Abstract
The aim of this study was to investigate S. aureus carriage among children with sickle cell disease (SCD), including the prevalence, risk factors, and antibiotic resistance. The study was cross-sectional, and involved 120 children with SCD recruited at the Princess Marie Louise Children’s Hospital (PML) in Accra and 100 apparently healthy children from environs of the hospital. Nasal swab samples were collected from the study participants and cultured for bacteria. Confirmation of S. aureus and methicillin-resistant Staphylococcus aureus (MRSA) isolates were done using the tube coagulase test and mecA polymerase chain reaction, respectively. All the S. aureus isolates were tested against standard antimicrobial agents using the Kirby-Bauer method. A structured questionnaire was used to obtain the socio-demographic and clinical data of the study participants. Binary logistic regression was used to identify determinants of S. aureus and MRSA carriage among the study participants. The nasal carriage prevalence of S. aureus was 33.3% (n = 40) and 10% (n = 10) among the participants of the SCD and control groups, respectively. As regards MRSA nasal carriage prevalence, the respective values were 3.33% (n = 4) and 0.00% (n = 0). SCD was significantly associated with S. aureus colonization (p < 0.0001, OR = 4.045), but not MRSA colonization (p = 0.128). In the SCD group, the significant predictors of S. aureus carriage were increasing age (p = 0.003; OR = 1.275) and living in self-contained apartments (p = 0.033; OR = 3.632), whereas male gender (p = 0.018; OR = 0.344) and the practice of self-medication (p = 0.039; OR = 0.233) were protective of S. aureus carriage. In the control group, a history of hospitalization in the past year was a risk factor for the carriage of S. aureus (p = 0.048; OR = 14.333). Among the participants of the SCD and control groups, respectively, the resistance prevalence recorded by S. aureus against the various antibiotics investigated were penicillin (100% each), cotrimoxazole (27.5% vs. 20%), tetracycline (25% vs. 50%), rifampicin (82.5% vs. 50%), erythromycin (30% vs. 20%), clindamycin (32.5% vs. 50%), gentamicin (7.5% vs. 20%), cefoxitin (27.5% vs. 20%), linezolid (30% vs. 40%), and fusidic acid (95% vs. 80%). The proportion of S. aureus isolates that were multidrug resistant (MDR) was 92.5% (37/40) in the SCD group and 100% (10/10) in the control group.
Keywords: S. aureus, MRSA, colonization, antibiotics, Accra
1. Introduction
Staphylococcus aureus is one of the predominantly isolated Gram-positive bacteria in clinical specimens, causing infections such as pneumonia, septicaemia, endocarditis, osteomyelitis, and meningitis [1,2,3,4]. It is also a commensal of various parts of the body, including the skin, perineum and pharynx, but preferentially colonizes the moist squamous epithelium of the anterior nares of the nose [5,6]. Consequently, different categories of S. aureus carriers—persistent carriers, intermittent carriers, and non-carriers—have been reported at rates 20%, 30%, and 50%, respectively [5,7,8,9].
The clinical significance of S. aureus has been accentuated by the emergence and spread of methicillin-resistant strains (methicillin-resistant Staphylococcus aureus [MRSA]), which are characteristically different from methicillin-sensitive strains (methicillin-sensitive Staphylococcus aureus [MSSA]) by virtue of their resistance to all beta-lactam antibiotics. MRSA strains were previously nosocomial, and bore the tag healthcare-associated MRSA (HA-MRSA) [10], but now bear additional tags—community-associated MRSA (CA-MRSA) and livestock-associated MRSA (LA-MRSA)—owing to their association with communities and livestock, respectively [11,12,13,14]. Over the years, the proportion of isolation of MRSA among S. aureus infections has increased to as high as 70% [15,16,17,18,19]. In the United States, in the year 2014, it accounted for an estimated 72,444 invasive infections, resulting in 9194 deaths [20]. Inpatient stays due to MRSA infections cost USD 14,000, relative to the financial burden for all other stays (USD 7600), with a two-fold increase in the length of hospitalization [21,22].
Sickle cell disease (SCD) refers to a couple of genetic disorders that are associated with structurally abnormal haemoglobin. The main genotypes that give rise to SCD include Hb SS, Hb SC, Hb Sβ+-thalassemia, and Hb Sβ0-thalassemia; other rare forms include haemoglobin SD and haemoglobin SE [23]. Owing to the impairment of their immune system, SCD patients are relatively predisposed to bacterial infections, resulting in frequent hospitalization and the intake of antibiotic treatment. This could invariably select for antibiotic resistant pathogens among these individuals, and potentially make them reservoirs for multidrug-resistant commensal and pathogenic microbes, such as MRSA. Indeed, MRSA carriage is an antecedent to subsequent infections [6,24,25]. Since 2012, MRSA has caused several outbreaks in Ghana [26], and a replication of these outbreaks in SCD patients could be fatal. Notwithstanding the public health threat that MRSA could pose to SCD patients, this immunologically-challenged population has received little attention from researchers studying MRSA carriage. Microbiology researchers studying sickle cell disease have usually focused on the occurrence of Streptococcus pneumoniae in SCD patients [27,28]. Yet, among SCD patients, S. aureus holds more potential in causing invasive diseases than S. pneumoniae [29,30,31,32,33]. Moreover, MRSA carriage studies seem to have focused on the general population [34,35,36] and a few other risk groups, such as children [37] and persons with HIV infection [38,39,40]. Hence, knowledge on the epidemiology of MRSA, including carriage rates, determinants of carriage, and the antibiotic resistance of colonizing strains, in relation to SCD patients, is limited. Therefore, conducting MRSA surveillance studies among these individuals would provide additional insights about the epidemiology of the pathogen and data to guide the management of its infections in this at-risk population. On this premise, this study aimed at investigating S. aureus and MRSA colonization among children with SCD at a paediatric hospital in Accra, Ghana, focusing on the prevalence, risk factors, and antimicrobial resistance patterns.
2. Methodology
2.1. Study Site, Design, and Sampling
This study was conducted at the Princess Marie Louise Children’s Hospital (PML) in Accra, Ghana. The city hosts about two million people, and has eight government or quasi-governmental hospitals (http://www.statsghana.gov.gh/). Apart from the Korle Bu Teaching Hospital, which offers specialized paediatric care as one of its specialties, PML is the only other major public hospital in the Accra Metropolis that focuses primarily on pediatric care. Its sickle cell clinic attends to SCD patients once a week on Thursdays. On average, about 30 children attend the clinic weekly. The study was cross-sectional, and involved 120 children with SCD (both HbSS and HbSC genotypes) and 100 children without the disease (control group) recruited between March and August 2018. The SCD participants were recruited from the sickle cell clinic of the hospital, and the control group participants were recruited from the environs of the hospital. The inclusion criteria for the selection of the participants constituting the SCD group were: being in a steady state with laboratory-proven HbSS or the HbSC genotype, being between 1 and 13 years of age, and being an outpatient. In the selection of the participants of the control group, the inclusion criteria satisfied were: being a child with laboratory-proven HbAA genotype, being apparently healthy, and being between 1 and 13 years of age. In both study groups, the exclusion criteria were: being on antimicrobials (other than penicillin, since in Ghana, penicillin is routinely administered to SCD individuals as prophylaxis) two weeks prior to sampling, having known co-morbidities, and an inability to determine the haemoglobin genotype. With the aid of a structured questionnaire, data on potential risk factors for S. aureus and MRSA carriage were collected from the study participants.
