Key Points
Question
What are the clinical and laboratory characteristics of critically ill children who developed an inflammatory multisystem syndrome during the coronavirus disease 2019 pandemic?
Findings
This case series included 58 hospitalized children, a subset of whom required intensive care, and met definitional criteria for pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 (PIMS-TS), including fever, inflammation, and organ dysfunction. Of these children, all had fever and nonspecific symptoms, such as abdominal pain (31 [53%]), rash (30 [52%]), and conjunctival injection (26 [45%]); 29 (50%) developed shock and required inotropic support or fluid resuscitation; 13 (22%) met diagnostic criteria for Kawasaki disease; and 8 (14%) had coronary artery dilatation or aneurysms. Some clinical and laboratory characteristics had important differences compared with Kawasaki disease, Kawasaki disease shock syndrome, and toxic shock syndrome.
Meaning
These findings help characterize the clinical features of hospitalized, seriously ill children with PIMS-TS and provide insights into this apparently novel syndrome.
Abstract
Importance
In communities with high rates of coronavirus disease 2019, reports have emerged of children with an unusual syndrome of fever and inflammation.
Objectives
To describe the clinical and laboratory characteristics of hospitalized children who met criteria for the pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (PIMS-TS) and compare these characteristics with other pediatric inflammatory disorders.
Design, Setting, and Participants
Case series of 58 children from 8 hospitals in England admitted between March 23 and May 16, 2020, with persistent fever and laboratory evidence of inflammation meeting published definitions for PIMS-TS. The final date of follow-up was May 22, 2020. Clinical and laboratory characteristics were abstracted by medical record review, and were compared with clinical characteristics of patients with Kawasaki disease (KD) (n = 1132), KD shock syndrome (n = 45), and toxic shock syndrome (n = 37) who had been admitted to hospitals in Europe and the US from 2002 to 2019.
Exposures
Signs and symptoms and laboratory and imaging findings of children who met definitional criteria for PIMS-TS from the UK, the US, and World Health Organization.
Main Outcomes and Measures
Clinical, laboratory, and imaging characteristics of children meeting definitional criteria for PIMS-TS, and comparison with the characteristics of other pediatric inflammatory disorders.
Results
Fifty-eight children (median age, 9 years [interquartile range {IQR}, 5.7-14]; 20 girls [34%]) were identified who met the criteria for PIMS-TS. Results from SARS-CoV-2 polymerase chain reaction tests were positive in 15 of 58 patients (26%) and SARS-CoV-2 IgG test results were positive in 40 of 46 (87%). In total, 45 of 58 patients (78%) had evidence of current or prior SARS-CoV-2 infection. All children presented with fever and nonspecific symptoms, including vomiting (26/58 [45%]), abdominal pain (31/58 [53%]), and diarrhea (30/58 [52%]). Rash was present in 30 of 58 (52%), and conjunctival injection in 26 of 58 (45%) cases. Laboratory evaluation was consistent with marked inflammation, for example, C-reactive protein (229 mg/L [IQR, 156-338], assessed in 58 of 58) and ferritin (610 μg/L [IQR, 359-1280], assessed in 53 of 58). Of the 58 children, 29 developed shock (with biochemical evidence of myocardial dysfunction) and required inotropic support and fluid resuscitation (including 23/29 [79%] who received mechanical ventilation); 13 met the American Heart Association definition of KD, and 23 had fever and inflammation without features of shock or KD. Eight patients (14%) developed coronary artery dilatation or aneurysm. Comparison of PIMS-TS with KD and with KD shock syndrome showed differences in clinical and laboratory features, including older age (median age, 9 years [IQR, 5.7-14] vs 2.7 years [IQR, 1.4-4.7] and 3.8 years [IQR, 0.2-18], respectively), and greater elevation of inflammatory markers such as C-reactive protein (median, 229 mg/L [IQR 156-338] vs 67 mg/L [IQR, 40-150 mg/L] and 193 mg/L [IQR, 83-237], respectively).
Conclusions and Relevance
In this case series of hospitalized children who met criteria for PIMS-TS, there was a wide spectrum of presenting signs and symptoms and disease severity, ranging from fever and inflammation to myocardial injury, shock, and development of coronary artery aneurysms. The comparison with patients with KD and KD shock syndrome provides insights into this syndrome, and suggests this disorder differs from other pediatric inflammatory entities.
This case series describes the clinical and laboratory characteristics of children hospitalized in England from March to May 2020 who met criteria for pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (PIMS-TS), and compares syndrome characteristics with historical cases of Kawasaki disease (KD), KD shock syndrome, and toxic shock syndrome (TSS).
Introduction
From March through May 2020, during the coronavirus disease 2019 (COVID-19) pandemic, pediatricians in the United Kingdom and elsewhere noted hospitalizations of children who developed fever and multisystem inflammation. Some of these children were critically ill with shock and multiorgan failure and required intensive care,1,2,3 and some had characteristics that were similar to Kawasaki disease (KD) or KD shock syndrome.4,5 The clinical evidence suggested the emergence of a pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (PIMS-TS).6,7,8 The purpose of this study was to describe the clinical and laboratory characteristics of patients who met criteria for PIMS-TS and to compare the characteristics with other pediatric inflammatory disorders.
Methods
The study of the children with PIMS-TS had approval from local clinical research offices in the United Kingdom. The review of patient records was registered as an audit (Great Ormond Street Hospital), and anonymized patient data were collated without informed consent.
Inclusion of patients with KD and KD shock syndrome was approved by the institutional review board at the University of San Diego following informed consent from parents/guardians. Inclusion of patients with toxic shock syndrome from the EUCLIDS and PERFORM studies was approved by the UK research ethics bodies and ethics board of individual partners. Informed consent from parents and guardians was obtained for these patient cohorts.
Case Ascertainment
Following the initial UK National Health Service alert and publication of the Royal College of Paediatrics and Child Health definition7 of PIMS-TS, the World Health Organization (WHO), US Centers for Disease Control and Prevention (CDC), and the European Centre for Disease Prevention and Control6,8,9 have all produced definitions for the childhood inflammatory disorder that has emerged as the COVID-19 pandemic evolved in different countries (Table 1). All definitions had been developed based on a limited number of unpublished cases. The CDC and WHO definitions include laboratory evidence of SARS-CoV-2 exposure or history of contact with SARS-CoV-2 in the preceding month.
