Key Points
Question
Is the incidence of intracerebral hemorrhage decreasing over time, and do risk factor profiles for intracerebral hemorrhage located in the deep vs lobar brain regions differ?
Findings
In this cohort study of 10 333 participants from the Framingham Heart Study, a generally stable age-adjusted intracerebral hemorrhage incidence rate was found between 1985 and 2016; an age-stratified analysis indicated a continued increase in the incidence rate among those 75 years and older, coinciding with a 3-fold increase in the use of anticoagulant medications. Hypertension was associated with intracerebral hemorrhage located in both the deep and lobar brain regions.
Meaning
Results of this study suggest that, although the intracerebral hemorrhage incidence rate has stabilized in the last 30 years in this cohort, it has not substantially decreased; the cumulative burden is likely to continue to increase.
Abstract
Importance
Intracerebral hemorrhage (ICH) has the highest mortality of all stroke types and is the most serious complication of anticoagulation. Data regarding trends in ICH incidence and location-specific risk factors on the population level are conflicting.
Objective
To assess long-term population-based trends in the incidence of ICH, examine incidence rates stratified by deep and lobar locations, and characterize location-specific risk factors.
Design, Setting, and Participants
This longitudinal prospective community-based cohort study comprised 10 333 original participants (n = 5209; age range, 28-62 years) and offspring participants (n = 5124; age range, 5-70 years) from the Framingham Heart Study who were followed up from January 1, 1948, to December 31, 2016. Original and offspring patient cohorts were confirmed to have experienced a spontaneous ICH event through imaging or pathologic testing. A total of 129 participants were identified with a primary incident of ICH. After exclusions, the remaining 99 patients were divided into 2 nested case-control samples, which were created by stratifying the first incident of ICH by brain region (lobar ICH or deep ICH), with 55 patients included in the lobar ICH sample and 44 patients included in the deep ICH sample. Patients were matched by age and sex (1:4 ratio) with 396 individuals without any stroke event (the control group). No participant in the patient samples was excluded or approached for consent, as their initial consent to participate in the Framingham Heart Study included consent to follow-up of cardiovascular outcomes. Data were analyzed in October 2019.
Main Outcomes and Measures
The unadjusted and age-adjusted ICH incidence rates, assessed in 3 periods (period 1, from 1948-1986; period 2, from 1987-1999; and period 3, from 2000-2016) to study incidence trends. Nested case-control samples were used to examine baseline risk factors and medication exposures with the incidence of ICH events located in the lobar and deep brain regions within the 10 years before participants experienced a stroke event.
Results
Of 10 333 original and offspring participants in the Framingham Heart Study, 129 patients (72 women [55.8%]; mean [SD] age, 77 [11] years) experienced a primary ICH incident during a follow-up period of 68 years (301 282 person-years), with an incidence rate of 43 cases per 100 000 person-years. The unadjusted incidence rate increased over time, but the age-adjusted incidence rate decreased slightly between periods 2 and 3. An age-stratified analysis indicated a continued increase in ICH incidence among patients 75 years and older, reaching 176 cases per 100 000 person-years in period 3. A concurrent 3-fold increase in the use of anticoagulant medications was observed, from 4.4% in period 2 to 13.9% in period 3. The incidence rate increased substantially with age for both lobar and deep ICH. Higher systolic and diastolic blood pressure and statin medication use (odds ratio [OR], 4.07; 95% CI, 1.16-14.21; P = .03) were associated with the incidence of deep ICH. Higher systolic blood pressure and apolipoprotein E ε4 allele homozygosity (OR, 3.66; 95% CI, 1.28-10.43; P = .02) were associated with the incidence of lobar ICH.
Conclusions and Relevance
This study found that the incidence of ICH increased in the oldest patients. Hypertension is a treatable risk factor for both deep and lobar ICH, while the use of statin medications is associated with the risk of a deep ICH event.
This cohort study examines data from original and offspring participants in the Framingham Heart Study to assess the incidence of intracerebral hemorrhage, incidence rates stratified by deep and lobar brain locations, and location-specific risk factors between 1948 and 2016.
Introduction
Intracerebral hemorrhage (ICH) has the highest mortality of all stroke types and is a leading cause of disability. Although numerous studies have reported a decrease in overall stroke incidence in high-income countries during the last 4 decades, the main factor in this trend is the decreased incidence of ischemic stroke. However, conflicting results have been published regarding ICH incidence. Some studies have suggested a decrease in ICH incidence,1,2 especially in persons younger than 75 years,3 and a decrease in hypertension-associated ICH.4 Other studies have suggested a stable overall ICH incidence rate5,6,7 or an increased ICH rate in patients 75 years and older,4 especially in the incidence of ICH located in the lobar region (lobar ICH).4 Higher incidence rates have also been reported among men.6,8
Furthermore, ICH is not a uniform disease, and ICH incidence may vary according to the presumed underlying pathophysiologic mechanisms. It has been traditionally proposed that the topographic location of ICH in the lobar vs deep (deep ICH) brain regions indicates different underlying pathophysiologic processes. Lobar ICH has been associated with cerebral amyloid angiopathy, while deep ICH is considered primarily a manifestation of hypertensive angiopathy.9 Observational studies within the past decade have suggested that inadequate blood pressure control has an independent association with ICH recurrence, regardless of ICH location,10 and is also likely implicated in the pathogenesis of lobar ICH.11 However, limited data are available from high-quality prospective longitudinal population-based cohort studies that describe trends in ICH rates and differences in risk factor profiles according to ICH location.