Furthermore, swabs were obtained from the anterior nares of the study participants by a qualified paediatrician; this was preceded by obtaining informed consent from their parents/guardians. For every participant, a sterile cotton swab was rotated five times in both anterior nares. Each sample was thereafter secured in a pre-labeled vial containing 1ml skim milk-tryptone-glucose-glycerin (STGG) medium. Subsequently, the samples were sent to the research laboratory of the Department of Medical Microbiology, University of Ghana Medical School, to be processed within four hours after collection. Upon arrival, these samples were each vortexed for about two minutes, followed by refrigeration at −80 °C, until needed.
2.2. Laboratory Analysis
The processing of the specimens, identification of S. aureus and MRSA, antimicrobial susceptibility testing, and molecular analysis were done following the procedures described by Donkor et al. [40]. The specimens were cultured on chocolate agar, blood agar, and MacConkey agar, and presumptive staphylococcal isolates were identified based on their reaction to the tube coagulase test. All isolates whose responses were negative were identified as coagulase-negative staphylococci (CoNS). Those isolates that displayed positive results to the test were presumptively identified as S. aureus, and were subjected to antimicrobial susceptibility testing. The testing was performed and interpreted following guidelines of the Clinical and Laboratory Standards Institute (www.clsi.org) using the following antimicrobials: linezolid (10 µg), cefoxitin (30 µg), fusidic acid (10 µg), clindamycin (2 µg), penicillin (10 µg), cotrimoxazole (1.25 µg trimethoprim + 23.75 µg sulphamethoxazole), rifampicin (5 µg), gentamicin (10 µg), erythromycin (15 µg), and tetracycline (30 µg). Subsequently, the cefoxitin-resistant isolates were confirmed as S. aureus via polymerase chain reaction (PCR) targeting the nucA gene and MRSA via PCR targeting the mecA gene.
2.3. Data Analysis
The data were analyzed with the help of STATA 14 (Strata Corp, College Station, TX, USA). Data on the resistance of S. aureus to the antimicrobials tested were summarized using descriptive statistics. Fisher’s exact tests were performed to determine the association between SCD and S. aureus and MRSA colonization. Within each group, independent samples Chi-square tests were performed to determine associations between individual categorical risk factors and S. aureus and MRSA colonization. Point biserial correlations were also performed to determine associations between risk factors that were continuous variables and S. aureus and MRSA colonization. Finally, risk factors that showed significant associations with colonization in the Chi-square and point biserial correlation tests were put in a binary logistic regression model to determine their predictive value of colonization. The significance of each predictor variable of colonization was assessed by determining the p value, odds ratio, and confidence interval; p values less than 0.05 were considered significant.
2.4. Ethical Approval
Approval for the conduction of this study was given by the Ethical and Protocol Review Committee of the School of Biomedical and Allied Health Sciences, College of Health Sciences, University of Ghana, with protocol identification number “SBAHS—MD.//AA/5A/2016-2017”.
3. Results
3.1. Demographic, Household, and Clinical Features of the Study Participants
In total, two hundred and twenty (220) individuals participated in this study. Of this number, the SCD participants comprised one hundred and twenty (120), whereas the participants of the control group were one hundred (100). The mean age and BMI of the SCD participants were 5.84 years and 15.37 Kg/m2, respectively, and the corresponding values within the control group were 6.36 years and 19.80 Kg/m2 respectively. In the SCD group, the gender distribution was uneven (48.3% males vs. 51.7% females), but was even in the control group (the males and females comprised 50% each of the population). In both study groups, the majority of the participants were enrolled in school (86.7% in the SCD group vs. 97% in the control group), resided in compound houses (69.2% in the SCD group vs. 61% in the control group), and often washed their hands with soap (58.3% in the SCD group vs. 55.0% in the control group), and at least 97% of the participants had no health worker present in their household. Table 1 presents the demographic and household characteristics of the study participants.
Table 1.
Demographic and household characteristics of the study participants.
| Demographic and Household Characteristics | SCD Group | Control Group | ||
|---|---|---|---|---|
| Number | % | Number | % | |
| Gender | ||||
| Male | 58 | 48.3 | 50 | 50.0 |
| Female | 62 | 51.7 | 50 | 50.0 |
| Current school enrolment | ||||
| Yes | 104 | 86.7 | 97 | 97.0 |
| No | 16 | 13.3 | 3 | 3.0 |
| Type of residence | ||||
| Self-contained | 37 | 30.8 | 38 | 38.0 |
| Compound | 83 | 69.2 | 61 | 61.0 |
| Presence of health worker in household | ||||
| Yes | 3 | 2.5 | 0 | 0 |
| No | 117 | 97.5 | 100 | 100 |
| Hand washing with soap | ||||
| Rarely | 50 | 41.7 | 45 | 45.0 |
| Often | 70 | 58.3 | 55 | 55.0 |
Age (SCD group = 5.84 ± 2.99 years; Control group = 6.36 ± 3.45); BMI (SCD group = 15.37 ± 10.91 Kg/m2; Control group = 19.80 ± 9.45 Kg/m2); Number of individuals per household (SCD group = 8.14 ± 3.32; Control group = 7.14 ± 2.80).
The clinical features of the study participants are presented in Table 2. As evident in the table, the SCD participants were predominantly (96.7%) on penicillin prophylaxis, whereas that attribute was absent among the participants of the control group. Moreover, relatively few of the study participants reported that they practiced self-medication (20% in the SCD group vs. 23% in the control group), had a history of hospitalization in the past year (40.8% in the SCD group vs. 8.0% in the control group), or a history of pneumonia (6.7% in the SCD group vs. 0.0% in the control group).
Table 2.
