Abstract
BACKGROUND
Metabolic syndrome is a cluster of cardiovascular risk factors, including central obesity, high blood pressure, elevated plasma glucose, reduced high-density lipoprotein and elevated triglyceride levels.
AIM
To investigate the relationship between metabolic biomarkers and long-term blood pressure variability (BPV) in young males.
METHODS
A cohort of 1112 healthy military males aged 18-40 years from the cardiorespiratory fitness and hospitalization events in armed forces study in eastern Taiwan was prospectively included. The following metabolic biomarkers were used: Waist circumference, serum uric acid (SUA), triglycerides, high density lipoprotein, triglycerides, and fasting glycose. BPV was assessed by average real variability (ARV) and standard deviation (SD) across 4 clinic visits during the study period (2012-14, 2014-15, 2015-16, and 2016-18). Multivariable linear regression analysis was used to determine the association after adjusting for age, body mass index, systolic and diastolic blood pressure (SBP and DBP), lipid profiles, physical activity, alcohol intake and tobacco smoking status.
RESULTS
In the unadjusted model, waist circumference was significantly and positively correlated with ARVDBP and SDDBP [β (standard errors) = 0.16 (0.049) and 0.22 (0.065), respectively], as was SUA [β = 0.022 (0.009) and 0.038 (0.012), respectively]. High-density lipoprotein was negatively correlated with ARVSBP [β = −0.13 (0.063)]. There were no associations with the other metabolic biomarkers. In contrast, only SUA was significantly correlated with SDSBP and SDDBP [β = 0.019 (0.011) and 0.027 (0.010), respectively] in the adjusted model.
CONCLUSION
Our findings showed that of traditional metabolic biomarkers, SUA had the strongest positive correlation with long-term systolic and diastolic BPV in young male adults, and the clinical relevance needs further investigation.
Keywords: Blood pressure variability, Metabolic syndrome, Serum uric acid, Young males
Core tip: This study examined the relationship between metabolic biomarkers and long-term blood pressure variability (BPV) in a military population of 1112 young males across 4 visits during a 7-year period. We found that serum uric acid and waist circumference were positively correlated with long-term BPV, whereas high-density lipoprotein was inversely correlated with long-term BPV in the unadjusted model. Only serum uric acid was positively correlated with long-term BPV in the fully adjusted model. Serum uric acid, which may reflect arterial stiffness, is likely one of the strongest metabolic biomarkers for long-term BPV and related cardiovascular risk in young male adults.
INTRODUCTION
Metabolic syndrome is a cluster of cardiovascular risk factors, including central obesity, high blood pressure, elevated plasma glucose, reduced high-density lipoprotein and elevated triglyceride levels. In 2004 and 2006, the National Cholesterol Education Program’s Adult Treatment Panel III[1] and the International Diabetes Federation[2] revised the definition of metabolic syndrome. In these fundamental statements, several other components, such as hyperuricemia, coagulation disorder and increased PAI-1, were described, though none of them were obligatory for diagnosing metabolic syndrome. Recent studies have shown that hyperuricemia could predict metabolic syndrome in elderly individuals, adolescents and even children[3-5]. In particular, the association of hyperuricemia with cardiovascular diseases has been reported since the 1950s in numerous epidemiological studies[6-10]. Whether serum uric acid (SUA) is merely a risk factor for cardiovascular disease or whether hypouricemic agents affect the outcome remains elusive[11].
Short-term and long-term blood pressure variability (BPV) are independently associated with cardiovascular events and renal damage[12]. In addition, long-term BPV, visit-to-visit variability in systolic blood pressure (SBP) and maximal SBP have been shown to be strongly prognostic for stroke and coronary events independent of mean blood pressure in a 2010 post hoc analysis of four large clinical trials on hypertension[12,13]. Of particular interest are that while several factors involved in short-term BPV have been recognized, information on the factors contributing to long-term BPV, particularly in younger adults, remains speculative[14,15]. Behavioral changes, seasonal changes, reduced dosing or adherence to antihypertensive treatment, and increased arterial stiffness have previously been proposed as possible mechanisms for long-term BPV[15-17]. These studies were conducted on middle-aged/older individuals or high-risk populations undergoing antihypertensive treatment, suggesting that BPV itself might be intertwined with these factors. Among young adults, greater body mass index levels were associated with higher long-term BPV in the Coronary Artery Risk Development in Young Adults study[18].
Despite a growing body of evidence revealing the benefits of long-term BPV control, before being integrated into clinical practices as an additional goal of antihypertensive treatment, an improved understanding of BPV is mandatory. Therefore, we aimed to investigate whether long-term BPV throughout young adulthood is associated with metabolic biomarkers, including the components of metabolic syndrome, in a cohort of young military males.
