Abstract
For the development of sustainable control of tick-borne diseases, insight is needed in biological factors that affect tick populations. Here, the ecological interactions among Ixodiphagus hookeri, Ixodes ricinus, and two vertebrate species groups were investigated in relation to their effects on tick-borne disease risk. In 1129 questing ticks, I. hookeri DNA was detected more often in I. ricinus nymphs (4.4%) than in larvae (0.5%) and not in adults. Therefore, we determined the infestation rate of I. hookeri in nymphs from 19 forest sites, where vertebrate, tick, and tick-borne pathogen communities had been previously quantified. We found higher than expected co-occurrence rates of I. hookeri with deer-associated Anaplasma phagocytophilum, and lower than expected rates with rodent-associated Borrelia afzelii and Neoehrlichia mikurensis. The prevalence of I. hookeri in nymphs varied between 0% and 16% and was positively correlated with the encounter probability of ungulates and the densities of all life stages of I. ricinus. Lastly, we investigated the emergence of I. hookeri from artificially fed, field-collected nymphs. Adult wasps emerged from seven of the 172 fed nymphs. From these observations, we inferred that I. hookeri is parasitizing I. ricinus larvae that are feeding on deer, rather than on rodents or in the vegetation. Since I. hookeri populations depend on deer abundance, the main propagation host of I. ricinus, these wasps have no apparent effect on tick populations. The presence of I. hookeri may directly interfere with the transmission cycle of A. phagocytophilum, but not with that of B. afzelii or N. mikurensis.
Keywords: parasitic wasp, biological control, tick-borne pathogen, host preference, parasitization, transmission cycle, Lyme borreliosis, human granulocytic anaplasmosis, neoehrlichiosis
1. Introduction
Lyme borreliosis poses serious health concerns in the northern hemisphere [1,2,3]. Increases in incidence have been observed in several countries in Europe. These increases are partially explained by geographical spread of its vector, Ixodes ricinus [4,5]. Other tick-borne diseases (TBDs), such as anaplasmosis and neoehrlichiosis, are also emerging [6,7]. Understanding which factors drive the population densities of ticks and the transmission cycles of tick-borne pathogens are important steps in assessing disease risk and formulating possible intervention strategies. In north-western Europe, TBDs are generally caused by a bite of an infected I. ricinus nymph [8]. Therefore, the density of infected nymphs (DIN) is an important ecological parameter that contributes to the overall disease risk of TBD [9,10,11].
Ixodes ricinus has a three-host life cycle and its survival depends on finding vertebrate hosts for feeding and propagation. Ixodes ricinus utilizes a multitude of host species, but these species differ in the number of ticks and in the life stages they feed [12]. In a typical north-western European forest, larvae predominantly feed on rodents, nymphs on rodents and birds, and adult ticks on deer, mostly Capreolus capreolus [13,14,15]. The presence of deer generally results in a high abundance of I. ricinus [15,16,17]. However, fluctuations in rodent density have also been associated with variations in the density of (infected) nymphs [18,19].
Control of I. ricinus-borne diseases primarily consists of the promotion of personal preventive actions, but its effectiveness is questionable [20,21,22]. Biological control of ticks is considered to be an environmentally friendly approach. However, so far, this has not been effectively applied in routine settings [23,24,25,26,27]. Interestingly, the parasitoid wasp, Ixodiphagus hookeri (Hymenoptera: Encyrtidae), is a natural enemy of I. ricinus, and has been a target of interest as a biological control agent [23,27,28].
Although the total lifecycle of I. hookeri generally takes one year, the adult life stage has a lifespan of only two to three days [23,29]. It is not well understood how female wasps find I. ricinus larvae and nymphs for oviposition [23,27]. We hypothesize that female I. hookeri randomly infest I. ricinus larvae and nymphs in the vegetation. We test this by calculating the infestation rate of the life stages of questing I. ricinus. If I. hookeri infests ticks in the vegetation, then we expect to find the wasp in nymphs together with horizontally transmitted tick-borne pathogens more or less randomly. The alternative hypothesis is that female I. hookeri infest I. ricinus larvae while feeding on a vertebrate host. By calculating the co-occurrence rates of the wasp with strictly horizontally transmitted tick-borne pathogens, we might infer a preference for a vertebrate host on which a female wasp would infest feeding ticks with her eggs.
The embryonic development of I. hookeri eggs is triggered by the attachment and engorgement of nymphs feeding on a vertebrate host [23,27]. Ixodiphagus hookeri larvae feed on the internal tissue along with the vertebrate blood ingested by nymphs [23,27]. Adult wasps emerge by making a hole in the nymph’s body, and kill the tick before molting [30]. Until now, the emergence of I. hookeri has only been observed from fully fed I. ricinus nymphs [27,30,31]. Although several studies on the interactions between I. hookeri and I. ricinus have been conducted [27,29,31,32,33,34,35], we lack estimates about the impact of this wasp on I. ricinus population dynamics. We hypothesize that the prevalence of I. hookeri in nymphs is driven by the abundance of immature stages of I. ricinus, and because of their predatory behavior, an increasing prevalence of wasps is negatively associated with the abundance of I. ricinus adults. For this, we determined the infestation rates of I. hookeri in questing nymphs, and correlated it with the abundances of the different life stages of I. ricinus from a cross-sectional study, in which tick density and vertebrate communities were quantified [13,36]. The latter allowed us to determine the relationship between I. hookeri and tick hosts such as ungulates and rodents.
Lastly, to determine whether the molecular detection of the wasp in ticks reflects the presence of viable wasp eggs, and to determine to what extent these infestations kill nymphs, an artificial tick feeding assay was performed.
2. Results
2.1. Prevalence of I. hookeri and Tick-Borne Pathogens in Different Life Stages of I. ricinus
To determine which life stage of I. ricinus was most often infested with I. hookeri, larvae (n = 367), nymphs (n = 684), and adults (n = 78) were tested (Table 1). Ixodiphagus hookeri DNA was found in two larvae (0.5%), 30 nymphs (4.4%), but not in adult I. ricinus. Thus, the highest prevalence of I. hookeri was detected in nymphs. The three horizontally transmitted tick-borne pathogens, Borrelia burgdorferi s.l., Anaplasma phagocytophilum, and Neoehrlichia mikurensis, were detected in nymphs and adults (Table 1), but not in larvae, except for one larva, which was positive of B. burgdorferi s.l. (Table 1).
Table 1.
