Abstract
A successful phase III trial for the combination of atezolizumab and bevacizumab (the IMbrave150 trial) in advanced hepatocellular carcinoma has recently been reported. This is groundbreaking because nivolumab and pembrolizumab, both programmed cell death-1 (PD-1) antibodies, have failed to show efficacy as first- and second-line therapeutics, respectively, in phase III clinical trials. Immunotherapy with a combination of atezolizumab and bevacizumab resulted in better survival than treatment with sorafenib for the first time since sorafenib was approved in 2007. The high efficacy of the combination of PD-1/programmed death ligand 1 (PD-L1) and vascular endothelial growth factor (VEGF) antibodies is not only due to their additive effects on tumor growth, but also to their reprogramming of the immunosuppressive microenvironment into an immunostimulatory microenvironment. These results were confirmed in a phase Ib trial that showed significantly longer progression-free survival in the atezolizumab plus bevacizumab group than in patients that received atezolizumab alone. These results demonstrate that immunotherapy with a combination of PD-1/PD-L1 and VEGF inhibitors is effective and may result in a reprogramming of the tumor microenvironment. The results of an ongoing phase III trial of a PD-1 antibody in combination with the VEGF receptor tyrosine kinase inhibitor (TKI) are highly anticipated.
Keywords: hepatocellular carcinoma, immune checkpoint inhibitor, PD-1 antibody, PD-L1 antibody, anti-VEGF inhibitor
1. Introduction
At the European Society for Medical Oncology (ESMO) Asia in November 2019, the positive results of the IMbrave150 study, a trial which compared the effects of the combination of atezolizumab and bevacizumab with those of sorafenib [1], drew attention to the possibility of immunotherapy with a combination of programmed cell death-1 (PD-1)/programmed death ligand 1 (PD-L1) and vascular endothelial growth factor (VEGF) inhibitors. This review outlines the scientific rationale for the therapeutic combination of PD-1/PD-L1 and VEGF antibodies, proof-of-concept results of the phase Ib trial, and results of other phase Ib trials for similar combination strategies.
2. The Rationale Underlying the Combination of PD-1/PD-L1 and VEGF Inhibitors
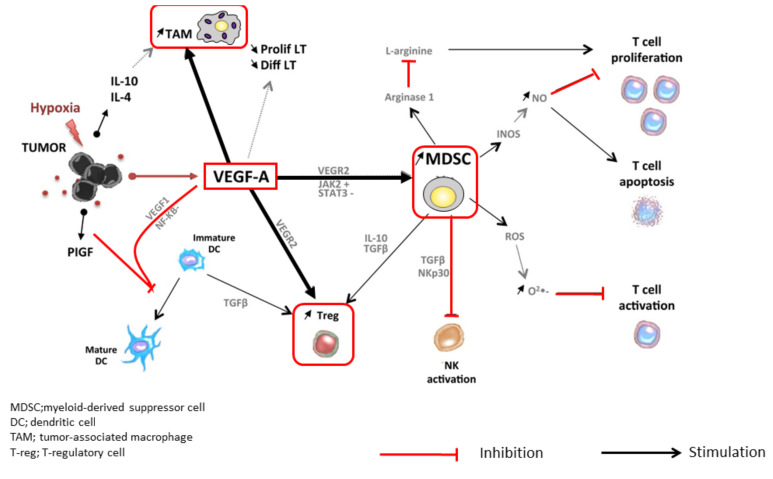
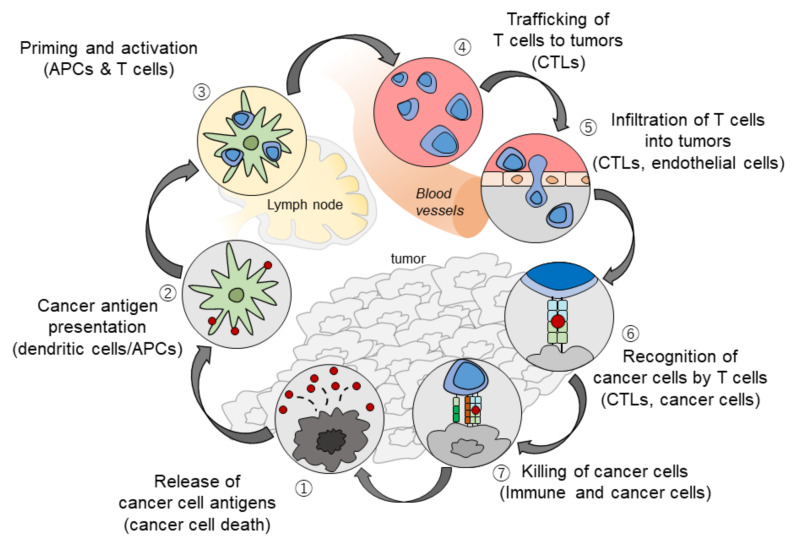
At tumor sites, VEGF released by hypoxic cancer cells and vascular endothelial cells promotes tumor growth, invasion, and metastasis by increasing neovascularization [2]. Simultaneously, VEGF enhances the mobilization and proliferation of various cells, including regulatory T cells (Tregs), and the release of immunosuppressive cytokines [2,3]. It also enhances the mobilization of tumor-associated macrophages (TAMs) and their polarization to an M2 phenotype. Tregs and TAMs promote tumor growth through the release of VEGF and angiopoietin-2, among other mechanisms [4]. VEGF can also activate myeloid-derived suppressor cells (MDSCs), which in turn release more VEGF [4]. Furthermore, VEGF inhibits dendritic cell maturation and antigen presentation in the priming phase. Thus, VEGF reduces the proliferation and activation of naive CD8+ cells by suppressing dendritic cell activity even in the presence of neoantigens [4] (Figure 1). VEGF-induced Tregs, TAMs, and MDSCs reduce the proliferation and function of CD8+ cells. VEGF also prevents antigen-activated CD8+ cells from infiltrating the tumor tissue through its effects on tumor angiogenesis. In addition, VEGF creates a microenvironment that inhibits the function of T cells in the tumor during the effector phase of the immune response [4]. Furthermore, immunosuppressive cells (Tregs, TAMs, and MDSCs) promote immune escape by releasing immunosuppressive cytokines, including interleukin (IL)-10 and transforming growth factor beta (TGF-β), and by inhibiting dendritic cell maturation and activation, NK cell activation, and T cell activation and proliferation [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25] (Figure 1). The cancer immunity cycle begins with the uptake and presentation of neoantigens released from necrotic tumor cells by dendritic cells. This is followed by seven steps: (1) tumor antigen release, (2) tumor antigen uptake and presentation by dendritic cells, (3) T cell priming and activation, (4) T cell migration to the tumor, (5) T cell invasion of the tumor, (6) cancer cell recognition by T cells, and (7) attack on tumor cells by T cells, which leads to cancer cell death and release of additional tumor antigens [5] (Figure 2). VEGF promotes immune escape at almost every step of the cancer immunity cycle [6,7,8,9]. Furthermore, hepatic interstitial cells such as Kupffer cells, liver endothelial cells, and hepatic stellate cells are involved in maintaining immune tolerance in the healthy liver and may contribute to the immunosuppressive microenvironment in hepatocellular carcinoma [26].
