This population-based cohort study assesses the association of testing in low-risk patients with subsequent care among low-risk primary care outpatients undergoing an annual health examination.
Key Points
Question
Are primary care patients who undergo low-value testing as part of an annual health examination (AHE) more likely to receive subsequent care than patients who do not?
Findings
In this population-based cohort study of low-risk patients undergoing an AHE, patients who received a low-value screening test (chest radiograph [n = 43 532], electrocardiogram [n = 245 686], or Papanicolaou test [n = 29 194]) on the date of or shortly after their AHE were at increased risk of subsequent specialist visits, diagnostic tests, and procedures in the following 90 and 180 days.
Meaning
These findings suggest that low-value testing of primary care outpatients contributes to further downstream care.
Abstract
Importance
The association of low-value testing with downstream care and clinical outcomes among primary care outpatients is unknown to date.
Objective
To assess the association of low-value testing with subsequent care among low-risk primary care outpatients undergoing an annual health examination (AHE).
Design, Setting, and Participants
This population-based retrospective cohort study used administrative health care claims from Ontario, Canada, for primary care outpatients undergoing an AHE between April 1, 2012, and March 31, 2016, to identify individuals who could be placed into one (or more) of the following 3 cohorts: adult patients (18 years or older) at low risk for cardiovascular and pulmonary disease, adult patients at low risk for cardiovascular disease, and female patients (aged 13-20 years or older than 69 years) at low risk for cervical cancer. The dates of analysis were June 3 to September 12, 2019.
Exposures
Low-value screening tests were defined per cohort as (1) a chest radiograph within 7 days, (2) an electrocardiogram (ECG) within 30 days, or (3) a Papanicolaou test within 7 days after an AHE.
Main Outcomes and Measures
Subsequent specialist visits, diagnostic tests, and procedures within 90 days after a low-value test (if the patient had a chest radiograph, ECG, or Papanicolaou test) or end of the exposure observation window (if not tested).
Results
Included in the chest radiograph, ECG, and Papanicolaou test cohorts of propensity score–matched pairs were 43 532 patients (mean [SD] age, 47.5 [14.4] years; 38.5% female), 245 686 patients (mean [SD] age, 49.9 [13.7] years; 51.1% female), and 29 194 patients (mean [SD] age, 45.5 [27.1] years; 100% female), respectively. At 90 days, chest radiographs in low-risk patients were associated with an additional 0.87 (95% CI, 0.69-1.05) and 1.96 (95% CI, 1.71-2.22) patients having an outpatient pulmonology visit or an abdominal or thoracic computed tomography scan per 100 patients, respectively, and ECGs in low-risk patients were associated with an additional 1.92 (95% CI, 1.82-2.02), 5.49 (95% CI, 5.33-5.65), and 4.46 (95% CI, 4.31-4.61) patients having an outpatient cardiologist visit, a transthoracic echocardiogram, or a cardiac stress test per 100 patients, respectively. At 180 days, Papanicolaou testing in low-risk patients was associated with an additional 1.31 (95% CI, 0.84-1.78), 52.8 (95% CI, 51.9-53.6), and 0.84 (95% CI, 0.66-1.01) patients having an outpatient gynecology visit, a follow-up Papanicolaou test, or colposcopy per 100 patients, respectively.
Conclusions and Relevance
Observed associations in this population-based cohort study suggest that testing in low-risk patients as part of an AHE increases the likelihood of subsequent specialist visits, diagnostic tests, and procedures.
Introduction
Low-value care, or health care services that do not improve patient outcomes or for which harms appear to outweigh the benefits, is estimated to cost the US health care system between $75.7 and $101.2 billion annually.1 Campaigns like Choosing Wisely have led to the publication of hundreds of recommendations to reduce low-value health care services; however, prior research has shown that low-value care continues to be frequent despite these recommendations, with substantial ordering variation observed across institutions and clinicians.1,2,3,4,5 In addition to direct patient harms and costs associated with low-value testing, abnormal results from these initial tests can initiate care cascades (ie, subsequent testing or treatment).6,7 Care cascades can increase costs to the health care system while also raising the burden of care on patients, including greater patient inconvenience and costs, and even exposing them to harms associated with subsequent, potentially unnecessary health care services.6,7
To facilitate timely and meaningful reductions in the use of low-value health care services, Kerr and colleagues5 suggest that Choosing Wisely campaigns shift their focus to identifying high-priority clinical targets for quality improvement efforts. These high-priority targets should be low-value tests, treatments, or procedures that (1) are frequently used despite recommendations and (2) are greatly compromising overall health care quality (eg, by subjecting patients to unnecessary health care services, including subsequent tests and treatments via care cascades).5 A recent study4 demonstrated that routine tests in low-risk patients conducted as part of an annual health examination (AHE) in primary care are performed frequently; however, whether these screening tests are associated with increased downstream clinical service use is unknown to date.