MATERIALS AND METHODS
Study population
The study cohort included 1112 military males aged 18-40 years from the cardiorespiratory fitness and hospitalization events in armed forces study in Taiwan who were not undergoing antihypertensive treatment[19]. All military participants self-reported their medical history and the status for tobacco smoking (current vs former or never), alcohol intake (current vs former or never) and physical activity. A physical examination including anthropometric and hemodynamic measurements and blood biochemical tests were performed at each clinic visit at the Hualien Armed Forces General Hospital of Taiwan every two years from 2012 to 2018 (2012-14, 2014-15, 2015-16, and 2016-18)[20].
Anthropometric measurements and biochemical blood tests
With regard to the anthropometric parameters, the body height, body weight and waist circumference of each participant were measured in a standing position. Body mass index was calculated as the ratio of body weight (kg) to body height squared (m2). Waist circumference was measured midway between the lower rib margin and the iliac crest. Blood biochemical tests regarding concentrations of total cholesterol, high-density lipoprotein, triglycerides, fasting plasma glucose, creatinine and SUA were enzymatically measured on an autoanalyzer (AU640, Olympus, Kobe, Japan). The estimated glomerular filtration rate (eGFR) was calculated according to the equation of chronic kidney disease epidemiology collaboration[21]. All blood samples were collected from the participants after an overnight 12-hour fast in the early morning by well-trained phlebotomists at the same blood draw station[22-26].
Measurements of long-term BPV
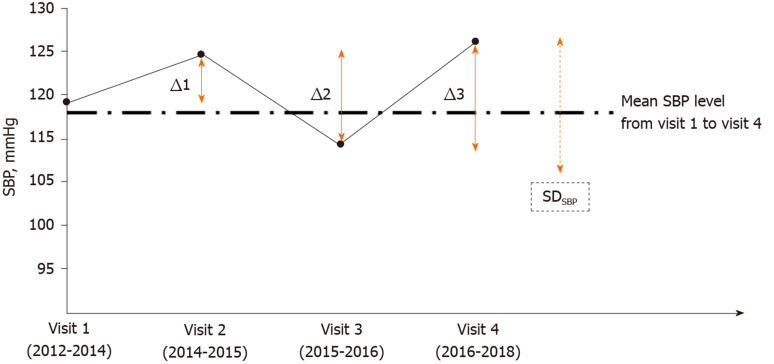
Sympathomimetic agents such as theophylline or caffeine-containing materials were prohibited for 12 h before each participant’s visit to the hospital. To avoid the temporary effects of pain, anxious mood, and cold temperature on blood pressure, the measurements of systolic and diastolic blood pressure (SBP and DBP, respectively) of each participant were performed in a room with an air conditioner, which maintained a constant indoor temperature of 24 °C. The blood pressure of each participant was checked once on the right upper arm in a sitting position after resting for at least 15 min using an FT-201 automated blood pressure monitor (Parama-Tech Co Ltd, Fukuoka, Japan) at each visit. Long-term BPV was assessed by standard deviation (SD) and average real variability (ARV) for both SBP and DBP across 4 visits for the period of 2012-2018. SDSBP and ARVSBP are illustrated in Figure 1 and are represented by dotted and solid lines, respectively. ARV is calculated as an average of the sum of absolute differences between successive measurements of blood pressure, which takes the order of measurements into account (|Δ1|+|Δ2|+|Δ3|)/3)[20].
Figure 1.
Long-term blood pressure variability was assessed by standard deviation and average real variability across 4 visits in the study period (2012-2018). (Standard deviation)SBP and [average real variability (ARV)]SBP were illustrated by dotted and solid lines, respectively. ARVSBP is calculated as an average of absolute differences between successive systolic blood pressure measurements, taking the order of measurements into account: (|Δ1| +|Δ2| +|Δ3|)/3). SD: Standard deviation; SBP: Systolic blood pressure.
Statistical analysis
Metabolic biomarkers in this study included SUA and the central components of metabolic syndrome: Waist circumference, serum triglycerides, high-density lipoprotein, and fasting glucose. Metabolic syndrome was defined according to the clinical criteria of the International Diabetes Federation[2]. The characteristics of participants with metabolic syndrome (n = 112) and those without (n = 1000) were expressed as numbers (%) and compared using a chi-square test for categorical data and as the mean ± SD and compared using a two-sample t test for continuous data. The range of each BPV index was divided by quantile to reveal the distribution of BPV in all study subjects. Differences in the levels of each metabolic biomarker among the quantile groups were assessed by analysis of covariance with adjustment for baseline age, body mass index, tobacco smoking status, alcohol intake status, total cholesterol levels, physical activity, SBP, and DBP. Unadjusted and multivariable adjusted linear regression models were used to determine the relationship between each biomarker and long-term BPV. Model 1 was adjusted for the baseline SBP and DBP. Model 2 was additionally adjusted for age, body mass index, smoking status, alcohol intake status, total cholesterol levels, and physical activity. This study protocol was reviewed and approved by the Institutional Review Board of Mennonite Christian Hospital (No. 16-05-008), and written informed consent was obtained from all participants. A P < 0.05 was considered significant. SAS statistical software (SAS version 9.4; SAS Institute, Cary, NC, United States) was used for all statistical analyses.
