared to participants with chronic neuropathic pain (3%) or osteoarthritis (1%), possibly hinting to a relative “resistance” to the placebo effect.26
NOCEBO AND BIOSIMILARS
In recent years, biosimilar biologic DMARDs (BSM) emerged as a preferred treatment for patients with a variety of rheumatic diseases over bio-originator biologic DMARDs (BO), because they are marketed at a significantly lower price, and switching patients from BO to BMS agents could result in significant healthcare fund savings (estimates reach £200 million/year for the NHS).27 Despite affirming results from several double-blind RCTs examining bio-equivalence and non-inferiority following open-label switch to a BSM versus remaining on the BO,28–33 results from nationwide switch observational studies and other real-world cohorts revealed alarming drug discontinuation rates of 15–30%, mostly for perceived lack of efficacy and subjective non-specific complains without increase in inflammatory markers.4,34–41
In a meta-analysis of all published BSM switch studies through 2018, the pooled drug discontinuation rate for 21 open-label studies (both RCT and real-world observational studies) was significantly higher than observed in 2 double-blind RCTs (15% vs. 7%).27 This discrepancy has been interpreted as a nocebo effect, adding to the long list of suboptimal outcomes associated with nocebo.42,43 It should be noted though, that other reasons may also play a role, such as a selection of biologic-naïve patients for RCTs, a less tight follow-up in the real-world setting, and causal misattributions on behalf of the physicians, who may discontinue BSMs in response to non-specific subjective complains more frequently that they would if the patient were on a BO.5 In the BIO-SWITCH multicentre prospective open-label study from the Netherlands, the investigators noted that although adverse events in the arm treated with BSMs was the same as in the blinded NOR-SWITCH from Norway (18 vs. 10%), the lead to BSM discontinuation more often (11% vs. 3%).4,28 This finding is congruent with negative preconceptions and uncertainty of prescribing physicians about the bio-equivalence of BSMs, which has been recorded in relevant studies.27,44–46
It is expected that patients with rheumatic disease will be even more prone to negative preconceptions about BSMs than physicians, due to previous adverse drug reactions and inability to interpret scientific medical data. Patients regard their rheumatologist as the most influential source of information and the most important contributor to agreeing to transition to a biosimilar, but objective and thorough patient education on BSMs seems to be lacking: in one study from Europe, scientific information on BSMs was inadequately provided to patients treated or about to be treated with a biosimilar, even though having a good understanding of BSMs lead to better adherence.46
SUGGESTIONS FOR CLINICAL PRACTICE
Clinical consequences of nocebo effects are far-reaching and include medication non-adherence and wasting, over-utilization of healthcare resources, polypharmacy, loss of patient trust, treatment with second-line therapies and suboptimal outcomes, and dropouts from clinical trials hindering interpretation and generalization of medical research findings.3,43,47–49 Assuming a rate of nocebo-related drug discontinuation of 3–10% supported by relevant literature in patients with rheumatic disease,5,27,41 implementation of strategies to diminish nocebo effects could result in higher rates of successful initial treatments and BSM transitions. Both individual physicians and healthcare organization administrators must take action to limit the impact of nocebo effects on clinical outcomes, focusing on providing patient education and addressing patient concerns.
Physicians must be able to identify patients with many risk factors for nocebo effects, in order to allocate more time for counselling and educating them about nocebo: it has been shown that high-risk patients who understand nocebo mechanisms are less likely to develop nocebo effects.50 Several questionnaires have been used in research settings to assess negative beliefs about medications (Q-No,51 Stanford Expectations of Treatment Scale,52 Beliefs About Medications53 ); however, more research is needed in order to identify a scale with satisfactory sensitivity and specificity for the prediction of nocebo effects. Regardless of specific tools used, healthcare professionals should take the time to elicit patient’s beliefs about treatment, prior history of adverse effects, and other stressful situations hindering adherence to medications.