The occurrence of tick symbionts in questing I. ricinus ticks.
| Symbiont | Larvae (n = 367) | Nymphs (n = 684) | Adults (n = 78) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | (Range) | n | % | (Range) | n | % | (Range) | |
| I. hookeri % | 2 | 0.5% | (0.1–2.5) | 30 | 4.4% | (3.0–6.2) | 0 | 0% | (0.0–4.6) |
| B. burgdorferi s.l. % | 1 | 0.3% | (0.0–1.5) | 82 | 12% | (9.6–14.7) | 13 | 16.7% | (9.2–26.8) |
| A. phagocytophilum % | 0 | 0% | (0.0–1.0) | 19 | 2.8% | (1.7–4.3) | 7 | 9% | (3.7–17.6) |
| N. mikurensis % | 0 | 0% | (0.0–1.0) | 30 | 4.4% | (3.0–6.2) | 4 | 5.1% | (1.4–12.6) |
Different life stages of questing I. ricinus were collected and tested for the presence of I. hookeri and tick-borne pathogens. Occurrence is presented as n (number of positive ticks), prevalence (%), and the 95% confidence intervals of the prevalence (range), which is calculated according to Armitage et al. [37].
2.2. Infestations with I. hookeri and Infection with Horizontally Transmitted Tick-Borne Pathogens
The presence of I. hookeri DNA was determined in 13,967 nymphs from the cross-sectional study, and which had already been tested for the prevalence of tick-borne pathogens [13,36]. Ixodiphagus hookeri was detected in nymphs from 18 of the 19 forest sites examined in the Netherlands, with prevalence varying from 0.1% to 15.9% (Table S1). The infestation with I. hookeri and infection with horizontally transmitted pathogens appeared not to be random (Table 2). The presence of I. hookeri DNA in nymphs was positively associated with the deer-associated A. phagocytophilum, and negatively associated with the rodent-borne B. afzelii and N. mikurensis (Table 2 and Table S2). Apparently, the infestation with I. hookeri appears somehow to be associated with the first blood meal of the questing nymphs.
Table 2.
Observed and expected co-occurrence of I. hookeri and tick-borne pathogens in I. ricinus nymphs.
| A. phagocytophilum | B. afzelii | N. mikurensis | |
|---|---|---|---|
| Observed co-occurrence | 72 | 4 | 9 |
| Expected co-occurrence | 26 | 17 | 46 |
| Odds ratio | 3.3 | 0.2 | 0.2 |
| p-value | <0.001 | <0.001 | <0.001 |
Questing nymphs (n = 13,967) from the 19 forest sites were tested for the presence of I. hookeri, A. phagocytophilum, B. afzelii, and N. mikurensis DNA. Odds ratio >1 and <1 indicates increased and decreased co-occurrence, respectively. A Fisher’s exact test was used to test the statistical significance for an association.
2.3. Association of I. hookeri Prevalence in Questing Nymphs with Density of I. ricinus
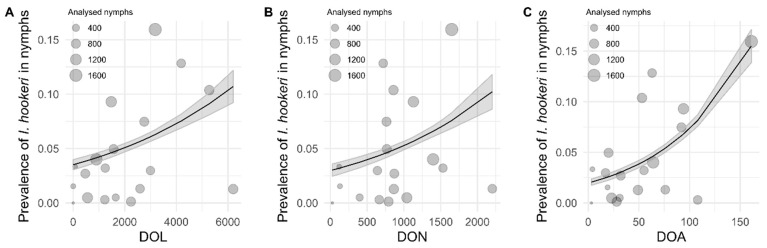
The relationship between prevalence (%) of I. hookeri in questing nymphs and the density of all life stages of I. ricinus of the 19 forests sites was investigated with generalized linear models. The prevalence (%) of I. hookeri was significantly positively associated with the density of questing I. ricinus larvae (DOL; p < 0.0001), nymphs (DON; p < 0.0001) and adults (DOA; p < 0.0001; Figure 1). Equations of all models, Akaike information criterion (AIC) values, and results of the likelihood ratio test are provided in Table S3.
Figure 1.
Associations of the prevalence of I. hookeri in questing nymphs with (A) the density of I. ricinus larvae (DOL), (B) density of nymphs (DON), and (C) density of adults (DOA) in the 19 forest sites (Table S4 and Figure S2). The density is presented per 1200 m2. Grey shading around the black regression line represents standard errors. All presented associations are significant (p < 0.0001). Equations of all models, AIC values and results of the likelihood ratio test are provided in Table S3.
2.4. Association of I. hookeri with Densities of Ungulates and Rodents
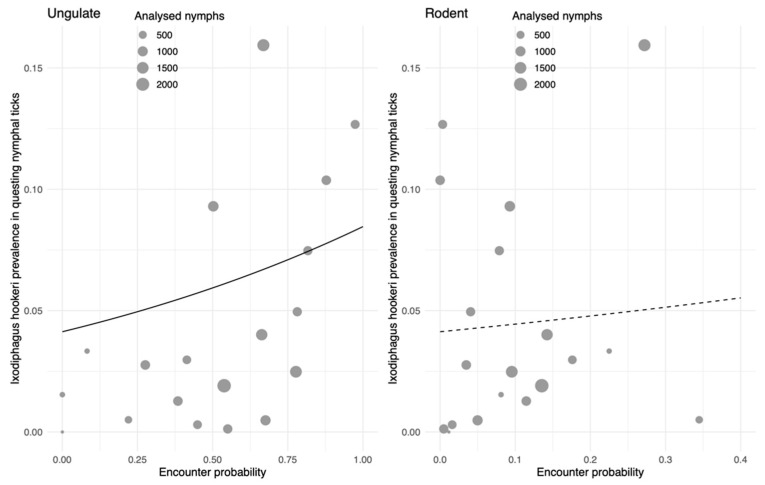
Previous analyses of the data from the cross-sectional study showed that the abundance of ungulates was positively associated with the density of the three life stages of I. ricinus [13], just like the prevalence of I. hookeri in our study (Figure 1). The observed positive association of I. hookeri with densities of the different life stages of I. ricinus might therefore be caused by the dominant role of deer in propagation of ticks. Indeed, the occurrence of I. hookeri in questing nymphs was positively correlated with the encounter probability with ungulates (Figure 2, left panel). No significant association between the encounter probability of rodents and I. hookeri occurrence was observed (Figure 2, right panel).
Figure 2.
Association of vertebrate species groups (ungulates and rodents) with the occurrence of I. hookeri in questing nymphs in the 19 forest sites. The occurrence is presented as the infestation prevalence of I. hookeri in questing nymphs (density of infected nymphs; DIN) at each site of 1200 m2. Horizontal axis: a function of encounter rates (see methods). A circle represents a single forest site. Best-fit beta binomial model is visualized by a solid line (a significant relationship to the host species, p = 0.018) or a dashed line (not significant, p = 0.7).