Table 1. Case Definitions for Emerging Inflammatory Condition During COVID-19 Pandemic From the World Health Organization, Royal College of Paediatrics and Child Health, and Centers for Disease Control and Prevention.
| World Health Organization8 | Royal College of Paediatrics and Child Health (United Kingdom)7 | Centers for Disease Control and Prevention (United States)9 |
|---|---|---|
|
|
|
Abbreviations: APTT, activated partial thromboplastin time; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ECHO, echocardiography; ESR, erythrocyte sedimentation rate; MIS-C, multisystem inflammatory syndrome in children; NT-proBNP, N-terminal pro–B-type natriuretic peptide; PT, prothrombin time; RT-PCR, reverse transcriptase–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Criteria for Kawasaki disease include persistent fever and 4 of 5 principal clinical features: erythema and cracking of lips, strawberry tongue, and/or erythema of oral and pharyngeal mucosa; bilateral bulbar conjunctival injection without exudate; rash (maculopapular, diffuse erythroderma); erythema and edema of the hands and feet and/or periungual desquamation; and cervical lymphadenopathy.
In this study, children were included who met the UK, CDC, or WHO definitions for PIMS-TS, without requiring proof of SARS-CoV-2 exposure, and investigated the value of this requirement in our analysis. Admission notes and transfer documents, including transfer letters and copies of referring hospital medical notes when available, were reviewed. Data were extracted from both electronic and paper records. Any report of mucocutaneous features during the course of the illness was recorded. Race/ethnicity was determined by parent report. The American Heart Association criteria for KD were used: persistent fever and 4 of 5 mucocutaneous features (erythema and cracking of lips, strawberry tongue, and/or erythema of oral and pharyngeal mucosa; bilateral bulbar conjunctival injection without exudate; rash [maculopapular, diffuse erythroderma]; erythema and edema of the hands and feet in acute phase and/or periungual desquamation in subacute phase; and cervical lymphadenopathy [>1.5 cm diameter]).10 Clinical patterns were established after review of numerous cases and included (1) a group with shock (inotrope use or fluid resuscitation >20 mL/kg); (2) a group that met criteria for KD; and (3) a group with fever and inflammation who did not have shock or did not meet the clinical criteria for KD. SARS-CoV-2 IgG was measured using EDI Novel Coronavirus COVID-19 IgG ELISA Kit (Epitope Diagnostics Inc).
Comparison With KD, KD Shock Syndrome, and Toxic Shock Syndrome
Because some features of the children who met criteria for PIMS-TS overlapped with features of KD and KD shock syndrome, clinical features of cases were compared with patients with KD and those with KD shock syndrome, seen between 2002 and 2019 at Rady Children’s Hospital San Diego. Clinical features also were compared with those of children with toxic shock syndrome from the PERFORM and EUCLIDS studies of febrile children in the European Union who were seen between 2012 and 2020 (details in eMethods in Supplement 2).
Data Management
Clinical characteristics and laboratory and other measurements were compared descriptively between the children who met criteria for PIMS-TS and children with KD, KD shock syndrome, and toxic shock syndrome from the previous cohorts. Because of the small number of cases, large number of comparisons, and differences in how the cohorts were assembled, formal statistical testing was not conducted; the findings should be interpreted as descriptive and exploratory.
Results
Between March 23 and May 16, 2020, 58 children who had been admitted to 8 hospitals in England were identified by invited survey and considered to meet the PIMS-TS criteria (Table 1). Eight of the children included in this study have previously been reported.2
Clinical Characteristics of Patients
The median age was 9 years (IQR, 5.7-14; range, 3 months-17 years), 20 were girls (34%), and 40 (69%) were of black or Asian race (Table 2). Most patients were previously healthy and only 7 had comorbidities, including 3 with asthma, 1 with neurodisability, 1 with epilepsy, 1 with sickle cell trait, and 1 with alopecia.
Table 2. Demographics and Clinical Features of the PIMS-TS Cohort.
| Characteristic | No. (%)a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All PIMS-TS cases (n = 58)b | Febrile and inflammatory (n = 23)c | Stratification by shockd | Stratification by Kawasaki diseasee | Stratification by Kawasaki clinical criteriae | Stratification by coronary artery aneurysmf | Stratification by evidence of SARS-CoV-2 infectiong | ||||||
| Shock present (n = 29) | Shock absent (n = 29) | Kawasaki disease (n = 13) | Not Kawasaki disease (n = 45) | Criteria met (n = 7) | Criteria not met (n = 51) | Present (n = 8) | Absent (n = 50) | Positive (n = 45) | Negative (n = 13) | |||
| Age, median (IQR), y | 9 (5.7-14) | 10 (5.5-14) | 10.5 (7-14) | 10 (3-14) | 8 (5-11) | 10.5 (5.7-14) | 6 (2-8) | 10 (6-14) | 9.5 (8-12.3) | 9 (5-11) | 10 (6-14) | 7 (2.5-14) |
| Sex | ||||||||||||
| Male | 38 (66) | 17 (74) | 16 (55) | 22 (76) | 10 (77) | 28 (62) | 6 (86) | 32 (63) | 6 (75) | 32 (64) | 30 (67) | 8 (61) |
| Female | 20 (34) | 6 (26) | 13 (45) | 7 (24) | 3 (23) | 17 (38) | 1 (14) | 19 (37) | 2 (25) | 18 (36) | 15 (33) | 5 (39) |
| Race/ethnicity | ||||||||||||
| Black | 22 (38) | 7 (30) | 14 (48) | 8 (28) | 8 (62) | 14 (31) | 2 (29) | 20 (39) | 7 (88) | 15 (30) | 18 (40) | 4 (31) |
| Asian | 18 (31) | 6 (26) | 6 (21) | 6 (21) | 0 | 12 (27) | 0 | 12 (24) | 0 | 12 (24) | 11 (24) | 1 (8) |
| White | 12 (21) | 8 (35) | 6 (21) | 12 (42) | 4 (31) | 14 (31) | 4 (57) | 14 (27) | 1 (13) | 17 (34) | 13 (29) | 5 (38) |
| Otherh | 6 (10) | 2 (9) | 3 (10) | 3 (10) | 1 (8) | 5 (11) | 1 (14) | 5 (10) | 0 | 6 (12) | 3 (7) | 3 (23) |
| Clinical features at presentationi | ||||||||||||
| Abdominal pain | 31 (53) | 13 (57) | 18 (62) | 13 (45) | 2 (15) | 29 (64) | 1 (14) | 30 (59) | 2 (33) | 29 (58) | 24 (55) | 7 (50) |
| Diarrhea | 30 (52) | 10 (44) | 19 (66) | 11 (38) | 7 (54) | 23 (51) | 2 (29) | 28 (55) | 6 (75) | 24 (48) | 25 (75) | 5 (36) |
| Rash | 30 (52) | 9 (39) | 15 (50) | 15 (50) | 10 (77) | 20 (44) | 7 (100) | 23 (45) | 5 (63) | 25 (50) | 21 (48) | 9 (64) |