When physicians suspect medication intolerance due to non-specific, pharmacologically implausible complains in high-risk patients, it may be worth attempting causal re-attributions or positive expectation induction, which has been shown to diminish and even reverse nocebo effects;50,54 frequent visits may be needed if patients are agreeable. A change in the time or place the patients take their medication may be warranted, if negative associations are identified. Physicians may also advise nocebo-prone patients to ignore patient information leaflets and provide individualized information on side effects, using graphic illustrations and positive framing. In a recent RCT, stating that a side effect was “uncommon, with 90% of people unaffected” with an inert substance led to 39% nocebo effects, versus 55% in the group where the same side effect was presented as “common, with 1 in 10 people affected”.55
Effective and positive patient-physician interaction is a key element toward the prevention of nocebo effects in all patients. Physicians who generate fear, dismiss patient concerns or seem emotionally unavailable cause negative patient anticipation; those who provide reassurance, show empathy and empower patients during their encounters forge a strong therapeutic alliance. Phrasing that avoids negative connotations and stresses expected benefits rather than safety concerns should be preferred in decision-making discussions and consent forms. Discussions about drug cost require caution, as patients tend to consider low-cost alternatives either less safe or less effective.56–58 Behavioural science data supports that face-to-face delivery of information provides stronger re-assurance than written material alone,16 which should be taken into account in appointment scheduling. “Soft skills” training aimed at enabling rheumatologists to maximize placebo effects and minimize nocebo effects should be applied, not only because it will make physicians and patients “feel better”, but in order to bring about a measurable positive impact on health outcomes for our patients.
CONFLICT OF INTEREST
EK declares no conflict of interest. G.D.K has received honoraria for lectures and advisory boards, and/or support for the organization of educational meetings and/or attendance to congresses from Abbvie, Aenorasis, BMS, Genesis, GSK, MSD, Novartis, Roche, Pfizer and UCB. P.P.S has received honoraria for lectures and/or advisory boards, and/or funding for research and congress attendances from Abbvie, Aenorasis, Amgen, BMS, Boehringer, Elli Lilly, Elpen, Genesis, Jannsen, Pfizer, MSD, Novartis, Roche, Sanofi and UCB.
DISCLAIMER
No part of this article contains recycled texts or graphics, or has been published elsewhere.
REFERENCES
- 1.Boone NW, Liu L, Romberg-Camps MJ, Duijsens L, Houwen C, van der Kuy PHM, et al. The nocebo effect challenges the non-medical infliximab switch in practice. Eur J Clin Pharmacol 2018;74(5):655–61. [ 10.1007/s00228-018-2418-4] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermans L, Nijs J, Calders P, De Clerck L, Moorkens G, Hans G, et al. Influence of Morphine and Naloxone on Pain Modulation in Rheumatoid Arthritis, Chronic Fatigue Syndrome/Fibromyalgia, and Controls: A Double-Blind, Randomized, Placebo-Controlled, Cross-Over Study. Pain Pract 2018;18(4):418–30. [ 10.1111/papr.12613] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 3.Nestoriuc Y, Orav EJ, Liang MH, Horne R, Barsky AJ. Prediction of nonspecific side effects in rheumatoid arthritis patients by beliefs about medicines. Arthritis Care Res (Hoboken) 2010;62(6):791–9. [ 10.1002/acr.20160] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tweehuysen L, van den Bemt BJF, van Ingen IL, de Jong AJL, van der Laan WH, van den Hoogen FHJ, et al. Subjective Complaints as the Main Reason for Biosimilar Discontinuation After Open-Label Transition From Reference Infliximab to Biosimilar Infliximab. Arthritis Rheumatol 2018; 70(1):60–8. [ 10.1002/art.40324] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 5.Kravvariti E, Kitas GD, Mitsikostas DD, Sfikakis PP. Nocebos in rheumatology: emerging concepts and their implications for clinical practice. Nat Rev Rheumatol 2018; 4(12):727–40. [ 10.1038/s41584-018-0110-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 6.Schedlowski M, Enck P, Rief W, Bingel U. Neuro-Bio-Behavioral Mechanisms of Placebo and Nocebo Responses: Implications for Clinical Trials and Clinical Practice. Pharmacol Rev 2015;67(3):697–730. [ 10.1124/pr.114.009423] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 7.