Abstract
Tanzania faces one of the highest cervical cancer burdens in the world. Recent work has suggested that the bacterial family Mycoplasmataceae is associated with higher levels of human papillomavirus (HPV), human immunodeficiency virus (HIV), and pre-cancerous cervical lesions. Mycoplasmataceae infection in Tanzania is not well understood, especially when considering the differences between sexually transmitted species of Mycoplasmataceae. To establish the prevalence of common Mycoplasmataceae cervical infections and evaluate their relationship with risk factors for cervical cancer, 1160 Tanzanian women responded to an epidemiological questionnaire and were tested for HIV, HPV, cervical lesions, Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma spp., and Lactobacillus iners. A subset of 134 women were used for 16s metagenomic sequencing of cervical DNA to establish the relative abundance of Mycoplasmataceae and Lactobacillus present. PCR detection of bacteria at the cervix found Ureaplasma spp. in 51.4% of women, M. hominis in 34%, M. genitalium in 2.3%, and L. iners in 75.6%. M. hominis and M. genitalium infection were significantly more prevalent among women with HPV and HIV. M. hominis prevalence was similar despite severity of cervical lesions; however, abundance of M. hominis increased significantly in women with cervical lesions. These results emphasize the importance of understanding the relationship between M. hominis and HPV-related cervical pathogenesis.
Keywords: cervical cancer, HPV, HIV, Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma, Lactobacillus iners, Sub-Saharan Africa, Tanzania
1. Introduction
Cervical cancer mortality is higher in Eastern Africa than in any other region of the world [1]. In Tanzania, cervical cancer is the most prevalent cancer in females [2]. Tanzania faces many issues which contribute to the burden of cervical cancer, including high human papillomavirus (HPV) prevalence, high human immunodeficiency virus (HIV) prevalence, low condom use, irregular preventative screening, and lack of full implementation of the pap smear. In Europe and the U.S., preventative screening for cervical cancer is usually done by HPV testing or checking for lesions in the cervical epithelium via a pap smear. In Tanzania however, cervical screening is mainly visual inspection with acetic acid (VIA), which is markedly less sensitive for early detection of cervical lesions than the pap smear and does not grade lesions by severity. Cervical lesions detected during screening are usually associated with HPV infection; however, recent studies have proposed that the cervical microbiome may be an important co-factor for the development of pre-cancerous and cancerous lesions [3]. Currently, it is not understood how, or which cervical microbiota contribute to cervical lesions, although in a previous study, we found that the bacterial family Mycoplasmataceae was the most significant differential cervical bacteria between women with normal cervical cytology and those with pre-cancerous lesions in Tanzania [4]. Mycoplasmataceae are the smallest known bacteria, in both physical and genomic size. During infection of the cervicovaginal epithelium, Mycoplasmataceae establish a persistent, intracellular infection which can lead to inflammatory-cytokine-mediated tissue injury. Although it is currently unknown if there is a mechanistic relationship between HPV and Mycoplasmataceae, the nature of Mycoplasma infection allows for direct interaction with HPV during co-infection of a single cell, and indirect interaction through cytokine responses.
Mycoplasmataceae is comprised of the genera Mycoplasma and Ureaplasma, which include several sexually transmitted species with global prevalence. Most notably, M. hominis, M. genitalium, and U. urealyticum are relatively common sexually transmitted infections (STIs) associated with cervical inflammation [3,5,6]. Among Mycoplasma, only M. genitalium is sometimes included in regular STI screening, although M. hominis is believed to have a similar pathogenesis. As a result, M. hominis has received significantly less study, and its relationship with HPV, HIV, and cervical lesions remains unclear. The prevalence of M. genitalium, M. hominis, and U. urealyticum in Tanzania has not previously been established in a large and diverse cohort, nor has it been considered alongside established risk factors for cervical dysplasia.
It has been suggested that high levels of cervicovaginal dysbiosis and transmission of Mycoplasma and other STIs in Eastern Africa is, in part, due to the commensal cervicovaginal bacteria in the region. Specifically, L. iners is the most prevalent cervicovaginal Lactobacillus in Eastern Africa, especially in HIV+ women, but has been shown to be less protective against cervicovaginal infection than other Lactobacillus [7]. Whether a cervical microbiome dominated by L. iners is conducive to infection and proliferation of Mycoplasmataceae, and the relationship between the bacteria and HPV pathogenesis, remains unclear. This study aims to establish the prevalence of common Mycoplasmataceae species in Tanzania and evaluate their relationship with L. iners and risk factors for cervical cancer, including HPV, HIV, and lifestyle factors.
2. Results
2.1. Cohort Demographics
DNA was successfully isolated from the cervical cytobrush samples of 1060 women. Complete data of cervical cytobrush DNA, pap-smear, VIA, HIV status, and epidemiological questionnaire response was available for 1002 women. Women with incomplete data were included in analyses where the missing data were not relevant. The cohort averaged 38.3 years of age, ranging from 18 to 73. A large majority (92.3%) of the women screened reported at least one previous pregnancy, and 84.1% were sexually active within the 3 months preceding sampling. A total of 67.4% of the cohort reported the use of at least one type of birth control, although it is unclear if they had recently used birth control at time of sampling. A total of 17.6% of the cohort had tested positive for HIV and these participants were on antiretroviral therapy at the time of sampling. Using a multiplex HPV genotyping PCR, we found that 46.1% of the cohort tested positive for at least one HPV genotype, and 38.2% of HPV positive women were coinfected with at least two genotypes.