| Shockd | 29 (50) | 0 | 29 (100) | 0 | 6 (46) | 23 (51) | 1 (14) | 28 (55) | 6 (75) | 23 (46) | 25 (56) | 4 (31) |
| Vomiting | 26 (45) | 10 (44) | 15 (52) | 11 (38) | 5 (38) | 21 (47) | 2 (29) | 24 (47) | 5 (63) | 21 (42) | 20 (45) | 6 (43) |
| Conjunctival injection | 26 (45) | 9 (39) | 11 (38) | 15 (52) | 11 (85) | 15 (33) | 7 (100) | 19 (37) | 5 (63) | 21 (42) | 20 (45) | 6 (43) |
| Mucous membrane changes | 17 (29) | 5 (22) | 6 (21) | 11 (38) | 6 (46) | 11 (24) | 6 (86) | 11 (22) | 1 (13) | 16 (32) | 11 (25) | 6 (43) |
| Headache | 15 (26) | 4 (17) | 11 (38) | 4 (14) | 4 (31) | 11 (24) | 1 (14) | 14 (27) | 4 (50) | 11 (22) | 13 (30) | 2 (14) |
| Respiratory symptoms | 12 (21) | 2 (13) | 9 (31) | 3 (10) | 3 (23) | 9 (20) | 1 (14) | 11 (22) | 3 (38) | 9 (18) | 9 (20) | 3 (21) |
| Lymphadenopathy | 9 (16) | 3 (13) | 2 (7) | 7 (24) | 5 (38) | 4 (9) | 4 (57) | 5 (10) | 2 (33) | 7 (14) | 8 (18) | 1 (7) |
| Swollen hands and feet | 9 (16) | 2 (13) | 4 (14) | 5 (17) | 4 (31) | 5 (11) | 4 (57) | 5 (10) | 1 (13) | 7 (14) | 7 (16) | 2 (14) |
| Sore throat | 6 (10) | 1 (4) | 5 (17) | 1 (3) | 0 | 6 (13) | 0 | 6 (12) | 1 (13) | 5 (10) | 6 (14) | 0 |
| Confusion | 5 (9) | 0 | 5 (17) | 0 | 1 (8) | 4 (9) | 0 | 5 (10) | 1 (13) | 4 (8) | 5 (11) | 0 |
Abbreviations: IQR, interquartile range; PIMS-TS, pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Clinical features are listed in order of frequency. In addition, pairwise comparison is included dividing the cohort by febrile and inflammatory, shock, Kawasaki disease, clinical diagnostic criteria of Kawasaki, presence of coronary artery aneurysm, and laboratory evidence for SARS-CoV-2 infection.
Fever >38 °C for >72 hours was an entry point to the study.
Febrile and inflammatory only: this cohort of children were those who did not meet the criteria for shock (footnote d) or the clinical diagnostic criteria for Kawasaki disease (footnote e).
Shock was defined as needing inotrope support or fluid resuscitation >20 mL/kg.
American Heart Association criteria for the definition of Kawasaki disease is to have persistent fever and 4 of the following 5 mucocutaneous features: erythema and cracking of lips, strawberry tongue, and/or erythema of oral and pharyngeal mucosa; bilateral bulbar conjunctival injection without exudate; rash (maculopapular, diffuse erythroderma); erythema and edema of the hands and feet in acute phase and/or periungual desquamation in subacute phase; and cervical lymphadenopathy (>1.5 cm diameter). Patients with fewer than 4 features were stratified as having Kawasaki disease if coronary artery aneurysms were present. In the absence of coronary artery changes, stratification by Kawasaki clinical criteria required 4 of 5 features to be present.
Coronary artery aneurysm is dilatation of any coronary artery seen on echocardiogram with a z score of >2.0 in the acute phase.
SARS-CoV-2 infection includes positive SARS-CoV-2 polymerase chain reaction or positive SARS-CoV-2 IgG serology results.
Other includes those of mixed race/ethnicity, Middle Eastern, or other ethnicity (https://www.ethnicity-facts-figures.service.gov.uk/ethnic-groups).
Presentation refers to the admission clerking to hospital, for example, the point at which the patient was considered to have a potential diagnosis of PIMS-TS.
All patients presented with persistent fever for 3 to 19 days and variable combinations of sore throat (n = 6 [10%]), headache (n = 15 [26%]), and abdominal pain (n = 31 [53%]). Erythematous rashes (1 patient had purpuric features) were present in 30 (52%). Conjunctival injection was noted in 26 (45%), lymphadenopathy in 9 (16%), mucus membrane changes and red cracked lips in 17 (29%), and swollen hands and feet in 9 (16%). Admission to pediatric critical care units was required in 29 patients (50%) and 13 (22%) developed acute kidney injury. Shock requiring inotropic support was present in 27 patients (47%). Mechanical ventilation was used for respiratory support in 25 patients (43%) (Table 3).
Table 3. Clinical Outcomes and Management.
| Characteristic | No. (%)a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All PIMS-TS cases (n = 58)b | Febrile and inflammatory (n = 23)c | Stratification by shockd | Stratification by Kawasaki diseasee | Stratification by Kawasaki clinical criteriae | Stratification by coronary artery aneurysmf | Stratification by evidence of SARS-CoV-2 infectiong | ||||||
| Shock present (n = 29) | Shock absent (n = 29) | Kawasaki disease (n = 13) | Not Kawasaki disease (n = 45) | Criteria met (n = 7) | Criteria not met (n = 51) | Present (n = 8) | Absent (n = 50) | Positive (n = 45) | Negative (n = 13) | |||
| Cardiac/circulatory/kidney | ||||||||||||
| Acute kidney injuryh | 13 (22) | 2 (9) | 11 (38) | 2 (7) | 3 (23) | 10 (22) | 0 | 13 (25) | 3 (38) | 10 (20) | 11 (24) | 2 (67) |
| Inotropic support | 27 (47) | 0 | 27 (93) | 0 | 6 (46) | 21 (47) | 1 (14) | 26 (51) | 6 (75) | 21 (42) | 23 (52) | 4 (29) |
| Extracorporeal membrane oxygenation | 3 (5) | 0 | 3 (10.3) | 0 | 0 | 3 (7) | 0 | 3 (60) | 0 | 3 (6) | 3 (7) | 0 |
| Respiratory | ||||||||||||
| Intubation | 25 (43) | 2 (9) | 23 (79) | 2 (7) | 5 (38) | 20 (44) | 1 (14) | 24 (47) | 5 (63) | 20 (40) | 20 (45) | 5 (36) |
| Pharmacotherapy | ||||||||||||
| Intravenous immunoglobulin | 41 (71) | 14 (61) | 21 (72) | 20 (69) | 13 (100) | 28 (62) | 7 (100) | 34 (68) | 8 (100) | 33 (66) | 33 (75) | 8 (57) |
| Corticosteroids | 37 (64) | 12 (52) | 19 (66) | 18 (62) | 12 (92) | 25 (56) | 7 (100) | 30 (59) | 7 (88) | 30 (60) | 33 (75) | 4 (29) |
| Anakinra (IL-1 receptor antagonist) | 3 (5) | 1 (4) | 2 (7) | 1 (3.4) | 0 | 3 (7) | 0 | 3 (6) | 0 | 3 (6) | 2 (5) | 1 (8) |
| Infliximab (TNF-α antagonist) | 8 (14) | 4 (17) | 2 (7) | 6 (21) | 4 (31) | 4 (9) | 3 (43) | 5 (19) | 3 (38) | 5 (10) | 7 (16) | 1 (8) |
| No. of immunomodulatory agents | ||||||||||||
| 2i | 35 (60) | 11 (48) | 18 (62) | 17 (59) | 12 (92) | 23 (51) | 7 (100) | 28 (55) | 7 (88) | 28 (56) | 32 (71) | 3 (23) |
| 3j | 9 (16) | 4 (17) | 3 (10) | 6 (21) | 4 (31) | 5 (11) | 3 (43) | 6 (12) | 3 (38) | 6 (12) | 8 (18) | 1 (8) |
| Outcomes | ||||||||||||
| Coronary artery aneurysm (z score >2) | 8 (14) | 1 (4) | 5 (17) | 3 (10) | 8 (62) | 0 | 1 (14) | 7 (14) | 8 (100) | 0 | 6 (13) | 2 (15) |
| Death | 1 (2) | 0 | 1 (3) | 0 | 0 | 1 (2) | 0 | 1 (2) | 0 | 1 (2) | 1 (2) | 0 |
Abbreviations: PIMS-TS, pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor.