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;9(4):463–84. [ 10.1016/j.ejpain.2004.11.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 8.Benedetti F, Lanotte M, Lopiano L, Colloca L. When words are painful: unraveling the mechanisms of the nocebo effect. Neuroscience 2007;147(2):260–71. [ 10.1016/j.neuroscience.2007.02.020] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 9.Jensen KB, Kaptchuk TJ, Chen X, Kirsch I, Ingvar M, Gollub RL, et al. A Neural Mechanism for Nonconscious Activation of Conditioned Placebo and Nocebo Responses. Cereb Cortex 2015;25(10):3903–10. [ 10.1093/cercor/bhu275] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palermo S, Benedetti F, Costa T, Amanzio M. Pain anticipation: an activation likelihood estimation meta-analysis of brain imaging studies. Hum Brain Mapp 2015;36(5):1648–61. [ 10.1002/hbm.22727] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Me 2010;16(11):1277–83. [ 10.1038/nm.2229] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 12.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 1998;20(5):937–45. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 13.Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol 2007;20(5):435–9. [ 10.1097/ACO.0b013e3282b972fb] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 14.Vambheim SM, Flaten MA. A systematic review of sex differences in the placebo and the nocebo effect. J Pain Res 2017;10:1831–9. [ 10.2147/JPR.S134745] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amanzio M, Palermo S, Skyt I, Vase L. Lessons Learned From Nocebo Effects in Clinical Trials for Pain Conditions and Neurodegenerative Disorders. J Clin Psychopharmacol 2016;36(5):475–82. [ 10.1097/JCP.0000000000000556] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 16.Barsky AJ, Orav EJ, Ahern DK, Rogers MP, Gruen SD, Liang MH. Somatic style and symptom reporting in rheumatoid arthritis. Psychosomatics 1999;40(5):396–403. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 17.Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol 2004;3(11):679–84. [ 10.1016/S1474-4422(04)00908-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 18.Klinger R, Blasini M, Schmitz J, Colloca L. Nocebo effects in clinical studies: hints for pain therapy. Pain Rep 2017;2(2). [ 10.1097/PR9.0000000000000586] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amanzio M, Corazzini LL, Vase L, Benedetti F. A systematic review of adverse events in placebo groups of anti-migraine clinical trials. Pain 2009;146(3):261–9. [ 10.1016/j.pain.2009.07.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 20.Colloca L, Finniss D. Nocebo effects, patient-clinician communication, and therapeutic outcomes. JAMA 2012;307(6):567–8. [ 10.1001/jama.2012.115] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Laarhoven AI, Vogelaar ML, Wilder-Smith OH, van Riel PL, van de Kerkhof PC, Kraaimaat FW, et al. Induction of nocebo and placebo effects on itch and pain by verbal suggestions. Pain 2011;152(7):1486–94. [ 10.1016/j.pain.2011.01.043] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 22.Zhang A, Lee YC. Mechanisms for Joint Pain in Rheumatoid Arthritis (RA): from Cytokines to Central Sensitization. Curr Osteoporos Rep 2018;16(5):603–10. [ 10.1007/s11914-018-0473-5] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potvin S, Marchand S. Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia and in healthy controls. Pain 2016;157(8):1704–10. [ 10.1097/j.pain.0000000000000573] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 24.Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum 2009;61(9):1226–34. [ 10.1002/art.24837] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 25.Mitsikostas DD, Chalarakis NG, Mantonakis LI, Delicha EM, Sfikakis PP. Nocebo in fibromyalgia: meta-analysis of placebo-controlled clinical trials and implications for practice. Eur J Neurol 2012;19(5):672–80. [ 10.1111/j.1468-1331.2011.03528.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 26.Cepeda MS, Lobanov V, Berlin JA. Use of ClinicalTrials.gov to estimate condition-specific nocebo effects and other factors affecting outcomes of analgesic trials. J Pain 2013;14(4):405–11. [ 10.1016/j.jpain.2012.12.