RESULTS
The baseline characteristics of the study participants (2012-2014) are shown in Table 1. Those males with metabolic syndrome had notably greater baseline levels of waist circumference, body mass index, total cholesterol, triglycerides, fasting glucose and blood pressure than those without metabolic syndrome. However, there were no differences in eGFR, physical activity, smoking status, or alcohol intake between those with metabolic syndrome and those without.
Table 1.
Clinical characteristics of study cohort, (n = 1112)
| Characteristics | With metabolic syndrome (n = 112) | Without metabolic syndromes (n = 1000) | P value |
| Age | 33.45 ± 3.93 | 32.08 ± 3.86 | < 0.001 |
| Body mass index (kg/m2) | 28.02 ± 2.03 | 24.82 ± 2.81 | < 0.001 |
| Waist circumference (cm) | 91.94 ± 5.04 | 83.49 ± 6.95 | < 0.001 |
| Systolic blood pressure (mm Hg) | 126.95 ± 14.90 | 117.39 ± 12.87 | < 0.001 |
| Diastolic blood pressure (mm Hg) | 78.12 ± 12.45 | 70.88 ± 9.53 | < 0.001 |
| Heart rate (beats per min) | 78.65 ± 10.55 | 74.62 ± 10.54 | < 0.001 |
| High blood pressure | 31 (27.7) | 47 (4.7) | < 0.001 |
| Total cholesterol (mg/dL) | 194.68 ± 38.61 | 178.53 ± 31.66 | < 0.001 |
| Serum triglyceride (mg/dL) | 250.67 ± 232.27 | 108.71 ± 66.01 | < 0.001 |
| Fasting plasma glucose (mg/dL) | 104.71 ± 27.08 | 93.36 ± 10.30 | < 0.001 |
| Serum uric acid (mg/dL) | 7.22 ± 1.42 | 6.64 ± 1.28 | < 0.001 |
| (Minimum-Maximum) | (3.9-12.7) | (2.8-13.2) | |
| High-density lipoprotein (mg/dL) | 47.12 ± 10.19 | 48.50 ± 9.77 | < 0.001 |
| Low-density lipoprotein (mg/dL) | 114.74 ± 28.51 | 109.56 ± 28.44 | 0.06 |
| eGFR (mL/min per 1.73 m2) | 97.18 ± 12.79 | 97.97 ± 13.74 | 0.56 |
| Physical activity | |||
| Never or occasionally | 23 (20.5) | 162 (16.2) | 0.40 |
| 1-2 times/wk | 38 (33.9) | 389 (38.9) | |
| ≥ 3-5 times/wk | 51 (45.6) | 449 (44.9) | |
| Current alcohol drinkers, n (%) | 53 (47.3) | 464 (46.4) | 0.85 |
| Current tobacco smokers, n (%) | 45 (40.2) | 386 (38.6) | 0.74 |
Continuous variables are expressed as mean ± standard deviation, and categorical variables as n (%); eGFR: Estimated glomerular filtration rate.
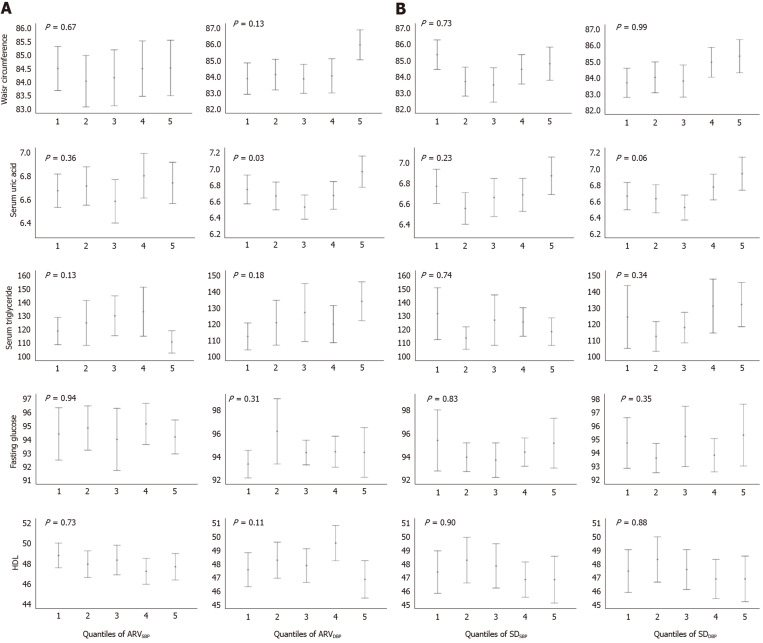
Figure 2 demonstrates the mean (95% confidence intervals) levels of each metabolic biomarker, with adjustment for the baseline SBP and DBP, in quantiles of SDBP and ARVBP. There was only a significant difference in the means of SUA among quartiles of ARVDBP (P = 0.03) and a borderline significant difference among quartiles of SDDBP (P = 0.06). In contrast, there were no significant differences in waist circumference, fasting glucose, triglycerides, or high-density lipoprotein against any indexes of BPV with adjustment.