There was a significant difference between the identification of cervical lesions between VIA and pap smear. Only 17% of women with pap smears graded HSIL had lesions identified by VIA. Additionally, although 88.9% of the cohort tested negative for cervical lesions by VIA, only 20.1% of the cohort was graded NILM by pap smear. The majority of women had pap smears exhibiting low-grade cervical dysplasia: ASCUS (24.8%) and LSIL (30.8%). More severe cervical dysplasia was apparent in 24.4% of women (14.9% ASC-H, 9.5% HSIL).
2.2. Mycoplasma Screen
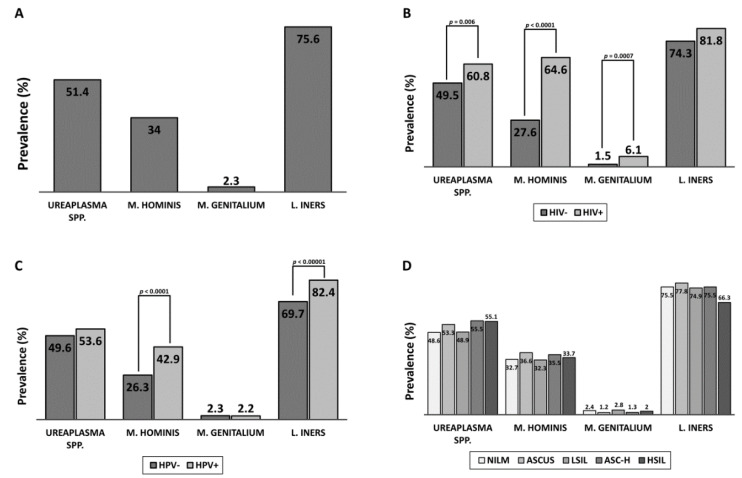
Table 1 shows the breakdown of all data collected in this study and the variation of cervical cancer risk factors and Mycoplasmataceae prevalence within each group. PCR detection of Mycoplasmataceae at the cervix found a high prevalence of the bacterial family among Tanzanian women—66% of whom tested positive for at least one Mycoplasmataceae. Ureaplasma spp. was the most prevalent Mycoplasmataceae, detectable in 51.4% of the cohort, followed by Mycoplasma hominis in 34%, and Mycoplasma genitalium in only 2.3% of women. Lactobacillus iners was more prevalent than Mycoplasmataceae—detectable in 75.6% of women. Detection of any Mycoplasmataceae significantly increased the likelihood of detection of other Mycoplasmataceae species in that individual (Figure S1B–E). Women with L. iners also had higher prevalence of Ureaplasma spp. and M. hominis than woman without L. iners. Both M. hominis and M. genitalium were more common in women who reported previously having been diagnosed with an STI, though it is unclear if the STI was Mycoplasma related (Figure S1C,D). Mycoplasma was prevalent amongst all age groups.
Table 1.
Prevalence of Mycoplasma, HPV, HIV, and epidemiological factors.
| Group | n | Ureaplasma spp. | M. hominis | M. genitalium | L. iners | HPV+ | HIV+ | NILM | LSIL+ | HSIL+ | Age |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 1060 | 51.4 | 34 | 2.3 | 75.6 | 46.1 | 17.6 | 20.1 | 55.5 | 24.4 | 38.3 |
| HPV | |||||||||||
| HPV+ | 489 | 53.6 | 42.9 * | 2.2 | 82.4 * | -- | 24.8 * | 17.3 | 52.6 | 30.1 * | 36.9 |
| HPV− | 571 | 49.6 | 26.3 * | 2.3 | 69.7 * | -- | 11.4 * | 22.4 | 58.1 | 19.5 * | 39.5 |
| 1 HPV | 302 | 55.3 | 38.1 | 1 * | 79.8 | -- | 19.4 | 20.7 | 51.7 | 27.6 | 37.2 |
| 2+ HPV | 187 | 50.8 | 50.8 * | 4.3 | 86.6 * | -- | 33.5 * | 11.9 * | 54 | 34.1 * | 36.5 |
| HIV | |||||||||||
| HIV+ | 181 | 60.8 * | 64.6 * | 6.1 * | 81.8 * | 65.2 * | -- | 16.9 | 60.1 | 23 | 39.2 |
| HIV− | 847 | 49.5 | 27.6 * | 1.5 | 74.3 | 42.3 * | -- | 20.8 | 54.6 | 24.6 | 38.1 |
| Cytology | |||||||||||
| NILM | 208 | 48.6 | 32.7 | 2.4 | 75.5 | 39.9 | 14.9 | -- | -- | -- | 38.4 |
| ASCUS | 257 | 53.3 | 36.6 | 1.2 | 77.8 | 34.6 * | 19.5 | -- | -- | -- | 37.6 |
| LSIL | 319 | 48.9 | 32.3 | 2.8 | 74.9 | 35.7 * | 18.8 | -- | -- | -- | 38.8 |
| ASC-H | 155 | 55.5 | 35.5 | 1.3 | 75.5 | 41.3 | 14.8 | -- | -- | -- | 37.6 |
| HSIL | 98 | 55.1 | 33.7 | 2 | 66.3 | 50 | 19.8 | -- | -- | -- | 39.5 |
| VIA | |||||||||||
| Normal | 873 | 51.1 | 33.7 | 2.3 | 75.6 | 44.3 | 14.7 * | 21 | 55.7 | 23.3 | 38 |
| Lesions | 109 | 49.5 | 35.8 | 2.8 | 79.8 | 63.3 * | 33 * | 15.7 | 55.6 | 28.7 | 37.1 |
| Age | |||||||||||
| 18–29 | 209 | 54.5 | 33.5 | 2.4 | 83.7 * | 52.6 | 10.6 * | 21.4 | 53.4 | 25.2 | 25.8 |
| 30–39 | 374 | 50.3 | 37.7 | 2.9 | 76.7 | 50 | 20.9 | 18.1 | 55.5 | 26.4 | 34.5 |