The objectives of this population-based retrospective cohort study were to assess the association of 3 low-value screening tests with downstream health care use and clinical outcomes among healthy patients undergoing an AHE. We hypothesized that low-risk patients who received one of these 3 tests would be more likely to receive subsequent health care services compared with patients who did not.
Methods
Study Design and Data Sources
This population-based retrospective cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. Claims from the following administrative databases between April 1, 2012, and March 31, 2017, in Ontario, Canada, were linked using unique encoded identifiers and analyzed at ICES: (1) the Ontario Health Insurance Plan (OHIP) database, which includes physician billings submitted to the Ontario Ministry of Health and Long-Term Care (MOHLTC) for OHIP-insured health care services; (2) the Registered Persons Database containing sociodemographic information on OHIP-eligible Ontario residents; (3) the National Ambulatory Care Reporting System database on hospital- and community-based ambulatory care; (4) the Discharge Abstract Database, which includes information on hospital discharges; (5) the ICES Physician Database containing demographic information on physicians in Ontario; and (6) the Client Agency Program Enrollment database that identifies patients rostered to primary care physicians. The dates of analysis were June 3 to September 12, 2019. The use of data for this study was authorized under §45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board.
Cohort Creation
This study focused on the use of 3 low-value screening tests as defined by the following Choosing Wisely recommendations: (1) chest radiographs for patients at low risk for cardiovascular and pulmonary disease,8 (2) electrocardiograms (ECGs) for patients at low risk for cardiovascular disease,8,9 and (3) Papanicolaou tests for women younger than 21 years or older than 69 years at low risk for cervical cancer.8 Patients were identified who underwent an AHE between April 1, 2012, and March 31, 2016, with a primary care clinician, which in Ontario is a family physician.4 The AHE was chosen as the index event for recruited patients because it is a routine service involving a “healthy patient with no apparent medical problems”4(p1) (presumably low-risk patients) and a primary care physician that is meant to be an opportunity “to discuss prevention…[eg, cancer screening] relevant to the individual patient’s medical history and lifestyle.”10(p1)
Three recommendation-specific cohorts were then derived by identifying AHEs involving patients who were eligible to have had 1 of the 3 low-value screening tests. Specifically, AHEs were identified involving (1) adult patients (18 years or older) at low risk for cardiovascular and pulmonary disease for the chest radiograph cohort, (2) adult patients at low risk for cardiovascular disease for the ECG cohort, and (3) female patients (aged 13-20 years or older than 69 years) at low risk for cervical cancer for the Papanicolaou test cohort. All eligibility criteria for each cohort are listed in eTable 1 in the Supplement.4 Any AHE involving patients with an invalid health card number, patients older than 105 years, or those with incomplete or missing information on sex, age, or postal code were excluded.4 We then excluded any AHE involving a primary care physician who conducted less than 50 AHEs in any of the 3 cohorts (ie, had <50 opportunities to order a test of interest) to ensure that the included physicians were representative of general practice.4 Last, random sampling was used to select 1 AHE per patient per cohort to facilitate model convergence by obviating the need to account for repeated measures.