Clinical features of the study participants.
| Clinical Features | SCD Group | Control Group | ||
|---|---|---|---|---|
| Number | % | Number | % | |
| Self-reported self-medication * | ||||
| Yes | 24 | 20 | 23 | 23.0 |
| No | 96 | 80 | 77 | 77.0 |
| Penicillin prophylaxis | ||||
| Yes | 116 | 96.7 | 0 | 0 |
| No | 4 | 3.3 | 100 | 100 |
| History of hospitalization in the past year | ||||
| Yes | 49 | 40.8 | 8 | 8.0 |
| No | 71 | 59.2 | 92 | 92.0 |
| Chronic skin condition | ||||
| Yes | 0 | 0 | 0 | 0 |
| No | 120 | 100 | 100 | 100 |
| History of pneumonia | ||||
| Yes | 8 | 6.7 | 0 | 0 |
| No | 112 | 93.3 | 100 | 100 |
| History of TB | ||||
| Yes | 0 | 0 | 0 | 0 |
| No | 120 | 100 | 100 | 100 |
| History of surgery | ||||
| Yes | 0 | 0 | 0 | 0 |
| No | 120 | 100 | 100 | 100 |
| Underlying disease | ||||
| Yes | 0 | 0 | 0 | 0 |
| No | 120 | 100 | 100 | 100 |
| History of blood transfusion | ||||
| Yes | 42 | 35 | 0 | 0 |
| No | 78 | 65 | 100 | 100 |
Frequency of hospitalization in the past year (SCD group = 0.70 ± 1.37 times; Control group = 0.10 ± 0.41); * Refers to participants’ use of medications without doctors’ prescription.
3.2. Relationship between Sickle Cell Disease and Staphylococcal Carriage
The distribution of Staphylococci isolated from the study participants—SCD group relative to the control group—were as follows: Staphylococcus aureus (33.3% vs. 10%), MRSA (3.33% vs. 0.00%), and coagulase-negative staphylococci (7.5% vs. 8.0%). Considering both study groups as a composite, the overall nasal carriage prevalence of S. aureus, MRSA, and coagulase-negative staphylococci were 22.73% (n = 50), 1.82% (n = 4), and 7.73% (n = 17) respectively. Moreover, a significant association was observed between the presence of sickle cell disease and S. aureus carriage (p < 0.0001), but this was not observed in relation to MRSA carriage (p = 0.128). The odds ratio for S. aureus carriage in relation to the presence of SCD was 4.045. This means that SCD children have more than a four-fold increased risk for S. aureus carriage.
PCR targeting the mecA gene did not yield evidence supporting the presence of the mecA gene in twelve (75%) of the sixteen cefoxitin-resistant isolates. It is noted that all these isolates are nucA gene-confirmed S. aureus isolates.
3.3. Risk Factors for Colonization with S. aureus and MRSA among the Study Participants
The results of the logistic regression analysis indicated that among the SCD children, male gender and the practice of self-medication were protective of S. aureus carriage, whereas increasing age, and living in self-contained apartments predisposed individuals to S. aureus carriage. In the control group, a history of hospitalization in the past year was a risk factor for S. aureus carriage. No risk factors of MRSA carriage were identified in either of the study groups. Details of the risk factor analysis are presented in Table 3.
Table 3.
Risk factors for S. aureus colonization.
| SCD Group | Control Group | |||
|---|---|---|---|---|
| Risk Factor | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Age | 1.275 (1.084–1.499) | 0.003 | N/A | N/A |
| Male gender | 0.344 (0.142–0.833) | 0.018 | N/A | N/A |
| Living in SC apartments | 3.632 (1.108–11.906) | 0.033 | N/A | N/A |
| Practice of self-medication | 0.233 (0.059–0.927) | 0.039 | N/A | N/A |
| Hospitalization in the past year | N/A | N/A | 14.333 (1.023–200.907) | 0.048 |
N/A = Not applicable; SC = Self-contained.
3.4. Patterns of Antimicrobial Resistance among the S. aureus and MRSA Isolates
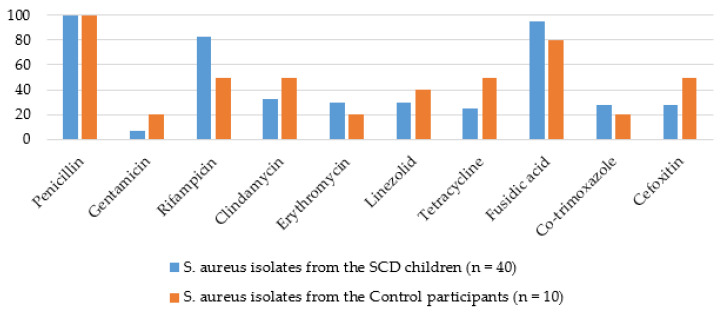
In both study groups, all the S. aureus isolates were penicillin-resistant. Moreover, relatively higher rates of resistance were observed for fusidic acid (95% in the SCD group vs. 80% in the control group) as well as rifampicin (82.5% in the SCD group vs. 50% in the control group), which was the only antibiotic whose differences in resistance rates reached statistical significance (z = 2.152; p = 0.03). The rates recorded against the other antimicrobials were: gentamicin (7.5% in the SCD group vs. 20% in the control group), clindamycin (32.5% in the SCD group vs. 50% in the control group), erythromycin (30% in the SCD group vs. 20% in the control group), linezolid (30% in the SCD group vs. 40% in the control group), tetracycline (25% in the SCD group vs. 50% in the control group), co-trimoxazole (27.5% in the SCD group vs. 20% in the control group), and cefoxitin (27.5% in the SCD group vs. 50% in the control group). The rates of multidrug resistance (resistance to more than two antimicrobial classes [41]) were 92.5% in the SCD group (n = 37) and 100% (n = 10) in the control group. In Figure 1, the prevalence rates of antimicrobial resistance are presented.
Figure 1.
Antibiotic resistance prevalence of the S. aureus isolates.
4. Discussion
This study aimed to investigate S. aureus and MRSA colonization among children with sickle cell disease and those without the disease in Accra. As far as we know, it is the second study on S. aureus and MRSA colonization among SCD children in Ghana, and one of the limited MRSA carriage studies conducted among this risk group globally. Overall, among the participants recruited in this study, the presence of SCD predisposed to the nasal carriage of S. aureus at a higher rate (33.3% vs. 10%). In a previous study that partly focused on comparing cohorts of children with and without SCD in the country on their predisposition to S. aureus carriage [42], the S. aureus carriage prevalence was not significantly different. In fact, the carriage prevalence was higher in the control group (50%) than the SCD group (48%). Another contrasting observation to that of this study is seen in one similar study conducted in Gabon [43], which reported a finding similar to that of Donkor et al. [42]—46.6% of the SCD children were S. aureus carriers as opposed to 46.9% in the control group. Consequently, this study is apparently the first to identify people with SCD as a risk group for S. aureus carriage, which could be due to changes in the epidemiology of prophylactic measures for SCD patients against S. pneumoniae, such as penicillin use and vaccination, which are known to affect S. aureus colonization [42]. Although additional studies could be conducted to ascertain this finding, this observation partly explains the high occurrence of invasive diseases caused by S. aureus in SCD patients [29,30,31,32,33]. The identification of individuals with SCD as an at-risk population for S. aureus carriage is an additional reason for worry, given their high intake of antibiotics, as this could make them reservoirs of multidrug-resistant S. aureus. Interestingly, in this study, this risk group for S. aureus carriage was not predisposed to MRSA carriage—the prevalence was 3.33% in the SCD group as opposed to 0.00% in the control group. It is noted that this is not the first time that S. aureus carriage, but not MRSA carriage, has been associated with a risk group. A previous study in the region identified HIV-infected individuals as a risk group for S. aureus carriage, but not MRSA carriage [40]. In a previous report by Schaumburg et al. [44], immunosuppression was noted to be associated with the carriage of PVL-positive S. aureus. As PVL characterization was not carried out in the current study and that of Donkor et al. [40], an exhaustive inference cannot be drawn between the PVL status of colonizing S. aureus strains and immunosuppression. However, as both studies were carried out in a PVL-endemic region, it is reasonable to hypothesize that PVL carriage could be a possible explanation for the linkage between immunosuppression and S. aureus carriage in the current study and the study conducted by Donkor et al. [40]. These observations reinforce the need for the continued surveillance of S. aureus and MRSA among at-risk populations.