2.5. Artificial Blood-Feeding of I. ricinus Nymphs
Lastly, to determine whether the detection of I. hookeri in ticks reflects the presence of viable wasp eggs, and to determine to what extent these infestations kill nymphs, an artificial tick feeding assay was performed. A total of 561 nymphs collected in the Amsterdamse Waterleidingduinen were placed on feeding membranes, of which 172 successfully blood-fed and engorged (Table 3). A total of 151 nymphs (88%) successfully molted into the adult stage. In total, 21 engorged nymphs did not molt and died (12%); 14 died without and seven died with the emergence of three to five wasps (Table 3). We detected wasp DNA in seven nymphs the wasps emerged from, as well as in nine of the 14 dead nymphs, and in ten of the 151 that engorged and successfully molted to adults (Table 3). In total, 15% of the nymphs were infested with I. hookeri, which is comparable to the infestation rate found previously in the Amsterdamse Waterleidingduinen (13%; Table S1). Thus, from the nymphs infested with I. hookeri (n = 26), sixteen (62%) died and ten (38%) survived and successfully molted into an adult tick.
Table 3.
The presence of pathogens and I. hookeri in nymphs, which successfully blood-fed in the assay.
| Ticks | I. hookeri % | A. phagocytophilum % | B. burgdorferi s.l. % | N. mikurensis % | |||||
|---|---|---|---|---|---|---|---|---|---|
| Female | (n = 64) | 7.8 | (2.6–17.3) | 15.6 | (7.8–26.9) | 0.0 | (0.0–5.6) | 0.0 | (0.0–5.6) |
| Male | (n = 87) | 5.7 | (1.9–12.9) | 19.5 | (11.8–29.4) | 0.0 | (0.0–4.2) | 0.0 | (0.0–4.2) |
| Not molted | (n = 14) | 64.3 | (35.1–87.2) | 14.3 | (1.8–42.8) | 0.0 | (0.0–23.2) | 0.0 | (0.0–23.2) |
| With wasps | (n = 7) | 100 | (59.0–100.0) | 28.6 | (3.7–71.0) | 0.0 | (0.0–41.0) | 0.0 | (0.0–41.0) |
| Total | (n = 172) | 15.1 | (10.1–21.4) | 18.0 | (12.6–24.6) | 0.0 | (0.0–2.1) | 0.0 | (0.0–2.1) |
Field-collected, questing nymphs were artificially fed in blood-feeding units in the laboratory, and the emergence of parasitoid wasps was monitored. After that, all ticks were analyzed by molecular methods for the presence of pathogens and I. hookeri. Between brackets are the 95% confidence intervals, as calculated according to Armitage et al. [37].
3. Discussion
In this study, we investigated the ecological interactions among the parasitoid wasp I. hookeri, the tick I. ricinus, and two vertebrate species groups (ungulates and rodents), and their combined effect on tick-borne disease risk. Significant differences in the prevalence of I. hookeri between questing I. ricinus larvae and nymphs (Table 1) were found as well as different associations of wasps with horizontally transmitted tick-borne pathogens in nymphs (Table 2). From these results, we inferred that female I. hookeri wasps infest engorging larvae while feeding on a deer, rather than on a rodent or in the vegetation. In addition, the positive association of the I. hookeri prevalence with the encounter probability of deer, but not rodents (Figure 2) supports the idea that female wasps are attracted to deer by their odor, and subsequently infest ticks that are feeding on these deer [38,39]. Since deer act as major propagation hosts for I. ricinus, we observed a positive association between I. hookeri prevalence and the density of all tick life stages, not only larvae and nymphs (Figure 1). Our findings further indicate that preferential infestation of I. ricinus larvae feeding on deer may directly interfere with the transmission cycles of tick-borne pathogens, such as A. phagocytophilum, that utilizes deer as amplification hosts (Table 2).
Here, we detected I. hookeri in ticks collected in 18 of the 19 locations around the Netherlands with a prevalence in questing I. ricinus nymphs ranging from 0.1% to 16% (Figure 1 and Table S1). This wasp has a widespread distribution in Europe [27,29,31,33,34,35] and various factors, including microclimate, tick density, and tick–host abundance, were proposed as possible determinants for the high variation in infestation rates [31,40,41]. A prospective study of ten years reported a decrease in I. scapularis larvae and nymphs together with a decrease in the I. hookeri prevalence in questing nymphs when deer populations sharply declined [40]. Similar observations were made in our cross-sectional study, where the encounter probability of ungulates, most notably roe deer, was positively associated with the prevalence of all the life stages of I. ricinus [13] and with the I. hookeri prevalence in questing nymphs (Figure 2, left panel). Interestingly, the prevalence was indifferent to the encounter probability of rodents (Figure 2, right panel). Fluctuating rodent populations have been shown to affect density of I. ricinus nymphs [18,19]; however, presence of deer is generally responsible for high density of ticks due to their role as propagation hosts [15,17,42].
In addition, deer play a role in attracting female I. hookeri over some distance [38,39]. In laboratory tests, it has been demonstrated that I. hookeri females preferred mostly unfed I. ricinus nymphs for oviposition over unfed larvae, engorged larvae and fully engorged nymphs [27], however, engorging ticks were not included in the experiment. In addition, it is not known how often wasps and questing nymphs are in spatial proximity in the wild. A strategy for female I. hookeri is to locate a nymph with help of chemical cues from a vertebrate host [32,43]. In laboratory experiments, I. hookeri females were attracted by carbon dioxide, which is an unspecific vertebrate cue, and by odors from roe deer feces, roe deer hair, and wild boar hair. In contrast, they were not attracted to odors derived from field mice, cattle, and rabbits [32]. Locating and parasitizing feeding ticks on their mammal hosts may be advantageous for I. hookeri wasps in several ways. First, the density of ticks feeding on a deer is expected to be (much) larger than the density of ticks in the vegetation [44]. Second, the probability of an engorging larva to successfully molt to a questing I. ricinus nymph is (much) higher than that of a questing larva [11,45].
Overall, the interaction of the wasp with the propagation host (deer) of its tick host ensures to a large extent the continuation of its lifecycle. This tri-trophic interaction makes it very difficult, if not impossible, to disentangle the effect of deer abundance on the tick and wasp populations, as well as the effect of the wasp on the tick population. Indeed, although the parasitic wasp is killing nymphs upon its emergence, hence interfering with the life cycle of ticks, our study was unable to detect any effect of the occurrence of the wasp on the population sizes (densities) of the different life stages of I. ricinus (Figure 1).