We undertook this observational longitudinal nested case-control study of original and offspring participants in the Framingham Heart Study with the aim to (1) characterize ICH incidence and incidence rates overall and stratified by brain topography, (2) examine temporal trends in ICH incidence rates during a follow-up period of more than 60 years, and (3) identify risk factors for an ICH incident according to brain topography.
Methods
Study Population
The Framingham Heart Study is a longitudinal community-based cohort study.12 The original cohort (generation 1) was enrolled in 1948 (n = 5209; age range, 28-62 years), and the participants have been reexamined biennially. The offspring cohort (generation 2)13 was enrolled in 1971 (n = 5124; age range, 5-70 years) and has been reexamined approximately every 4 years. An estimated 99% of the participants in the cohorts were white with European ancestry. From a sociodemographic perspective, the participants were in the middle class and had high educational levels (approximately 70% of participants were high school graduates, and 18% of participants were college graduates). At each follow-up visit, participants’ interim history, lifestyle risk factors, comorbidities, and medication use were reviewed and recorded. Blood pressure, height, and weight were also measured.
The institutional review board of Boston University Medical Center approved the consent form and the study design of the original Framingham Heart Study, and written informed consent was obtained from all participants. In the present study, no participant in the patient samples was excluded or approached for consent, as their initial consent to participate in the Framingham Heart Study included consent to follow-up of cardiovascular outcomes and subsequent analyses. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Definitions, Surveillance, and Ascertainment
The present study spans 68 years, during which some of the definitions of cardiovascular risk factors have changed. The presence or absence of a cardiovascular risk factor was defined in accordance with the accepted definition at the time of data recording.12 Hypertension was defined as systolic blood pressure (SBP) of more than 140 mm Hg, diastolic blood pressure (DBP) of more than 90 mm Hg, or the use of an antihypertensive medication.14 In addition to recording SBP and DBP, we calculated pulse pressure, which was defined as the difference between the SBP and DBP.
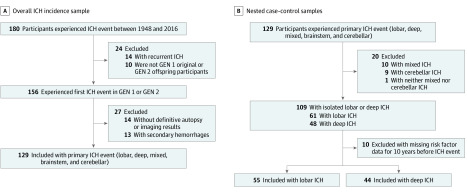
The Framingham Heart Study cohort was actively surveilled for possible end points of interest beginning at the date of the study’s inception.15 From January 1, 1948, to December 31, 2016, 180 total participants experienced an ICH event; of those, 24 participants were excluded (14 participants had recurrent ICH, and 10 participants were not original or offspring participants). The remaining 156 original and offspring participants from generations 1 and 2 were identified by the stroke review panel (V.-A.L., H.J.A., J.R.R., and S.S.) to have experienced an initial ICH event between 1948 and 2016; the medical records of these patients were further reviewed by a stroke neurologist (V.-A.L.). Participants with secondary hemorrhages (n = 13) and those without definitive autopsy results or imaging confirmation of intraparenchymal hemorrhage (n = 14) were excluded. The remaining 129 patients comprised the sample for this study, which was classified into patients who experienced a supratentorial deep ICH event, a lobar or mixed (ie, lobar and deep) ICH event, or an infratentorial (ie, cerebellar or brainstem) ICH event (Figure 1A). Incidents of deep ICH (n = 48; 24 men [50.0%]; mean [SD] age, 78 [12] years) were defined as those sparing the cortex and selectively involving the thalamus, basal ganglia, or corona radiata; incidents of brainstem ICH (n = 4) were also included within the deep ICH cases for the purpose of the subtype-specific analyses. Supratentorial hemorrhages involving the cortex or corticosubcortical areas and sparing the deep ICH locations were classified as lobar ICH (n = 61; 25 men [41.0%]; mean [SD] age, 76 [13] years).
Figure 1. Study Flowchart .
A, Overall ICH incidence sample. B, Nested case-control samples. Isolated deep ICH events included isolated brainstem hemorrhages in the pons, midbrain, or medulla regions (n = 4). ICH indicates intracerebral hemorrhage; GEN, generation.
Nested Case-Control Samples
We created 2 nested case-control samples from the subset of 129 participants who experienced a first incident of deep or lobar ICH between 1948 and 2016. Patients with a cerebellar ICH incident (n = 9) were not included owing to the small number of cases and the ambiguity with regard to the exact underlying pathophysiologic mechanisms. Patients with a mixed ICH incident (n = 10) or an ICH that was neither cerebellar nor mixed (n = 1) were also excluded from this part of the analysis owing to the small number of cases. We included only those participants who had attended a Framingham Heart Study clinical examination within the 10 years before they experienced a stroke event and excluded the 10 participants who had not. The remaining 99 patients were categorized into 2 nested case-control samples, which were created by stratifying the first incident of ICH by brain region, with 55 patients included in the lobar ICH sample and 44 patients included in the deep ICH sample (Figure 1B).