Figure 1.
Immune suppressive microenvironment induced by VEGF (modified from ref. [4] with permission).
Figure 2.
The Cancer-Immunity Cycle (modified from ref. [5] with permission).
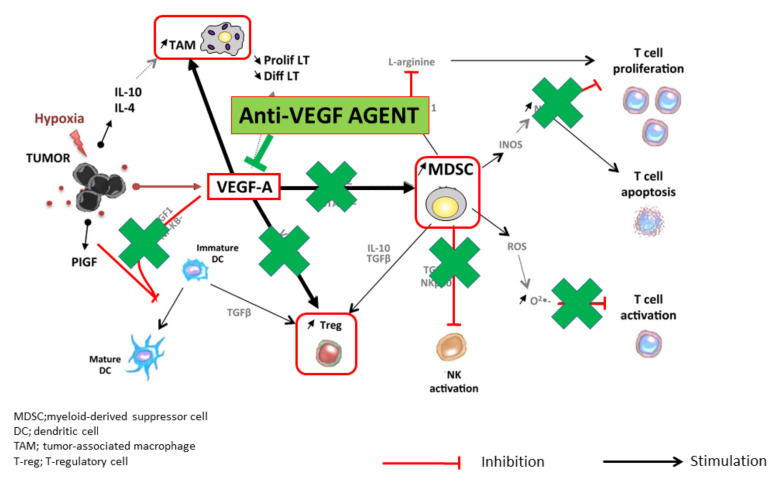
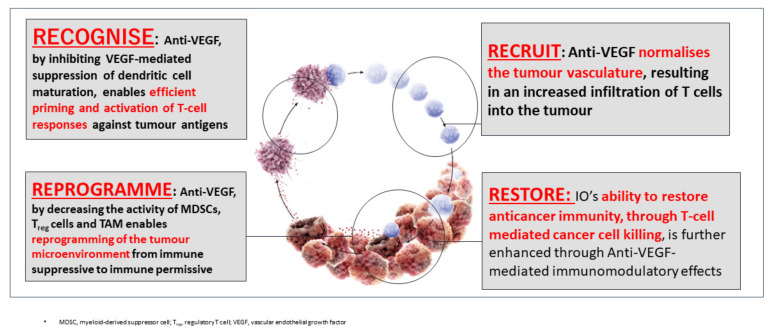
The administration of molecular targeted drugs that inhibit VEGF activity, such as multi-kinase inhibitors that inhibit VEGF receptors, leads to an increase in antigen presentation by dendritic cells [8]. These drugs also promote T cell activation in the priming phase [8] and improve the migration of T cells from the lymph nodes to the tumor site by normalizing the tumor vasculature [15]. In addition, these drugs have been found to suppress the generation of Tregs, TAMs, and MDSCs at the tumor site, and to negatively regulate the expression of immunosuppressive cytokines such as TGF-β and IL-10 [10]. VEGF inhibitors therefore reprogram the immunosuppressive tumor microenvironment into an immunostimulatory environment [6,8]. The administration of PD-1/PD-L1 antibodies under such conditions enhances the antitumor activity of T cells (Figure 3 and Figure 4). As described above, the combination of VEGF and PD-1/PD-L1 inhibitors promotes antitumor immunity according to the four Rs. First, a reversal of the VEGF-mediated inhibition of dendritic cell maturation results in the effective priming and activation of T cells (Recognition) [9]. Second, anti-VEGF antibodies normalize the tumor vasculature and promote the effective infiltration of T cells into the tumor (Recruitment) [15]. Third, anti-VEGF antibodies inhibit the activity of MDSCs, Tregs, and TAMs, leading to the reprogramming of the immunosuppressive microenvironment into an immunostimulatory microenvironment (Reprogramming) [6]. Fourth, PD-1/PD-L1 antibodies enhance the ability of T cells to attack tumor cells (Restoration) (Figure 3). These four Rs lead to efficient cancer immunity and tumor growth inhibition. Proteins released by the killed tumor cells are taken up by dendritic cells, and then processed into tumor antigen peptides that are presented on major histocompatibility complex (MHC) class I molecules, leading to a progression through the cancer immunity cycle and further tumor attacks [5] (Figure 2). As described above, normalization of the VEGF-suppressed tumor microenvironment with molecular targeted agents against VEGF leads to the efficient attack on tumors by activated T cells [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,27] (Figure 2 and Figure 4). In addition, non-clinical study of lenvatinib, a tyrosine kinase inhibitor (TKI), showed that the inhibition of VEGF activity reduced TAMs and Tregs in the tumor microenvironment, leading to a decrease in TGF-β and IL-10, a decreased expression of T cell exhaustion markers such as PD-1 and TIM-3, and an increased expression of immunostimulatory cytokines such as IL-12 [28,29,30,31]. These findings form the rationale for a trial of the combination of TKIs and anti-PD-1/PD-L1 antibodies.