| 40–49 | 321 | 51.1 | 33.6 | 2.2 | 74.8 | 41.7 | 19.2 | 21.6 | 58.1 | 20.3 | 44.1 |
| 50+ | 133 | 51.9 | 27.1 | 0.8 | 59.4 * | 36.1 * | 16 | 21.4 | 53.4 | 25.2 | 54.8 |
| Last Sex | |||||||||||
| <3 months | 876 | 52.9 | 35.3 | 2.5 | 77.4 | 47.1 | 15.7 | 19.6 | 56.7 | 23.7 | 37.4 |
| 4–12 months | 81 | 48.1 | 32.1 | 1.2 | 65.4 | 38.3 | 29.1 * | 17.5 | 50 | 32.5 | 39.6 |
| >2 months | 85 | 42.4 | 23.5 * | 1.2 | 64.7 * | 44.7 | 26.2 | 28.2 | 50.6 | 21.2 | 46.6 |
| Sex Partners | |||||||||||
| 1–2 | 455 | 47.9 | 25.5 * | 1.5 | 71.4 * | 38.5 * | 8.9 * | 20 | 59.1 | 20.9 | 38.6 |
| 3–5 | 465 | 54.8 | 40.2 * | 2.8 | 77.4 | 52.9 * | 21.4 * | 19.2 | 54.3 | 26.5 | 37.7 |
| >5 | 102 | 52 | 44.1 * | 2.9 | 82.4 | 50 | 36.6 * | 26.2 | 47.5 | 26.3 | 39.3 |
| Pregnancies | |||||||||||
| 0 | 80 | 47.5 | 32.5 | 2.5 | 76.3 | 52.5 | 8.9 * | 20.3 | 58.2 | 21.5 | 32.5 |
| 1–2 | 319 | 53.9 | 36.4 | 2.8 | 82.8 * | 50.5 | 19.2 | 21 | 57.1 | 21.9 | 32.9 |
| 3–5 | 492 | 51 | 33.3 | 2 | 72.8 | 45.3 | 19.3 | 21.1 | 55.7 | 23.2 | 39.8 |
| >5 | 151 | 51 | 32.5 | 2 | 68.2 | 37.1 * | 13.2 | 15.4 | 51 | 33.6 * | 47.7 |
| Birth Control | |||||||||||
| No | 340 | 49.7 | 33.8 | 3.2 | 73.2 | 47.1 | 21.4 | 20.7 | 56.8 | 22.5 | 38.3 |
| Yes | 702 | 52.6 | 34.2 | 1.9 | 76.5 | 45.9 | 15.8 | 19.9 | 55.1 | 25 | 38.3 |
| Birth Control Type | |||||||||||
| Pills | 370 | 51.6 | 34.6 | 1.4 | 75.1 | 42.7 | 17 | 19 | 56.6 | 24.4 | 40.7 |
| Injection | 416 | 54.6 | 35.8 | 2.6 | 78.1 | 48.8 | 15.9 | 20 | 50 * | 30 * | 37.4 |
| Condom | 44 | 43.2 | 40.9 | 4.5 | 81.8 | 61.4 * | 47.6 * | 27.3 | 50 | 22.7 | 35.9 |
| Implant | 124 | 57.3 | 37.9 | 1.6 | 78.2 | 42.7 | 12.9 | 19.1 | 64.2 * | 16.7 * | 33.9 |
| Loop | 70 | 47.1 | 24.3 | 2.9 | 67.1 | 42.9 | 2.9 * | 20.6 | 47.1 | 32.3 | 43.8 |
| Natural | 12 | 41.7 | 33.3 | 0 | 91.7 * | 41.7 | 16.7 | 25 | 58.3 | 16.6 | 40.1 |
| STI Self-Report | |||||||||||
| No recent | 939 | 51.8 | 33.2 | 1.9 | 75.5 | 45.4 | 15.1 * | 19.7 | 57 | 23.3 | 38.2 |
| Yes recent | 73 | 53.4 | 38.4 | 4.1 | 75.3 | 50.7 | 37.5 * | 27.4 | 42.5 * | 30.1 | 39 |
| PCR Detection | |||||||||||
| Ureaplasma spp. | 545 | -- | 38 | 2.9 | 80.6 * | 48.1 | 20.8 | 18.9 | 54.9 | 26.2 | 38.1 |
| M. hominis | 360 | 57.5 * | -- | 3.9 | 83.6 * | 58.3 * | 33.3 * | 19.3 | 55.8 | 24.9 | 37.7 |
| M. genitalium | 24 | 66.7 | 58.3 * | -- | 83.3 | 45.8 | 45.8 * | 23.8 | 57.1 | 19.1 | 35.5 |
| L. iners | 801 | 54.8 | 37.6 * | 2.5 | -- | 50.3 * | 19 | 20.2 | 56.4 | 23.4 | 37.4 |
Values are listed as percentage of women positive for the condition labeled in each column. The cohort is broken down into sub-groups in each row, depending on results from testing or survey. A one-proportion Z-test was used to identify prevalence in subgroups that differ significantly from the cohort average. Values were considered significant when p < 0.05 and are bolded and labeled with a ‘*’. The column ‘LSIL+’ includes LSIL and ASCUS pap smear results for ease of interpretation. Similarly, the column ‘HSIL+’ includes HSIL and ASC-H pap smear results. Abbreviations: HPV (Human Papillomavirus), HIV (Human Immunodeficiency Virus), NILM (Normal for Intraepithelial Lesion or Malignancy), LSIL (Low-Grade Squamous Intraepithelial Lesion), ASCUS (Atypical Squamous Cells of Unknown Significance), ASC-H (Atypical Squamous Cells-Cannot Rule Out High Grade), HSIL (High-Grade Squamous Intraepithelial Lesion).