In our study, among the SCD participants, none of the factors studied were predictive of MRSA carriage. However, male gender and the practice of self-medication were protective of S. aureus carriage, while increasing age and living in self-contained apartments were identified as risk factors for S. aureus nasal carriage. In the control group, a history of hospitalization in the past year was a risk factor for S. aureus nasal carriage. Some studies have failed to demonstrate any significant association between gender and S. aureus carriage [34,45,46]. Be that as it may, male gender has been associated with S. aureus carriage in other studies [24]. Consistent with these reports, female gender, by virtue of estrogen production, has been linked with better immune function [47]. It is just a few earlier studies that reported female gender as a risk factor for S. aureus carriage [48]. Hence, the reason why male gender emerged to be protective of S. aureus carriage among the SCD participants in the current study is unclear. It is possible that the female participants in this study engaged more frequently in nose picking, a phenomenon that is known to predispose to S. aureus carriage [6]. At present, this only remains a hypothesis, as the frequency of nose picking was not evaluated in this study. Another possibility is that the nasal secretions of the male participants in this study were more bactericidal. It has been reported that nasal fluids from non-carriers are more bactericidal to S. aureus than are those from carriers [49]. Also likely, the SCD participants’ serum levels of 25-hydroxyvitamin D, a marker of total levels of vitamins in the body, played a role. An inverse relationship has been reported between 25-hydroxyvitamin D and S. aureus carriage [50], and SCD patients are known to be frequently vitamin D-deficient [51]. Perhaps, the male SCD participants in the current study had significantly higher levels of this marker than did their female counterparts, and this likely accounted for their low levels of S. aureus carriage. Some of these hypotheses may be additional dynamics that the presence of SCD introduces in terms of gender-contingent S. aureus carriage. Additional research could help improve clarity on this atypical finding.
It is quite interesting that the practice of self-medication emerged to be protective of S. aureus colonization among the SCD children. This is especially so, given that a previous study by Lemma et al. [52] reported a history of antibiotic use over the previous 3 months to be a significant risk factor for S. aureus colonization among children aged between 7 and 15 years. However, the researchers failed to indicate whether those individuals with a history of antibiotic usage administered the drugs by themselves (self-medication) or with doctors’ prescription. Hence, it does not seem legitimate to compare the findings of this current study with that reported by Lemma et al. [52]. Moreover, even though antibiotic use has been reported to predispose to MRSA carriage [53], it is noted that the current study evaluated whether or not the study participants practiced self-medication, but did not evaluate the drugs used in the practice. Moreover, the variable “self-medication” is limited by the fact that it only encompasses what was reported by the study participants, and hence may not reflect self-medication in the absolute sense. Hence, a measure of caution is warranted in the interpretation of this finding. Regardless of the aforementioned technicalities, this finding underpins the need to evaluate self-medication practices in an exhaustive manner when carrying out S. aureus and MRSA surveillance studies, as well as the need to focus more studies on self-medication.
Furthermore, age has been noted to have an inverse relationship with S. aureus carriage [54,55,56,57,58]. For example, carriage rates of 28.4% and 35% have been reported in children aged from four to six years [56] and three to eleven years, respectively [57]. In a more recent study, Halablab et al. [58] reported carriage rates of 57.1%, 34.9%, 24.8%, 37.0%, and 45.8%, respectively, for individuals aged between 6–10 years, 11–17 years, 18–25 years, 26–40 years, and 41–65 years. In summary, they reported carriage rates of 43.9% among individuals aged between six and seventeen years as opposed to 29.3% among those aged between eighteen and sixty-five years). Hence, the contrasting finding that increasing age is a risk factor for S. aureus carriage among individuals with SCD is difficult to explain. It may be a chance finding, or could probably be attributed to differences in population dynamics, and there may be a need to rely on future research to improve insights on the observation.
Similar to the phenomenon with age in the current study, it is difficult to pinpoint why living in self-contained apartments would predispose to S. aureus carriage, as was observed among the SCD participants in this study. One possible explanation for the phenomenon could be that the S. aureus carriers among the SCD participants may have acquired their carrier states from colonized household members. Past studies have demonstrated the importance of close contacts within households and with parents in the spread of S. aureus carriage among children [59,60]. Nonetheless, as this study was not designed to include an evaluation of S. aureus carriage among household members of the study participants, these attempts at explanations are at best, educated guesses.
Finally, the observed predisposition of individuals with a history of hospitalization in the control group to S. aureus nasal carriage was not surprising. Yet, it is important to highlight that it has not been universally established as a risk factor. To illustrate, although some studies have failed at demonstrating previous hospitalization as a risk factor for S. aureus carriage [24,52], others have demonstrated that it predisposes to MRSA carriage [23,61,62]. Thus, this study seems to be the first to report a history of hospitalization as a risk factor for S. aureus colonization. It provides additional insights into the predisposition of individuals whose household members are employed in the healthcare setting to S. aureus carriage [53,63].
In both study groups, all the S. aureus isolates were penicillin-resistant, and the majority of them, at least, 80%, displayed resistance towards fusidic acid. The high rates of resistance recorded against penicillin was expected, given the high rates of resistance (>80%) recorded against the antibiotic by S. aureus in several studies [34,35,38,42,64]. These studies additionally reported S. aureus resistance to fusidic acid at rates of 0%–12%, and the high rates of fusidic acid resistance observed in this study could either be an isolated case or could predict the beginning of rendering fusidic acid ineffective as a therapeutic agent against S. aureus infections. For erythromycin, linezolid, and co-trimoxazole, the rates of resistance against them were similar in both groups, and generally ranged between 20% and 40%. Earlier studies conducted within the country [34,35,38,42,64] have reported comparable rates for erythromycin, but higher rates for co-trimoxazole. With regard to linezolid resistance, although the rates of 30% and 20% reported in the SCD and control groups, respectively, might seem moderate in this study, they are cause for worry. This is because generally, S. aureus resistance to linezolid is rare [65,66,67,68], and the situation is no different in Ghana [34,40]. Moreover, the antibiotic is one of the few therapeutic options for treating MRSA infections. Besides, it has a low coverage in the country. Evidently then, it may be necessary to design studies to screen for the plasmid-borne cfr gene as well as other linezolid resistance determinants among pathogens in the country. Although there seemed to be disparities between the two study groups with regard to the rates of resistance of the S. aureus isolates to gentamicin, clindamycin, and tetracycline, the apparent disparities may constitute a chance finding. Nonetheless, the rates of resistance are consistent with what have been reported in previous studies [39,40,64]. Resistance to rifampicin was significantly higher in the isolates emanating from the SCD group than those of the control group. This high rate (82.5%) warrants attention, particularly owing to the drawback it could pose to tuberculosis (TB) management should this resistance trait be transferred to etiologic agents of TB, given that rifampicin is a backbone to TB management [69].