In this study, the wasp was detected in questing larvae and nymphs, but not in adult ticks (Table 1). Wasp DNA has previously been detected in questing nymphs and adults of I. ricinus with significantly higher prevalence in nymphs [33]. Our results are in line with the idea that nymphs are the main developmental stage affected by the parasitoid wasp. In addition, until now, the emergence of I. hookeri has been observed only in fully fed I. ricinus nymphs [27,30]. Nevertheless, parasitization itself might already occur at the larval stage of questing ticks to some extent. Although we detected the wasp DNA in two larvae (Table 1), we can imagine that they might have been parasitized while briefly feeding on a vertebrate rather than while questing. Some larvae in the vegetation already fed on a host and inadvertently detached due to grooming. The same explanation is used for finding B. burgdorferi s.l. infected questing larvae (Table 1, [46]). The results on co-occurrence of both I. hookeri and tick-borne pathogens in questing nymphs are contrary to what we expected (that I. hookeri parasitize ticks in the vegetation). Instead, our results support the idea that parasitization takes place when a larva feeds on a vertebrate. We observed a positive association between infestation with I. hookeri and infection with A. phagocytophilum, which is a pathogen that a I. ricinus larva acquires while feeding on a deer, an A. phagocytophilum-competent host (Table 2; [47,48,49]). Thus, both acquisition of a bacterium and parasitization by I. hookeri occurs simultaneously. Interestingly, we detected a strong negative association of I. hookeri with B. afzelii and N. mikurensis, rodent-associated pathogens (Table 2). The absence of Lyme spirochetes and Babesia microti in I. scapularis nymphs infested with parasitic wasps has been observed before [50]. These results suggest that wasps rarely parasitize larvae feeding on rodents [51,52] and questing nymphs infected with these pathogens. Our results further imply that I. hookeri, which infests feeding I. ricinus larva, can survive its molting to the nymphal stage (transstadial transmission). The efficiency of the transstadial transmission of the wasp as well as the effects on the survival on the infested tick is unknown, and warrants further investigation.
One of the questions of this study was whether I. hookeri decreases disease risk by reducing tick and/or their associated pathogen communities. Obviously, a fraction of the nymphs will not complete its life cycle, because of predation by the wasp, and therefore the abundance of the wasp has a negative impact on the life cycle of I. ricinus. Given that survival of wasps depends on its interaction with deer (the propagation hosts of I. ricinus), a diminishing or mitigating effect by the wasp on the tick population is rather unlikely (Figure 1). The parasitic wasp may influence tick-borne pathogen cycles to various extent, as it co-occurs in questing nymphal ticks less frequently with B. afzelii and N. mikurensis and more frequently with A. phagocytophilum. Future studies could investigate the potential long-lasting effect of I. hookeri on A. phagocytophilum and other deer-associated pathogens, such as Babesia spp. Investigation into comprehensive tick-borne disease risk ultimately requires the estimate DIN of these and other tick-borne pathogens.
The results from the artificial tick feeding assay show that molecular detection of I. hookeri DNA indicates presence of viable wasps as adult wasps successfully emerged from artificially blood-fed nymphs (Table 3). A total of 7 of the 172 nymphs (4.1%) were killed by emerging wasps (Figure 3), which is in concordance with one previous study [27], but not another [34]. The number of emerged parasitoids from a single I. ricinus nymph in our assay ranged from 3 to 5 wasps per tick, which were 1–15 in a German study and 2–20 in a Slovak study [27,34]. About 8% of the nymphs did not molt, and no wasp emerged (Table 3). From these not molted nymphs, 64% was infested with I. hookeri, which is comparable to a previous study [27]. The nymphs of that study were dissected one year later and dead I. hookeri wasps were found inside [27]. Adult parasitoids may not be able to emerge if they cannot consume all the material inside the nymphs [25]. Interestingly, I. hookeri DNA was also detected in 10 of 151 (6.6%) engorged ticks that successfully molted to adults and thus survived infestation with the parasitic wasp. This observation indicates that the development of wasp eggs was suppressed, but the mechanism behind it remains unknown. In other arthropods, it has been shown that some facultative symbionts may enhance survival of their host infested by a parasitic wasp [53,54].
Figure 3.
Ixodiphagus hookeri (left) and an engorged nymph of I. ricinus, which the parasitoid wasp emerged from (right). The field-collected nymph was fed to repletion using an artificial blood-feeding assay. During the molting process, a wasp emerged in the test tube. Photos were taken with a mobile phone and three images were processed in photo editing software to one. Original photos are in Figure S1.
The infection rate of the tick-borne pathogens in the ticks from the feeding assay is not in line with the observations in the field (Table 3). The absence of B. burgdorferi s.l. and N. mikurensis could be explained by antibiotics in the assay or by their incompatibility with the innate immune components in bovine blood [55]. Admittedly, little is known about antibiotic susceptibility, immune (in) compatibility, and tissue tropism of N. mikurensis. In contrast, A. phagocytophilum prevalence in I. ricinus nymphs that fed in the feeding assay was significantly higher (17.6%) than nymphs from the vegetation (Table 1 and Table S1). Perhaps, the intracellular location of A. phagocytophilum in ticks might prevent killing by gentamycin [56]. Further improvements of the artificial feeding system are necessary to enable studies of the microbial interactions with the parasitic wasp in more detail. Results from the artificial feeding assays indicate that the molecular detection of the wasp is indicative for the presence of viable wasps in ticks.
4. Materials and Methods
4.1. Tick Collection
Ixodes ricinus of all life stages were collected in 2019 by dragging a blanket of 1 m2 from two locations: Buunderkamp and Amsterdamse Waterleidingduinen (Table S4 and Figure S2), the Netherlands. Ticks were identified to species level using morphological keys [57]. A proportion of nymphs collected in Amsterdamse Waterleidingduinen was used in an artificial blood-feeding assay (see below). The remainder of nymphs were tested by PCR-based methods for the presence of tick-borne pathogens and I. hookeri.
4.2. Cross-Sectional Study
Extensive field surveys had been carried out previously in 19 sites located in forested areas in the Netherlands in 2013 and 2014 [13,36]. Data were collected on the density of questing I. ricinus (blanket dragging), vertebrate communities (camera and live trapping), and on the infection rates of tick-borne pathogens (qPCR detection). Only data on A. phagocytophilum, N. mikurensis, and B. afzelii were used in this study. Details from these forest sites are provided in Table S4 and Figure S2. All handling procedures of this study were approved by the Animal Experiments Committee of Wageningen University (WUR-2013055 and WUR-2014019) and by the Netherlands Ministry of Economic Affairs (FF/75A/2013/003).