Each patient in the lobar ICH and deep ICH sample was matched by cohort, sex, and age (within 2 years) with 4 individuals in the control group (n = 396) who had not experienced a stroke and who had also attended a clinical examination within the 10 years before matching was performed.
Statistical Analyses
Our study sample comprised the combined group of 10 333 original and offspring cohort participants. Participants who did not experience an ICH event were censored at the date on which they were last known to be ICH free through 2016. We analyzed the ICH incidence overall and by period (period 1, from 1948-1986; period 2, from 1987-1999; and period 3, from 2000-2016). The second period began in 1987, which was chosen to reflect the more widespread adoption of computed tomographic imaging, which has been associated with increases in recorded stroke incidence, and to consider the changes in primary and secondary prevention practices, especially with regard to blood pressure control. The beginning of the third period (2000) was chosen to reflect further changes in secondary prevention practices, especially the widespread use of statin and anticoagulant medications and the introduction of non–vitamin K antagonist medications.
We estimated the unadjusted ICH incidence by age group (45-74 years, 75-84 years, and 85-99 years, dichotomized into groups aged <75 years and aged ≥75 years), sex, and period, and we used log-linear Poisson regression analyses to compare the age group–specific incidences. The age distribution varied by sex and period; hence, we used direct standardization to estimate incidence rates for sex and period adjusted by age group, and we used log-linear Poisson regression analyses to compare the levels of sex and period adjusted by age group. We repeated these analyses separately for deep ICH and lobar ICH using the nested case-control subset of 99 patients. We then compared the 99 patients with the 396 matched individuals in the control group; patients with deep ICH and lobar ICH were compared separately.
Summaries of categorical variables were reported as proportions, and continuous variables were reported as means and SDs. In each patient subset (deep ICH and lobar ICH), we used multivariable conditional logistic regression models to investigate the association of demographic and clinical characteristics with the risk of ICH, adjusted for age and time between the date of the clinical assessment and the date of the ICH event (or the matching dates for persons in the control group). In the subset of patients with deep ICH and lobar ICH only, we used multivariable logistic regression analyses to examine the associations between the risk factors and the type of ICH. We compared patients with deep ICH and lobar ICH with respect to all-cause mortality using Cox proportional hazards regression analyses. All tests were 2-sided and unpaired with a significance threshold of α < .05. Data analyses were conducted in October 2019 using SAS software, version 9.4 (SAS Institute Inc).
Results
Overall and Stratified ICH Incidence
The selection process and reasons for exclusion of patients with ICH are presented in the study flowchart (Figure 1A). Over a follow-up period of 68 years and 301 282 person-years, 129 patients (72 women [55.8%]; mean [SD] age, 77 [11] years) experienced a primary ICH event, with an incidence rate of 43 cases per 100 000 person-years. The overall ICH incidence is presented in Table 1 and eFigure 1 in the Supplement. The ICH incidence increased substantially with age in both sexes (Table 1; eFigure 1 in the Supplement); this increase was also observed when the ICH incidence was stratified by location (eTable 1, eTable 2, and eFigure 2 in the Supplement).
Table 1. Overall Intracerebral Hemorrhage Incidence Rates From 1948 to 2016, by Sex and Age.
| Variable | Women | Men | Combined women and men | Poisson regression | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Person-years | Incidence ratea | No. | Person-years | Incidence ratea | No. | Person-years | Incidence ratea | Estimate (95% CI) | P value | |
| Unadjusted | 72 | 171 072 | 42 | 57 | 130 210 | 44 | 129 | 301 282 | 43 | NA | NA |
| Age adjusted | NA | NA | 38 | NA | NA | 50 | NA | NA | NA | NA | NA |
| Age group, y | |||||||||||
| 45-74 | 21 | 133 306 | 16 | 25 | 109 000 | 23 | 46 | 242 306 | 19 | 1 [Reference] | Reference |
| 75-84 | 26 | 26 329 | 99 | 19 | 16 643 | 114 | 45 | 42 972 | 105 | 5.52 (3.66-8.32) | <.001 |
| 85-99 | 25 | 11 437 | 219 | 13 | 4567 | 285 | 38 | 16 004 | 237 | 12.51 (8.14-19.22) | <.001 |
Abbreviation: NA, not applicable.
Per 100 000 person-years.
The unadjusted incidence rates were comparable in women and men (42 cases per 100 000 person-years in women vs 44 cases per 100 000 person-years in men). Although the age-adjusted incidence rate was higher in men (50 cases per 100 000 person-years) compared with women (38 cases per 100 000 person-years) (Table 1), this difference was not statistically significant (eTable 3 in the Supplement). The incidence of lobar ICH was slightly more frequent than deep ICH, with an incidence rate of 18 cases per 100 000 person-years and 15 cases per 100 000 person-years, respectively (eTable 1 and eTable 2 in the Supplement).