Figure 2.
Levels of metabolic biomarkers in quantiles of (average real variability)SBP and (standard deviation)SBP. Bars represent means (95% confidence intervals) with adjustment for systolic blood pressure and diastolic blood pressure, age, body mass index, total cholesterol levels, physical activity, tobacco smoking status, alcohol intake status. HDL: High-density lipoprotein; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; ARV: Average real variability; SD: Standard deviation. P values were calculated by analysis of covariance.
The results of the linear regression models are shown in Table 2. In the unadjusted model, waist circumference and SUA were positively and significantly correlated with ARVDBP [β and standard errors = 0.16 (0.049) and 0.022 (0.009), respectively] and SDDBP [β = 0.22 (0.065) and 0.038 (0.012), respectively]. In contrast, high-density lipoprotein was negatively and significantly correlated with ARVSBP [β = -0.13 (0.063)]. With adjustment for the baseline SBP and DBP in model 1, the positive correlation between waist circumference and ARVDBP decreased and was not significant, and neither was in the fully adjusted model 2. The positive correlation of SUA with SDDBP and the inverse correlation of high-density lipoprotein with ARVSBP remained significant in model 1. In the fully adjusted model 2, SUA was positively and significantly correlated with SDSBP and SDDBP [β = 0.019 (0.010) and 0.027 (0.012), respectively], whereas the inverse correlation of high-density lipoprotein with ARVSBP was no longer significant.
Table 2.
Association of metabolic biomarkers with long-term blood pressure variability indexes in multivariable liner regression
|
Unadjusted |
Model 1 |
Model 2 |
|||||||
| β (SE) | P value | R2, % | β (SE) | P value | R2, % | β (SE) | P value | R2, % | |
| Waist circumference | |||||||||
| ARVSBP | 0.046 (0.046) | 0.31 | 0.0 | 0.039 (0.044) | 0.38 | 7.9 | −0.002 (0.026) | 0.95 | 68.6 |
| ARVDBP | 0.157 (0.049) | < 0.01 | 0.9 | 0.078 (0.048) | 0.10 | 8.3 | −0.004 (0.028) | 0.88 | 68.9 |
| SDSBP | 0.071 (0.060) | 0.23 | 0.1 | 0.050 (0.058) | 0.38 | 8.1 | −0.021 (0.034) | 0.53 | 68.9 |
| SDDBP | 0.220 (0.065) | < 0.01 | 1.0 | 0.100 (0.064) | 0.11 | 8.3 | −0.003 (0.037) | 0.92 | 68.9 |
| Serum uric acid | |||||||||
| ARVSBP | 0.009 (0.008) | 0.27 | 0.1 | 0.009 (0.008) | 0.30 | 1.6 | 0.011 (0.008) | 0.18 | 11.0 |
| ARVDBP | 0.022 (0.009) | 0.01 | 0.5 | 0.016 (0.009) | 0.07 | 1.8 | 0.012 (0.008) | 0.16 | 11.0 |
| SDSBP | 0.020 (0.011) | 0.06 | 0.3 | 0.019 (0.011) | 0.08 | 1.8 | 0.019 (0.010) | 0.05 | 11.1 |
| SDDBP | 0.038 (0.012) | < 0.01 | 1.0 | 0.030 (0.012) | 0.01 | 2.1 | 0.027 (0.011) | 0.01 | 11.3 |
| Serum triglycerides | |||||||||
| ARVSBP | 0.031 (0.673) | 0.96 | 0.0 | −0.014 (0.663) | 0.98 | 3.0 | 0.310 (0.615) | 0.61 | 18.2 |
| ARVDBP | 1.155 (0.712) | 0.10 | 0.2 | 0.473 (0.715) | 0.50 | 3.0 | 0.343 (0.660) | 0.60 | 18.2 |
| SDSBP | −0.003 (0.871) | 0.99 | 0.0 | −0.121 (0.860) | 0.88 | 3.0 | 0.141 (0.796) | 0.86 | 18.2 |
| SDDBP | 1.545 (0.946) | 0.10 | 0.2 | 0.493 (0.954) | 0.60 | 3.0 | 0.478 (0.881) | 0.58 | 18.2 |
| Fasting glucose | |||||||||
| ARVSBP | −0.008 (0.086) | 0.92 | 0.0 | −0.011 (0.086) | 0.90 | 0.5 | 0.006 (0.084) | 0.94 | 5.2 |
| ARVDBP | 0.024 (0.091) | 0.79 | 0.0 | −0.014 (0.092) | 0.88 | 0.5 | −0.033 (0.090) | 0.71 | 5.2 |
| SDSBP | 0.036 (0.111) | 0.74 | 0.0 | 0.029 (0.111) | 0.79 | 0.5 | 0.028 (0.109) | 0.79 | 5.2 |
| SDDBP | 0.126 (0.120) | 0.29 | 0.0 | 0.072 (0.123) | 0.55 | 0.2 | 0.058 (0.121) | 0.62 | 4.5 |
| High-density lipoprotein | |||||||||
| ARVSBP | −0.134 (0.063) | 0.03 | 0.4 | −0.131 (0.063) | 0.03 | 0.9 | −0.080 (0.060) | 0.18 | 11.3 |
| ARVDBP | −0.062 (0.067) | 0.36 | 0.1 | −0.045 (0.068) | 0.51 | 0.6 | 0.004 (0.065) | 0.95 | 11.2 |
| SDSBP | −0.130 (0.082) | 0.11 | 0.2 | −0.121 (0.082) | 0.14 | 0.7 | −0.058 (0.078) | 0.45 | 11.2 |
| SDDBP | −0.120 (0.089) | 0.18 | 0.1 | −0.095 (0.091) | 0.29 | 0.3 | −0.022 (0.087) | 0.79 | 10.4 |