2.3. Effects of HIV Infection
Being HIV+ increased the odds of detection of all Mycoplasmataceae and L. iners. Mycoplasma hominis and genitalium infections were especially prevalent among HIV+ women when compared to HIV− (odds ratio (OR) 4.8 and 4.2, respectively), while Ureaplasma spp. and L. iners were only slightly more common (OR 1.6 for both). This data supports previous research suggesting the HIV+ population acts as a reservoir for M. hominis infection [8]. Mycoplasma was still quite prevalent among HIV− women (49.5%, 27.6%, 1.5% prevalence for Ureaplasma spp., M. hominis, and M. genitalium, respectively).
2.4. Effects of HPV Infection
Women infected with at least one HPV genotype were significantly more likely to have cervical dysplasia, especially high-grade lesions (OR 1.3773 for non-NILM, OR 2.7108 for HSIL). HPV+ women were also more likely to be infected with M. hominis (OR 2.1, p < 0.0001), while M. genitalium and Ureaplasma did not have a significant increase in prevalence associated with HPV (Figure 1). Commensal bacteria L. iners, was more likely to be present in HPV+ women (OR 2.0, p < 0.00001). Co-infection with two or more different HPV genotypes was associated with higher prevalence of M. hominis and M. genitalium than women infected by one HPV genotype. Multiple HPV infections were much more common amongst HIV+ women; however, this increase in Mycoplasma prevalence was also apparent in HIV− women with multiple HPV when compared to HIV−, single HPV women.
Figure 1.
Relationship between Mycoplasmataceae abundance and cervical cancer risk factors. (A) Overall prevalence of the screened Mycoplasmataceae and Lactobacillus species in the cohort; (B) Comparison of prevalence between HIV+ and HIV− women; (C) Comparison of prevalence between HPV+ and HPV− women; (D) Comparison of prevalence between women based on cervical cytology.
2.5. Effects of Cervical Cytology
Mycoplasmataceae were not significantly more or less prevalent among women with cervical dysplasia (Figure 1). Multivariate analysis of cervical cytology found that prevalence of HPV, number of pregnancies, use of injection-based birth control, and self-reporting of a previous STI varied significantly between cytology groups (Figure S1A). Only HPV prevalence had an obvious positive relationship with severity of cervical lesions, while having more than five pregnancies or using injection-based birth control were associated with increased odds of high-grade cervical lesions.
2.6. Effects of Other Factors
Sexual history was an important factor for detection of Mycoplasmataceae and L. iners. Women with three or more unique previous sex partners were significantly more likely to be infected with M. hominis, HPV, and HIV and were more likely to test HSIL. Prevalence of M. hominis and L. iners was significantly higher among women who had been sexually active during the 3 months prior to sampling (Figure S1C,E). Self-reported condom use was very low, especially for HIV− women (2.6%), contributing to increased transmission of Mycoplasma among sexually active women. Aging was associated with a significant decrease (p = 0.0004) in L. iners prevalence, decreasing from 83.7% in women 18–29 to 59.4% in women 50+. Age did not appear to be related with a shift in Mycoplasmataceae prevalence, although women aged 50+ did have somewhat lower prevalence of M. hominis and M. genitalium, possibly related to menopause or decreased sexual activity. L. iners prevalence also decreased in women with three or more previous pregnancies; however, this may have been influenced by a higher average age among high-gravidity women.