Trends in ICH Incidence
The unadjusted incidence rate increased steadily over time, from 25 cases per 100 000 person-years in period 1 to 73 cases per 100 000 person-years in period 3. The unadjusted odds ratio (OR) of the incidence rate in period 3 compared with period 1 (the referent period) was 2.96 (95% CI, 1.94-4.51; P < .001) (Table 2 and Figure 2A). However, after age adjustment, the incidence rate decreased slightly (from 51 cases per 100 000 person-years to 46 cases per 100 000 person-years) between periods 2 and 3, after an initial increase between periods 1 and 2. The age-adjusted OR of the incidence rate in period 3 compared with period 1 was 1.45 (95% CI, 0.92-2.27; P = .11). The age-stratified analysis revealed that the incidence rate remained low in the group aged 45 to 74 years and increased slightly in the group aged 75 to 84 years (from 96 cases per 100 000 person-years to 113 cases per 100 000 person-years between periods 1 and 3); however, the incidence rate increased substantially between periods 1 and 3 in the group 85 years and older, from 39 cases per 100 000 person-years to 287 cases per 100 000 person-years (Figure 2B).
Table 2. Between-Period Comparison of Intracerebral Hemorrhage Incidence Rates From 1948 to 2016.
| Period | Cases, No. | Person-years | Unadjusted | Age adjusted | ||||
|---|---|---|---|---|---|---|---|---|
| Incidence ratea | Poisson regression | Incidence ratea | Poisson regression | |||||
| Estimate (95% CI) | P value | Estimate (95% CI) | P value | |||||
| 1 | 40 | 161 970 | 25 | 1 [Reference] | Reference | 30 | 1 [Reference] | Reference |
| 2 | 42 | 69 658 | 60 | 2.44 (1.58-3.76) | <.001 | 51 | 1.50 (0.95-2.34) | .08 |
| 3 | 47 | 64 353 | 73 | 2.96 (1.94-4.51) | <.001 | 46 | 1.45 (0.92-2.27) | .11 |
Per 100 000 person-years.
Figure 2. Intracerebral Hemorrhage Incidence From 1948 to 2016.
A, Overall incidence. Incidence per period is centered at the median year for each period. B, Age-stratified incidence. Incidence per period is centered at the median year for each period.
Given that only 1 event occurred in the oldest age group (≥85 years) in period 1, we dichotomized our cohort with a cutoff age of 75 years. For all patients 75 years and older, the ICH incidence rate increased from 88 cases per 100 000 person-years to 176 cases per 100 000 person-years between periods 1 and 3. The ICH incidence rate also increased between periods 2 and 3, from 158 cases per 100 000 person-years to 176 cases per 100 000 person-years. The pattern was similar for both patients with deep ICH and lobar ICH (data not shown).
Risk Factors and Mortality
Patients with deep ICH and individuals in the control group had comparable cardiovascular risk factor profiles, with the exception of hypertension (Table 3). Hypertension had a higher prevalence among patients (37 of 43 patients [86.3%]) compared with matched individuals (1267 of 174 individuals [72.4%]) in the control group, although the difference was not statistically significant. Patients with deep ICH had significantly higher SBP (mean [SD], 154 [26] mm Hg) compared with the control group (mean [SD], 143 [22] mm Hg; P = .008) and DBP (mean [SD], 81 [17] mm Hg) compared with the control group (mean [SD], 76 [13] mm Hg; P = .01); however, patients did not have significantly higher pulse pressure. Patients with deep ICH were more likely to be receiving a statin medication compared with individuals in the control group (7 of 21 patients [33.3%] vs 12 of 88 matched individuals [13.6%]; OR, 4.07; 95% CI, 1.16-14.21; P = .03). All-cause mortality was higher in patients with deep ICH (hazard ratio [HR], 5.84; 95% CI, 3.88-8.79; P < .001) compared with matched individuals in the control group.
Table 3. Nested Case-Control Analyses of Baseline Characteristics in Patients With Deep and Lobar Intracerebral Hemorrhage.
| Characteristic | Deep ICH | Lobar ICH | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient group, No./total No. (%) | Control group, No./total No. (%) | OR (95% CI)a,b | P value | Patient group, No./total No. (%) | Control group, No./total No. (%) | OR (95% CI)a,b | P value | |
| Total No. | 44 | 176 | NA | NA | 55 | 220 | NA | NA |
| Age at matching, mean (SD), y | 78 (12) | 78 (12) | NA | NA | 76 (13) | 76 (12) | NA | NA |
| Male sex | 21/44 (47.7) | 84/176 (47.7) | NA | NA | 24/55 (43.6) | 96/220 (43.6) | NA | NA |
| Educational level | ||||||||