Figure 3.
Anti-VEGF antibody reprograms the tumor microenvironment from immune suppressive to immune permissive (modified from ref. [4] with permission).
Figure 4.
Scientific rationale of Immune-checkpoint Inhibitors plus Anti-VEGF: 4 Roles of anti-VEGF inhibitors in Cancer Immunity cycle, Recognise, Recruitment, Reprogramme, and Restore (original Figure).
3. Classification of the Tumor Microenvironment and Determination of Immunotherapeutic Strategies
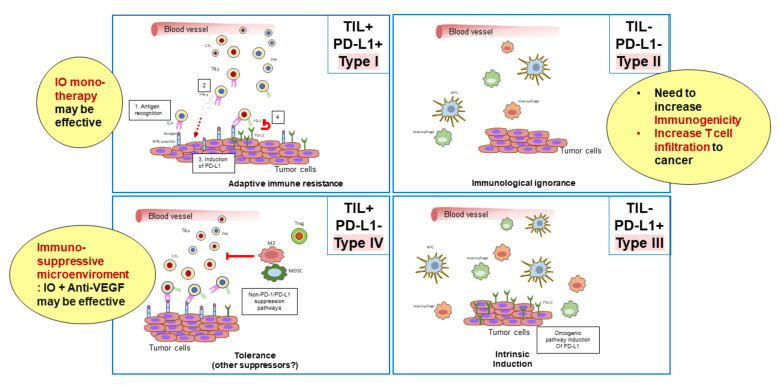
Cancers are classified into four types based on the presence of tumor-infiltrating CD8+ T cells and the expression of PD-L1 [32] (Figure 5). Type I tumors contain tumor-infiltrating lymphocytes and express PD-L1. Type I cancers generally show an adequate response to monotherapy with immune checkpoint inhibitors. By contrast, type IV tumors lack PD-L1 expression, although they do contain tumor-infiltrating lymphocytes. Type IV tumors are not responsive to immune checkpoint inhibitors because the immunosuppressive tumor microenvironment inhibits the proliferation and activity of CD8+ cells in these tumors. In type I, there is an initial antitumor immune response, in which perforin, granzyme, and interferon gamma (IFN-γ) are released by activated CD8+ cells, resulting in an immune attack on the cancer cells [32]. However, IFN-γ binds to IFN-γ receptors on the cancer cell surface and upregulates the expression of PD-L1 through the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway [31]. This leads to immune escape, whereby cancer cells evade the attack by activated CD8+ cells. Therefore, type I cancers are responsive to monotherapy with PD-1/PD-L1 antibodies. By contrast, type IV tumors do not show an initial local immune response, even though CD8+ cells are present and the tumor expression of PD-L1 is low. These tumors are never attacked by CD8+ cells because T cell activity is inhibited by the immunosuppressive microenvironment. Therefore, induction of IFN-γ and PD-L1 expression is not observed [28,32]. As expected, such cancers are not responsive to anti-PD-1/PD-L1 antibody monotherapy due to the absence of immune escape through the PD-1/PD-L1 axis. Thus, PD-1 antibody monotherapy is not predicted to be effective in cancers without PD-L1 expression, even if there are large numbers of tumor-infiltrating lymphocytes. In such tumors, anti-VEGF antibodies or inhibitors may reprogram the immunosuppressive microenvironment into an immunostimulatory microenvironment by targeting Tregs, TAMs, and MDSCs, leading to an attack by antigen-specific T cells. This, in turn, would lead to the induction of PD-L1 on the cancer cell surface by IFN-γ. In this scenario, PD-1/PD-L1 antibodies could inhibit immune escape through the PD-1/PD-L1 axis [28,32]. Therefore, this combination therapy could be effective in tumors that are unresponsive to anti-PD-1/PD-L1 monotherapy. Dramatic tumor inhibition could therefore result from the concomitant administration of PD-1/PD-L1 antibodies and VEGF antibodies or TKIs in type IV tumors (Figure 3 and Figure 4) [32]. However, in Type II and III tumors, where no tumor-infiltrating lymphocytes are present, another strategy to increase immunogenicity may be necessary.
Figure 5.
Cancer is classified into 4 types depending on immune microenvironment (TIL: CD8+ cell and PD-L1 expression) (Type I-IV) (modified from ref. [32] with permission).
4. The Results of a Phase Ib Trial of the Combination of Atezolizumab and Bevacizumab (Clinical Trials.Gov Identifier NCT02715531)
4.1. The Use of the Combination of Atezolizumab (a PD-L1 Antibody) and Bevacizumab (a VEGF Antibody) in Unresectable Hepatocellular Carcinoma (Arm A)
Arm A of NCT02715531 was a single-arm phase Ib study of the combination of atezolizumab (a PD-L1 antibody) and bevacizumab (a VEGF antibody) in unresectable hepatocellular carcinoma. Updated results from the 104 unresectable hepatocellular carcinoma patients in Arm A were presented at the annual meeting of the European Society for Medical Oncology (ESMO) in Barcelona, in the fall of 2019 [33]. Fifty-three percent of patients had macroscopic vascular invasion (MVI), of whom 88% were hepatocellular carcinoma patients with highly advanced extrahepatic spread (EHS). Although these were highly advanced cases, evaluation by an independent imaging facility (IRF) based on Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) showed an overall response rate (ORR) of 36% (95% confidence interval [CI], 26–46%). The ORR based on the modified RECIST (mRECIST) was 39%. The percentage of patients achieving a complete response (CR) based on RECIST 1.1 was 12%. Moreover, the partial response (PR) rate and disease control rate (DCR) were 24% and 71%, respectively. The median duration of response was not reached (95% CI, 11.8–not estimated [NE]). There were 20 patients (54%) with a duration of response ≥ 9 months and 11 patients (30%) with long-term responses (duration of response ≥ 12 months).