Data are presented as β (SE) using Pearson’s correlation coefficient for Model 2: SBP and DBP adjustments; Model 3: The covariates in Model 2, age, BMI, total cholesterol, exercise frequency, current drinker and current smoker adjustments. SBP: Systolic blood pressure; DBP: Diastolic blood pressure; ARV: Average real variability; SD: Standard deviation; SE: Standard errors.
DISCUSSION
Our principal findings were that among young males who were not undergoing antihypertensive therapy, SUA and waist circumference were associated with a greater long-term diastolic BPV index, whereas high-density lipoprotein was associated with a lower systolic BPV index. With adjustment for the baseline confounders, SUA was the only metabolic biomarker associated with higher systolic and diastolic BPV indexes, indicating that SUA might be one of the strongest metabolic biomarkers related to long-term BPV.
Uric acid is the final enzymatic product of purine metabolism in the human body. Elevated SUA is mostly due to reduced urinary excretion or comes from high oral intake of purine-rich foods[27]. Uric acid, which is partly produced by vascular endothelial cells, has been regarded as an antioxidant in plasma with anti-atherosclerotic effects[28]. In contrast, higher SUA may also increase peroxynitrite-mediated oxidation of low-density lipoprotein and stimulate the production of free radicals that have proinflammatory effects on vascular smooth muscle cells[29], resulting in insulin resistance and arterial stiffness[30,31]. Hyperuricemia is a potent risk factor for gout flares[32], and several previous population studies have shown that there is an association with hypertension[33] and other metabolic disorders, such as type 2 diabetes, metabolic syndrome, and nonalcoholic fatty liver disease[29,30,32]. Given that greater SUA has been shown to be longitudinally associated with increased arterial stiffness and the development of hypertension in men or women without hypertension[34,35], the implication of the role of hyperuricemia in BPV would remain.
The improving interMediAte RisK management (MARK) study revealed that waist size was linearly correlated with the cardio-ankle vascular index (CAVI), a marker of central arterial stiffness, but not with brachial-ankle pulse wave velocity (baPWV), a marker of peripheral arterial stiffness, among middle- and old-aged males and females with intermediate risk for cardiovascular disease[36], which was in line with the present study finding that waist circumference was not an independent risk factor for long-term peripheral BPV in young male adults. In contrast, high-density lipoprotein was linearly correlated with baPWV but not CAVI among middle- and old-aged male adults in the MARK study[36], which could be the reason why the relationship between high-density lipoprotein and peripheral BPV remained after adjustment for the baseline SBP and DBP in young male adults in the present study. With regard to other components of metabolic syndrome, such as serum triglycerides and fasting plasma glucose, although both of them were linearly correlated with baPWV among middle- and old-aged male adults, high serum triglycerides and fasting glucose were not significantly associated with a higher risk of criteria-defined peripheral arterial stiffness in the MARK study[36]. We could speculate that among young male adults, the ranges of serum triglyceride and fasting glucose levels were relatively limited, and thus, the relationship with the peripheral arterial stiffness index or related BPV might not be significant. In another study of southern Chinese adults[37], the baseline BP level was found to be the strongest predictor of baPWV among the metabolic biomarkers, and only high-density lipoprotein and SUA were not correlated with baPWV. This finding could also be explained by our result that after adjustment for the baseline BP, the association between waist circumference and BPV was absent.