It is noted that the phenotypic testing (cefoxitin resistance trait) upon which methicillin resistance was presumptively evaluated yielded results that were inconsistent with the mecA PCR results. Only four of the total sixteen cefoxitin-resistant S. aureus isolates, all emanating from the SCD participants, were confirmed to carry the mecA gene. Those isolates in which carriage of the mecA gene could not be demonstrated likely harbored other determinants of methicillin resistance, such as the newly discovered mecC gene [70,71].
The rates of multidrug resistance were high in both study groups (SCD group (92.5%, n = 37); control group (100%, n = 10)). These rates are higher than those recorded in previous studies [35,39,40,64], and may be due to the extensive marketing of antimicrobials and indiscriminate antibiotic use [54,55,72,73]. Evidently, policy makers and implementers would have to step up public health campaigns against antimicrobial abuse and institute more stringent policies on antimicrobial acquisition.
There are a few potential limitations to our study. Firstly, we sampled only the anterior nares (the main site of S. aureus colonization) and, therefore, our data does not cover other colonization sites, such as the skin, throat, and rectum. Secondly, the PCR analysis did not cover the newly discovered mecC gene.
5. Conclusions
This study concludes that among children in Accra, sickle cell disease predisposed to the carriage of S. aureus, but not MRSA. The risk factors for S. aureus colonization appeared to be quite different in the SCD children compared with the control group. Both the SCD children and the control group harbored multidrug-resistant S. aureus, and this may be due to extensive antimicrobial use in the country. Further research involving longitudinal studies are needed to provide insights into the dynamics of S. aureus and MRSA carriage among SCD patients in Ghana. Such studies could also be extended to other at-risk populations that have not been previously studied, such as stroke patients [74].
Acknowledgments
We thank the children who enrolled as participants in this study as well as their parents/guardians for providing their consent. We also recognize the efforts of the staff of the PML Hospital. The efforts of the technical staff of the Department of Medical Microbiology and the Department of Biochemistry, Cell and Molecular Biology are also appreciated.
Author Contributions
Conceptualization, E.S.D.; methodology, E.S.D., G.A.P., F.C.N.K., A.N.B., V.A.A., M.Y.N. and E.M.A.T.; software, E.S.D., F.C.N.K., A.N.B., S.D., and G.A.P.; validation, E.S.D., G.A.P., F.C.N.K., A.N.B., S.D., and E.M.A.T.; formal analysis, E.S.D., F.C.N.K., A.N.B., V.A.A., E.M.A.T., M.Y.N. and G.A.P.; investigation, A.N.B., V.A.A., E.S.D., G.A.P., F.C.N.K., M.Y.N. and S.D.; resources, E.S.D., A.N.B., S.D., V.A.A., G.A.P., F.C.N.K., and E.M.A.T.; data curation, E.S.D., F.C.N.K., V.A.A., S.D., A.N.B., and G.A.P.; writing—original draft preparation, E.S.D. and F.C.N.K.; writing—review and editing, E.S.D., F.C.N.K., M.Y.N., A.N.B., V.A.A., G.A.P, S.D., and E.M.A.T.; visualization, A.N.B., E.S.D., F.C.N.K., S.D., V.A.A., and G.A.P.; supervision, E.S.D., G.A.P, F.C.N.K., S.D., A.N.B., and E.M.A.T.; project administration, E.S.D., A.N.B., F.C.N.K., M.Y.N., G.A.P., S.D., and V.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicting interests.
References
- 1.Fowler V., Olsen M.K., Corey G.R., Woods C.W., Cabell C.H., Reller L.B., Cheng A., Dudley T., Oddone E.Z. Clinical Identifiers of Complicated Staphylococcus aureus Bacteremia. Arch. Intern. Med. 2003;163:2066. doi: 10.1001/archinte.163.17.2066. [DOI] [PubMed] [Google Scholar]
- 2.Donkor E.S., Newman M.J., Oliver-Commey J., Bannerman E., Dayie N.T.K.D., Badoe E.V. Invasive disease and paediatric carriage of Streptococcus pneumoniae in Ghana. Scand. J. Infect. Dis. 2010;42:254–259. doi: 10.3109/00365540903490000. [DOI] [PubMed] [Google Scholar]
- 3.Paulsen J., Mehl A., Askim Å., Solligård E., Åsvold B.O., Damås J.K. Epidemiology and outcome of Staphylococcus aureus bloodstream infection and sepsis in a Norwegian county 1996–2011: An observational study. BMC Infect. Dis. 2015;15:116. doi: 10.1186/s12879-015-0849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todar K. Todar’s Online Textbook of Bacteriology. Department of Bacteriology, University of Wisconsin-Madison; Madison, WI, USA: 2006. [Google Scholar]
- 5.Kluytmans J., van Belkum A., Verbrugh H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997;10:505–520. doi: 10.1128/CMR.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wertheim H.F.L., Melles D.C., Vos M.C., Van Leeuwen W., Van Belkum A., Verbrugh H.A., Nouwen J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 7.Eriksen N.H., Espersen F., Rosdahl V., Jensen K. Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol. Infect. 1995;115:51–60. doi: 10.1017/S0950268800058118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu L., Umeda A., Kondo S., Amako K. Typing of Staphylococcus aureus colonising human nasal carriers by pulsed-field gel electrophoresis. J. Med. Microbiol. 1995;42:127–132. doi: 10.1099/00222615-42-2-127. [DOI] [PubMed] [Google Scholar]
- 9.Nouwen J., Boelens H., Van Belkum A., Verbrugh H. Human Factor in Staphylococcus aureus Nasal Carriage. Infect. Immun. 2004;72:6685–6688. doi: 10.1128/IAI.72.11.6685-6688.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran G.J., Krishnadasan A., Gorwitz R.J., Fosheim G.E., McDougal L.K., Carey R.B., Talan D.A. Methicillin-ResistantS. aureusInfections among Patients in the Emergency Department. N. Engl. J. Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 11.Haenni M., Saras E., Chatre P., Médaille C., Bes M., Madec J.-Y., Laurent F. A USA300 variant and other human-related methicillin-resistant Staphylococcus aureus strains infecting cats and dogs in France. J. Antimicrob. Chemother. 2011;67:326–329. doi: 10.1093/jac/dkr499. [DOI] [PubMed] [Google Scholar]