| High school graduate | 31/44 (70.5) | 111/172 (64.5) | 1.32 (0.63-2.76) | .47 | 36/52 (69.2) | 155/212 (73.1) | 0.90 (0.43-1.88) | .79 |
| College graduate | 8/44 (18.2) | 25/172 (14.5) | 1.20 (0.49-2.92) | .70 | 7/52 (13.5) | 43/212 (20.2) | 0.57 (0.24-1.38) | .21 |
| Blood pressure, mean (SD), mm Hgc | ||||||||
| Systolic | 154 (26) | 143 (22) | 1.02 (1.01-1.04) | .008 | 148 (26) | 138 (23) | 1.02 (1.01-1.04) | .002 |
| Diastolic | 81 (17) | 76 (13) | 1.04 (1.01-1.07) | .01 | 77 (14) | 75 (13) | 1.02 (0.99-1.04) | .20 |
| Pulse pressure, mean (SD), mm Hg | 73 (23) | 67 (19) | 1.01 (1.00-1.03) | .18 | 71 (21) | 63 (20) | 1.03 (1.01-1.05) | .002 |
| Hypertension | 37/43 (86.0) | 126/174 (72.4) | 2.65 (0.98-7.16) | .06 | 42/53 (79.2) | 132/216 (61.1) | 2.89 (1.30-6.43) | .009 |
| Cardiovascular disease | 19/44 (43.2) | 52/176 (29.5) | 1.78 (0.87-3.64) | .11 | 18/55 (32.7) | 62/220 (28.2) | 1.13 (0.58-2.18) | .72 |
| Diabetes | 9/35 (25.7) | 17/133 (12.3) | 2.74 (0.98-7.67) | .06 | 4/43 (9.3) | 29/170 (17.1) | 0.40 (0.13-1.28) | .12 |
| Atrial fibrillation | 9/44 (20.5) | 21/176 (11.9) | 2.05 (0.79-5.35) | .14 | 6/55 (10.9) | 14/220 (6.4) | 1.68 (0.60-4.69) | .32 |
| Smoking | 3/41 (7.3) | 18/166 (10.8) | 0.74 (0.20-2.77) | .66 | 8/51 (15.7) | 30/208 (14.4) | 1.09 (0.45-2.63) | .85 |
| Total cholesterol, mean (SD), mg/dLc | 198 (42) | 204 (43) | 1.00 (0.99-1.01) | .75 | 203 (47) | 202 (41) | 1.00 (0.99-1.01) | .95 |
| High-density lipoprotein, mean (SD), mg/dLc | 52 (14) | 20 (14) | 1.00 (0.96-1.05) | .85 | 53 (16) | 55 (17) | 0.98 (0.95-1.02) | .33 |
| Statin usec | 7/21 (33.3) | 12/88 (13.6) | 4.07 (1.16-14.21) | .03 | 5/20 (25.0) | 21/84 (25.0) | 0.99 (0.29-3.38) | .98 |
| Anticoagulant usec | 4/31 (12.9) | 10/136 (7.4) | 2.37 (0.60-9.35) | .22 | 3/33 (9.1) | 4/137 (2.9) | 3.05 (0.66-14.06) | .15 |
| APOE4 c | 6/27 (22.2) | 25/132 (18.9) | 1.15 (0.40-3.29) | .80 | 11/28 (39.3) | 28/143 (19.6) | 3.66 (1.28-10.43) | .02 |
| APOE4 homozygous vs APOE3 homozygous, %c | 6/21 (28.6) | 24/109 (22.0) | 1.19 (0.39-3.64) | .76 | 10/23 (43.5) | 25/123 (20.3) | 5.64 (1.42-22.31) | .01 |
Abbreviations: APOE3, apolipoprotein E ε3 allele; APOE4, apolipoprotein E ε4 allele; ICH, intracerebral hemorrhage, NA, not applicable; OR, odds ratio.
OR for patient group.
OR was adjusted for age and time between clinical assessment and stroke event or matching date, respectively.
Missing data in more than 10% of participants.
Patients with lobar ICH did not differ from matched individuals in the control group in any demographic or cardiovascular risk factors, with the exception of hypertension and blood pressure components (Table 3). Compared with individuals in the control group, patients with lobar ICH had a higher hypertension prevalence (42 of 53 patients [79.2%] vs 132 of 216 matched individuals [61.1%]; OR, 2.89; 95% CI, 1.30-6.43; P = .009), elevated SBP (mean [SD], 148 [26] mm Hg in patients vs 138 [23] mm Hg in matched individuals; P = .002) and elevated pulse pressure (mean [SD], 71 [21] mm Hg in patients vs 63 [20] mm Hg in matched individuals; P = .002), but not a greater prevalence of elevated DBP. The presence of apolipoprotein E ε4 (APOE4; OMIM 107741) allele homozygosity was associated with a higher risk of lobar ICH (11 of 28 patients [39.3%] vs 28 of 143 matched individuals [19.6%]; OR, 3.66; 95% CI, 1.28-10.43; P = .02). This association was strengthened when the ε2/ε2, ε2/ε3, and ε2/ε4 allele genotypes were excluded (10 of 23 patients [43.5%] among those with APOE4 homozygosity vs 25 of 123 matched individuals [20.3%] among those without APOE4 homozygosity; OR, 5.64; 95% CI, 1.42-22.31; P = .01). All-cause mortality was higher in patients with lobar ICH (HR, 4.00; 95% CI, 2.81-5.72; P < .001) compared with matched individuals in the control group.
A direct comparison of risk factors in patients with deep ICH vs lobar ICH yielded no significant differences, although the comparison was underpowered owing to the small number of cases.
Anticoagulant Medication Use
Trends in the use of anticoagulant medications in the entire cohort and stratified by ICH location are summarized in eTable 4 in the Supplement. An overall significant increase was observed in anticoagulant medication use over time. A 3-fold increase in the use of anticoagulant medications was observed between periods 2 and 3, from 4.4% in period 2 to 13.9% in period 3. The adjusted rate of anticoagulant medication use in period 3 compared with period 2 was 3.28 (95% CI, 1.20-8.95; P = .02).