Exposures
Patients within cohorts were followed up to identify whether they had the corresponding low-value screening test of interest on the date of or shortly after their AHE. Receipt of a low-value chest radiograph, ECG, or Papanicolaou test was defined within their respective cohorts as: (1) at least 1 billing claim for a chest radiograph on the date of or within 7 days after the AHE, (2) at least 1 billing claim for an ECG on the date of or within 30 days after the AHE, and (3) at least 1 billing claim for a Papanicolaou test on the date of or within 7 days after the AHE (eTable 2 in the Supplement).4 These time windows are based on previously observed and published post-AHE testing distributions.3,4,7,11
Outcomes
The main outcomes for this study encompassed subsequent specialist visits, diagnostic tests, and procedures. We observed whether patients in the chest radiograph cohort had an outpatient pulmonology visit (ie, visit or consultation with an internist, pulmonologist, or general thoracic surgeon) or bronchoscopy within 90 days after the index AHE. In addition, to accommodate provincial wait times, patients in the chest radiograph cohort were observed over 180 days to identify whether they had an abdominal or thoracic computed tomography (CT) scan.11,12 For patients in the ECG cohort, we identified whether they had any of the following within 90 days after the index AHE: (1) outpatient cardiology visit, (2) transthoracic echocardiogram (TTE), (3) cardiac stress test, or (4) cardiac catheterization with or without coronary angiogram.7 Last, we identified whether patients in the Papanicolaou test cohort had any of the following within 180 days after the index AHE: (1) outpatient gynecology visit, (2) follow-up Papanicolaou test, or (3) colposcopy. The lengthier outcome observation window for subsequent testing in the Papanicolaou test cohort was informed by current guidelines regarding Papanicolaou test follow-up.13
Secondary outcomes were surgical procedures within 1 year after the AHE, including pneumonectomy or lobectomy procedure for the chest radiograph cohort, coronary revascularization procedure for the ECG cohort, and hysterectomy for the Papanicolaou test cohort. In addition, for all patients regardless of cohort, we identified whether they experienced a hospitalization, an emergency department visit, or death within 1 year after the AHE.
For all outcomes, a patient’s outcome observation window began either the day after the AHE-associated screening test if screened or the day after the end of the exposure observation window if not screened (eg, at 8 days after the AHE for the chest radiograph and Papanicolaou test cohorts). Full outcome definitions are listed in eTable 3 in the Supplement.
Covariates
Baseline sociodemographic characteristics were measured for patients (sex, age, neighborhood income quintile, rurality, and postal code region)14 and for the physician responsible for their AHE (age, years since medical school graduation, location of medical school graduation [Canada; United States; United Kingdom, Ireland, Australia, or New Zealand; or other], whether more than 50% of payments were fee-for-service, workload [measured in full-time equivalents15 and based on their billings relative to other physicians in that specialty], and rural practice location). In addition, billing group characteristics were measured, including the primary care reimbursement model of unique physicians’ billing group (defined as a group of 2 or more physicians submitting joint billings to the Ontario MOHLTC) and the practice population size (the number of patients per billing group divided by the number of physicians in the billing group).4 Primary care reimbursement models in Ontario are defined by their primary mechanism for reimbursement (fee-for-service for family health groups vs capitation for family health networks, organizations, and teams16,17). All measured characteristics for patients, physicians, and billing groups were chosen based on their prior association with the use of low-value testing.4,7,11,18
Statistical Analysis
To minimize confounding due to measured baseline covariates, we used propensity score matching before estimating associations between low-value screening and study outcomes.18 Within a recommendation-specific cohort, we estimated a patient’s probability of being screened with the corresponding low-value test conditional on all measured baseline covariates (ie, their propensity score) by regressing an indicator of screening (exposure) as a dichotomous outcome on all previously listed covariates using logistic regression. Screened patients were matched 1:1 to a patient in the same cohort who did not receive that test on the logit of their propensity score with greedy nearest-neighbor matching (without replacement) using calipers of width equal to 0.2 times the SD of the logit of the propensity score.19
The distributions of continuous and dichotomous baseline characteristics (mean and prevalence, respectively) were compared between screened and unscreened patients using standardized differences before and after matching. We interpreted an absolute standardized difference of at least 0.10 to indicate potentially meaningful baseline imbalances in the mean or prevalence of a measured characteristic between exposure groups.18,20 In the event of differences at or above this threshold in the matched sample, the propensity score model was iteratively modified by including nonlinear terms (and/or interactions with other covariates) or hard-matching for problematic continuous and categorical variables, respectively.18
For all outcomes in the matched samples, we compared the marginal probabilities of the corresponding event between screened and unscreened patients via the McNemar test for correlated proportions. The difference in marginal probabilities was expressed as both a risk difference (RD) and relative risk (RR) with corresponding 95% CI using Wald methods for estimating SEs from paired data.21 We calculated the attributable fraction among the exposed (AFe) for each outcome by dividing the risk difference between exposed and unexposed patients (numerator) by the risk in the exposed (denominator). The AFe was not reported if the corresponding numerator (estimated risk difference) was statistically insignificant. The AFe for a given outcome (expressed as a percentage) can be interpreted as the proportion of events among those who were exposed that are attributable to the exposure itself.