- 12.Camargo C., Cunha M., Bonesso M.F., Da Cunha F.P., Barbosa A.N., Fortaleza C.M.C.B. Systemic CA-MRSA infection following trauma during soccer match in inner Brazil: Clinical and molecular characterization. Diagn. Microbiol. Infect. Dis. 2013;76:372–374. doi: 10.1016/j.diagmicrobio.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Quitoco I.M.Z., Ramundo M.S., Silva-Carvalho M.C., Souza R.R., Beltrame C.O., De Oliveira T.F., Araújo R., Del Peloso P.F., Coelho L., Figueiredo A.M.S. First report in South America of companion animal colonization by the USA1100 clone of community-acquired meticillin-resistant Staphylococcus aureus (ST30) and by the European clone of methicillin-resistant Staphylococcus pseudintermedius (ST71) BMC Res. Notes. 2013;6:336. doi: 10.1186/1756-0500-6-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey J.A., Cosgrove S.E., Stewart W.F., Pollak J., Schwartz B.S. A population-based study of the epidemiology and clinical features of methicillin-resistant Staphylococcus aureus infection in Pennsylvania, 2001-2010. Epidemiol. Infect. 2013;141:1166–1179. doi: 10.1017/S0950268812001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsueh P.-R., Liu C.-Y., Luh K.-T. Current Status of Antimicrobial Resistance in Taiwan. Emerg. Infect. Dis. 2002;8:132–137. doi: 10.3201/eid0802.010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Nosocomial Infections Surveillance System National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control. 2004;32:470–485. doi: 10.1016/j.ajic.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Tiemersma E.W., Bronzwaer S.L., Lyytikäinen O., Degener J.E., Schrijnemakers P., Bruinsma N., Monen J., Witte W., Grundmann H. Methicillin-resistant Staphylococcus aureus in Europe, 1999–2002. Emerg. Infect. Dis. 2004;10:1627–1634. doi: 10.3201/eid1009.040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amaral M.M., Coelho L., Flores R.P., Souza R.R., Silva-Carvalho M.C., Teixeira L., Ferreira-Carvalho B.T., Figueiredo A.M.S. The Predominant Variant of the Brazilian Epidemic Clonal Complex of Methicillin?Resistant Staphylococcus aureus Has an Enhanced Ability to Produce Biofilm and to Adhere to and Invade Airway Epithelial Cells. J. Infect. Dis. 2005;192:801–810. doi: 10.1086/432515. [DOI] [PubMed] [Google Scholar]
- 19.European Antimicrobial Resistance Surveillance System: Annual Report 2006. [(accessed on 11 October 2016)]; Available online: http://www.rivm.nl/earss/Images/EARSS%202006%20Def_tcm61-44176.pdf.
- 20.Centers for Disease Control and Prevention Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Methicillin-Resistant Staphylococcus Aureus. [(accessed on 26 September 2016)];2014 Available online: www.cdc.gov/abcs/reports-findings/survreports/mrsa14.html.
- 21.Elixhauser A., Steiner C. Infections with Methicillin-Resistant Staphylococcus Aureus (MRSA) in U.S. Hospitals, 1993–2005: Statistical Brief #35. Agency for Health Care Policy and Research; Rockville, MD, USA: 2007. [PubMed] [Google Scholar]
- 22.Klevens R.M., Morrison M.A., Nadle J., Petit S., Gershman K., Ray S., Harrison L.H., Lynfield R., Dumyati G., Townes J.M., et al. Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States. JAMA. 2007;298:1763. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 23.Saraf S.L., Molokie R.E., Nouraie M., Sable C.A., Luchtman-Jones L., Ensing G.J., Campbell A., Rana S.R., Niu X.M., Machado R., et al. Differences in the clinical and genotypic presentation of sickle cell disease around the world. Paediatr. Respir. Rev. 2013;15:4–12. doi: 10.1016/j.prrv.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seybold U., Supthut-Schröder B., Draenert R., Hogardt M., Bogner M.J. Prevalence and risk factors of nasal colonization with Staphylococcus aureus–Association with HIV infection in older patients. Scand. J. Infect. Dis. 2009;41:63–66. doi: 10.1080/00365540802460000. [DOI] [PubMed] [Google Scholar]
- 25.Popovich K.J., Hota B., Aroutcheva A., Kurien L., Patel J., Lyles-Banks R., Grasso A.E., Spec A., Beavis K.G., Hayden M.K., et al. Community-Associated Methicillin-Resistant Staphylococcus aureus Colonization Burden in HIV-Infected Patients. Clin. Infect. Dis. 2013;56:1067–1074. doi: 10.1093/cid/cit010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donkor E.S., Jamrozy D., Mills R.O., Dankwah T., Amoo P.K., Egyir B., Badoe E.V., Twasam J., Bentley S.D. A genomic infection control study for Staphylococcus aureus in two Ghanaian hospitals. Infect. Drug Resist. 2018;11:1757–1765. doi: 10.2147/IDR.S167639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kateete D., Kajumbula H., Kaddu-Mulindwa D.H., Ssevviri A.K. Nasopharyngeal carriage rate of Streptococcus pneumoniae in Ugandan children with sickle cell disease. BMC Res. Notes. 2012;5:28. doi: 10.1186/1756-0500-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dayie N.T.K.D., Tetteh-Ocloo G., Labi A.-K., Olayemi E., Slotved H.-C., Lartey M., Donkor E.S. Pneumococcal carriage among sickle cell disease patients in Accra, Ghana: Risk factors, serotypes and antibiotic resistance. PLoS ONE. 2018;13:e0206728. doi: 10.1371/journal.pone.0206728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuonghae H.O., Nwankwo M.U., Offor E.C. Pattern of bacteremia in febrile children with sickle cell anemia. Ann. Trop. Pediatr. 1993;13:55–64. doi: 10.1080/02724936.1993.11747625. [DOI] [PubMed] [Google Scholar]
- 30.Akuse R.M. Variation in the pattern of bacterial infection in patients with sickle cell disease requiring admission. J. Trop. Pediatr. 1996;42:318–323. doi: 10.1093/tropej/42.6.318. [DOI] [PubMed] [Google Scholar]
- 31.Aken’Ova Y.A., Bakare R.A., Okunade M.A. Septicaemia in sickle cell anaemia patients: The Ibadan experience. Central Afr. J. Med. 1998;44:102–104. [PubMed] [Google Scholar]