A pairwise comparison is included dividing the cohort by febrile and inflammatory, shock, Kawasaki disease, clinical diagnostic criteria of Kawasaki, presence of coronary artery aneurysm, and laboratory evidence for SARS-CoV-2 infection.
Fever >38 °C for >72 hours was an entry point to the study.
Febrile and inflammatory only: this cohort of children were those who did not meet the criteria for shock (footnote d) or the clinical diagnostic criteria for Kawasaki disease (footnote e).
Shock was defined as needing inotrope support or fluid resuscitation >20 mL/kg.
American Heart Association criteria for the definition of Kawasaki disease is to have persistent fever and 4 of the following 5 mucocutaneous features: erythema and cracking of lips, strawberry tongue, and/or erythema of oral and pharyngeal mucosa; bilateral bulbar conjunctival injection without exudate; rash (maculopapular, diffuse erythroderma); erythema and edema of the hands and feet in acute phase and/or periungual desquamation in subacute phase; and cervical lymphadenopathy (>1.5 cm diameter). Patients with fewer than 4 features were stratified as having Kawasaki disease if coronary artery aneurysms were present. In the absence of coronary artery changes, stratification by Kawasaki clinical criteria required 4 of 5 features to be present.
Coronary artery aneurysm is dilatation of any coronary artery seen on echocardiogram with a z score of >2.0 in the acute phase.
SARS-CoV-2 infection includes positive SARS-CoV-2 polymerase chain reaction or positive SARS-CoV-2 IgG serology results.
Acute kidney injury defined by creatinine level greater than the upper limit for age.
Two agents of intravenous immunoglobulin, corticosteroids, anakinra, or infliximab were given to manage inflammation.
Three agents of intravenous immunoglobulin, corticosteroids, anakinra, or infliximab were given to manage inflammation.
SARS-CoV-2 Test Results
Results from polymerase chain reaction (PCR) tests to detect SARS-CoV-2 were positive in 26% (n = 15) (Table 4). IgG antibody against SARS-CoV-2 was positive in 40 of 46 patients (87%) (IgG antibody was not tested in 21% [12/58] and was negative in 13% [6/46]). In total, 45 of 58 patients (78%) had evidence of current or prior SARS-CoV-2 infection. There were no meaningful differences in clinical and laboratory features between patients who either were not tested for SARS-CoV-2 antibody, who had negative results on both antibody and PCR tests compared with patients who tested positive for SARS-CoV-2, or between patients with or without confirmed exposure to SARS-CoV-2 (eFigure 1 and eTable 1 in Supplement 2).
Table 4. Laboratory Results.
| Reference range | Median (IQR)a | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All PIMS-TS cases (n = 58)b | Febrile and inflammatory (n = 23)c | Stratification by shockd | Stratification by Kawasaki diseasee | Stratification by Kawasaki clinical criteriae | Stratification by coronary artery aneurysmf | Stratification by evidence of SARS-CoV-2 infectiong | |||||||
| Shock present (n = 29) | Shock absent (n = 29) | Kawasaki disease (n = 13) | Not Kawasaki disease (n = 45) | Criteria met (n = 7) | Kawasaki criteria not met (n = 51) | Present (n = 8) | Absent (n = 50) | Positive (n = 45) | Negative (n = 13) | ||||
| Virology, No. (%) | |||||||||||||
| SARS-CoV-2 respiratory PCR positive | 15 (26) | 5/23 (22) | 10 (35) | 5 (17) | 0 | 15 (33) | 0 | 15 (29) | 0 | 15 (30) | 15 (33) | ||
| SARS-CoV-2 IgG antibody | 40/46 (83) | 15/18 (83) | 22/25 (88) | 18/23 (78) | 8/12 (67) | 32/36 (89) | 4/6 (67) | 36/42 (86) | 6 (75) | 34/40 (75) | 40/42 (95) | ||
| Any SARS-CoV-2 PCR or IgG positive | 45/58 (78) | 17 (74) | 25 (86) | 20 (69) | 8 (62) | 37 (82) | 4 (57) | 41 (80) | 6 (75) | 39 (78) | 45 (100) | ||
| No positive test result | 13 (22) | 6 (26) | 4 (14) | 9 (31) | 5 (39) | 8 (18) | 3 (43) | 10 (20) | 2 (25) | 11 (22) | 0 | 13/13 (100) | |
| Laboratory values | |||||||||||||
| Hematology | |||||||||||||
| Total white blood cell count, ×109/L | 4-13.5 | 17 (12-22) [n = 58] | 16 (11.2-19) [n = 23] | 18 (14-28) [n = 29] | 17 (11.3-18.8) [n = 29] | 17 (13.5-26.4) [n = 13] | 17 (12.15-22.6) [n = 45] | 17 (11-17) [n = 7] | 17.4 (12.5-22.4) [n = 51] | 20 (15-29) [n = 8] | 17 (11.6-21.7) [n = 50] | 17 (12-23) [n = 45] | 17 (13-21) [n = 13] |
| Neutrophil count, ×109/L | 1.5-7 | 13 (10-19) [n = 58] | 10.7 (7.4-16) [n = 23] | 16 (11-25) [n = 29] | 10.8 (6.8-16) [n = 29] | 13.2 (10.2-16.4) [n = 13] | 12.5 (8.5-19.5) [n = 45] | 12.5 (6-14) [n = 7] | 14 (10.1-19.2) [n = 51] | 16 (13-26) [n = 8] | 12 (7.9-18.9) [n = 50] | 14 (9-20) [n = 45] | 13 (8-18) [n = 13] |