There were several strengths in this study. First, since all the procedures were performed in a standard manner in the same referral hospital, the physical and laboratory examinations were performed uniformly. Second, lifestyles, such as dietary intake and physical training, were similar in the military troops, and thus, many unmeasured factors were controlled at baseline. In contrast, there were some limitations in this study. First, to our knowledge, there are remarkable sex differences in the association of metabolic biomarkers with arterial stiffness[36], and the present results were only obtained from young male adults and might not be appropriate for young female adults. Second, although many confounders were adjusted for in the model at baseline, we could not avoid the possibility of potential confounders, which might lead to bias. Third, we did not have data for the amount and frequency of alcohol consumption and tobacco smoking for each subject, which might be inversely associated with long-term BPV.
In conclusion, our findings showed that of the traditional metabolic biomarkers, high-density lipoprotein might have an inverse correlation with long-term BPV, regardless of no significance in the fully adjusted model. SUA levels rather than waist circumference had the strongest positive correlation with long-term systolic and diastolic BPV in young male adults, and the clinical relevance needs further investigation.
ARTICLE HIGHLIGHTS
Research background
Short-term and long-term blood pressure variability (BPV) are associated with metabolic syndrome and cardiovascular events in middle- and old-aged adults.
Research motivation
It is unclear regarding the relationships between various metabolic biomarkers and BPV in young adults.
Research objectives
To examine the relationship of metabolic biomarkers with long-term BPV in young males.
Research methods
A cohort of 1112 healthy military males aged 18-40 years from the cardiorespiratory fitness and hospitalization events in armed forces study in eastern Taiwan was prospectively included. The following metabolic biomarkers were used: Waist circumference, serum uric acid (SUA), triglycerides, high density lipoprotein, triglycerides, and fasting glycose. BPV was assessed by average real variability (ARV) and standard deviation (SD) across 4 clinic visits during the study period (2012-14, 2014-15, 2015-16, and 2016-18). Multivariable linear regression analysis was used to determine the association after adjusting for age, body mass index, systolic and diastolic blood pressure (SBP and DBP), lipid profiles, physical activity, alcohol intake and tobacco smoking status.
Research results
In the unadjusted model, waist circumference was significantly and positively correlated with ARVDBP and SDDBP [β (standard errors) = 0.16 (0.049) and 0.22 (0.065), respectively], as was SUA [β = 0.022 (0.009) and 0.038 (0.012), respectively]. High-density lipoprotein was negatively correlated with ARVSBP [(β = -0.13 (0.063)). There were no associations with the other metabolic biomarkers. In contrast, only SUA was significantly correlated with SDSBP and SDDBP [β = 0.019 (0.011) and 0.027 (0.010), respectively] in the adjusted model.
Research conclusions
Our findings showed that of traditional metabolic biomarkers, SUA had the strongest positive correlation with long-term systolic and diastolic BPV in young male adults.
Research perspectives
Future studies should highlight the mechanism for the interplay between SUA, arterial stiffness and long-term BPV.
Footnotes
Institutional review board statement: This prospective study was approved by the Institutional Review Board of the Mennonite Christian Hospital (No. 16-05-008) in the Hualien City of Taiwan.
Informed consent statement: The written informed consent was obtained from all participants.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Manuscript source: Invited manuscript
Peer-review started: February 25, 2020
First decision: April 29, 2020
Article in press: May 21, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Raikou VD S-Editor: Wang J L-Editor: A E-Editor: Liu JH
Contributor Information
Yu-Kai Lin, Department of Medicine, Hualien Armed Forces General Hospital, Hualien 970, Taiwan; Departments of Neurology, Tri-Service General Hospital, and Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei 114, Taiwan.
Pang-Yen Liu, Department of Medicine, Tri-Service General Hospital and National Defense Medical Center, Taipei 114, Taiwan.
Chia-Hao Fan, Department of Medicine, Hualien Armed Forces General Hospital, Hualien 970, Taiwan.
Kun-Zhe Tsai, Department of Medicine, Hualien Armed Forces General Hospital, Hualien 970, Taiwan.
Yen-Po Lin, Department of Critical Care Medicine, Taipei Tzu-Chi General Hospital, New Taipei City 231, Taiwan.
Ju-Mi Lee, Department of Preventive Medicine, Eulji College of Medicine, Daejeon 34824, South Korea.