- 32.Thanni L.O. Bacterial osteomyelitis in major sickling haemoglobinopathies: Geographic difference in pathogen prevalence. Afr. Health Sci. 2006;6:236–239. doi: 10.5555/afhs.2006.6.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kizito M.E., Mworozi E., Ndugwa C., Serjeant G.R. Bacteraemia in homozygous sickle cell disease in Africa: Is pneumococcal prophylaxis justified? Arch. Dis. Child. 2006;92:21–23. doi: 10.1136/adc.2005.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egyir B., Guardabassi L., Nielsen S.S., Larsen J., Addo K.K., Newman M.J., Larsen A.R. Prevalence of nasal carriage and diversity of Staphylococcus aureus among inpatients and hospital staff at Korle Bu Teaching Hospital, Ghana. J. Glob. Antimicrob. Resist. 2013;1:189–193. doi: 10.1016/j.jgar.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Egyir B., Guardabassi L., Esson J., Nielsen S.S., Newman M.J., Addo K.K., Larsen A.R. Insights into Nasal Carriage of Staphylococcus aureus in an Urban and a Rural Community in Ghana. PLoS ONE. 2014;9:e96119. doi: 10.1371/journal.pone.0096119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eibach D., Nagel M., Hogan B., Azuure C., Krumkamp R., Dekker D., Gajdiss M., Brunke M., Sarpong N., Owusu-Dabo E., et al. Nasal Carriage of Staphylococcus aureus among Children in the Ashanti Region of Ghana. PLoS ONE. 2017;12:e0170320. doi: 10.1371/journal.pone.0170320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donkor E.S., Nartey E. Nasal colonisation of antibiotic resistant bacteria in Ghanaian children less than five years of age. Internet J. Microbiol. 2007;5:1–5. [Google Scholar]
- 38.Egyir B., Oteng A.A., Owusu E., Newman M.J., Addo K.K., Larsen A.R. Characterization of Staphylococcus aureus from Human Immunodeficiency Virus (HIV) patients in Accra, Ghana. J. Infect. Dev. Ctries. 2016;10:453–456. doi: 10.3855/jidc.7428. [DOI] [PubMed] [Google Scholar]
- 39.Donkor E.S., Badoe E.V., Annan J.A., Nii-Trebi N. Colonisation of antibiotic resistant bacteria in a cohort of HIV infected children in Ghana. Pan Afr. Med. J. 2017;26:1–7. doi: 10.11604/pamj.2017.26.60.10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donkor E.S., Kotey F.C.N., Dayie N.T.K.D., Duodu S., Tetteh-Quarcoo P.B., Osei M.-M., Tette E.M.A. Colonization of HIV-Infected Children with Methicillin-Resistant Staphylococcus aureus. Pathogens. 2019;8:35. doi: 10.3390/pathogens8010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labi A.-K., Obeng-Nkrumah N., Addison N.O., Donkor E.S. Salmonella blood stream infections in a tertiary care setting in Ghana. BMC Infect. Dis. 2014;14:3857. doi: 10.1186/s12879-014-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donkor E.S., Foster-Nyarko E., Enweronu-Laryea C.C. Relationship between antibiotic resistance and sickle cell anemia: Preliminary evidence from a pediatric carriage study in Ghana. Infect. Drug Resist. 2013;6:71–77. doi: 10.2147/IDR.S40062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaumburg F., Biallas B., Feugap E.N., Alabi A., Mordmüller B., Kremsner P., Grobusch M., Lell B., Van Der Linden M., Peters G., et al. Carriage of encapsulated bacteria in Gabonese children with sickle cell anaemia. Clin. Microbiol. Infect. 2013;19:235–241. doi: 10.1111/j.1469-0691.2012.03771.x. [DOI] [PubMed] [Google Scholar]
- 44.Schaumburg F., Alabi A., Peters G., Becker K. New epidemiology of Staphylococcus aureus infection in Africa. Clin. Microbiol. Infect. 2014;20:589–596. doi: 10.1111/1469-0691.12690. [DOI] [PubMed] [Google Scholar]
- 45.Ngoa U.A., Schaumburg F., Adegnika A.A., Kösters K., Möller T., Fernandes J.F., Alabi A., Issifou S., Becker K., Grobusch M.P., et al. Epidemiology and population structure of Staphylococcus aureus in various population groups from a rural and semi urban area in Gabon, Central Africa. Acta Trop. 2012;124:42–47. doi: 10.1016/j.actatropica.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Ebruke C., Dione M., Walter B., Worwui A., Adegbola R.A., Roca A., Antonio M. High genetic diversity of Staphylococcus aureus strains colonising the nasopharynx of Gambian villagers before widespread use of pneumococcal conjugate vaccines. BMC Microbiol. 2016;16:38. doi: 10.1186/s12866-016-0661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marriott I., Huet-Hudson Y.M. Sexual Dimorphism in Innate Immune Responses to Infectious Organisms. Immunol. Res. 2006;34:177–192. doi: 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- 48.Luzar M.A., Coles G.A., Faller B., Slingeneyer A., Dah G.D., Briat C., Wone C., Knefati Y., Kessler M., Peluso F. Staphylococcus aureus Nasal Carriage and Infection in Patients on Continuous Ambulatory Peritoneal Dialysis. N. Engl. J. Med. 1990;322:505–509. doi: 10.1056/NEJM199002223220804. [DOI] [PubMed] [Google Scholar]
- 49.Cole A.M., Dewan P., Ganz T. Innate Antimicrobial Activity of Nasal Secretions. Infect. Immun. 1999;67:3267–3275. doi: 10.1128/IAI.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olsen K., Falch B.M., Danielsen K., Johannessen M., Sollid J.U.E., Thune I., Grimnes G., Jorde R., Simonsen G.S., Furberg A.-S. Staphylococcus aureus nasal carriage is associated with serum 25-hydroxyvitamin D levels, gender and smoking status. The Tromsø Staph and Skin Study. Eur. J. Clin. Microbiol. Infect. Dis. 2011;31:465–473. doi: 10.1007/s10096-011-1331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winters A.C., Kethman W., Kruse-Jarres R., Kanter J. Vitamin D Insufficiency is a Frequent Finding in Pediatric and Adult Patients with Sickle Cell Disease and Correlates with Markers of Cell Turnover. J. Nutr. Disord. Ther. 2014;4:140. doi: 10.4172/2161-05091000140. [DOI] [Google Scholar]
- 52.Lemma M.T., Zenebe Y., Tulu B., Mekonnen D., Mekonnen Z. Methicillin Resistant Staphylococcus aureus among HIV Infected Pediatric Patients in Northwest Ethiopia: Carriage Rates and Antibiotic Co-Resistance Profiles. PLoS ONE. 2015;10:e0137254. doi: 10.1371/journal.pone.0137254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fritz S.A., Garbutt J., Elward A., Shannon W., Storch G.A. Prevalence of and Risk Factors for Community-Acquired Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Colonization in Children Seen in a Practice-Based Research Network. Pediatrics. 2008;121:1090–1098. doi: 10.1542/peds.2007-2104. [DOI] [PubMed] [Google Scholar]