In addition, the progression-free survival (PFS) and overall survival (OS) were extremely good (PFS, 7.3 months [95% CI, 5.4–9.9]; OS, 17.1 months [95% CI, 13.8–not reached]). The result is very promising considering the fact that 53%, 88%, and 36% of patients had MVI, EHS with or without MVI, and alpha-fetoprotein (AFP) > 400 ng/mL, respectively.
4.2. Randomized Controlled Arm Comparing the Combination of Atezolizumab Plus Bevacizumab Versus Atezolizumab Alone (Arm F)
Arm F of the study compared PFS in unresectable hepatocellular carcinoma between the combination of atezolizumab (1200 mg) and bevacizumab (15 mg/kg) (every 3 weeks), and atezolizumab alone (1200 mg) as a first-line therapy. This was a proof-of-concept study to determine whether the favorable outcomes observed in Arm A were due to atezolizumab alone or to the combined effect of bevacizumab plus atezolizumab. Importantly, the ORR of the combination of atezolizumab and bevacizumab was slightly higher (20%) than that of atezolizumab alone (17%), which is consistent with data from other trials on the ORR of immune checkpoint inhibitors alone (about 15–18.3% [34,35,36,37,38,39]). In fact, the median PFS was 5.6 months (95% CI, 3.6–7.4) for atezolizumab plus bevacizumab, and 3.4 months (95% CI, 1.9–5.2) for atezolizumab alone. The hazard ratio was 0.55 (95% CI, 0.40–0.74; p = 0.0108). These data clearly showed the beneficial effect of bevacizumab on atezolizumab therapy. The PFS of atezolizumab plus bevacizumab in Arm F (5.6 months) was shorter than that in Arm A (7.3 months). However, this result may be due to the fact that the median follow-up period of Arm F was shorter (6.6 months vs. 12.4 months). With extended follow-up, the PFS in Arm F may have been equivalent to that of Arm A. In any case, the results of Arm F clearly supported the hypothesis that bevacizumab reprograms the immunosuppressive microenvironment into an immunostimulatory environment, enhancing the efficacy of atezolizumab (Figure 4).
5. Results of Phase Ib Studies of Other Combinations of PD-1/PD-L1 Antibodies and VEGF Inhibitors
In addition to the trial of atezolizumab and bevacizumab described above, other studies are examining the efficacy of combined PD-1/PD-L1 and VEGF inhibition. One such study, the LEAP-002 study, is a phase III clinical trial of pembrolizumab and lenvatinib [40,41]. This trial is ongoing and the results are highly anticipated. In addition, multiple other clinical trials of immune checkpoint inhibitors and VEGF inhibitors have been completed (Table 1). The number of patients who received pembrolizumab and lenvatinib (n = 67) was lower than the number of patients who received atezolizumab and bevacizumab in Arm A of the phase Ib trial described above (n = 104). The ORR (40.3%), DCR (85.1%), PFS (9.7 months), and OS (20.4 months) of the combination of pembrolizumab and lenvatinib were higher than those of the combination of atezolizumab and bevacizumab [42]. Furthermore, the efficacy of the combination of nivolumab and lenvatinib (evaluated by an independent imaging committee based on RECIST 1.1), which was recently reported at the annual meeting of the American Society of Clinical Oncology, Gastrointestinal Cancers (ASCO GI), was higher than that of the other two combination therapies (ORR, 54.2%; DCR, 91.7%; PFS, 7.4 months; and OS, not reached) [43]. Of course, it is not adequate to compare the results of single-arm trials with different patient populations, small sample sizes, and short observation periods. However, the results are very promising. The ORR and PFS of the combination of camerelizumab and apatinib were 38.9% and 7.2 months, respectively [44]. However, there have been no updated reports on this combination. Moreover, the reported results of the combination of avelumab and axitinib [45] were slightly inferior to those of other combination therapies (ORR, 13.6%; PFS, 5.5 months; and OS, 12.7 months, based on RECIST 1.1). Therefore, at present, the most promising ongoing trial is the LEAP-002 study [40,41]. The decision whether or not to proceed to phase III trials of the combination of nivolumab and lenvatinib has currently drawn attention. In any case, the efficacy of all other combinations of anti-PD-1/PD-L1 antibodies and TKIs or anti-VEGF antibodies, except for the combination of avelumab and axitinib, is higher than that of nivolumab (a PD-1 antibody) alone (ORR, 15%; DCR, 55%; PFS, 3.7 months; and OS, 16.4 months) [34] or pembrolizumab alone (ORR, 18.3%; DCR, 62.2%; PFS, 3.0 months; OS, 13.9 months) [36]. Therefore, combined immunotherapy is expected to shift the paradigm as a first-line treatment option in advanced hepatocellular carcinoma [41,46].
Table 1.
Efficacy of Immune Checkpoint Inhibitors and Combination Immunotherapy with VEGF Antibodies/Tyrosine Kinase Inhibitors in Phase 1b Trials according to RECIST 1.1.
| Efficacy | Anti-PD-1 Monotherapy (Phase 3 Trial) | Anti-PD-1/PD-L1 plus TKI/Anti-VEGF (Phase 1b Trial) | |||||
|---|---|---|---|---|---|---|---|
| Nivolumab [34] (n = 214) |
Pembrolizumab [36] (n = 278) |
Atezolizumab + bevacizumab [33] (n = 104) |
Pembrolizumab + Lenvatinib [42] (n = 67) |
Camrelizumab + apatinib [44] (n = 18) |
Avelumab + axitinib [45] (n = 22) |
Nivolumab + Lenvatinib [43] (n = 24) |
|
| ORR (95% CI) | 15% | 18.3% (14.0–23.4) | 36% (26–46) | 40.3% (28.5–53.0) | 38.9% | 13.6% (2.9–34.9) | 54.2% (32.8–74.4) |
| DCR (95% CI) | 55% | 62.2% | 71% | 85.1% (74.3–92.6) | 83.3% | 68.2% (45.1–86.1) | 91.7% (73.0–99.0) |
| PFS, months (95% CI) | 3.7 (3.1–3.9) | 3.0 (2.8–4.1) | 7.4 (5.6–10.7) | 9.7 (5.3–13.8) | 7.2 (2.6–NE) | 5.5 (1.9–7.4) | 7.4 (3.7–NE) |
| OS, months (95% CI) | 16.4 (13.9–18.4) | 13.9 (11.6–16.0) | 17.1 (13.8–NE) | 20.4 (11.0–NE) | NR | 12.7 (8.0–NE) | NR |
| DOR, months (M) | 23.3 (3.1–34.5+) | 13.8 (1.5–23.6) | NE (11.7–NE) | 11.0 (5.6–11.0) | NA | 5.5 (3.7–7.3) | NA |
DCR, disease control rate; DOR, duration of response; NA, not available; NE; not evaluable; NR, not reached; ORR, objective response rate (RECIST 1.1); OS, overall survival; PFS, progression-fee survival. TKI, tyrosine kinase inhibitor.