4.3. Detection of I. hookeri and Tick-Borne Pathogens
DNA extraction from the individual questing ticks was achieved by alkaline lysis in ammonium hydroxide [58]. The lysates were stored at 4 °C. Samples were tested with qPCRs for presence of A. phagocytophilum [47], B. burgdorferi s.l. [59], and N. mikurensis [60]. For the study described here, the presence of I. hookeri DNA was detected by qPCR targeting a 104-bp fragment of the Cytochrome Oxidase I gene using primers 5′-AGA TGT TGA TAC TCG AGC TT-3′, 5′- AAT TTT ATT CCA TTT ATT GAA GCT A-3′ and a probe 5′-ATTO647- TGC TGT TCC AAC AGG AGT AAA AGT TTT TAG ATG A-BHQ2-3′. The specificity of this newly developed qPCR was determined as described previously [31,59]. In short, qPCR-positive samples were subjected to conventional PCR targeting a fragment of the 16S rRNA gene [31]. Both strands of PCR products were sequenced using Sanger Sequencing (Baseclear, Leiden, Netherlands). Resulting sequences were compared with sequences in Genbank using BLAST. A DNA lysate from an I. hookeri specimen, which was both morphologically and genetically identified, was used as positive control [31]. All qPCRs were carried out on a Light Cycler 480 (Roche Diagnostics Nederland B.V, Almere, the Netherlands) in a final volume of 20 μL with iQ multiplex Powermix, 3 μL of sample, and 0.2 μM for all primers and different concentrations for probes (30176908). Positive controls and negative water controls were used on every plate tested. To minimize contamination and false-positive samples, the DNA extraction, PCR mix preparation, sample addition, and qPCR analyses were performed in separated air locked dedicated labs.
4.4. Co-Infection Analysis
A Fisher’s exact test was applied to explore correlations between tick-borne pathogens and I. hookeri. For this, the expected co-occurrence was calculated assuming independent acquisition of tick-borne pathogens and of parasitoid wasps by multiplying their prevalence estimates and the observed density of nymphal ticks [13].
4.5. Association of I. hookeri Prevalence in Questing Nymphs with the Density of I. ricinus
We performed regression analyses to investigate associations of I. hookeri prevalence in questing I. ricinus nymphs with densities of I. ricinus larvae (DOL), nymphs (DON), and adults (DOA) of I. ricinus. Because I. hookeri prevalence is represented as proportional data, we chose a binomial generalized linear model taking into account sample size with the logit link transform. A likelihood ratio test was performed to assess the goodness of fit of all models. The ranges of DOL, DON, DOA, as well as I. hookeri prevalence in questing nymphs are provided in Table S1. The prevalence of I. hookeri was calculated based on a subset of samples tested. Model building was performed in R version 3.6.1 “Action of the Toes” [61].
4.6. Association of I. hookeri Prevalence with Vertebrate Encounter Probability
In order to find out whether I. hookeri displays the observed vertebrate host preference as inferred from the co-occurrence data, we considered a scenario in which numbers of I. hookeri presence in the local tick collections follow the beta binomial distribution. Furthermore, the beta mean was considered to relate (via the logit link) to the local ungulate population as measured by encounter probability [13]. The ungulate population consisted of four species: roe deer (Capreolus capreolus), fallow deer (Dama dama), red deer (Cervus elaphus), and wild boar (Sus scrofa). The rodent population consisted of two (major) species, namely wood mouse (Apodemus sylvaticus) and bank vole (Myodes glarelolus). Other rodent species, which occurred to a negligible extent, were: field vole (Microtus agrestis), common shrew (Sorex araneus), and pygmy shrew (Sorex minutus). The model was fitted by maximizing the beta binomial likelihood to the numbers of I. hookeri presence and absence per forest site. The likelihood-ratio test was applied to test whether I. hookeri prevalence is significantly associated with the local ungulate population. In addition to the ungulates, an association of rodents to I. hookeri prevalence was tested by following the same procedure. The calculations were performed using R [62].
4.7. Artificial Blood-Feeding of I. ricinus Nymphs and Examination of Wasp Emergence
In order to determine the wasp emergence rate, I. ricinus nymphs were placed on artificial blood-feeding units which were prepared according to previously described methods [63,64]. Between 50 and 60 ticks were placed in each of the six blood-feeding units. The nymphs were fed on heparinized bovine blood, which was supplemented with glucose (4 g/L), fungizone (2.5 μg/mL), gentamycin (50 μg/mL), and 5 μL of 100 mM ATP solution per 4 mL of blood. Cow blood was obtained from Carus (Wageningen University, The Netherlands) under animal ethics protocol no. AVD1040020173624 and from the Faculty of Veterinary Medicines, Utrecht University.
Blood was replaced every 24 h. Engorged and detached ticks were collected and stored individually in 2 mL Eppendorf tubes with pierced lids, which were kept in a desiccator, with approximately 90% relative humidity at room temperature and observed daily for parasitic wasp emergence. After the blood-feeding experiment, all engorged ticks were also tested for presence of tick-borne pathogens and I. hookeri with PCR-based methods.
5. Conclusions
Ungulates, particularly deer, are important drivers of tick populations and facilitate the infestation of I. ricinus by I. hookeri. This double role of deer diminishes the negative effect of the wasp on the tick abundance by killing I. ricinus. As this wasp infests I. ricinus larvae feeding on deer rather than on rodents, it has no direct interference with the transmission cycle of B. afzelii. Taken together, natural I. hookeri populations have a minimal impact on TBD risk. Insights in these ecological interactions might provide new food for thoughts on the biological control of Ixodes ricinus-borne diseases.
Acknowledgments
The authors thank Ankje de Vries (RIVM) and Vitalij Kuzkin for their help in collecting ticks, and Kamil Krawczyk for the construction and modification of Figure 3. We are also grateful to Hanke Bons-Clements from the Faculty of Veterinary Medicines, Utrecht University, for providing heparinized bovine blood. We thank Willem Takken (WUR) for his careful and constructive comments.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/5/339/s1. Table S1: Density of I. ricinus larvae, nymphs, and adults, and prevalence of A. phagocytophilum, N. mikurensis, B. afzelii, and I. hookeri per study site, Table S2: Observed and expected co-occurrence of I. hookeri with tick-borne pathogens, Table S3: Full equations of all models for prediction of prevalence of I. hookeri in questing nymphs, AIC values and results of the likelihood ratio test, Table S4: Summary description of the 19 locations from the cross-sectional study, Figure S1: Original photographs used for the fabrication of Figure 3, Figure S2: A map of the Netherlands with forest sites in which ticks were sampled.