| Lymphocyte count, ×109/L | 1.5-4 | 0.8 (0.5-1.5) [n = 58] | 1.2 (0.7-2.9) [n = 23] | 0.7 (0.4-0.9) [n = 29] | 1.3 (0.7-2.8) [n = 29] | 1.2 (0.5-1.6) [n = 13] | 0.8 (0.6-1.5) [n = 45] | 1.3 (0.5-1.8) [n = 7] | 0.8 (0.5-1.4) [n = 51] | 0.6 (0.4-1.3) [n = 8] | 0.8 (0.6-1.6) [n = 50] | 0.8 (0.4-1.4) [n = 45] | 0.8 (0.5-2.9) [n = 13] |
| Hemoglobin, g/L | 111-147 | 92 (83-103) [n = 51] | 97 (87-108) [n = 19] | 85 (74-100) [n = 27] | 99.5 (88-109) [n = 24] | 88.5 (72-109) [n = 12] | 92.5 (83-102) [n = 39] | 109 (84-110) [n = 6] | 91 (83-101.5) [n = 45] | 80 (70-95) [n = 8] | 93 (83-106) [n = 43] | 93 (83-103) [n = 42] | 88 (79-106) [n = 9] |
| Platelet count, ×109/L | 200-450 | 151 (104-210) [n = 55] | 175.5 (101-209) [n = 22] | 136 (75-214) [n = 28] | 176 (118-210) [n = 27] | 176 (125-262) [n = 12] | 147.5 (93-195) [n = 43] | 176 (106-302) [n = 6] | 150 (101-210) [n = 49] | 173 (123-230) [n = 8] | 151 (97-209) [n = 47] | 142 (91-201) [n = 42] | 180 (129-332) [n = 13] |
| Inflammatory markers | |||||||||||||
| C-reactive protein, mg/L | 0-5 | 229 (156-338) [n = 58] | 176 (82-192) [n = 23] | 321 (223-371) [n = 29] | 176 (83-229) [n = 29] | 295 (173-357) [n = 13] | 206 (151-331) [n = 45] | 238 (106-339) [n = 7] | 220 (156-338) [n = 51] | 301 (205-361) [n = 8] | 191 (132-330.5) [n = 50] | 251 (158-342) [n = 45] | 220 (131-323) [n = 13] |
| Ferritin, μg/L | 7-140 | 610 (359-1280) [n = 52] | 379.5 (195-831) [n = 20] | 888 (556-1530) [n = 28] | 378 (180-907) [n = 25] | 620 (306.3-1254) [n = 12] | 592 (373-1443) [n = 41] | 357 (146-1078) [n = 6] | 631 (381-1342) [n = 47] | 637 (376-1076) [n = 8] | 574 (355-1378) [n = 45] | 679 (374-1249) [n = 42] | 495 (190-1627) [n = 11] |
| Biochemistry | |||||||||||||
| Lactate dehydrogenase, U/L | 125-243 | 419 (319-887) [n = 41] | 327 (274-463) [n = 15] | 764 (291-989) [n = 23] | 327 (273.5-451.8) [n = 18] | 373 (309-828) [n = 9] | 448 (319-912.5) [n = 32] | 359 (246-373) [n = 3] | 434 (323-906) [n = 38] | 615 (371-905) [n = 6] | 408 (311-900) [n = 35] | 414 (310-915) [n = 34] | 1104 (327-1209) [n = 7] |
| ALT, U/L | 0-34 | 42 (26-95) [n = 56] | 40 (21-79) [n = 23] | 47 (30-107) [n = 28] | 31.5 (20-77) [n = 28] | 36.5 (18.75-117.8) [n = 12] | 42 (27-97) [n = 44] | 26 (12-141) [n = 6] | 43 (28-96) [n = 50] | 86 (34-129) [n = 8] | 40 (25-77) [n = 48] | 42 (30-95) [n = 43] | 28 (22-273) [n = 13] |
| Albumin, g/L | 35-54 | 24 (21-27) [n = 51] | 27 (24-33) [n = 19] | 22 (20-24) [n = 27] | 27 (25-32) [n = 24] | 24 (20-27) [n = 12] | 24 (21-29) [n = 39] | 27 (23-28) [n = 6] | 24 (21-28) [n = 45] | 21 (18-26) [n = 8] | 25 (21-29) [n = 43] | 24 (21-27) [n = 41] | 27 (21-31) [n = 10] |
| Creatinine, μmol/L | 30-80 (varies with age) | 71 (43-108) [n = 48] | 62 (42-93) [n = 19] | 78 (42-104) [n = 32] | 61 (45-92) [n = 20] | 72 (46-123) [n = 8] | 71 (41-102) [n = 33] | 42 (40-46) [n = 3] | 76 (40-118) [n = 25] | 72 (46-122) [n = 8] | 71 (40-101) [n = 33] | 67 (44-116) [n = 30] | 76 (40-96) [n = 11] |
| Cardiac markers | |||||||||||||
| Troponin, ng/L | 0-15 | 45 (8-294) [n = 56] | 8 (5-45) [n = 17] | 124 (45-497) [n = 26] | 8 (5-45) [n = 22] | 19.3 (7-153) [n = 12] | 45.1 (8-355) [n = 38] | 10 (5-38) [n = 6] | 47.5 (11-353) [n = 44] | 100 (25-379) [n = 7] | 45 (7-278) [n = 43] | 45 (8-202) [n = 41] | 256 (9-598) [n = 9] |
| NT-proBNP, pg/mL | <100 | 788 (174-10 548) [n = 29] | 310.5 (106-1354) [n = 17] | 14 017 (7004-35 000) [n = 11] | 212.5 (70-876) [n = 18] | 788 (56-32 169) [n = 7] | 921.5 (180-9962) [n = 22] | 118 (23-636) [n = 4] | 1833 (213-12 868) [n = 25] | 32 169 (1994-35 000) [n = 3] | 629 (155-7597) [n = 26] | 1140 (184-11 719) [n = 27] | 11 (10-12) [n = 2] |
| Coagulation | |||||||||||||
| Fibrinogen, g/L | 1.99-4.09 | 5.7 (4.4-7) [n = 51] | 4.8 (3.5-5.8) [n = 18] | 6.1 (5-7.3) [n = 27] | 4.9 (3.9-6.7) [n = 24] | 7.1 (4.8-7.6) [n = 13] | 5.7 (4.3-6.8) [n = 38] | 6 (4.7-7.4) [n = 7] | 5.7 (4.3-6.9) [n = 44] | 6.9 (5.7-7.8) [n = 8] | 5.5 (4.3-6.8) [n = 43] | 5.8 (4.4-7.1) [n = 42] | 5.5 (3.8-7.6) [n = 9] |
| D-dimer, ng/mL | 100-560 | 3578 (2085-8235) [n = 53] | 2402 (1336-4248) [n = 20] | 5935 (3548-12 842) [n = 28] | 2383 (1357-4360) [n = 25] | 3238 (969-6262) [n = 11] | 3578 (2205-10 000) [n = 42] | 3494 (1733-6650) [n = 6] | 3578 (2205-8729) [n = 47] | 4375 (2662-6906) [n = 6] | 3564 (1964-10 000) [n = 47] | 3910 (2563-10 000) [n = 27] | 2094 (1379-5815) [n = 10] |
Abbreviations: ALT, alanine aminotransferase; IQR, interquartile range; NT-proBNP, N-terminal pro–B-type natriuretic peptide; PIMS-TS, pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SI conversion factors: To convert ALT and lactate dehydrogenase to μkat/L, multiply by 0.0167; to convert creatinine to mg/dL, divide by 88.4.