Jiunn-Tay Lee, Departments of Neurology, Tri-Service General Hospital, and Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei 114, Taiwan.
Gen-Min Lin, Department of Medicine, Hualien Armed Forces General Hospital, Hualien 970, Taiwan; Department of Medicine, Tri-Service General Hospital and National Defense Medical Center, Taipei 114, Taiwan; Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL 60611, United States. farmer507@yahoo.com.tw.
Data sharing statement
As the study materials were obtained from the military in Taiwan, the data were confidential and not allowed to be opened in public. If there are any needs for clarification, the readers can contact Colonel Dr. Gen-Min Lin, the corresponding author for sharing the data.
References
- 1.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 3.Cicero AFG, Fogacci F, Giovannini M, Grandi E, Rosticci M, D'Addato S, Borghi C. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella Heart Study. Sci Rep. 2018;8:11529. doi: 10.1038/s41598-018-29955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun HL, Pei D, Lue KH, Chen YL. Uric Acid Levels Can Predict Metabolic Syndrome and Hypertension in Adolescents: A 10-Year Longitudinal Study. PLoS One. 2015;10:e0143786. doi: 10.1371/journal.pone.0143786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang L, Kubota M, Nagai A, Mamemoto K, Tokuda M. Hyperuricemia in obese children and adolescents: the relationship with metabolic syndrome. Pediatr Rep. 2010;2:e12. doi: 10.4081/pr.2010.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GERTLER MM, GARN SM, LEVINE SA. Serum uric acid in relation to age and physique in health and in coronary heart disease. Ann Intern Med. 1951;34:1421–1431. doi: 10.7326/0003-4819-34-6-1421. [DOI] [PubMed] [Google Scholar]
- 7.Brand FN, McGee DL, Kannel WB, Stokes J, 3rd, Castelli WP. Hyperuricemia as a risk factor of coronary heart disease: The Framingham Study. Am J Epidemiol. 1985;121:11–18. doi: 10.1093/oxfordjournals.aje.a113972. [DOI] [PubMed] [Google Scholar]
- 8.Li YH, Lin GM, Lin CL, Wang JH, Chen YJ, Han CL. Relation of serum uric acid and body mass index to mortality in high-risk patients with established coronary artery disease: a report from the ET-CHD registry, 1997-2006. J Cardiol. 2013;62:354–360. doi: 10.1016/j.jjcc.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Lin GM, Li YH, Zheng NC, Lai CP, Lin CL, Wang JH, Jaiteh LE, Han CL. Serum uric acid as an independent predictor of mortality in high-risk patients with obstructive coronary artery disease: a prospective observational cohort study from the ET-CHD registry, 1997-2003. J Cardiol. 2013;61:122–127. doi: 10.1016/j.jjcc.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Lin YK, Lin YP, Lee JT, Lin CS, Wu TJ, Tsai KZ, Su FY, Kwon Y, Hoshide S, Lin GM. Sex-specific association of hyperuricemia with cardiometabolic abnormalities in a military cohort: The CHIEF study. Medicine (Baltimore) 2020;99:e19535. doi: 10.1097/MD.0000000000019535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu AH, Gladden JD, Ahmed M, Ahmed A, Filippatos G. Relation of serum uric acid to cardiovascular disease. Int J Cardiol. 2016;213:4–7. doi: 10.1016/j.ijcard.2015.08.110. [DOI] [PubMed] [Google Scholar]
- 12.Parati G, Ochoa JE, Bilo G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr Hypertens Rep. 2012;14:421–431. doi: 10.1007/s11906-012-0290-7. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 15.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–155. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 16.Okada H, Fukui M, Tanaka M, Inada S, Mineoka Y, Nakanishi N, Senmaru T, Sakabe K, Ushigome E, Asano M, Yamazaki M, Hasegawa G, Nakamura N. Visit-to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2012;220:155–159. doi: 10.1016/j.atherosclerosis.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 17.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: new independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. J Am Soc Hypertens. 2011;5:184–192. doi: 10.1016/j.jash.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Yano Y, Ning H, Allen N, Reis JP, Launer LJ, Liu K, Yaffe K, Greenland P, Lloyd-Jones DM. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. 2014;64:983–988. doi: 10.1161/HYPERTENSIONAHA.114.03978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin GM, Li YH, Lee CJ, Shiang JC, Lin KH, Chen KW, Chen YJ, Wu CF, Lin BS, Yu YS, Lin F, Su FY, Wang CH. Rationale and design of the cardiorespiratory fitness and hospitalization events in armed forces study in Eastern Taiwan. World J Cardiol. 2016;8:464–471. doi: 10.4330/wjc.v8.i8.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin GM, Tsai KZ, Lin CS, Han CL. Physical Fitness and Long-Term Blood Pressure Variability of Young Male Military Personnel. Curr Hypertens Rev. 2019 doi: 10.2174/1573402115666191023111351. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai KZ, Lin JW, Lin F, Su FY, Li YH, Lin YP, Lin YK, Han CL, Hsieh CB, Lin GM. Association of betel nut chewing with exercise performance in a military male cohort: the CHIEF study. J R Army Med Corps. 2018;164:399–404. doi: 10.1136/jramc-2017-000899. [DOI] [PubMed] [Google Scholar]