2.7. Relative Abundance
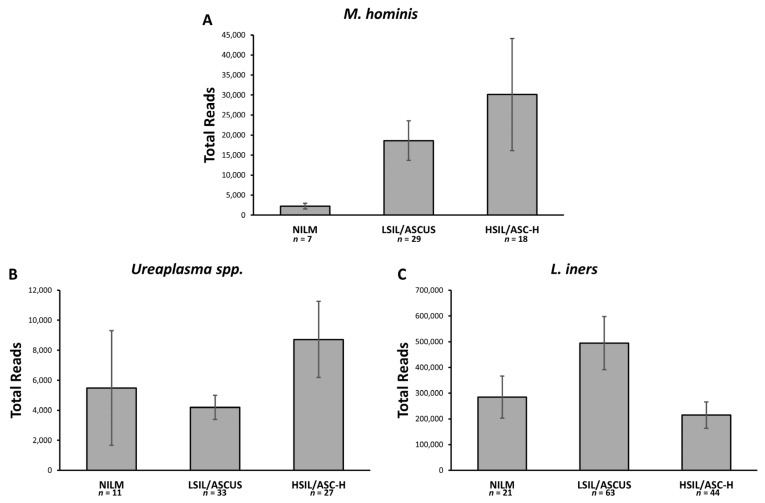
A subset of 104 cervical samples was analyzed via 16s metagenomic sequencing to establish the relative abundance of Mycoplasmataceae and L. iners present. Each sample was rarefied to an even depth of 1000 reads. After rarefication, women with more than 5 reads from Ureaplasma spp., M. hominis, M. genitalium, or L. iners were considered positive for that bacteria. The prevalence of each bacteria was similar to results from PCR screening, although no M. genitalium reads were present among the subset of samples tested. By using the number of reads generated, we were able to determine the relative abundance of each bacteria in each woman’s cervical microbiome. Using this, we estimated the relative abundance of the screened bacteria within cervical cytology groups by adjusting the prevalence of a bacteria by the average relative abundance of that bacteria in positive samples of each cytology grade. When looking at the relative abundance of Mycoplasmataceae in women with cervical dysplasia, it becomes apparent that a significantly larger portion of the cervical microbiota is M. hominis (Figure 2). M. hominis is the only Mycoplasmataceae which increases linearly with the development of more severe cervical lesions. Ureaplasma spp. were most abundant among HSIL women; however, LSIL had a lower abundance than NILM women. L. iners was least abundant among HSIL women, but significantly more abundant in LSIL than NILM women. Lactobacillus crispatus is considered to be the most protective cervicovaginal microbe. Although we did not PCR screen for L. crispatus, no women with L. crispatus reads by 16s had M. hominis, suggesting L. crispatus protects against Mycoplasma infection, while L. iners does not.
Figure 2.
Relative abundance of Mycoplasma based on cervical cytology. Abundance among infected is the mean of positive 16s samples (n) adjusted by prevalence determined by PCR screen. Error bars represent standard error of the mean. (A) Expected number of M. hominis 16s DNA reads for 100 Tanzanian women of varying cervical cytology; (B) Expected number of Ureaplasma spp. 16s DNA reads for 100 Tanzanian women of varying cervical cytology; (C) Expected number of L. iners 16s DNA reads for 100 Tanzanian women of varying cervical cytology.
3. Discussion
In this study, we found that cervical Mycoplasma infection is prevalent among Tanzanian women. Even though M. genitalium is more often screened for as a cervicovaginal infection, we found that M. hominis and Ureaplasma spp. were significantly more common in Tanzania. Similar results have been found in M. hominis and M. genitalium screens from other sub-Saharan African countries [9,10,11,12]. The primers we used to detect Ureaplasma spp. included both U. urealyticum and U. parvum. U. urealyticum is known to be a cervicovaginal pathogen; however, U. parvum is sometimes commensal in the uterus. Because we took our samples partially from the endocervix, it is likely U. parvum originating from the cervical opening may also have been detected. For this reason, we did not consider Ureaplasma spp. as a non-commensal infection and focus on the importance of M. hominis as a common, poorly understood cervical infection in Tanzania.
Women who reported having had an STI were more likely to have an M. hominis or M. genitalium infection; however, most women with such an infection did not report any history of STIs. This indicates that most M. hominis and M. genitalium infections are asymptomatic, and thus go untreated. Currently, it is unclear how long a Mycoplasma infection of the cervix can persist while untreated. We detected higher prevalence of M. hominis among sexually active women, even those with a single long-term partner, suggesting sex may be important for persistence of M. hominis infection. Despite similar prevalence, significantly higher abundance of M. hominis in the presence of cervical lesions, especially high-grade cervical lesions, suggests that proliferation of M. hominis and development of cervical lesions have some form of mechanistic relationship. It is possible proliferation of M. hominis drives the formation of cervical lesions, or that cervical lesions create a microenvironment that favors proliferation of M. hominis. Longitudinal sampling of M. hominis abundance and cervical cytology would help to clarify this relationship. M. hominis may also contribute to HPV-driven cervical lesion formation by increasing persistence of the pathogens during co-infection. We found that prevalence of M. hominis was significantly higher among HPV+ women, which could result from prolonged persistence increasing the likelihood of sampling an infection. This idea is supported by previous studies which have identified cervical pathogens, including Mycoplasma, as cofactors in the persistence of HPV infection [13,14,15,16]. The intracellular nature of Mycoplasma infection is particularly interesting when considering its relationship with HPV. Intracellular bacterial infections may directly interact with HPV replication in epithelial cells, while also contributing to the epithelium’s immune microenvironment by influencing cytokine expression. The data presented here highlights the need for further research into M. hominis prevalence and pathogenesis, especially related to HPV, HIV, and cervical cancer.
Our data support previous research suggesting L. iners is an especially common commensal cervical bacteria in Sub-Saharan African countries. Increased prevalence of commensal L. iners among HIV+, HPV+, and Mycoplasmataceae+ women suggests that L. iners does not protect the cervix from infection, as other Lactobacillus species are believed to do. This is further evidenced by our 16s data, where co-detection of M. hominis and L. iners was common, but M. hominis and L. crispatus were never detected together.