6. Conclusions
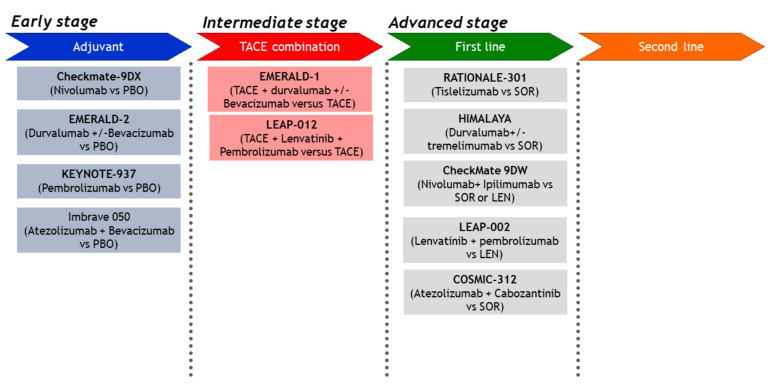
This article described the scientific rationale for the combination of PD-1/PD-L1 antibodies plus VEGF inhibitors, and discussed the results of a phase Ib trial of this combination. We also described the results of Arm F of a randomized phase Ib trial of the combination of atezolizumab and bevacizumab, a combination that also achieved positive results in the phase III IMbrave150 study. The results of the phase Ib trial (Arm F) and the success of the phase III IMbrave150 study suggest that the tumor microenvironment was changed by bevacizumab, enabling greater responses to the immune checkpoint blockade, as hypothesized. In addition to the improvement in PFS, in the phase III IMbrave150 study, the OS was also improved, which was an unexpected finding [1]. These results are paradigm-changing as well as practice-changing. This study suggested that the immunosuppressive tumor microenvironment was successfully reprogrammed into an immunostimulatory microenvironment that was responsive to an immune checkpoint blockade. Therefore, the promising results that have been reported with combinations of anti-PD-1/PD-L1 antibodies and VEGF inhibitors (bevacizumab or TKIs) may be due to a normalization of the tumor microenvironment. In addition to the combination of atezolizumab and bevacizumab, therapies with other combinations targeting the same pathways (Table 1), especially the combinations of penbrolizumab and lenvatinib (the LEAP-002 study) and atezolizumab and cabozantinib (the COSMIC-312 trial), are highly promising (Figure 6 and Table 2) [1,34,36,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]. Furthermore, other phase III trials of combinations with CTLA-4 inhibitors [66] (durvalumab plus tremelimumab [HIMALAYA study] and nivolumab plus ipilimumab [the CheckMate 9DW study]) are currently being conducted (Figure 1 and Table 2). In the era of combination immunotherapy, the treatment of hepatocellular carcinoma, including the proper use of molecular targeted drugs after progression on immunotherapy [67,68], has entered a period of a major paradigm shift.
Figure 6.
Ongoing Phase III trials in HCC (original Figure).
Table 2.
Phase III Clinical Trials of Advanced Stage HCC.
| Target Population | Design | Trial Name | Result | Presentation | Publication | 1st Author | |
|---|---|---|---|---|---|---|---|
| Advanced | First line | 1. Sorafenib vs. Sunitinib | SUN1170 | Negative | ASCO 2011 | JCO 2013 | Cheng AL [47] |
| 2. Sorafenib ± Erlotinib | SEARCH | Negative | ESMO 2012 | JCO 2015 | Zhu AX [48] | ||
| 3. Sorafenib vs. Brivanib | BRISK-FL | Negative | AASLD 2012 | JCO 2013 | Johnson PJ [49] | ||
| 4. Sorafenib vs. Linifanib | LiGHT | Negative | ASCO-GI 2013 | JCO 2015 | Cainap C [50] | ||
| 5. Sorafenib ± Doxorubicin | CALGB 80802 | Negative | ASCO-GI 2016 | ||||
| 6. Sorafenib ±- HAIC | SILIUS | Negative | EASL 2016 | Lancet GH 2018 | Kudo M [51] | ||
| 7. Sorafenib ± Y90 | SARAH | Negative | EASL 2017 | Lancet-O 2017 | Vilgrain V [52] | ||
| 8. Sorafenib ± Y90 | SIRveNIB | Negative | ASCO 2017 | JCO 2018 | Chow PKH [53] | ||
| 9. Sorafenib vs. Lenvatinib | REFLECT | Positive | ASCO 2017 | Lancet 2018 | Kudo M [54] | ||
| 10. Sorafenib vs. Nivolumab | CheckMate-459 | Negative | ESMO 2019 | Yau T [34] | |||
| 11. Sorafenib ± Y90 | SORAMIC | Negative | EASL 2018 | J Hepatol 2019 | Ricke J [55] | ||
| 12. Sorafenib vs. Atezolizumab + Bevacizumab | IMbrave150 | Positive | ESMO-Asia 2019 | Cheng AL [1] | |||
| 13. Sorafenib vs. Durvalumab + Tremelimumab vs. Durva | HIMALAYA | Ongoing | |||||
| 14. Sorafenib vs. Tislelizumab | Rationale301 | Ongoing | |||||
| 15. Lenvatinib ± Pembrolizumab | LEAP002 | Ongoing | |||||
| 16. Lenvatinib or Sorafenib vs. Nivolumab + Ipilimumab | CheckMate 9DW | Ongoing | |||||
| 17. Sorafenib vs. Atezolizumab + Cabozantinib | COSMIC-312 | Ongoing | |||||
| Second line | 1. Brivanib vs. Placebo | BRISK-PS | Negative | EASL 2012 | JCO 2013 | Llovet JM [56] | |
| 2. Everolimus vs. Placebo | EVOLVE-1 | Negative | ASCO-GI 2014 | JAMA 2014 | Zhu AX [57] | ||
| 3. Ramucirumab vs. Placebo | REACH | Negative | ESMO 2014 | Lancet-O 2015 | Zhu AX [58] | ||
| 4. S-1 vs. Placebo | S-CUBE | Negative | ASCO 2015 | Lancet GH 2017 | Kudo M [59] | ||
| 5. ADI-PEG 20 vs. Placebo | NA | Negative | ASCO 2016 | Ann Oncol 2018 | Abou-Alfa GK [60] | ||
| 6. Regorafenib vs. Placebo | RESORCE | Positive | WCGC 2016 | Lancet 2017 | Bruix J [61] | ||
| 7. Tivantinib vs. Placebo | METIV-HCC | Negative | ASCO 2017 | Lancet-O 2018 | Rimassa L [62] | ||
| 8. Tivantinib vs. Placebo | JET-HCC | Negative | ESMO 2017 | ||||
| 9. DT# vs. Placebo | ReLive | Negative | ILCA 2017 | Lancet Gastroenterol Hepatol | Merle P [63] | ||
| 10. Cabozantinib vs. Placebo | CELESTIAL | Positive | ASCO-GI 2018 | NEJM 2018 | Abou-Alfa G [64] | ||
| 11. Ramucirumab vs. Placebo | REACH-2 | Positive | ASCO 2018 | Lancet-O 2019 | Zhu AX [65] | ||
| 12. Pembrolizumab vs. Placebo | KEYNOTE-240 | Negative | ASCO 2019 | JCO 2020 | Finn RS [36] | ||