Author Contributions
A.I.K.: Conceptualization, Investigation, Formal analysis, Writing—Original Draft, Visualization. J.W.B.: Methodology, Investigation, Writing—Review & Editing. C.J.M.K.: Resources, Writing—Review & Editing, Supervision, Project administration, Funding acquisition. M.F.: Methodology, Data Curation, Investigation, Writing—Review & Editing. K.T.: Methodology, Formal analysis, Writing—Review & Editing, Visualization. H.S.: Conceptualization, Resources, Writing—Review & Editing, Supervision, Project administration, Funding acquisition. S.D.: Conceptualization, Investigation, Writing—Original Draft, Supervision. All authors read and approved the final manuscript.
Funding
This study was funded by the Dutch Ministry of Health, Welfare and Sport (VWS), and by a grant from the European Interreg North Sea Region program, as part of the NorthTick project.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.Stanek G., Wormser G.P., Gray J., Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 2.Sprong H., Azagi T., Hoornstra D., Nijhof A.M., Knorr S., Baarsma M.E., Hovius J.W. Control of Lyme borreliosis and other Ixodes ricinus-borne diseases. Parasites Vectors. 2018;11:145. doi: 10.1186/s13071-018-2744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisen R.J., Eisen L. The blacklegged tick, Ixodes scapularis: An increasing public health concern. Trends Parasitol. 2018;34:295–309. doi: 10.1016/j.pt.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindgren E., Andersson Y., Suk J.E., Sudre B., Semenza J.C. Public health. Monitoring EU emerging infectious disease risk due to climate change. Science. 2012;336:418–419. doi: 10.1126/science.1215735. [DOI] [PubMed] [Google Scholar]
- 5.Medlock J.M., Hansford K.M., Bormane A., Derdakova M., Estrada-Pena A., George J.C., Golovljova I., Jaenson T.G., Jensen J.K., Jensen P.M., et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors. 2013;6:1. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matei I.A., Estrada-Pena A., Cutler S.J., Vayssier-Taussat M., Varela-Castro L., Potkonjak A., Zeller H., Mihalca A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors. 2019;12:599. doi: 10.1186/s13071-019-3852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azagi T., Hoornstra D., Kremer K., Hovius J.W.R., Sprong H. Evaluation of disease causality of rare Ixodes ricinus-borne infections in Europe. Pathogens. 2020;9:150. doi: 10.3390/pathogens9020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofhuis A., van de Kassteele J., Sprong H., van den Wijngaard C.C., Harms M.G., Fonville M., van Leeuwen A.D., Simoes M., van Pelt W. Predicting the risk of Lyme borreliosis after a tick bite, using a structural equation model. PLoS ONE. 2017;12:e0181807. doi: 10.1371/journal.pone.0181807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannelli A., Boggiatto G., Grego E., Cinco M., Murgia R., Stefanelli S., De Meneghi D., Rosati S. Acarological risk of exposure to agents of tick-borne zoonoses in the first recognized Italian focus of Lyme borreliosis. Epidemiol. Infect. 2003;131:1139–1147. doi: 10.1017/S0950268803001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coipan E.C., Jahfari S., Fonville M., Maassen C.B., van der Giessen J., Takken W., Takumi K., Sprong H. Spatiotemporal dynamics of emerging pathogens in questing Ixodes ricinus. Front. Cell. Infect. Microbiol. 2013;3:36. doi: 10.3389/fcimb.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randolph S.E. Tick ecology: Processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology. 2004;129(Suppl. 1):S37–S65. doi: 10.1017/S0031182004004925. [DOI] [PubMed] [Google Scholar]
- 12.Jaenson T.G., Talleklint L., Lundqvist L., Olsen B., Chirico J., Mejlon H. Geographical distribution, host associations, and vector roles of ticks (Acari: Ixodidae, Argasidae) in Sweden. J. Med. Entomol. 1994;31:240–256. doi: 10.1093/jmedent/31.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takumi K., Sprong H., Hofmeester T.R. Impact of vertebrate communities on Ixodes ricinus-borne disease risk in forest areas. Parasites Vectors. 2019;12:434. doi: 10.1186/s13071-019-3700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmeester T.R., van der Lei P.B., van Leeuwen A.D., Sprong H., van Wieren S.E. New foci of Haemaphysalis punctata and Dermacentor reticulatus in the Netherlands. Ticks Tick Borne Dis. 2016;7:367–370. doi: 10.1016/j.ttbdis.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Hofmeester T.R., Sprong H., Jansen P.A., Prins H.H.T., van Wieren S.E. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in Dutch forests. Parasites Vectors. 2017;10:433. doi: 10.1186/s13071-017-2370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rand P.W., Lubelczyk C., Lavigne G.R., Elias S., Holman M.S., Lacombe E.H., Smith R.P., Jr. Deer density and the abundance of Ixodes scapularis (Acari: Ixodidae) J. Med. Entomol. 2003;40:179–184. doi: 10.1603/0022-2585-40.2.179. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert L., Maffey G.L., Ramsay S.L., Hester A.J. The effect of deer management on the abundance of Ixodes ricinus in Scotland. Ecol. Appl. 2012;22:658–667. doi: 10.1890/11-0458.1. [DOI] [PubMed] [Google Scholar]
- 18.Ostfeld R.S., Schauber E.M., Canham C.D., Keesing F., Jones C.G., Wolff J.O. Effects of acorn production and mouse abundance on abundance and Borrelia burgdorferi infection prevalence of nymphal Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:55–63. doi: 10.1089/153036601750137688. [DOI] [PubMed] [Google Scholar]