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 27.Odinet JS, Day CE, Cruz JL, Heindel GA. The Biosimilar Nocebo Effect? A Systematic Review of Double-Blinded Versus Open-Label Studies. J Manag Care Spec Pharm 2018;24(10):952–9. [ 10.18553/jmcp.2018.24.10.952] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to bio-similar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017;389(10086):2304–16. [ 10.1016/S0140-6736(17)30068-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 29.Park W, Suh CH, Shim SC, Molina FFC, Jeka S, Medina-Rodriguez FG, et al. Efficacy and Safety of Switching from Innovator Rituximab to Biosimilar CT-P10 Compared with Continued Treatment with CT-P10: Results of a 56-Week Open-Label Study in Patients with Rheumatoid Arthritis. BioDrugs 2017;31(4):369–77. [ 10.1007/s40259-017-0233-6] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinblatt ME, Baranauskaite A, Dokoupilova E, Zielinska A, Jaworski J, Racewicz A, et al. Switching From Reference Adalimumab to SB5 (Adalimumab Biosimilar) in Patients With Rheumatoid Arthritis: Fifty-Two-Week Phase III Randomized Study Results. Arthritis Rheumatol 2018;70(6):832–40. [ 10.1002/art.40444] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo DH, Prodanovic N, Jaworski J, Miranda P, Ramiterre E, Lanzon A, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis 2017;76(2):355–63. [ 10.1136/annrheumdis-2015-208786] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen S, Genovese MC, Choy E, Perez-Ruiz F, Matsumoto A, Pavelka K, et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomised, double-blind, phase III equivalence study. Ann Rheum Dis 2017;76(10):1679–87. [ 10.1136/annrheumdis-2016-210459] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smolen JS, Choe JY, Prodanovic N, Niebrzydowski J, Staykov I, Dokoupilova E, et al. Safety, immunogenicity and efficacy after switching from reference infliximab to biosimilar SB2 compared with continuing reference infliximab and SB2 in patients with rheumatoid arthritis: results of a randomised, double-blind, phase III transition study. Ann Rheum Dis 2018;77(2):234–40. [ 10.1136/annrheumdis-2017-211741] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdalla A, Byrne N, Conway R, Walsh T, Mannion G, Hanly M, et al. Long-term safety and efficacy of biosimilar infliximab among patients with inflammatory arthritis switched from reference product. Open Access Rheumatol 2017;9:29–35. [ 10.2147/OARRR.S124975] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avouac J, Molto A, Abitbol V, Etcheto A, Salcion A, Gutermann L, et al. Systematic switch from innovator infliximab to biosimilar infliximab in inflammatory chronic diseases in daily clinical practice: The experience of Cochin University Hospital, Paris, France. Semin Arthritis Rheum 2018;47(5):741–8. [ 10.1016/j.semarthrit.2017.10.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 36.Benucci M, Gobbi FL, Bandinelli F, Damiani A, Infantino M, Grossi V, et al. Safety, efficacy and immunogenicity of switching from innovator to biosimilar infliximab in patients with spondyloarthritis: a 6-month real-life observational study. Immunol Res 2017;65(1):419–22. [ 10.1007/s12026-016-8843-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 37.Glintborg B, Sorensen IJ, Loft AG, Lindegaard H, Linauskas A, Hendricks O, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis 2017;76(8):1426–31. [ 10.1136/annrheumdis-2016-210742] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 38.Holroyd CR, Parker L, Bennett S, Zarroug J, Underhill C, Davidson B, et al. Switching to biosimilar infliximab: real world data in patients with severe inflammatory arthritis. Clin Exp Rheumatol 2018;36(1):171–2. [PMID: ] [PubMed] [Google Scholar]
- 39.Nikiphorou E, Kautiainen H, Hannonen P, Asikainen J, Kokko A, Rannio T, et al. Clinical effectiveness of CT-P13 (Infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther 2015;15(12):1677–83. [ 10.1517/14712598.2015.1103733] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 40.Scherlinger M, Germain V, Labadie C, Barnetche T, Truchetet ME, Bannwarth B, et al. Switching from originator infliximab to biosimilar CT-P13 in real-life: The weight of patient acceptance. Joint Bone Spine 2018;85(5):561–7. [ 10.1016/j.jb-spin.2017.10.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 41.Bakalos G, Zintzaras E. Drug Discontinuation in Studies Including a Switch From an Originator to a Biosimilar Monoclonal Antibody: A Systematic Literature Review. Clin Ther 2019;41(1):155–73 e13. [ 10.1016/j.clinthera.2018.11.