Red: Positive trials, Blue: Ongoing trials, Black: Negative trials.
Funding
This research received no external funding.
Conflicts of Interest
Masatoshi Kudo has received grants from Taiho Pharmaceuticals, Chugai Pharmaceuticals, Otsuka, Takeda, Sumitomo Dainippon-Sumitomo, Daiichi Sankyo, AbbVie, Astellas Pharma, and Bristol-Myers Squibb. He has also received grants and personal fees from Merck Sharpe and Dohme (MSD), Eisai, and Bayer, and is an adviser for MSD, Eisai, Bayer, Bristol-Myers Squibb, Eli Lilly, and ONO Pharmaceuticals.
References
- 1.Cheng A.L., Qin S., Ikeda M., Galle P.R., Ducreux M., Zhu A.X., Kim T.Y., Kudo M., Breder V., Merle P., et al. IMbrave150: Efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC) Ann. Oncol. 2019;30(Suppl. 9):ix186–ix187. doi: 10.1093/annonc/mdz446.002. [DOI] [Google Scholar]
- 2.Fukumura D., Kloepper J., Amoozgar Z., Duda D.G., Jain R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018;15:325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chouaib S., Messai Y., Couve S., Escudier B., Hasmim M., Noman M.Z. Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Front. Immunol. 2012;3:21. doi: 10.3389/fimmu.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voron T., Marcheteau E., Pernot S., Colussi O., Tartour E., Taieb J., Terme M. Control of the immune response by pro-angiogenic factors. Front. Oncol. 2014;4:70. doi: 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen D.S., Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Hegde P.S., Wallin J.J., Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin. Cancer Biol. 2018;52:117–124. doi: 10.1016/j.semcancer.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N., Hillan K.J., Gerber H.P., Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 8.Gabrilovich D.I., Chen H.L., Girgis K.R., Cunningham H.T., Meny G.M., Nadaf S., Kavanaugh D., Carbone D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 9.Gabrilovich D., Ishida T., Oyama T., Ran S., Kravtsov V., Nadaf S., Carbone D.P. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. doi: 10.1182/blood.V92.11.4150. [DOI] [PubMed] [Google Scholar]
- 10.Elovic A.E., Ohyama H., Sauty A., McBride J., Tsuji T., Nagai M., Weller P.F., Wong D.T. IL-4-dependent regulation of TGF-alpha and TGF-beta1 expression in human eosinophils. J. Immunol. 1998;160:6121–6127. [PubMed] [Google Scholar]
- 11.Guermonprez P., Valladeau J., Zitvogel L., Thery C., Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 12.Villadangos J.A., Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 13.Griffioen A.W., Damen C.A., Blijham G.H., Groenewegen G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood. 1996;88:667–673. doi: 10.1182/blood.V88.2.667.bloodjournal882667. [DOI] [PubMed] [Google Scholar]
- 14.Griffioen A.W., Damen C.A., Martinotti S., Blijham G.H., Groenewegen G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: The role of angiogenic factors. Cancer Res. 1996;56:1111–1117. [PubMed] [Google Scholar]
- 15.Goel S., Duda D.G., Xu L., Munn L.L., Boucher Y., Fukumura D., Jain R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motz G.T., Santoro S.P., Wang L.P., Garrabrant T., Lastra R.R., Hagemann I.S., Lal P., Feldman M.D., Benencia F., Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodi F.S., Lawrence D., Lezcano C., Wu X., Zhou J., Sasada T., Zeng W., Giobbie-Hurder A., Atkins M.B., Ibrahim N., et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol. Res. 2014;2:632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallin J.J., Bendell J.C., Funke R., Sznol M., Korski K., Jones S., Hernandez G., Mier J., He X., Hodi F.S., et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C., Li J., Song L., Zhang D., Tong Q., Ding M., Bowman L., Aziz R., Stoner G.D. Black raspberry extracts inhibit benzo(a)pyrene diol-epoxide-induced activator protein 1 activation and VEGF transcription by targeting the phosphotidylinositol 3-kinase/Akt pathway. Cancer Res. 2006;66:581–587. doi: 10.1158/0008-5472.CAN-05-1951. [DOI] [PubMed] [Google Scholar]
- 21.Ko S.Y., Guo H., Barengo N., Naora H. Inhibition of ovarian cancer growth by a tumor-targeting peptide that binds eukaryotic translation initiation factor 4E. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:4336–4347. doi: 10.1158/1078-0432.CCR-08-2924. [DOI] [PubMed] [Google Scholar]
- 22.Kusmartsev S., Eruslanov E., Kubler H., Tseng T., Sakai Y., Su Z., Kaliberov S., Heiser A., Rosser C., Dahm P., et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: Link to tumor-induced immune suppression in renal cell carcinoma. J. Immunol. 2008;181:346–353. doi: 10.4049/jimmunol.181.1.346. [DOI] [PubMed] [Google Scholar]