- 19.Krawczyk A.I., van Duijvendijk G.L.A., Swart A., Heylen D., Jaarsma R.I., Jacobs F.H.H., Fonville M., Sprong H., Takken W. Effect of rodent density on tick and tick-borne pathogen populations: Consequences for infectious disease risk. Parasites Vectors. 2020;13:34. doi: 10.1186/s13071-020-3902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazquez M., Muehlenbein C., Cartter M., Hayes E.B., Ertel S., Shapiro E.D. Effectiveness of personal protective measures to prevent Lyme disease. Emerg. Infect. Dis. 2008;14:210–216. doi: 10.3201/eid1402.070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisen L., Dolan M.C. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol. 2016;53:1063–1092. doi: 10.1093/jme/tjw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster E., Fleshman A.C., Ford S.L., Levin M.L., Delorey M.J., Eisen R.J., Eisen L. Preliminary evaluation of human personal protective measures against the nymphal stage of the Asian longhorned tick (Acari: Ixodidae) J. Med. Entomol. 2020 doi: 10.1093/jme/tjaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu R., Hyland K.E., Oliver J.H. A review on the use of Ixodiphagus wasps (Hymenoptera: Encyrtidae) as natural enemies for the control of ticks (Acari:Ixodidae) Syst. Appl. Acarol. 1988;3:19–28. doi: 10.11158/saa.3.1.3. [DOI] [Google Scholar]
- 24.Smith C.N., Cole M.M. Studies of parasites of the American dog tick. J. Econ. Entomol. 1943;36:569–572. doi: 10.1093/jee/36.4.569. [DOI] [Google Scholar]
- 25.Mwangi E.N., Hassan S.M., Kaaya G.P., Essuman S. The impact of Ixodiphagus hookeri, a tick parasitoid, on Amblyomma variegatum (Acari: Ixodidae) in a field trial in Kenya. Exp. Appl. Acarol. 1997;21:117–126. doi: 10.1023/B:APPA.0000031790.30821.57. [DOI] [PubMed] [Google Scholar]
- 26.Hu R., Hyland K.E. Effects of the feeding process of Ixodes scapularis (Acari: Ixodidae) on embryonic development of its parasitoid, Ixodiphagus hookeri (Hymenoptera: Encyrtidae) J. Med. Entomol. 1998;35:1050–1053. doi: 10.1093/jmedent/35.6.1050. [DOI] [PubMed] [Google Scholar]
- 27.Collatz J., Selzer P., Fuhrmann A., Oehme R.M., Mackenstedt U., Kahl O., Steidle J.L.M. A hidden beneficial: Biology of the tick-wasp Ixodiphagus hookeri in Germany. J. Appl. Entomol. 2011;135:351–358. doi: 10.1111/j.1439-0418.2010.01560.x. [DOI] [Google Scholar]
- 28.Larrousse F., King A.G., Wolbach S.B. The over-wintering in Massachusetts of Ixodiphagous caucurteri. Science. 1928;67:351–353. doi: 10.1126/science.67.1735.351. [DOI] [PubMed] [Google Scholar]
- 29.Plantard O., Bouju-Albert A., Malard M.A., Hermouet A., Capron G., Verheyden H. Detection of Wolbachia in the tick Ixodes ricinus is due to the presence of the hymenoptera endoparasitoid Ixodiphagus hookeri. PLoS ONE. 2012;7:e30692. doi: 10.1371/journal.pone.0030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood H.P. Notes on the life history of the tick parasite Hunterellus hookeri Howard. J. Econ. Entomol. 1911;4:425–431. doi: 10.1093/jee/4.5.425. [DOI] [Google Scholar]
- 31.Tijsse-Klasen E., Braks M., Scholte E.J., Sprong H. Parasites of vectors--Ixodiphagus hookeri and its Wolbachia symbionts in ticks in The Netherlands. Parasites Vectors. 2011;4:228. doi: 10.1186/1756-3305-4-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collatz J., Fuhrmann A., Selzer P., Oehme R.M., Hartelt K., Kimmig P., Meiners T., Mackenstedt U., Steidle J.L.M. Being a parasitoid of parasites: Host finding in the tick wasp Ixodiphagus hookeri by odours from mammals. Entomol. Exp. Appl. 2010;134:131–137. doi: 10.1111/j.1570-7458.2009.00943.x. [DOI] [Google Scholar]
- 33.Ramos R.A., Campbell B.E., Whittle A., Lia R.P., Montarsi F., Parisi A., Dantas-Torres F., Wall R., Otranto D. Occurrence of Ixodiphagus hookeri (Hymenoptera: Encyrtidae) in Ixodes ricinus (Acari: Ixodidae) in southern Italy. Ticks Tick Borne Dis. 2015;6:234–236. doi: 10.1016/j.ttbdis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Bohacsova M., Mediannikov O., Kazimirova M., Raoult D., Sekeyova Z. Arsenophonus nasoniae and Rickettsiae infection of Ixodes ricinus due to parasitic wasp Ixodiphagus hookeri. PLoS ONE. 2016;11:e0149950. doi: 10.1371/journal.pone.0149950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sormunen J.J., Sippola E., Kaunisto K.M., Vesterinen E.J., Saaksjarvi I.E. First evidence of Ixodiphagus hookeri (Hymenoptera: Encyrtidae) parasitization in Finnish castor bean ticks (Ixodes ricinus) Exp. Appl. Acarol. 2019;79:395–404. doi: 10.1007/s10493-019-00437-6. [DOI] [PubMed] [Google Scholar]
- 36.Hofmeester T.R., Jansen P.A., Wijnen H.J., Coipan E.C., Fonville M., Prins H.H.T., Sprong H., van Wieren S.E. Cascading effects of predator activity on tick-borne disease risk. Proc. Biol. Sci. 2017;284:20170453. doi: 10.1098/rspb.2017.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armitage P., Berry G., Matthews J.N.S. Statistical Methods in Medical Research. 4th ed. Blackwell Publishers; Malden, MA, USA: 2001. p. 817. [Google Scholar]
- 38.Demas F.A., Hassanali A., Mwangi E.N., Kunjeku E.C., Mabveni A.R. Cattle and Amblyomma variegatum odors used in host habitat and host finding by the tick parasitoid, Ixodiphagus hookeri. J. Chem. Ecol. 2000;26:1079–1093. doi: 10.1023/A:1005497201074. [DOI] [Google Scholar]
- 39.Hu R., Hyland K.E. Prevalence and seasonal activity of the wasp parasitoid, Ixodiphagus hookeri (Hymenoptera: Encyrtidae) in its tick host, Ixodes scapularis (Acari: Ixodidae) Syst. Appl. Acarol. 1997;2:95–100. doi: 10.11158/saa.2.1.12. [DOI] [Google Scholar]
- 40.Stafford K.C., 3rd, Denicola A.J., Kilpatrick H.J. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) and the tick parasitoid Ixodiphagus hookeri (Hymenoptera: Encyrtidae) with reduction of white-tailed deer. J. Med. Entomol. 2003;40:642–652. doi: 10.1603/0022-2585-40.5.642. [DOI] [PubMed] [Google Scholar]
- 41.Hu R., Hyland K.E., Mather T.N. Occurrence and distribution in Rhode Island of Hunterellus hookeri (Hymenoptera: Encyrtidae), a wasp parasitoid of Ixodes dammini. J. Med. Entomol. 1993;30:277–280. doi: 10.1093/jmedent/30.1.277. [DOI] [PubMed] [Google Scholar]