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 42.Chavarria V, Vian J, Pereira C, Data-Franco J, Fernandes BS, Berk M, et al. The Placebo and Nocebo Phenomena: Their Clinical Management and Impact on Treatment Outcomes. Clin Ther 2017;39(3):477–86. [ 10.1016/j.clinthera.2017.01.031] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 43.Gupta A, Thompson D, Whitehouse A, Collier T, Dahlof B, Poulter N, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet 2017;389(10088):2473–81. [ 10.1016/S0140-6736(17)31075-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 44.Cohen H, Beydoun D, Chien D, Lessor T, McCabe D, Muenzberg M, et al. Awareness, Knowledge, and Perceptions of Biosimilars Among Specialty Physicians. Adv Ther 2017;33(12):2160–72. [ 10.1007/s12325-016-0431-5] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peyrin-Biroulet L, Lonnfors S, Roblin X, Danese S, Avedano L. Patient Perspectives on Biosimilars: A Survey by the European Federation of Crohn’s and Ulcerative Colitis Associations. J Crohns Colitis 2017;11(1):128–33. [ 10.1093/ecco-jcc/jjw138] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 46.Frantzen L, Cohen JD, Trope S, et al. Patients’ information and perspectives on biosimilars in rheumatology: a French nation-wide survey. Joint Bone Spine 2019. [ 10.1016/j.jbspin.2019.01.001] [DOI] [PubMed] [Google Scholar]
- 47.Cepeda MS, Lobanov V, Berlin JA. Using Sherlock and Clinical-Trials.gov data to understand nocebo effects and adverse event dropout rates in the placebo arm. J Pain;14(9):999. [ 10.1016/j.jpain.2013.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 48.Desai RJ, Sarpatwari A, Dejene S, Khan NF, Lii J, Rogers JR, et al. Differences in rates of switchbacks after switching from branded to authorized generic and branded to generic drug products: cohort study. BMJ 2018;361:k1180. [ 10.1136/bmj.k1180] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitsikostas DD. Nocebo in headaches: implications for clinical practice and trial design. Curr Neurol Neurosci Rep 2012;12(2):132–7. [ 10.1007/s11910-011-0245-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 50.Tweehuysen L, Huiskes VJ, van den Bemt BJ, Vriezekolk JE, Teerenstra S, van den Hoogen FHJ, et al. Open-Label Non-Mandatory Transitioning From Originator Etanercept to Biosimilar SB4: 6-Month Results From a Controlled Cohort Study. Arthritis Rheumatol 2018;70(9):1408–18. [ 10.1002/art.40516] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 51.Mitsikostas DD, Deligianni CI. Q-No: a questionnaire to predict nocebo in outpatients seeking neurological consultation. Neurol Sci 2015;36(3):379–81. [ 10.1007/s10072-014-1959-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 52.Younger J, Gandhi V, Hubbard E, Mackey S. Development of the Stanford Expectations of Treatment Scale (SETS): a tool for measuring patient outcome expectancy in clinical trials. Clin Trials 2012;9(6):767–76. [ 10.1177/1740774512465064] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 53.Neame R, Hammond A. Beliefs about medications: a questionnaire survey of people with rheumatoid arthritis. Rheumatology (Oxford) 2005;44(6):762–7. [ 10.1093/rheumatology/keh587] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 54.Bartels DJP, van Laarhoven AIM, Stroo M, Hijne K, Peerdeman KJ, Donders ART, et al. Minimizing nocebo effects by conditioning with verbal suggestion: A randomized clinical trial in healthy humans. PLoS One 2017; 12(9): e0182959. [ 10.1371/journal.pone.0182959] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Webster RK, Weinman J, Rubin GJ. Positively Framed Risk Information in Patient Information Leaflets Reduces Side Effect Reporting: A Double-Blind Randomized Controlled Trial. Ann Behav Med 2018;52(11):920–9. [ 10.1093/abm/kax064] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 56.Colgan S, Faasse K, Martin LR, Stephens MH, Grey A, Petrie KJ. Perceptions of generic medication in the general population, doctors and pharmacists: a systematic review. BMJ Open 2015;5(12):e008915. [ 10.1136/bmjopen-2015-008915] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ringe JD, Moller G. Differences in persistence, safety and efficacy of generic and original branded once weekly bisphosphonates in patients with postmenopausal osteoporosis: 1-year results of a retrospective patient chart review analysis. Rheumatol Int 2009;30(2):213–21. [ 10.1007/s00296-009-0940-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 58.Tinnermann A, Geuter S, Sprenger C, Finsterbusch J, Buchel C. Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science 2017;358(6359):105–8. [ 10.1126/science.aan1221] [PMID: ] [DOI] [PubMed] [Google Scholar]