- 23.Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S., Sosman J.A., McDermott D.F., Powderly J.D., Gettinger S.N., et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X., Zeng Q., Wu M.X. Improved efficacy of dendritic cell-based immunotherapy by cutaneous laser illumination. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18:2240–2249. doi: 10.1158/1078-0432.CCR-11-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou W., Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 26.Tiegs G., Lohse A.W. Immune tolerance: What is unique about the liver. J Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Oyama T., Ran S., Ishida T., Nadaf S., Kerr L., Carbone D.P., Gabrilovich D.I. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J. Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 28.Kato Y., Tabata K., Kimura T., Yachie-Kinoshita A., Ozawa Y., Yamada K., Ito J., Tachino S., Hori Y., Matsuki M., et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS ONE. 2019;14:e0212513. doi: 10.1371/journal.pone.0212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kudo M. Combination Cancer Immunotherapy in Hepatocellular Carcinoma. Liver Cancer. 2018;7:20–27. doi: 10.1159/000486487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudo M. Targeted and immune therapies for hepatocellular carcinoma: Predictions for 2019 and beyond. World J. Gastroenterol. 2019;25:789–807. doi: 10.3748/wjg.v25.i7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudo M. Combination Cancer Immunotherapy with Molecular Targeted Agents/Anti-CTLA-4 Antibody for Hepatocellular Carcinoma. Liver Cancer. 2019;8:1–11. doi: 10.1159/000496277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teng M.W., Ngiow S.F., Ribas A., Smyth M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu C.H., Lee M.S., Lee K.H., Numata K., Stein S., Verret W., Hack S., Spahn J., Liu B., Huang C., et al. Randomised efficacy and safety results for atezolizumab + bevacizumab in patients with previously untreated, unresectable hepatocellular carcinoma. Ann. Oncol. 2019;30(Suppl. 9):ix187. doi: 10.1093/annonc/mdz446.006. [DOI] [Google Scholar]
- 34.Yau T., Park J.W., Finn R.S., Cheng A.L., Mathurin P., Edeline J., Kudo M., Han K.H., Harding J.J., Merle P., et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab vs sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. Ann. Oncol. 2019;30(Suppl. 5):v874–v875. doi: 10.1093/annonc/mdz394.029. [DOI] [Google Scholar]
- 35.Zhu A.X., Finn R.S., Edeline J., Cattan S., Ogasawara S., Palmer D., Verslype C., Zagonel V., Fartoux L., Vogel A., et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 36.Finn R.S., Ryoo B.Y., Merle P., Kudo M., Bouattour M., Lim H.Y., Breder V., Edeline J., Chao Y., Ogasawara S., et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 37.Kudo M. Pembrolizumab for the Treatment of Hepatocellular Carcinoma. Liver Cancer. 2019;8:143–154. doi: 10.1159/000500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kudo M. Immune checkpoint blockade in hepatocellular carcinoma: 2017 update. Liver Cancer. 2017;6:1–12. doi: 10.1159/000449342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.Y., Choo S.P., Trojan J., Welling T.H.R., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Llovet J.M., Kudo M., Cheng A.L., Finn R.S., Galle P.R., Kaneko S., Meyer T., Qin S., dutcus C.E., Chen E., et al. First-Line Combination Therapy With Lenvatinib Plus Pembrolizumab for Patients with Advanced Hepatocellular Carcinoma: Phase 3 LEAP-002 Study; Proceedings of the ILCA 13th Annual Conference; Chicago, IL, USA. 20–22 September 2019. [Google Scholar]
- 41.Kudo M. Immuno-Oncology Therapy for Hepatocellular Carcinoma: Current Status and Ongoing Trials. Liver Cancer. 2019;8:221–238. doi: 10.1159/000501501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Llovet J.M., Finn R.S., Ikeda M., Sung M.W., Baron A.D., Kudo M., Okusaka T., Kobayashi M., Kumada H., Kaneko S., et al. A phase 1b trial of lenvatinib plus pembrolizumab in unresectable hepatocellular carcinoma: Upated results. Ann. Oncol. 2019;30(Suppl. 5):v253–v324. doi: 10.1093/annonc/mdz247.073. [DOI] [Google Scholar]
- 43.Kudo M., Ikeda K., Motomura K., Okusaka T., Kato N., Dutcus C.E., Hisai T., Suzuki M., Ikezawa H., Iwata T., et al. A Phase 1b Study of Lenvatinib Plus Nivolumab in Patients With Unresectable Hepatocellular Carcinoma; Proceedings of the ASCO-GI; San Francisco, CA, USA. 23–25 January 2020. [Google Scholar]
- 44.Xu J.M., Zhang Y., Jia R., Wang Y., Liu R., Zhang G., Zhao C., Zhang Y., Zhou J., Wang Q. Anti-programmed death-1 antibody SHR-1210 (S) combined with apatinib (A) for advanced hepatocellular carcinoma (HCC), gastric cancer (GC) or esophagogastric junction (EGJ) cancer refractory to standard therapy: A phase 1 trial. J. Clin. Oncol. 2018;36:4075. doi: 10.1200/JCO.2018.36.15_suppl.4075. [DOI] [Google Scholar]