A similar trend was observed in both patients with lobar ICH and deep ICH but was more pronounced in those with deep ICH, in which the proportion of patients using anticoagulant medications increased from 4.8% in period 2 to 18.0% in period 3.
Discussion
In this longitudinal community-based study with a follow-up period of more than 60 years, we documented an increasing ICH incidence with age among participants of both sexes and among patients with deep ICH and lobar ICH. The unadjusted ICH incidence was higher in women, but no difference was observed between men and women in the age-adjusted incidence rates. The unadjusted ICH incidence has steadily increased over time, while the age-adjusted ICH incidence has decreased insignificantly in the last 30 years. In the age-stratified analyses, we observed a continued increase in ICH incidence in those 75 years and older. This increase has coincided with the increased use of statin and anticoagulant medications. Hypertension, especially systolic hypertension, was associated with both deep ICH and lobar ICH. Patients with deep ICH had a 4-fold higher likelihood of using statin medications, whereas higher pulse pressure and APOE4 homozygous carrier status were associated with the risk of lobar ICH.
The results of our investigation are consistent with previous studies in several important ways.6,8 We observed higher age-adjusted incidence rates of ICH in men compared with women, although the difference was not statistically significant, likely owing to lack of power. Unlike previous studies that reported a decrease in the incidence of deep ICH with a concurrent increase in the incidence of lobar ICH in those 75 years and older,4 we observed an increasing incidence of deep ICH and lobar ICH with advancing age. In accordance with studies conducted in high-income countries, we found a generally stable age-adjusted incidence of ICH during the past 30 years.4,7 This finding was likely associated with improvements in primary and secondary preventive practices, which is reflected in the well-documented decrease in the prevalence of risk factors in the Framingham Heart Study cohort; both hypertension prevalence and BP levels, which are the most important ICH risk factors, decreased steadily.16,17
The initial substantial increase in ICH incidence between periods 1 and 2 is likely associated with improvements in the diagnosis of ICH with the advent of neuroimaging, which has been associated with increases in case discovery and reported incidence.18 Despite the stabilization in age-adjusted ICH incidence, the overall incidence continues to increase. This increase is more substantial in those 75 years and older, among whom we observed an increase in ICH incidence over the last 30 years. These findings, in conjunction with the expected increase in life expectancy, suggest that the absolute number of individuals who experience an ICH event, particularly at an older age, will likely continue to increase despite improvements in primary and secondary preventive interventions. Such a trend has already been documented in other high-income countries.19
These ICH incidence trends also contrast with ischemic stroke trends, in which preventive practices have been associated with a substantial decrease in the age-standardized incidence of ischemic stroke.2,17,20 The increased use of antithrombotic medications and well-established ischemic stroke risk reduction therapies, which have been associated with adverse hemorrhagic effects, might account for part of this trend in ICH incidence. Several studies have indicated that the proportion of patients with ICH who use anticoagulant medications is steadily increasing.4,21 Our study indicated an increasing trend in anticoagulant medication use over time, especially among patients in the deep ICH subcohort, in which the proportion of those who used anticoagulant medications increased from 4.8% in period 2 to 18.0% in period 3.
The use of statin medications has also substantially increased in the last 20 years.22 Contrary to the association between anticoagulant medications and increases in the risk of hemorrhage, the association between statin medications and ICH is controversial. An association between statin medications and ICH was first reported in post hoc analyses of randomized clinical trials23,24 and was reported to be owing to their pleiotropic effects.25 Subsequent observational and epidemiologic data, however, are conflicting.26,27 In our cohort, patients with deep ICH had a 4-fold higher likelihood of using statin medications compared with matched individuals in the control group despite no significant differences in cardiovascular disease prevalence. However, we approach this finding with caution given the relatively low number of exposed individuals. In addition to hemorrhagic risk, an alternative way in which antithrombotic and statin medications may be associated with ICH is through their reported efficacy in reducing cardiovascular disease risk and associated mortality.28 By prolonging life expectancy and eliminating competing cardiovascular risks earlier in the life course, these medications might have indirect associations with the increased incidence of ICH.
Our study reaffirms the associations between elevated BP and deep ICH and between the APOE4 genotype and lobar ICH,29 with the latter indicating the association of cerebral amyloid angiopathy with lobar ICH. In addition, our findings support the importance of hypertension,10 especially systolic hypertension, as a risk factor for lobar ICH and highlight the complexity of the pathophysiologic underpinnings of lobar ICH. While cerebral amyloid angiopathy is substantially more prevalent in patients with lobar ICH compared with those with deep ICH, it is not ubiquitous.11 In a recent clinocopathologic and imaging study of ICH, approximately 50% of patients with lobar ICH had mild or absent cerebral amyloid angiopathy but had coexistent moderate or severe disease of other small blood vessels.11 The association between elevated pulse pressure and lobar ICH is a novel finding. Pulse pressure is a measure of arterial stiffness30 which has been associated with end-organ damage, such as coronary disease, cerebral atrophy, white matter changes, and the resultant slowing of processing speed and worsening of executive function.31 These associations are independent of SBP and DBP levels, and pulsatile stress is thought to play an integral role.