A pairwise comparison is included dividing the cohort by febrile and inflammatory, shock, Kawasaki disease, clinical diagnostic criteria of Kawasaki, presence of coronary artery aneurysm, and laboratory evidence for SARS-CoV-2 infection.
Fever >38 °C for >72 hours was an entry point to the study.
Febrile and inflammatory only: this cohort of children were those who did not meet the criteria for shock (footnote d) or the clinical diagnostic criteria for Kawasaki disease (footnote e).
Shock was defined as needing inotrope support or fluid resuscitation >20 mL/kg.
American Heart Association criteria for the definition of Kawasaki disease is to have persistent fever and 4 of the following 5 mucocutaneous features: erythema and cracking of lips, strawberry tongue, and/or erythema of oral and pharyngeal mucosa; bilateral bulbar conjunctival injection without exudate; rash (maculopapular, diffuse erythroderma); erythema and edema of the hands and feet in acute phase and/or periungual desquamation in subacute phase; and cervical lymphadenopathy (>1.5 cm diameter). Patients with fewer than 4 features were stratified as having Kawasaki disease if coronary artery aneurysms were present. In the absence of coronary artery changes, stratification by Kawasaki clinical criteria required 4 of 5 features to be present.
Coronary artery aneurysm is dilatation of any coronary artery seen on echocardiogram with a z score of >2.0 in the acute phase.
SARS-CoV-2 infection includes positive SARS-CoV-2 polymerase chain reaction or positive SARS-CoV-2 IgG serology results.
Laboratory Investigations
All patients had evidence of a marked inflammatory state (Table 4), for example, C-reactive protein (CRP) (median, 229 mg/L [IQR, 156-338]), neutrophilia (13 × 109/L [IQR, 10-19]), and ferritin (610 μg/L [IQR, 359-1280]). Troponin concentrations were elevated in 68% (34/50), and N-terminal pro–B-type natriuretic peptide (NT-proBNP) in 83% (24/29). This included 2 children who required extracorporeal membrane oxygenation for severe myocardial dysfunction.
Microbiological and Virological Investigations
Blood cultures, surface swabs, and other site cultures to detect staphylococci and streptococci in all patients were negative. Respiratory viral screening, undertaken using a multiplex panel for a range of common respiratory viruses, identified adenovirus and enterovirus in 1 patient. One patient had significant Epstein-Barr virus viremia; genetic and functional screening test results for familial hemophagocytic lymphocytic histiocytosis in this patient were negative.
Clinical Course Following Admission
Examination of the clinical course suggested 3 provisional clinical patterns (Table 2; eFigure 2 in Supplement 2): First, 23 children had persistent fever and elevated inflammatory markers, but no features of organ failure or mucocutaneous features suggestive of KD or toxic shock syndrome.
Second, 29 children developed shock, often associated with evidence of left ventricular dysfunction on echocardiography (62%; 18/29) and with elevation of troponin (66%; 19/29) and NT-proBNP (100%; 11/11 tested). Four patients developed arrhythmia: 1 patient had first-degree atrioventricular block with frequent supraventricular ectopic beats; 1 had intractable broad complex tachycardia, associated with low cardiac output, necessitating extra corporeal membrane oxygenation; 1 had atrial fibrillation managed with amiodarone; and 1 had second-degree heart block, which resolved without treatment.
Third, 7 children fulfilled the American Heart Association diagnostic criteria for KD. Of these, 1 progressed to shock. A total of 13 children met the criteria for KD when coronary artery aneurysms were included.
Only 55 children underwent echocardiography to assess for coronary artery aneurysms. Eight children had abnormally dilated coronary arteries (z score >2), including 7 with z scores greater than 2.5 (Table 3). Giant coronary artery aneurysms (z score >10) were documented in 2 patients. Coronary artery aneurysms developed in 8 children: 1 with fever and inflammation, 5 with shock alone, 1 with mucocutaneous features of KD alone, and 1 with both shock and mucocutaneous features of KD.
Comparison of Laboratory Findings in Patients With Shock and Coronary Artery Aneurysms
Children with PIMS-TS who developed shock (n = 29) had numerically higher CRP and neutrophil counts, lower albumin, lower lymphocyte counts, and elevated troponin and NT-proBNP concentrations compared with those without shock (Table 4; eFigure 3 and eTable 1 in Supplement 2). Laboratory findings among children who developed coronary artery dilatation or aneurysms were not meaningfully different from those without coronary artery aneurysms (eFigure 4 and eTable 1 in Supplement 2), neither were those in children who did and did not meet the clinical diagnostic criteria for KD (eFigure 5 and eTable 1 in Supplement 2).
Treatment
Inotropic support was required in 47%; 71% were treated with intravenous immunoglobulin and 64% with corticosteroids. Three patients received anakinra and eight infliximab (Table 3); 22% of the patients recovered with supportive care alone.
Comparison With Other Childhood Inflammatory Diseases
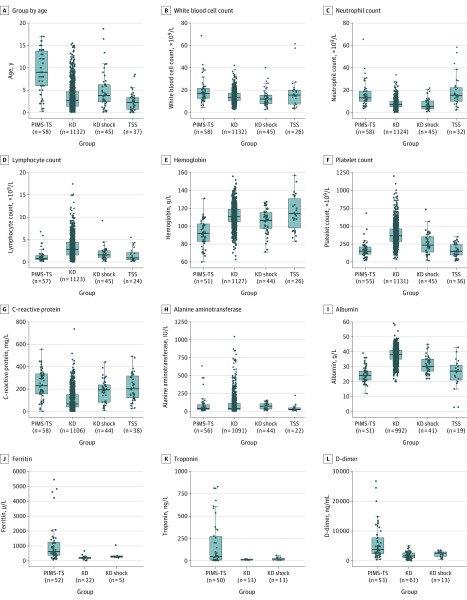
The comparison groups of children from cohorts with other inflammatory diseases included 1132 patients with KD (mean age, 2.7 years [IQR, 1.4-4.7]), 45 with KD shock syndrome (mean age, 3.8 years [IQR, 0.2-18]), and 37 with toxic shock syndrome (mean age, 7.4 years [IQR, 2.4-15.4]). Patients with PIMS-TS were generally older than those with KD or KD shock syndrome and had higher white blood cell count, neutrophil count, and CRP, as well as more profound lymphopenia and anemia (Figure; eTables 1 and 2 in Supplement 2). They also tended to have lower platelet counts, higher fibrinogen levels, and greater elevation of troponin. Alanine aminotransferase levels and D-dimer levels were similar between those with PIMS-TS and KD and also between those with PIMS-TS and KD shock syndrome. Patients with PIMS-TS tended to be older than those with toxic shock syndrome. Hemoglobin levels were lower, while CRP and alanine aminotransferase levels were higher. Ferritin and troponin results were not available in the toxic shock syndrome group.