All analyses were conducted using SAS, version 9.4 (SAS Institute).
Results
Participant Characteristics
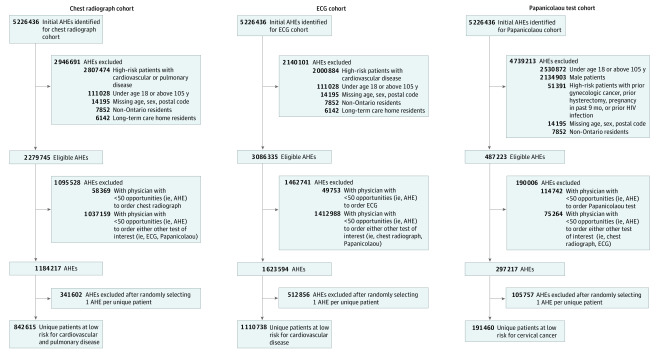
The Figure shows the cohort creation process. Before matching, we identified 842 615 patients who could have received a low-value chest radiograph (2.6% received the test), 1 110 738 patients who could have received a low-value ECG (11.2% received the test), and 191 460 patients who could have received a low-value Papanicolaou test (7.7% received the test) as part of an AHE. After matching, the chest radiograph, ECG, and Papanicolaou test cohorts of propensity score–matched pairs consisted of 43 532 patients (mean [SD] age, 47.5 [14.4] years; 38.5% female), 245 686 patients (mean [SD] age, 49.9 [13.7] years; 51.1% female), and 29 194 patients (mean [SD] age, 45.5 [27.1] years; 100% female), respectively.
Figure. Flowchart of Participants Placed Into Recommendation-Specific Cohorts.
AHEs indicates annual health examinations; ECG, electrocardiogram.
Table 1 and Table 2 compare the distribution of measured baseline covariates between patients who did and did not have testing both before and after matching.22 They demonstrate that matching effectively balanced all measured baseline characteristics between patients groups (ie, all absolute standardized differences were <0.10) within each cohort.
Table 1. Baseline Characteristics by Low-Value Screening Test Receipt Within Cohorts Before Matching.
| Characteristic | Chest radiograph cohort | ECG cohort | Papanicolaou test cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Not screened (n = 820 845) | Screened (n = 21 770) | D | Not screened (n = 986 756) | Screened (n = 123 982) | D | Not screened (n = 176 752) | Screened (n = 14 708) | D | |
| Patient | |||||||||
| Female, No. (%) | 511 815 (62.4) | 8387 (38.5) | 0.49a | 631 581 (64.0) | 63 329 (51.1) | 0.26a | 176 752 (100) | 14 708 (100) | 0.00 |
| Age, mean (SD), y | 43.0 (14.2) | 47.7 (14.4) | 0.32a | 42.6 (14.6) | 50.0 (13.7) | 0.52a | 59.1 (28.0) | 45.5 (27.1) | 0.49a |
| Neighborhood income quintile, No. (%) | |||||||||
| 1, Lowest | 121 264 (14.8) | 4762 (21.9) | 0.18a | 146 137 (14.8) | 20 170 (16.3) | 0.04 | 31 793 (18.0) | 2100 (14.3) | 0.10a |
| 2 | 149 768 (18.2) | 4965 (22.8) | 0.11a | 180 288 (18.3) | 23 559 (19.0) | 0.02 | 34 705 (19.6) | 2554 (17.4) | 0.06 |
| 3 | 165 973 (20.2) | 4109 (18.9) | 0.03 | 199 599 (20.2) | 24 907 (20.1) | 0.00 | 34 617 (19.6) | 2904 (19.7) | 0.00 |