Strengths and Limitations
Our study has several strengths. It used a longitudinal design with a follow-up period of more than 60 years, which allowed for the analysis of temporal trends. Continuous surveillance ensured the reliable capture of clinical events. The Framingham Heart Study has a low loss to follow-up, with more than 95% of participants followed up until death. Case ascertainment is particularly rigorous. The longitudinal prospective nature of the cohort facilitated the creation of nested case-control samples, which allowed for the examination of risk factors for ICH in the 10 years before the end-point events.
Our study also has limitations. The study population comprised almost exclusively white individuals with European ancestry. Extrapolation of the findings to different socioeconomic environments should take into account the context of our study variables, which included risk factor distribution, access to health care, adherence to prevention practices, and medication use that reflected the standards and behaviors of a high-income western country. Although we obtained data on statin and anticoagulant medication use, we lacked consistent and reliable data on other antithrombotic medications, such as antiplatelet drugs, which have been associated with ICH risk. We lacked data on neuroimaging markers of ICH risk, such as cerebral microbleeds, which can add valuable information to risk stratification. We did not have sufficient power to address long-term cognitive implications and corroborate previous findings that suggested a higher risk of dementia after a lobar ICH event.32 We view our findings on trends in anticoagulant and statin medication use and the contemporaneous increasing patterns in overall ICH trends with caution; an association with increasing ICH incidence, especially in the oldest participants, is suggested, but causation cannot be inferred. Our sample size did not allow exploration of the possible interactions between medication classes and other risk factors.
Conclusions
We documented an overall stabilization in age-adjusted ICH incidence during the last 30 years but an increasing ICH incidence among patients in the older age group, which suggests that the cumulative incidence and prevalence are likely to continue to increase given the aging of the population and the increase in life expectancy. Therefore, health care systems may experience an increase in the overall number of patients with ICH in the future. We provided support for the importance of hypertension, especially systolic hypertension, as a risk factor for both deep and lobar ICH. Given the lack of therapy to treat ICH, emphasis should be placed on prevention, which needs to be optimized and intensified. The aging of the population and the more widespread use of anticoagulant medications suggest the need for more precise and accurate risk stratification strategies to mitigate the competing risks of ischemia and hemorrhage, especially in older individuals.
eTable 1. Age-Specific and Sex-Specific Deep Intracerebral Hemorrhage Incidence Rates in the Framingham Heart Study, 1948-2016
eTable 2. Age-Specific and Sex-Specific Lobar Intracerebral Hemorrhage Incidence Rates in the Framingham Heart Study, 1948-2016
eTable 3. Unadjusted and Age-Adjusted Incidence Rate Ratios of Intracerebral Hemorrhage in Men vs Women
eTable 4. Anticoagulant Use Trends
eFigure 1. Age-Stratified and Sex-Stratified Incidence of Intracerebral Hemorrhage
eFigure 2. Incidence Rate of Lobar and Deep Intracerebral Hemorrhage Stratified by Age
References
- 1.Krishnamurthi RV, Moran AE, Forouzanfar MH, et al. ; Global Burden of Diseases, Injuries, and Risk Factors 2010 Study Stroke Expert Group . The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob Heart. 2014;9(1):101-106. doi: 10.1016/j.gheart.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 2.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. ; Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group . Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1(5):e259-e281. doi: 10.1016/S2214-109X(13)70089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolink WM, Klijn CJ, Brouwers PJ, Kappelle LJ, Vaartjes I. Time trends in incidence, case fatality, and mortality of intracerebral hemorrhage. Neurology. 2015;85(15):1318-1324. doi: 10.1212/WNL.0000000000002015 [DOI] [PubMed] [Google Scholar]
- 4.Lovelock CE, Molyneux AJ, Rothwell PM; Oxford Vascular Study . Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol. 2007;6(6):487-493. doi: 10.1016/S1474-4422(07)70107-2 [DOI] [PubMed] [Google Scholar]
- 5.Carlsson M, Wilsgaard T, Johnsen SH, et al. Temporal trends in incidence and case fatality of intracerebral hemorrhage: the Tromso Study 1995-2012. Cerebrovasc Dis Extra. 2016;6(2):40-49. doi: 10.1159/000447719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giroud M, Delpont B, Daubail B, et al. Temporal trends in sex differences with regard to stroke incidence: the Dijon Stroke Registry (1987-2012). Stroke. 2017;48(4):846-849. doi: 10.1161/STROKEAHA.116.015913 [DOI] [PubMed] [Google Scholar]
- 7.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167-176. doi: 10.1016/S1474-4422(09)70340-0 [DOI] [PubMed] [Google Scholar]
- 8.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082-1090. doi: 10.1161/STROKEAHA.108.540781 [DOI] [PubMed] [Google Scholar]