- 45.Kudo M., Motomura K., Wada Y., Inaba Y., Sakamoto Y., Kurosaki M., Umeyama Y., Kamei Y., Yoshimitsu J., Fujii Y., et al. First-line avelumab + axitinib in patients with advanced hepatocellular carcinoma: Results from a phase 1b trial (VEGF Liver 100) J. Clin. Oncol. 2019;37(Suppl. 15) doi: 10.1200/JCO.2019.37.15_suppl.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng A.L., Hsu C., Chan S.L., Choo S.P., Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J. Hepatol. 2020;72:307–319. doi: 10.1016/j.jhep.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 47.Cheng A.L., Kang Y.K., Lin D.Y., Park J.W., Kudo M., Qin S., Chung H.C., Song X., Xu J., Poggi G., et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J. Clin. Oncol. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 48.Zhu A.X., Rosmorduc O., Evans T.R., Ross P.J., Santoro A., Carrilho F.J., Bruix J., Qin S., Thuluvath P.J., Llovet J.M., et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015;33:559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 49.Johnson P.J., Qin S., Park J.W., Poon R.T., Raoul J.L., Philip P.A., Hsu C.H., Hu T.H., Heo J., Xu J., et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 50.Cainap C., Qin S., Huang W.T., Chung I.J., Pan H., Cheng Y., Kudo M., Kang Y.K., Chen P.J., Toh H.C., et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J. Clin. Oncol. 2015;33:172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kudo M., Ueshima K., Yokosuka O., Ogasawara S., Obi S., Izumi N., Aikata H., Nagano H., Hatano E., Sasaki Y., et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018;3:424–432. doi: 10.1016/S2468-1253(18)30078-5. [DOI] [PubMed] [Google Scholar]
- 52.Vilgrain V., Pereira H., Assenat E., Guiu B., Ilonca A.D., Pageaux G.P., Sibert A., Bouattour M., Lebtahi R., Allaham W., et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 53.Chow P.K.H., Gandhi M., Tan S.B., Khin M.W., Khasbazar A., Ong J., Choo S.P., Cheow P.C., Chotipanich C., Lim K., et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J. Clin. Oncol. 2018;36:1913–1921. doi: 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- 54.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 55.Ricke J., Klumpen H.J., Amthauer H., Bargellini I., Bartenstein P., de Toni E.N., Gasbarrini A., Pech M., Peck-Radosavljevic M., Popovic P., et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J. Hepatol. 2019;71:1164–1174. doi: 10.1016/j.jhep.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Llovet J.M., Decaens T., Raoul J.L., Boucher E., Kudo M., Chang C., Kang Y.K., Assenat E., Lim H.Y., Boige V., et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J. Clin. Oncol. 2013;31:3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 57.Zhu A.X., Kudo M., Assenat E., Cattan S., Kang Y.K., Lim H.Y., Poon R.T., Blanc J.F., Vogel A., Chen C.L., et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 58.Zhu A.X., Park J.O., Ryoo B.Y., Yen C.J., Poon R., Pastorelli D., Blanc J.F., Chung H.C., Baron A.D., Pfiffer T.E., et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 59.Kudo M., Moriguchi M., Numata K., Hidaka H., Tanaka H., Ikeda M., Kawazoe S., Ohkawa S., Sato Y., Kaneko S., et al. S-1 versus placebo in patients with sorafenib-refractory advanced hepatocellular carcinoma (S-CUBE): a randomised, double-blind, multicentre, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017;2:407–417. doi: 10.1016/S2468-1253(17)30072-9. [DOI] [PubMed] [Google Scholar]
- 60.Abou-Alfa G.K., Qin S., Ryoo B.Y., Lu S.N., Yen C.J., Feng Y.H., Lim H.Y., Izzo F., Colombo M., Sarker D., et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann. Oncol. 2018;29:1402–1408. doi: 10.1093/annonc/mdy101. [DOI] [PubMed] [Google Scholar]
- 61.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., Pracht M., Yokosuka O., Rosmorduc O., Breder V., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 62.Rimassa L., Assenat E., Peck-Radosavljevic M., Pracht M., Zagonel V., Mathurin P., Rota Caremoli E., Porta C., Daniele B., Bolondi L., et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19:682–693. doi: 10.1016/S1470-2045(18)30146-3. [DOI] [PubMed] [Google Scholar]
- 63.Merle P., Blanc J.F., Phelip J.M., Pelletier G., Bronowicki J.P., Touchefeu Y., Pageaux G., Gerolami R., Habersetzer F., Nguyen-Khac E., et al. Doxorubicin-loaded nanoparticles for patients with advanced hepatocellular carcinoma after sorafenib treatment failure (RELIVE): a phase 3 randomised controlled trial. Lancet Gastroenterol. Hepatol. 2019;4:454–465. doi: 10.1016/S2468-1253(19)30040-8. [DOI] [PubMed] [Google Scholar]
- 64.Abou-Alfa G.K., Meyer T., Cheng A.L., El-Khoueiry A.B., Rimassa L., Ryoo B.Y., Cicin I., Merle P., Chen Y., Park J.W., et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu A.X., Kang Y.K., Yen C.J., Finn R.S., Galle P.R., Llovet J.M., Assenat E., Brandi G., Pracht M., Lim H.Y., et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 66.Kudo M. Scientific rationale for combination immunotherapy of hepatocellular carcinoma with anti-PD-1/PD-L1 and anti-CTLA-4 antibodies. Liver Cancer. 2019;8:413–426. doi: 10.1159/000503254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bouattour M., Mehta N., He A.R., Cohen E.I., Nault J.C. Systemic Treatment for Advanced Hepatocellular Carcinoma. Liver Cancer. 2019;8:341–358. doi: 10.1159/000496439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rimassa L., Pressiani T., Merle P. Systemic Treatment Options in Hepatocellular Carcinoma. Liver Cancer. 2019;8:427–446. doi: 10.1159/000499765. [DOI] [PMC free article] [PubMed] [Google Scholar]