Figure. Comparison of Age and Laboratory Results in 4 Different Patient Groups.
The pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 (PIMS-TS) group included children meeting the case definition (n = 58). The Kawasaki disease (KD) cohort included 1132 children; the KD shock syndrome cohort included 45 children; and the toxic shock syndrome (TSS) included 37 children. The horizontal lines in the boxes indicate medians; lower and upper edges of boxes indicate interquartile range and the bars extend to the highest and lowest value within 1.5 times the interquartile ranges. Details available in eTable 1 and eTable 2 in Supplement 2.
eFigure 6 in Supplement 2 shows that, overall, the children meeting the diagnostic criteria of KD in the PIMS-TS group (n = 13) differed from those with KD pre–COVID-19; the PIMS-TS group who met the diagnostic criteria for KD tended to be older and have higher neutrophil, CRP, ferritin, fibrinogen, and troponin levels and lower lymphocyte counts.
Discussion
In this case series of 58 hospitalized children who met broad definitions for childhood multisystem inflammatory disorders recently proposed in the United Kingdom, United States, or by the WHO,7,8,9 there was a wide spectrum of presenting signs and symptoms, including fever, gastrointestinal symptoms, and rash, as well as disease severity, including myocardial injury, shock, and development of coronary artery aneurysms. Comparison with patients from cohorts with KD, KD shock syndrome, and toxic shock syndrome provides additional insights into this syndrome, and suggests that PIMS-TS differs from these pediatric inflammatory entities.
Since the first reports of an unusual inflammatory illness in children that emerged in the months following the onset of COVID-19, there have been additional reports from many countries of children with fever and inflammation, for which no cause could be identified, first in health alerts and web exchanges between professional groups, and then in case reports and small case series in rapid publications.2,3,4 As these cases have emerged in temporal association with the pandemic, a link with SARS-CoV-2 is likely.
The cases reported in this study provide evidence of a wider spectrum of illness than identified in the initial UK definition and the early reports. In addition, there provisionally appears to be 3 patterns of disease among children hospitalized with PIMS-TS. One group of children had persistent fever and elevated levels of inflammatory markers, but without features of KD, shock, or organ failure. A second group fulfilled the diagnostic criteria for KD. A third group had shock and clinical, echocardiographic, and laboratory evidence of myocardial injury. The clinical and laboratory features of these groups may provide useful insights to the new syndrome.
The current study provides information that may be helpful in addressing various questions that have arisen with respect to PIMS-TS. Cases of PIMS-TS generally occurred in children older than those with KD and KD shock syndrome, and with different laboratory features. When PIMS-TS cases with coronary artery aneurysms were compared with pre–COVID-19 KD cases that developed coronary artery aneurysms, children with PIMS-TS tended to be older, have more intense inflammation, and have higher levels of markers of cardiac injury, suggesting that these are 2 separate entities and that treatment for PIMS-TS may need to be different than that for KD. Various biomarkers, including CRP, ferritin, troponin, and NT-proBNP levels may be helpful in predicting progression of disease.
However, comparison of children with PIMS-TS who developed coronary artery dilatation or aneurysms with those who did not failed to identify any differences in clinical or laboratory markers. Of particular concern was the finding that coronary artery aneurysms were found in a subset of all 3 groups of PIMS-TS. The lack of association either between the levels of inflammation in these groups or markers of cardiac injury and development of coronary artery aneurysms suggests that the coronary changes are not solely a consequence of severity of inflammation. The lack of any clinical and laboratory markers that identify patients who develop coronary artery aneurysms and the occurrence of coronary aneurysms in all 3 groups has implications for treatment and cardiac investigation. Children with KD require coronary echocardiography to detect coronary artery aneurysms, and the echocardiographic changes may either worsen or resolve, leading to recommendations for both acute echocardiographic studies, as well as sequential follow-up at 2 and 6 weeks.10 The high NT-proBNP and troponin levels raise concern as to myocardial cell injury, and follow-up of cardiac function as well echocardiographic studies to detect coronary artery aneurysms are warranted across the spectrum of PIMS-TS in both the acute and convalescent phases.
As an uncontrolled case series, this study does not provide evidence on effectiveness of treatment of PIMS-TS. Patients were treated with a range of immunomodulatory medications, according to local practice. Further studies will be needed to establish optimal treatment, and whether the same agents that show benefit in KD reduce both risk of coronary artery aneurysms and progression to severe illness or whether other agents targeting specific inflammatory pathways or cells may be preferable.
This study also cannot address the mechanisms underlying PIMS-TS. However, the timing of the disorder emerging in relation to the epidemic, and the finding that most patients were negative for detection of the virus but positive for antibody against SARS-CoV-2, raises the possibility that the disorder may involve an aberrant development of acquired immunity. There is evidence from SARS-CoV-1 that antibodies accentuate disease either through antibody enhancement of viral entry or replication as has been observed in dengue11 or through triggering of a host inflammatory response either through formation of immune complexes or direct antitissue or cellular activation. Antispike antibodies against SARS-CoV-1 have been shown to accentuate inflammation in primates and in human macrophages12 and it is therefore possible that as antibodies develop against SARS-CoV-2 they may trigger an inflammatory process through a similar mechanism. The possibility that PIMS-TS arises from an unusual acquired immune response to SARS-CoV-2 (either antibody or T cell) has implications for the development of vaccines, and thus the mechanisms require additional investigation.
Although this study has shown that PIMS-TS has differences from pre–COVID-19 KD, the similarity in clinical features in some cases and development of coronary artery aneurysms in both disorders may provide clues to the underlying mechanisms of both. Immune complexes have been well documented in KD13,14 and may also mediate vascular injury, through activation of inflammatory responses through the Fc gamma receptor15 or neutrophil activation.16 In this study, most patients with PIMS-TS were treated with intravenous immunoglobulin and/or corticosteroids, and fewer patients received a range of other immunomodulating agents. In view of the extremely high CRP levels, IL-6 may be involved in the myocardial depression.17,18 However, the effect of anti-inflammatory agents, including anti–IL-6 agents, needs further evaluation in both observational and trial settings to determine the effect on inflammation and coronary artery aneurysm development.19
Limitations
This study has several limitations. First, it was based on retrospective data collection from a number of hospitals during a period before and during the development of the case definition. Investigations and management were individualized by center and patient rather than following a standardized protocol.