| 4 | 189 496 (23.1) | 4398 (20.2) | 0.07 | 227 227 (23.0) | 27 709 (22.3) | 0.02 | 36 337 (20.6) | 3419 (23.2) | 0.07 |
| 5, Highest | 193 662 (23.6) | 3521 (16.2) | 0.19a | 232 653 (23.6) | 27 546 (22.2) | 0.03 | 39 119 (22.1) | 3719 (25.3) | 0.07 |
| Unknown | 682 (0.1) | 15 (0.1) | 0.01 | 852 (0.1) | 91 (0.1) | 0.00 | 181 (0.1) | 12 (0.1) | 0.01 |
| Patient rural residence, No. (%) | |||||||||
| Yes | 39 662 (4.8) | 688 (3.2) | 0.09 | 49 495 (5.0) | 3757 (3.0) | 0.10a | 8170 (4.6) | 1176 (8.0) | 0.14a |
| Unknown | 522 (0.1) | 13 (0.1) | 0.00 | 633 (0.1) | 76 (0.1) | 0.00 | 155 (0.1) | 6 (<0.1) | 0.02 |
| Postal code region, No. (%) | |||||||||
| Eastern Ontario | 80 844 (9.8) | 1011 (4.6) | 0.20a | 102 511 (10.4) | 7304 (5.9) | 0.17a | 19 636 (11.1) | 1837 (12.5) | 0.04 |
| Central Ontario | 419 890 (51.2) | 9490 (43.6) | 0.15a | 500 761 (50.7) | 68 412 (55.2) | 0.09 | 84 863 (48.0) | 6999 (47.6) | 0.01 |
| Metropolitan Toronto | 188 640 (23.0) | 8551 (39.3) | 0.36a | 219 408 (22.2) | 37 709 (30.4) | 0.19a | 45 403 (25.7) | 2225 (15.1) | 0.26a |
| Southwestern Ontario | 112 872 (13.8) | 2254 (10.4) | 0.10a | 140 238 (14.2) | 8755 (7.1) | 0.23a | 21 805 (12.3) | 3051 (20.7) | 0.23a |
| Northern Ontario | 18 574 (2.3)b | 464 (2.1) | 0.01 | 23 808 (2.4)c | 1796 (1.4)d | 0.07 | 5035 (2.8)e | 596 (4.1) | 0.07 |
| Charlson Comorbidity Index based on past 2 y, mean (SD) | 0.00 (0.06) | 0.00 (0.07) | 0.01 | 0.01 (0.18) | 0.01 (0.18) | 0.00 | 0.12 (0.57) | 0.06 (0.39) | 0.13 |
| Frequency of hospitalizations in past year, mean (SD) | 0.02 (0.16) | 0.01 (0.12) | 0.07 | 0.02 (0.15) | 0.01 (0.11) | 0.07 | 0.07 (0.33) | 0.05 (0.27) | 0.08 |
| Frequency of ED visits in past year, mean (SD) | 0.19 (0.60) | 0.16 (0.53) | 0.06 | 0.22 (0.67) | 0.18 (0.56) | 0.08 | 0.33 (0.92) | 0.42 (1.01) | 0.10 |
| Frequency of physician visits in past year, mean (SD) | 6.27 (6.78) | 6.56 (5.88) | 0.05 | 7.28 (7.42) | 8.14 (7.16) | 0.12 | 12.63 (13.40) | 10.84 (10.94) | 0.15 |
| Physician | |||||||||
| Time since medical school graduation, mean (SD), y | 26.03 (10.02) | 29.91 (9.88) | 0.39a | 25.84 (9.96) | 28.61 (10.36) | 0.27a | 27.18 (10.25) | 26.01 (9.89) | 0.12a |
| Female physician, No. (%) | 390 164 (47.5) | 5358 (24.6) | 0.49a | 481 437 (48.8) | 42 228 (34.1) | 0.30a | 85 238 (48.2) | 9215 (62.7) | 0.29a |
| Location of medical school graduation, No. (%) | |||||||||
| Canada | 479 774 (58.4) | 12 423 (57.1) | 0.03 | 585 065 (59.3) | 68 608 (55.3) | 0.08 | 106 363 (60.2) | 10 004 (68.0) | 0.16a |