- 42.Tagliapietra V., Rosa R., Arnoldi D., Cagnacci F., Capelli G., Montarsi F., Hauffe H.C., Rizzoli A. Saturation deficit and deer density affect questing activity and local abundance of Ixodes ricinus (Acari, Ixodidae) in Italy. Vet. Parasitol. 2011;183:114–124. doi: 10.1016/j.vetpar.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 43.Takasu K., Takano S., Sasaki M., Yagi S., Nakamura S. Host recognition by the tick parasitoid Ixodiphagus hookeri (Hymenoptera: Encyrtidae) Environ. Entomol. 2003;32:614–617. doi: 10.1603/0046-225X-32.3.614. [DOI] [Google Scholar]
- 44.Hofmeester T.R., Coipan C., van Wieren S., Prins H.H.T., Takken W., Sprong H. Few vertebrate species dominate the Borrelia burgdorferi s.l. life cycle. Environ. Res. Lett. 2016;11:043001. doi: 10.1088/1748-9326/11/4/043001. [DOI] [Google Scholar]
- 45.Hartemink N.A., Randolph S.E., Davis S.A., Heesterbeek J.A. The basic reproduction number for complex disease systems: Defining R(0) for tick-borne infections. Am. Nat. 2008;171:743–754. doi: 10.1086/587530. [DOI] [PubMed] [Google Scholar]
- 46.van Duijvendijk G., Coipan C., Wagemakers A., Fonville M., Ersoz J., Oei A., Foldvari G., Hovius J., Takken W., Sprong H. Larvae of Ixodes ricinus transmit Borrelia afzelii and B. miyamotoi to vertebrate hosts. Parasites Vectors. 2016;9:97. doi: 10.1186/s13071-016-1389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jahfari S., Coipan E.C., Fonville M., van Leeuwen A.D., Hengeveld P., Heylen D., Heyman P., van Maanen C., Butler C.M., Foldvari G., et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasites Vectors. 2014;7:365. doi: 10.1186/1756-3305-7-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stuen S., Granquist E.G., Silaghi C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013;3:31. doi: 10.3389/fcimb.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamsikova Z., Silaghi C., Takumi K., Rudolf I., Gunar K., Sprong H., Kazimirova M. Presence of roe deer affects the occurrence of Anaplasma phagocytophilum ecotypes in questing Ixodes ricinus in different habitat types of Central Europe. Int. J. Environ. Res. Public Health. 2019;16:4725. doi: 10.3390/ijerph16234725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mather T.N., Piesman J., Spielman A. Absence of spirochaetes (Borrelia burgdorferi) and piroplasms (Babesia microti) in deer ticks (Ixodes dammini) parasitized by chalcid wasps (Hunterellus hookeri) Med. Vet. Entomol. 1987;1:3–8. doi: 10.1111/j.1365-2915.1987.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 51.Hanincova K., Schafer S.M., Etti S., Sewell H.S., Taragelova V., Ziak D., Labuda M., Kurtenbach K. Association of Borrelia afzelii with rodents in Europe. Parasitology. 2003;126:11–20. doi: 10.1017/S0031182002002548. [DOI] [PubMed] [Google Scholar]
- 52.Coipan C.E., van Duijvendijk G.L.A., Hofmeester T.R., Takumi K., Sprong H. The genetic diversity of Borrelia afzelii is not maintained by the diversity of the rodent hosts. Parasites Vectors. 2018;11:454. doi: 10.1186/s13071-018-3006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliver K.M., Russell J.A., Moran N.A., Hunter M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA. 2003;100:1803–1807. doi: 10.1073/pnas.0335320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie J., Vilchez I., Mateos M. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS ONE. 2010;5:e12149. doi: 10.1371/journal.pone.0012149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhide M.R., Travnicek M., Levkutova M., Curlik J., Revajova V., Levkut M. Sensitivity of Borrelia genospecies to serum complement from different animals and human: A host-pathogen relationship. FEMS Immunol. Med. Microbiol. 2005;43:165–172. doi: 10.1016/j.femsim.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Chen G., Severo M.S., Sakhon O.S., Choy A., Herron M.J., Felsheim R.F., Wiryawan H., Liao J., Johns J.L., Munderloh U.G., et al. Anaplasma phagocytophilum dihydrolipoamide dehydrogenase 1 affects host-derived immunopathology during microbial colonization. Infect. Immun. 2012;80:3194–3205. doi: 10.1128/IAI.00532-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hillyard P.D. Ticks of North-West Europe: Keys and Notes for Identification of the Species. Field Studies Council; Shrewsbury, UK: 1996. [Google Scholar]
- 58.Wielinga P.R., Gaasenbeek C., Fonville M., de Boer A., de Vries A., Dimmers W., Akkerhuis Op Jagers G., Schouls L.M., Borgsteede F., van der Giessen J.W. Longitudinal analysis of tick densities and Borrelia, Anaplasma, and Ehrlichia infections of Ixodes ricinus ticks in different habitat areas in The Netherlands. Appl. Environ. Microbiol. 2006;72:7594–7601. doi: 10.1128/AEM.01851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heylen D., Tijsse E., Fonville M., Matthysen E., Sprong H. Transmission dynamics of Borrelia burgdorferi s.l. in a bird tick community. Environ. Microbiol. 2013;15:663–673. doi: 10.1111/1462-2920.12059. [DOI] [PubMed] [Google Scholar]
- 60.Jahfari S., Fonville M., Hengeveld P., Reusken C., Scholte E.J., Takken W., Heyman P., Medlock J.M., Heylen D., Kleve J., et al. Prevalence of Neoehrlichia mikurensis in ticks and rodents from North-west Europe. Parasites Vectors. 2012;5:74. doi: 10.1186/1756-3305-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2019. R Version 3.6.1 “Action of the Toes”. [Google Scholar]
- 62.R Core Team . R Studio: Integrated Development for R. R Studio, Inc.; Boston, MA, USA: 2015. 0.99.903. [Google Scholar]
- 63.Oliver J.D., Lynn G.E., Burkhardt N.Y., Price L.D., Nelson C.M., Kurtti T.J., Munderloh U.G. Infection of immature Ixodes scapularis (Acari: Ixodidae) by membrane feeding. J. Med. Entomol. 2016;53:409–415. doi: 10.1093/jme/tjv241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krull C., Bohme B., Clausen P.H., Nijhof A.M. Optimization of an artificial tick feeding assay for Dermacentor reticulatus. Parasites Vectors. 2017;10:60. doi: 10.1186/s13071-017-2000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.