This study highlights the need to account for significant regional differences in cervicovaginal microbiota, especially Mycoplasma. The high prevalence of M. hominis and its association with risk factors for cervical cancer (HPV, HIV, and cervical lesions) demonstrates the importance of better understanding M. hominis pathogenesis. Our results suggest screening for Mycoplasma is especially important in Tanzania, particularly among women at high risk for cervical cancer. Establishing a screening and treatment protocol to address the prevalence of asymptomatic Mycoplasma infection could reduce transmission of HPV and HIV by reducing susceptibility to infection, and potentially prevent progression of cervical lesions. Long-term, longitudinal studies are needed to clarify whether Mycoplasma becomes abundant at the cervix preceding or following the development of lesions, which would help to clarify if Mycoplasma is driving formation of cervical lesions or benefitting from the microenvironment associated with lesions.
4. Materials and Methods
4.1. Participants and Ethical Precautions
This study reports findings derived from an ongoing cross-sectional cohort study analyzing demographics of HPV and cervical cancer in HIV-positive and -negative women from rural and urban Tanzania. Between March 2015 and February 2017, female patients undergoing cervical cancer screening were approached for enrollment in the study. Those who were pregnant, menstruating, under 18, reported being sick in the past 30 days, or had a preexisting, non-HIV, immunologic defect were excluded from the study. Disease histories and physical examinations were used to rule out any clinical symptoms or visible signs for these conditions. Samples were collected at three sites in Tanzania: Ocean Road Cancer Institute (ORCI) in Dar es Salaam and rural clinics in Chalinze and Bagamoyo. After collection of cervical samples and demographic data, samples from 1060 women were screened for Mycoplasma species and Lactobacillus iners. A subset of 132 women were also used for 16s metagenomic sequencing.
4.2. Demographic Data Collection
This study was approved for human subjects work by the University of Nebraska-Lincoln Institutional Review Board (IRB) under protocol ID: 14709. All study participants gave informed consent and were evaluated by study clinicians. A set of pretested, standardized questionnaires was used to gather demographic data. All personal identifiers were removed from samples to ensure patient confidentiality. With the permission of the patients, medical history was retrospectively retrieved from hospital medical records. More than 30 variables were identified and assessed in the questionnaire, including time since last sexual intercourse, number of sexual partners, number of pregnancies, use and type of birth control, and self-reported history of STI infections.
4.3. Specimen Collection, HIV and Pap Tests
Blood samples were collected via venipuncture into acid-citrate-dextrose tubes (Streck, Omaha, NE, USA) and processed using centrifugation at the on-site study laboratory within 6 h of being drawn. The separated plasma was tested at the ORCI, as part of standard of care, using Standard Diagnostics HIV-1/2 3.0 detection kit (Chembio, Medford, NY, USA). Cervical cytobrush samples and pap smears were collected from the cervical transformation zone of all patients. Pap smears were examined by at least three trained cytologists and classified according to the pap classification protocol: negative for intraepithelial lesion or malignancy (NILM); atypical squamous cells of undetermined significance (ASC-US); low-grade squamous intraepithelial lesions (LSIL); atypical squamous cells but cannot exclude high-grade lesions (ASC-H); high-grade squamous intraepithelial lesions (HSIL). Cervical cytobrush specimens were placed in lysis buffer (Qiagen, Redwood City, CA, USA) and then shipped to the Nebraska Center for Virology at the University of Nebraska-Lincoln (UNL) for processing.
4.4. DNA Isolation
Cervical cytobrush samples were vortexed and separated from the brush with lysis buffer. DNA was extracted from the lysis buffer using the DNeasy Tissue extraction kit (Qiagen, Redwood City, CA, USA) according to the manufacturer’s protocol. The DNA concentration was determined by UV spectrophotometer (Thermofisher, Waltham, MA, USA) at 260/280 nm.
4.5. HPV Genotyping
To determine HPV status, DNA samples were genotyped for HR-HPVs (types 16, 18, 30, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66) and LR-HPVs (types 6 and 11) using a low-cost multiplex PCR assay [17].
4.6. Mycoplasmataceae and L. iners Screen
A multiplex PCR targeting M. genitalium, M. hominis, and Ureaplasma spp. was adapted from Stellrecht et al. [18], with the addition of primers targeting L. iners established in [19]. Primers were mixed with sample DNA and Qiagen Multiplex PCR Master Mix according to the manufacturer’s protocol.
The PCRs were performed in a final volume of 25 μL. The cycling conditions were as follows: an initial denaturation of 95 °C for 15 min, followed by 35 cycles, with 1 cycle consisting of denaturation at 94 °C for 15 s, and annealing and extension at 60 °C for 1 min, then a final elongation of 72 °C for 5 min. After amplification, DNA samples were run in 0.5% agarose gels containing Ethidium Bromide at 95 volts for 1 h. Gels were then imaged using a Bio Rad ChemiDoc MP Imaging System (Biorad, Hercules, CA, USA) to visualize bands.
4.7. Statistical Analyses
Multivariate analysis of variance (MANOVA) using one variable selected as fixed versus the other remaining dependent variables collected (Ureaplasma spp., M. hominis, M. genitalium, L. iners, HPV, HIV, Age, time since last sexual activity, number of sex partners, number of pregnancies, self-reporting of STI infection, use of birth control, and type of birth control used) was used to identify significant differences between women with different cervical cytology, HIV, or HPV status. The birth control types considered were pills, injections, condoms, implants, loop, and natural. Odds ratios were calculated to identify groups with significantly increased odds of HPV, HIV, or Mycoplasmataceae. A p value of 0.05 was the maximum considered to be significant throughout the study.