Second, during this time, PCR testing of stool was not routinely undertaken; hence, this was measured only in a small group of children in this cohort. It is possible that viral replication is occurring in the gastrointestinal tract, endothelium, or myocardial tissue, but because these samples were not available, this mechanism cannot be explored.
Third, seroprevalence data in children within the United Kingdom are unavailable, so it is not possible to be certain of the background rate of SARS-CoV-2 IgG positivity in the population.
Fourth, there is no diagnostic test for KD, so it is not possible to exclude that the cohort includes children who have KD rather than a newer emerging condition associated with SARS-CoV-2. This study attempted to distinguish between the cohort who met the criteria of KD from other children in the cohort.
Fifth, there is no national registry of KD or toxic shock syndrome in England, so comparing numbers of children with PIMS-TS vs usual prevalence of KD is not possible.
Conclusions
In this case series of hospitalized children who met criteria for PIMS-TS, there was a wide spectrum of presenting signs and symptoms and disease severity, ranging from fever and inflammation to myocardial injury, shock, and development of coronary artery aneurysms. The comparison with patients with KD and KD shock syndrome provides insights into this syndrome, and suggests this disorder differs from other pediatric inflammatory entities.
List of Investigators
eMethods
eTable 1. Differences in Median Values for All Categories
eTable 2. Summary of Laboratory Parameters of the Whole PIMS-TS Cohort Compared With the Historic Cohorts
eFigure 1. Comparison of Age and Laboratory Features in 58 Children With PMIS-TS and Either No Evidence of SARS-CoV-2 Infection or Evidence of SARS-CoV-2
eFigure 2. Characteristics of the Patients in the PMIS-TS Group
eFigure 3. Comparison of PMIS-TS Children With Shock vs Nonshock
eFigure 4. Comparison of Age and Laboratory Features in 58 Children With PMIS-TS and Either No Coronary Artery Aneurysms or Coronary Artery Aneurysms
eFigure 5. Comparison of Age and Laboratory Features in 58 Children With PMIS-TS Who Either Didn’t Fulfill the Clinical Diagnostic Criteria for Kawasaki Disease of Did Fulfill the Clinical Criteria
eFigure 6. Comparison of Age and Laboratory Results in 3 Different Patient Groups
eAppendix. RCPCH Case definition
eReferences
References
- 1.Toubiana J, Poirault C, Corsia A, et al. Outbreak of Kawasaki disease in children during COVID-19 pandemic: a prospective observational study in Paris, France. medRxiv. Preprint posted May 2020. doi: 10.1101/2020.05.10.20097394 [DOI] [PMC free article] [PubMed]
- 2.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607-1608. doi: 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;382:1370-22. doi: 10.1161/CIRCULATIONAHA.120.048360 [DOI] [PubMed] [Google Scholar]
- 4.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. Published online May 13, 2020. doi: 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic comment. Lancet. Published online May 13, 2020. doi: 10.1016/S0140-6736(20)31129-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control . Rapid risk assessment: paediatric inflammatory multisystem syndrome and SARS -CoV-2 infection in children. Published May 15, 2020. Accessed May 22, 2020. https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment
- 7.Royal College of Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. Accessed May 22, 2020. https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19
- 8.World Health Organization . Multisystem inflammatory syndrome in children and adolescents with COVID-19. Published May 15, 2020. Accessed May 22, 2020. https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
- 9.Centers for Disease Control and Prevention . Emergency preparedness and response: health alert network. Published May 14, 2020. Accessed May 22, 2020. https://emergency.cdc.gov/han/2020/han00432.asp
- 10.McCrindle BW, Rowley AH, Newburger JW, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention . Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927-e999. doi: 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 11.Katzelnick LC, Gresh L, Halloran ME, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358(6365):929-932. doi: 10.1126/science.aan6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Wei Q, Lin Q, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4):S6. doi: 10.1172/jci.insight.123158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin M, Holland PC, Nokes TJ, et al. Platelet immune complex interaction in pathogenesis of Kawasaki disease and childhood polyarteritis. Br Med J (Clin Res Ed). 1985;290(6480):1456-1460. doi: 10.1136/bmj.290.6480.1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menikou S, Langford PR, Levin M. Kawasaki disease: the role of immune complexes revisited. Front Immunol. 2019;10:1156. doi: 10.3389/fimmu.2019.01156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagelkerke SQ, Kuijpers TW. Immunomodulation by IVIg and the role of Fc-gamma receptors: classic mechanisms of action after all? Front Immunol. 2015;5(8232):674. doi: 10.3389/fimmu.2014.00674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayadas TN, Tsokos GC, Tsuboi N. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation. 2009;120(20):2012-2024. doi: 10.1161/CIRCULATIONAHA.108.771170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pathan N, Franklin JL, Eleftherohorinou H, et al. Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Crit Care Med. 2011;39(7):1692-1711. doi: 10.1097/CCM.0b013e3182186d27 [DOI] [PubMed] [Google Scholar]
- 18.Pathan N, Hemingway CA, Alizadeh AA, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363(9404):203-209. doi: 10.1016/S0140-6736(03)15326-3 [DOI] [PubMed] [Google Scholar]
- 19.Nozawa T, Imagawa T, Ito S. Coronary-artery aneurysm in tocilizumab-treated children with Kawasaki’s disease. N Engl J Med. 2017;377(19):1894-1896. doi: 10.1056/NEJMc1709609 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of Investigators
eMethods
eTable 1. Differences in Median Values for All Categories
eTable 2. Summary of Laboratory Parameters of the Whole PIMS-TS Cohort Compared With the Historic Cohorts
eFigure 1. Comparison of Age and Laboratory Features in 58 Children With PMIS-TS and Either No Evidence of SARS-CoV-2 Infection or Evidence of SARS-CoV-2
eFigure 2. Characteristics of the Patients in the PMIS-TS Group
eFigure 3. Comparison of PMIS-TS Children With Shock vs Nonshock
eFigure 4. Comparison of Age and Laboratory Features in 58 Children With PMIS-TS and Either No Coronary Artery Aneurysms or Coronary Artery Aneurysms
eFigure 5. Comparison of Age and Laboratory Features in 58 Children With PMIS-TS Who Either Didn’t Fulfill the Clinical Diagnostic Criteria for Kawasaki Disease of Did Fulfill the Clinical Criteria
eFigure 6. Comparison of Age and Laboratory Results in 3 Different Patient Groups
eAppendix. RCPCH Case definition
eReferences