- 54.Bogaert D., van Belkum A., Sluijter M., Luijendijk A., de Groot R., Rumke H.C., Verbrugh H.A., Hermans P.W. Colonization by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 55.Donkor E.S., Dayie N.T., Tette E.M.A. Methicillin-Resistant Staphylococcus aureus in Ghana: Past, Present, and Future. Microb. Drug Resist. 2019;25:717–724. doi: 10.1089/mdr.2018.0115. [DOI] [PubMed] [Google Scholar]
- 56.Ciftci I.H., Koken R., Bukulmez A., Özdemir M., Safak B., Cetinkaya Z. Nasal carriage ofStaphylococcus aureusin 4-6 age groups in healthy children in Afyonkarahisar, Turkey. Acta Paediatr. 2007;96:1043–1046. doi: 10.1111/j.1651-2227.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- 57.Zanelli G., Sansoni A., Zanchi A., Cresti S., Pollini S., Rossolini G.M., Cellesi C. Staphylococcus aureus nasal carriage in the community: A survey from central Italy. Epidemiol. Infect. 2002;129:417–420. doi: 10.1017/S0950268802007434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halablab M.A., Hijazi S.M., Fawzi M.A., Araj G.F. Staphylococcus aureus nasal carriage rate and associated risk factors in individuals in the community. Epidemiol. Infect. 2009;138:702–706. doi: 10.1017/S0950268809991233. [DOI] [PubMed] [Google Scholar]
- 59.Miller M., Cook H.A., Furuya E.Y., Bhat M., Lee M.-H., Vavagiakis P., Visintainer P., Vasquez G., Larson E., Lowy F.D. Staphylococcus aureus in the Community: Colonization Versus Infection. PLoS ONE. 2009;4:e6708. doi: 10.1371/journal.pone.0006708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Regev-Yochay G., Raz M., Carmeli Y., Shainberg B., Navon-Venezia S., Pinco E., Leavitt A., Keller N., Rahav G., Malley R., et al. Parental Staphylococcus aureus Carriage is Associated With Staphylococcal Carriage in Young Children. Pediatr. Infect. Dis. J. 2009;28:960–965. doi: 10.1097/INF.0b013e3181a90883. [DOI] [PubMed] [Google Scholar]
- 61.Madani T.A., Al-Abdullah N.A., Al-Sanousi A.A., Ghabrah T.M., Afandi S.Z., Bajunid H.A. Methicillin-Resistant Staphylococcus aureus in Two Tertiary-Care Centers in Jeddah, Saudi Arabia. Infect. Control. Hosp. Epidemiol. 2001;22:211–216. doi: 10.1086/501891. [DOI] [PubMed] [Google Scholar]
- 62.Köck R., Winner K., Schaumburg F., Jurke A., Rossen J.W., Friedrich A.W. Admission prevalence and acquisition of nasal carriage of methicillin-resistant Staphylococcus aureus (MRSA) in German rehabilitation centres. J. Hosp. Infect. 2014;87:115–118. doi: 10.1016/j.jhin.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Creech C.B., Kernodle D.S., Alsentzer A., Wilson C., Edwards K.M. Increasing Rates of Nasal Carriage of Methicillin-Resistant Staphylococcus aureus in Healthy Children. Pediatr. Infect. Dis. J. 2005;24:617–621. doi: 10.1097/01.inf.0000168746.62226.a4. [DOI] [PubMed] [Google Scholar]
- 64.Dekker D., Wolters M., Mertens E., Boahen K.G., Krumkamp R., Eibach D., Schwarz N.G., Adu-Sarkodie Y., Rohde H., Chistner M., et al. Antibiotic resistance and clonal diversity of invasive Staphylococcus aureus in the rural Ashanti Region, Ghana. BMC Infect. Dis. 2016;16:720. doi: 10.1186/s12879-016-2048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howe R.A., Noel A., Bowker K.E., Enne V.I., Walsh T.R., MacGowan A.P. Program and Abstracts of the Forty-Second Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 2002. American Society for Microbiology; Washington, DC, USA: 2002. Emergence of linezolid (LIN) resistance in Staphylococcus aureus can be associated with loss of erythromycin (ERY) resistance; p. 75. [Google Scholar]
- 66.Morales G., Baos E., Arribi A., Andrade R., Picazo J.J., Candel F.J., Peláez B., De La Torre M.-Á., Fereres J., Sánchez-García M. Resistance to Linezolid Is Mediated by the cfr Gene in the First Report of an Outbreak of Linezolid?Resistant Staphylococcus aureus. Clin. Infect. Dis. 2010;50:821–825. doi: 10.1086/650574. [DOI] [PubMed] [Google Scholar]
- 67.Long K.S., Vester B. Resistance to Linezolid Caused by Modifications at Its Binding Site on the Ribosome. Antimicrob. Agents Chemother. 2011;56:603–612. doi: 10.1128/AAC.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shore A.C., Lazaris A., Kinnevey P., Brennan O.M., Brennan G.I., O’Connell B., Feßler A.T., Schwarz S., Coleman D.C. First Report of cfr-Carrying Plasmids in the Pandemic Sequence Type 22 Methicillin-Resistant Staphylococcus aureus Staphylococcal Cassette Chromosome mec Type IV Clone. Antimicrob. Agents Chemother. 2016;60:3007–3015. doi: 10.1128/AAC.02949-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.A Mitchison D. Role of individual drugs in the chemotherapy of tuberculosis. Int. J. Tuberc. Lung Dis. 2000;4:796–806. [PubMed] [Google Scholar]
- 70.Cartwright E., Paterson G.K., Raven K.E., Harrison E.M., Gouliouris T., Kearns A., Pichon B., Edwards G., Skov R.L., Larsen A.R., et al. Use of Vitek 2 antimicrobial susceptibility profile to identify mecC in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2013;51:2732–2734. doi: 10.1128/JCM.00847-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paterson G.K., Morgan F.J.E., Harrison E.M., Cartwright E.J.P., Torok E., Zadoks R.N., Parkhill J., Peacock S.J., Holmes M.A. Prevalence and characterization of human mecC methicillin-resistant Staphylococcus aureus isolates in England. J. Antimicrob. Chemother. 2013;69:907–910. doi: 10.1093/jac/dkt462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donkor E.S., Tetteh-Quarcoo P.B., Nartey P., Agyeman I.O. Self-Medication Practices with Antibiotics among Tertiary Level Students in Accra, Ghana: A Cross-Sectional Study. Int. J. Environ. Res. Public Health. 2012;9:3519–3529. doi: 10.3390/ijerph9103519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donkor E.S., Newman M.J., Yeboah-Manu D. Epidemiological aspects of non-human antibiotic usage and resistance: Implications for the control of antibiotic resistance in Ghana. Trop. Med. Int. Health. 2012;17:462–468. doi: 10.1111/j.1365-3156.2012.02955.x. [DOI] [PubMed] [Google Scholar]
- 74.Donkor E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018;2018:1–10. doi: 10.1155/2018/3238165. [DOI] [PMC free article] [PubMed] [Google Scholar]