4.8. 16S rRNA Library Preparation, and Sequencing of the V4 Region
DNA samples were used for tag sequencing of the V4 hypervariable region of the 16S rRNA gene. A 250-bp section of the V4 region was amplified using universal primers described in reference 40. The PCRs were performed in 25 μL. The cycling conditions were as follows: an initial denaturation of 98 °C for 3 min, followed by 25 cycles, with 1 cycle consisting of denaturation at 98 °C for 30 s, annealing at 55 °C for 30 s, and extension at 68 °C for 45 s, and then a final elongation of 68 °C for 4 min. Following amplification, PCR products were analyzed on a 2% agarose gel to confirm correct product size. Normalized amplicons (1 to 2 ng/μL) from 144 samples were pooled together using an epMotion M5073 liquid handler (Eppendorf AG, Hamburg, Germany). Pooled libraries were sequenced using the Illumina MiSeq platform using the dual-index sequencing strategy outlined by Kozich et al. [20].
4.9. 16S Data Processing and Bacterial Community Analysis.
The sequencing data obtained from the sequencer were subsequently analyzed using the Illumina MiSeq data analysis pipeline developed by the Fernando lab (described in detail at https://github.com/FernandoLab). Briefly, initial quality filtering was carried out to remove sequences that had ambiguous bases, incorrect lengths, and inaccurate assemblies. Subsequently, the quality-filtered reads were run through the UPARSE pipeline (http://www.drive5.com/uparse/) and subjected to chimera filtering and OTU clustering (at a similarity threshold of 97%), followed by the generation of an OTU table. Taxonomy was assigned to the OTUs using the assign_taxonomy.py command available in QIIME using the Greengenes database (May 2013). The OTU table was rarefied across samples to the lowest sample depth (1000 reads) using QIIME based on the Mersenne Twister pseudorandom number generator. All statistical analyses were performed with samples at an even depth.
4.10. Ethics Statement
All human subject protocols were approved by safety committees at the Ocean Road Cancer Institute (ORCI) and UNL in accordance with the Helsinki Declaration. Participation by patients was entirely voluntary, and written patient consent was required for inclusion in the study.
5. Conclusions
In this study, we analyzed the cervical microbiota of 1160 Tanzanian women. We found that the bacterial family of Mycoplasmataceae was detected at the cervix at 66% of women tested. Among the Mycoplasmataceae family, Ureaplasma spp. was the most common, at about 51.4% of the cohort. Mycoplasma hominis was found in 34% of the cohort, while Mycoplasma genitalium was found in only 2.3% of the cohort. We found that HIV+ women had increased odds for all Mycoplasmataceae and L. iners. Mycoplasma hominis and genitalium infections were especially prevalent among HIV+ women compared HIV−. Those women who were HPV+ were more likely to have M. hominis (OR 2.1, p < 0.0001). Co-infection with multiple HPV genotypes was associated with higher a prevalence for M. hominis and M. genitalium. Multiple HPV genotype infections were more common amongst HIV+ women (Figure 1).
The prevalence of Mycoplasmataceae was not associated with cervical dysplasia. However, we found that the abundance of Mycoplasmataceae bacteria, such as M. hominis, increased with more severe cervical lesions, LSIL and HSIL. Ureaplasma spp. abundance was also associated with HSIL women (Figure 2). These data suggest that the abundance of M. hominis and M. Ureaplasma are associated with cervical dysplasia. We suggest that M. hominis infection of the cervix could lead to chronic inflammation, which favors HPV-related neoplasias.
Acknowledgments
We thank the Angeletti, and Wood laboratory members and other members of the National Center for Virology (NCV) for critical discussions of this work.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/5/1093/s1, Figure S1: MANOVA Results. (A) MANOVA analysis of factors in relationship cervical cytology; (B) MANOVA analysis of factors in relationship to Ureaplasma spp. detection; (C) MANOVA analysis of factors in relationship to M. hominis detection; (D) MANOVA analysis of factors in relationship to M. genitalium detection; (E) MANOVA analysis of factors in relationship to L. iners detection.
Author Contributions
Conceptualization, P.C.A.; methodology, C.K. (Cameron Klein) and P.C.A.; investigation, C.K. (Cameron Klein) and P.C.A.; resources, P.C.A.; data curation, K.S., C.K. (Crispin Kahesa), P.C.A.; writing—original draft preparation, C.K. (Cameron Klein) and P.C.A. writing—review and editing, C.K. (Cameron Klein) and P.C.A.; supervision, C.K. (Crispin Kahesa), J.M., P.C.A.; project administration, P.C.A.; funding acquisition, P.C.A., J.T.W., C.W. All authors have read and agreed to the published version of the manuscript.