| United States | 4271 (0.5) | 168 (0.8) | 0.03 | 5380 (0.5) | 412 (0.3) | 0.03 | 1068 (0.6) | 80 (0.5) | 0.01 |
| United Kingdom, Ireland, Australia, or New Zealand | 33 442 (4.1) | 989 (4.5) | 0.02 | 39 017 (4.0) | 7281 (5.9) | 0.09 | 8158 (4.6) | 707 (4.8) | 0.01 |
| Other | 303 358 (37.0) | 8190 (37.6) | 0.01 | 357 294 (36.2) | 47 681 (38.5) | 0.05 | 61 163 (34.6) | 3917 (26.6) | 0.17a |
| More than 50% of payments fee-for-service | 415 525 (50.6) | 14 539 (66.8) | 0.33a | 493 062 (50.0) | 72 947 (58.8) | 0.18a | 91 156 (51.6) | 6788 (46.2) | 0.11a |
| Workload measured in FTEs based on payment, mean (SD) | 1.35 (0.43) | 1.50 (0.44) | 0.35a | 1.34 (0.43) | 1.41 (0.43) | 0.17a | 1.34 (0.43) | 1.27 (0.39) | 0.17a |
| Rural practice location, No. (%) | 24 582 (3.0) | 387 (1.8) | 0.08 | 31 308 (3.2) | 1509 (1.2) | 0.13a | 5550 (3.1) | 844 (5.7) | 0.13a |
| Billing group | |||||||||
| Primary care payment model (primary method of reimbursement), No. (%) | |||||||||
| Family health group (fee-for-service) | 451 513 (55.0) | 15 138 (69.5) | 0.30a | 537 757 (54.5) | 77 742 (62.7) | 0.17a | 99 389 (56.2) | 7108 (48.3) | 0.16a |
| Family health network (capitation) | 277 (<0.1) | ≤5 (<0.1)f | 0.01 | 338 (<0.1) | 7 (<0.1) | 0.02 | 42 (<0.1) | 21 (0.1) | 0.04 |
| Family health organization (capitation) | 206 721 (25.2) | 3900 (17.9) | 0.18a | 249 262 (25.3) | 29 301 (23.6) | 0.04 | 43 318 (24.5) | 4293 (29.2) | 0.11a |
| Family health team (capitation) | 161 168 (19.6) | 2714 (12.5) | 0.20a | 197 883 (20.1) | 16 875 (13.6) | 0.17a | 33 631 (19.0) | 3269 (22.2) | 0.08 |
| Other | 1166 (0.1) | ≤15 (<0.1)f | 0.02 | 1516 (0.2) | 57 (<0.1) | 0.03 | 372 (0.2) | 17 (0.1) | 0.02 |
| Practice population size, mean (SD) No. of patients per billing group | 1826 (911) | 2141 (1142) | 0.31a | 1815 (904) | 1881 (934) | 0.07 | 1748 (852) | 1682 (764) | 0.08 |
| No. of physicians in billing group, mean (SD) | 58.3 (93.1) | 94.9 (120.0) | 0.34a | 57.7 (92.9) | 67.8 (101.0) | 0.10 | 57.9 (93.0) | 46.7 (79.5) | 0.13a |
Abbreviations: D, standardized difference; ECG, electrocardiogram; ED, emergency department; FTEs, full-time equivalents.
D ≥ 0.10.
Twenty-five had unknown postal code region.
Thirty had unknown postal code region.
Six had unknown postal code region.
Ten had unknown postal code region.
Values suppressed to prevent back-calculation of small cells (ie, cells with <6 observations) within a column as per ICES guidelines.22
Table 2. Baseline Characteristics by Low-Value Screening Test Receipt Within Cohorts After Matching.