- 9.Martini SR, Flaherty ML, Brown WM, et al. Risk factors for intracerebral hemorrhage differ according to hemorrhage location. Neurology. 2012;79(23):2275-2282. doi: 10.1212/WNL.0b013e318276896f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biffi A, Anderson CD, Battey TW, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA. 2015;314(9):904-912. doi: 10.1001/jama.2015.10082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues MA, Samarasekera N, Lerpiniere C, et al. The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol. 2018;17(3):232-240. doi: 10.1016/S1474-4422(18)30006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsao CW, Vasan RS. Cohort profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44(6):1800-1813. doi: 10.1093/ije/dyv337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. the Framingham offspring study. Am J Epidemiol. 1979;110(3):281-290. doi: 10.1093/oxfordjournals.aje.a112813 [DOI] [PubMed] [Google Scholar]
- 14.Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med. 1993;153(2):154-183. doi: 10.1001/archinte.1993.00410020010002 [DOI] [PubMed] [Google Scholar]
- 15.Andersson C, Johnson AD, Benjamin EJ, Levy D, Vasan RS. 70-Year legacy of the Framingham Heart Study. Nat Rev Cardiol. 2019;16(11):687-698. doi: 10.1038/s41569-019-0202-5 [DOI] [PubMed] [Google Scholar]
- 16.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523-532. doi: 10.1056/NEJMoa1504327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carandang R, Seshadri S, Beiser A, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296(24):2939-2946. doi: 10.1001/jama.296.24.2939 [DOI] [PubMed] [Google Scholar]
- 18.Drury I, Whisnant JP, Garraway WM. Primary intracerebral hemorrhage: impact of CT on incidence. Neurology. 1984;34(5):653-657. doi: 10.1212/WNL.34.5.653 [DOI] [PubMed] [Google Scholar]
- 19.Bejot Y, Bailly H, Graber M, et al. Impact of the ageing population on the burden of stroke: the Dijon Stroke Registry. Neuroepidemiology. 2019;52(1-2):78-85. doi: 10.1159/000492820 [DOI] [PubMed] [Google Scholar]
- 20.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8(4):355-369. doi: 10.1016/S1474-4422(09)70025-0 [DOI] [PubMed] [Google Scholar]
- 21.Liotta EM, Prabhakaran S. Warfarin-associated intracerebral hemorrhage is increasing in prevalence in the United States. J Stroke Cerebrovasc Dis. 2013;22(7):1151-1155. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.015 [DOI] [PubMed] [Google Scholar]
- 22.Salami JA, Warraich H, Valero-Elizondo J, et al. National trends in statin use and expenditures in the US adult population from 2002 to 2013: insights from the Medical Expenditure Panel Survey. JAMA Cardiol. 2017;2(1):56-65. doi: 10.1001/jamacardio.2016.4700 [DOI] [PubMed] [Google Scholar]
- 23.Goldstein LB, Amarenco P, Szarek M, et al. ; SPARCL Investigators . Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24, pt 2):2364-2370. doi: 10.1212/01.wnl.0000296277.63350.77 [DOI] [PubMed] [Google Scholar]
- 24.Collins R, Armitage J, Parish S, Sleight P, Peto R; Heart Protection Study Collaborative Group . Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20 536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363(9411):757-767. doi: 10.1016/S0140-6736(04)15690-0 [DOI] [PubMed] [Google Scholar]
- 25.Park HS, Gu JY, Yoo HJ, et al. Thrombin generation assay detects moderate-intensity statin-induced reduction of hypercoagulability in diabetes. Clin Appl Thromb Hemost. 2018;24(7):1095-1101. doi: 10.1177/1076029618766254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergouwen MD, de Haan RJ, Vermeulen M, Roos YB. Statin treatment and the occurrence of hemorrhagic stroke in patients with a history of cerebrovascular disease. Stroke. 2008;39(2):497-502. doi: 10.1161/STROKEAHA.107.488791 [DOI] [PubMed] [Google Scholar]
- 27.Gaist D, Goldstein LB, Cea Soriano L, Garcia Rodriguez LA. Statins and the risk of intracerebral hemorrhage in patients with previous ischemic stroke or transient ischemic attack. Stroke. 2017;48(12):3245-3251. doi: 10.1161/STROKEAHA.117.019141 [DOI] [PubMed] [Google Scholar]
- 28.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934. doi: 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 29.Biffi A, Sonni A, Anderson CD, et al. ; International Stroke Genetics Consortium . Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68(6):934-943. doi: 10.1002/ana.22134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franklin SS, Gustin W IV, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure: the Framingham Heart Study. Circulation. 1997;96(1):308-315. doi: 10.1161/01.CIR.96.1.308 [DOI] [PubMed] [Google Scholar]
- 31.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility—Reykjavik study. Brain. 2011;134(pt 11):3398-3407. doi: 10.1093/brain/awr253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moulin S, Labreuche J, Bombois S, et al. Dementia risk after spontaneous intracerebral haemorrhage: a prospective cohort study. Lancet Neurol. 2016;15(8):820-829. doi: 10.1016/S1474-4422(16)00130-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Age-Specific and Sex-Specific Deep Intracerebral Hemorrhage Incidence Rates in the Framingham Heart Study, 1948-2016
eTable 2. Age-Specific and Sex-Specific Lobar Intracerebral Hemorrhage Incidence Rates in the Framingham Heart Study, 1948-2016
eTable 3. Unadjusted and Age-Adjusted Incidence Rate Ratios of Intracerebral Hemorrhage in Men vs Women
eTable 4. Anticoagulant Use Trends
eFigure 1. Age-Stratified and Sex-Stratified Incidence of Intracerebral Hemorrhage
eFigure 2. Incidence Rate of Lobar and Deep Intracerebral Hemorrhage Stratified by Age