- 23.Tsai KZ, Lai SW, Hsieh CJ, Lin CS, Lin YP, Tsai SC, Chung PS, Lin YK, Lin TC, Ho CL, Han CL, Kwon Y, Hsieh CB, Lin GM. Association between mild anemia and physical fitness in a military male cohort: The CHIEF study. Sci Rep. 2019;9:11165. doi: 10.1038/s41598-019-47625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao WH, Su FY, Lin F, Yu YS, Lin GM. Association of electrocardiographic left and right ventricular hypertrophy with physical fitness of military males: The CHIEF study. Eur J Sport Sci. 2019;19:1214–1220. doi: 10.1080/17461391.2019.1595741. [DOI] [PubMed] [Google Scholar]
- 25.Su FY, Wang SH, Lu HH, Lin GM. Association of Tobacco Smoking with Physical Fitness of Military Males in Taiwan: The CHIEF Study. Can Respir J. 2020;2020:5968189. doi: 10.1155/2020/5968189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen KW, Meng FC, Shih YL, Su FY, Lin YP, Lin F, Lin JW, Chang WK, Lee CJ, Li YH, Hsieh CB, Lin GM. Sex-Specific Association between Metabolic Abnormalities and Elevated Alanine Aminotransferase Levels in a Military Cohort: The CHIEF Study. Int J Environ Res Public Health. 2018;15:545. doi: 10.3390/ijerph15030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 28.Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Durme C, van Echteld IA, Falzon L, Aletaha D, van der Heijde DM, Landewé RB. Cardiovascular risk factors and comorbidities in patients with hyperuricemia and/or gout: a systematic review of the literature. J Rheumatol Suppl. 2014;92:9–14. doi: 10.3899/jrheum.140457. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med. 2012;125:679–687.e1. doi: 10.1016/j.amjmed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 31.Singh JA, Reddy SG, Kundukulam J. Risk factors for gout and prevention: a systematic review of the literature. Curr Opin Rheumatol. 2011;23:192–202. doi: 10.1097/BOR.0b013e3283438e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rho YH, Choi SJ, Lee YH, Ji JD, Choi KM, Baik SH, Chung SH, Kim CG, Choe JY, Lee SW, Chung WT, Song GG. The prevalence of metabolic syndrome in patients with gout: a multicenter study. J Korean Med Sci. 2005;20:1029–1033. doi: 10.3346/jkms.2005.20.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barros RP, Gustafsson JÅ. Estrogen receptors and the metabolic network. Cell Metab. 2011;14:289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Tomiyama H, Shiina K, Vlachopoulos C, Iwasaki Y, Matsumoto C, Kimura K, Fujii M, Chikamori T, Yamashina A. Involvement of Arterial Stiffness and Inflammation in Hyperuricemia-Related Development of Hypertension. Hypertension. 2018;72:739–745. doi: 10.1161/HYPERTENSIONAHA.118.11390. [DOI] [PubMed] [Google Scholar]
- 35.Choi HY, Kim SH, Choi AR, Kim SG, Kim H, Lee JE, Kim HJ, Park HC. Hyperuricemia and risk of increased arterial stiffness in healthy women based on health screening in Korean population. PLoS One. 2017;12:e0180406. doi: 10.1371/journal.pone.0180406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Sanchez L, Garcia-Ortiz L, Patino-Alonso MC, Recio-Rodriguez JI, Fernando R, Marti R, Agudo-Conde C, Rodriguez-Sanchez E, Maderuelo-Fernandez JA, Ramos R, Gomez-Marcos MA MARK Group. Association of metabolic syndrome and its components with arterial stiffness in Caucasian subjects of the MARK study: a cross-sectional trial. Cardiovasc Diabetol. 2016;15:148. doi: 10.1186/s12933-016-0465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L, Zhu W, Mai L, Fang L, Ying K. The association of metabolic syndrome and its components with brachial-ankle pulse wave velocity in south China. Atherosclerosis. 2015;240:345–350. doi: 10.1016/j.atherosclerosis.2015.03.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As the study materials were obtained from the military in Taiwan, the data were confidential and not allowed to be opened in public. If there are any needs for clarification, the readers can contact Colonel Dr. Gen-Min Lin, the corresponding author for sharing the data.