| Characteristic | Chest radiograph cohort | ECG cohort | Papanicolaou test cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Not screened (n = 21 766) | Screened (n = 21 766) | D | Not screened (n = 122 843) | Screened (n = 122 843) | D | Not screened (n = 14 597) | Screened (n = 14 597) | D | |
| Patienta | |||||||||
| Female, No. (%) | 8386 (38.5) | 8386 (38.5) | 0.00 | 62 817 (51.1) | 62 817 (51.1) | 0.00 | 14 597 (100) | 14 597 (100) | 0.00 |
| Age, mean (SD), y | 47.3 (14.7) | 47.7 (14.4) | 0.02 | 49.9 (13.7) | 49.9 (13.7) | 0.00 | 45.5 (27.1) | 45.5 (27.1) | 0.00 |
| Neighborhood income quintile, No. (%) | |||||||||
| 1, Lowest | 4286 (19.7) | 4761 (21.9) | 0.05 | 19 402 (15.8) | 19 984 (16.3) | 0.01 | 2204 (15.1) | 2082 (14.3) | 0.02 |
| 2 | 4725 (21.7) | 4965 (22.8) | 0.03 | 23 492 (19.1) | 23 346 (19.0) | 0.00 | 2384 (16.3) | 2539 (17.4) | 0.03 |
| 3 | 4277 (19.6) | 4107 (18.9) | 0.02 | 24 949 (20.3) | 24 664 (20.1) | 0.01 | 3037 (20.8) | 2885 (19.8) | 0.03 |
| 4 | 4463 (20.5) | 4397 (20.2) | 0.01 | 27 300 (22.2) | 27 457 (22.4) | 0.00 | 3422 (23.4) | 3400 (23.3) | 0.00 |
| 5, Highest | 4001 (18.4) | 3521 (16.2) | 0.06 | 27 612 (22.5) | 27 307 (22.2) | 0.01 | 3529 (24.2) | 3679 (25.2) | 0.02 |
| Unknown | 14 (0.1) | 15 (0.1) | 0.00 | 88 (0.1) | 85 (0.1) | 0.00 | 21 (0.1) | 12 (0.1) | 0.02 |
| Patient rural residence, No. (%) | |||||||||
| Yes | 643 (3.0) | 688 (3.2) | 0.01 | 3961 (3.2) | 3658 (3.0) | 0.01 | 958 (6.6) | 1168 (8.0) | 0.06 |
| Unknown | 14 (0.1) | 13 (0.1) | 0.00 | 69 (0.1) | 73 (0.1) | 0.00 | 18 (0.1) | 6 (<0.1) | 0.03 |
| Postal code region, No. (%) | |||||||||
| Eastern Ontario | 1011 (4.6) | 1011 (4.6) | 0.00 | 7140 (5.8) | 7140 (5.8) | 0.00 | 1814 (12.4) | 1814 (12.4) | 0.00 |
| Central Ontario | 9489 (43.6) | 9489 (43.6) | 0.00 | 68 280 (55.6) | 68 280 (55.6) | 0.00 | 6998 (47.9) | 6998 (47.9) | 0.00 |
| Metropolitan Toronto | 8549 (39.3) | 8549 (39.3) | 0.00 | 37 427 (30.5) | 37 427 (30.5) | 0.00 | 2224 (15.2) | 2224 (15.2) | 0.00 |
| Southwestern Ontario | 2253 (10.4) | 2253 (10.4) | 0.00 | 8280 (6.7) | 8280 (6.7) | 0.00 | 3014 (20.6) | 3014 (20.6) | 0.00 |
| Northern Ontario | 464 (2.1) | 464 (2.1) | 0.00 | 1716 (1.4) | 1716 (1.4) | 0.00 | 547 (3.7) | 547 (3.7) | 0.00 |
| Charlson Comorbidity Index based on past 2 y, mean (SD)b | 0.00 (0.06) | 0.00 (0.07) | 0.00 | 0.01 (0.21) | 0.01 (0.18) | 0.02 | 0.06 (0.40) | 0.06 (0.39) | 0.00 |
| Frequency of hospitalizations in past year, mean (SD)b | 0.01 (0.13) | 0.01 (0.12) | 0.02 | 0.01 (0.12) | 0.01 (0.11) | 0.02 | 0.05 (0.25) | 0.05 (0.27) | 0.01 |
| Frequency of ED visits in past year, mean (SD)b | 0.16 (0.51) | 0.16 (0.53) | 0.00 | 0.20 (0.63) | 0.18 (0.56) | 0.04 | 0.33 (0.89) | 0.42 (1.01) | 0.09 |