Funding
The work described in this paper was supported by a grant from the National Cancer Institute (U54CA190155).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Formana D., de Martel C., Lacey C.J., Soerjomatarama I., Lortet-Tieulent J., Bruni L., Vignat J., Ferlay J., Bray F., Plummer M., et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 2.Bruni L., Albero G., Serrano B., Mena M., Gómez D., Muñoz J., Bosch F.X., de Sanjosé S. Human Papillomavirus and Related Diseases in the World. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre); Barcelona, Spain: Jun 17, 2019. Summary Report. [Google Scholar]
- 3.Mitra A., MacIntyre D.A., Marchesi J.R., Lee Y.S., Bennett P.R., Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome. 2016;4:1–15. doi: 10.1186/s40168-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein C., Gonzalez D., Samwel K., Kahesa C., Mwaiselage J., Aluthge N., Fernando S., West J.T., Wood C., Angeletti P.C. Relationship between the cervical microbiome, HIV Status, and precancerous lesions. MBio. 2019;10:e02785-18. doi: 10.1128/mBio.02785-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Audirac-Chalifour A., Torres-Poveda K., Bahena-Román M., Téllez-Sosa J., Martínez-Barnetche J., Cortina-Ceballos B., López-Estrada G., Delgado-Romero K., Burguete-García A.I., Cantú D., et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: A pilot study. PLoS ONE. 2016;11:e0153274. doi: 10.1371/journal.pone.0153274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Histological Evidence of Chronic Mycoplasma genitalium–Induced Cervicitis in HIV-Infected Women: A Retrospective Cohort Study | The Journal of Infectious Diseases | Oxford Academic. [(accessed on 2 March 2020)]; doi: 10.1093/infdis/jiw025. Available online: https://academic.oup.com/jid/article/213/11/1828/2459303. [DOI] [PMC free article] [PubMed]
- 7.Jespers V., van de Wijgert J., Cools P., Verhelst R., Verstraelen H., Delany-Moretlwe S., Mwaura M., Ndayisaba G.F., Mandaliya K., Menten J., et al. The significance of Lactobacillus crispatus and L. vaginalis for vaginal health and the negative effect of recent sex: A cross-sectional descriptive study across groups of African women. BMC Infect. Dis. 2015;15:115. doi: 10.1186/s12879-015-0825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djigma F., Ouedraogo C., Sagna T., Ouermi D., Sanogo K., Bisseye C., Kabre A., Pietra V., Simpore J., Nikiema J.B., et al. HIV-infected women of Burkina Faso: A “reservoir” of mycoplasma infection. J. Infect. Dev. Ctries. 2011;5:176–181. doi: 10.3855/jidc.950. [DOI] [PubMed] [Google Scholar]
- 9.Bacterial Etiology of Sexually Transmitted Infections at a STI Clinic in Ghana; Use of Multiplex Real Time PCR | Sylverken | Ghana Medical Journal. [(accessed on 2 March 2020)]; Available online: https://www.ajol.info/index.php/gmj/article/view/145764. [PMC free article] [PubMed]
- 10.Kouegnigan Rerambiah L., Ndong J.C., Medzegue S., Elisee-Ndam M., Djoba Siawaya J.F. Genital Mycoplasma infections and their resistance phenotypes in an African setting. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:1087–1090. doi: 10.1007/s10096-015-2326-9. [DOI] [PubMed] [Google Scholar]
- 11.Redelinghuys M.J., Ehlers M.M., Dreyer A.W., Lombaard H.A., Kock M.M. Comparison of the new Mycofast Revolution assay with a molecular assay for the detection of genital mycoplasmas from clinical specimens. BMC Infect. Dis. 2013;13:453. doi: 10.1186/1471-2334-13-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agbakoba N., Adetosoye A.I., Adewole I.F. Presence of mycoplasma and ureaplasma species in the vagina of women of reproductive age. West Afr. J. Med. 2007;26:28–31. doi: 10.4314/wajm.v26i1.28299. [DOI] [PubMed] [Google Scholar]
- 13.Vriend H.J., Bogaards J.A., van Bergen J.E.A.M., Brink A.A.T.P., van den Broek I.V.F., Hoebe C.J.P.A., King A.J., van der Sande M.A.B., Wolffs P.F.G., de Melker H.E., et al. Incidence and persistence of carcinogenic genital human papillomavirus infections in young women with or without Chlamydia trachomatis co-infection. Cancer Med. 2015;4:1589–1598. doi: 10.1002/cam4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y.L., You K., Qiao J., Zhao Y.M., Geng L. Bacterial vaginosis is conducive to the persistence of HPV infection. Int. J. STD AIDS. 2012;23:581–584. doi: 10.1258/ijsa.2012.011342. [DOI] [PubMed] [Google Scholar]
- 15.Clarke M.A., Rodriguez A.C., Gage J.C., Herrero R., Hildesheim A., Wacholder S., Burk R., Schiffman M. A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection. BMC Infect. Dis. 2012;12:33. doi: 10.1186/1471-2334-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillet E., Meys J.F.A., Verstraelen H., Bosire C., De Sutter P., Temmerman M., Broeck D.V. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: A meta-analysis. BMC Infect. Dis. 2011;11:10. doi: 10.1186/1471-2334-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samwel K., Kahesa C., Mwaiselage J., Gonzalez D., West J.T., Wood C., Palefsky J., Angeletti P.C. Analytical performance of a low-cost multiplex polymerase chain reaction human papillomavirus genotyping assay for use in Sub-Saharan Africa. J. Med. Virol. 2019;91:308–316. doi: 10.1002/jmv.25329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stellrecht K.A., Woron A.M., Mishrik N.G., Venezia R.A. Comparison of Multiplex PCR Assay with Culture for Detection of Genital Mycoplasmas. J. Clin. Microbiol. 2004;42:1528–1533. doi: 10.1128/JCM.42.4.1528-1533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusters J.G., Reuland E.A., Bouter S., Koenig P., Dorigo-Zetsma J.W. A multiplex real-time PCR assay for routine diagnosis of bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:1779–1785. doi: 10.1007/s10096-015-2412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozich J.J., Westcott S.L., Baxter N.T., Highlander S.K., Schloss P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl. Environ. Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.