| Frequency of physician visits in past year, mean (SD)b | 6.03 (6.27) | 6.56 (5.88) | 0.09 | 7.86 (7.69) | 8.14 (7.16) | 0.09 | 10.34 (11.40) | 10.85 (10.96) | 0.05 |
| Physician | |||||||||
| Time since medical school graduation, mean (SD), y | 28.98 (9.84) | 29.91 (9.89) | 0.09 | 27.64 (10.13) | 28.57 (10.37) | 0.09 | 26.05 (9.87) | 25.98 (9.87) | 0.01 |
| Female physician, No. (%) | 5358 (24.6) | 5358 (24.6) | 0.00 | 41 968 (34.2) | 41 968 (34.2) | 0.00 | 9153 (62.7) | 9153 (62.7) | 0.00 |
| Location of medical school graduation, No. (%) | |||||||||
| Canada | 12 892 (59.2) | 12 422 (57.1) | 0.04 | 68 535 (55.8) | 67 834 (55.2) | 0.01 | 9491 (65.0) | 9901 (67.8) | 0.06 |
| United States | 136 (0.6) | 168 (0.8) | 0.02 | 584 (0.5) | 407 (0.3) | 0.02 | 105 (0.7) | 80 (0.5) | 0.02 |
| United Kingdom, Ireland, Australia, or New Zealand | 974 (4.5) | 989 (4.5) | 0.00 | 6093 (5.0) | 7197 (5.9) | 0.04 | 630 (4.3) | 704 (4.8) | 0.02 |
| Other | 7764 (35.7) | 8187 (37.6) | 0.04 | 47 631 (38.8) | 47 405 (38.6) | 0.00 | 4371 (29.9) | 3912 (26.8) | 0.07 |
| More than 50% of payments fee-for-service | 13 770 (63.3) | 14 535 (66.8) | 0.07 | 68 632 (55.9) | 72 049 (58.7) | 0.06 | 6571 (45.0) | 6712 (46.0) | 0.02 |
| Workload measured in FTEs based on payment, mean (SD) | 1.49 (0.43) | 1.50 (0.44) | 0.03 | 1.40 (0.43) | 1.41 (0.43) | 0.02 | 1.27 (0.39) | 1.27 (0.39) | 0.00 |
| Rural practice location, No. (%) | 397 (1.8) | 387 (1.8) | 0.00 | 2001 (1.6) | 1495 (1.2) | 0.03 | 637 (4.4) | 842 (5.8) | 0.06 |
| Billing group | |||||||||
| Primary care payment model (primary method of reimbursement), No. (%) | |||||||||
| Family health group (fee-for-service) | 14 509 (66.7) | 15 134 (69.5) | 0.06 | 73 680 (60.0) | 76 817 (62.5) | 0.05 | 7178 (49.2) | 7032 (48.2) | 0.02 |
| Family health network (capitation) | ≤5 (<0.1)c | ≤5 (<0.1)c | 0.00 | 9 (<0.1) | 7 (<0.1) | 0.00 | 18 (0.1) | 21 (0.1) | 0.01 |
| Family health organization (capitation) | 4193 (19.3) | 3900 (17.9) | 0.03 | 28 638 (23.3) | 29 184 (23.8) | 0.01 | 4130 (28.3) | 4263 (29.2) | 0.02 |
| Family health team (capitation) | 3037 (14.0) | 2714 (12.5) | 0.04 | 20 342 (16.6) | 16 778 (13.7) | 0.08 | 3231 (22.1) | 3264 (22.4) | 0.01 |
| Other | ≤25 (<1.0)c | ≤15 (<1.0)c | 0.01 | 174 (0.1) | 57 (<0.1) | 0.03 | 40 (0.3) | 17 (0.1) | 0.04 |
| Practice population size, mean (SD) No. of patients per billing group | 2169 (1125) | 2141 (1141) | 0.02 | 1875 (907) | 1868 (913) | 0.01 | 1671 (751) | 1674 (755) | 0.00 |
| No. of physicians in billing group, mean (SD) | 88.1 (116.0) | 94.9 (120.0) | 0.06 | 64.5 (98.5) | 67.9 (101.0) | 0.03 | 48.0 (82.2) | 46.7 (79.5) | 0.02 |
Abbreviations: D, standardized difference; ECG, electrocardiogram; ED, emergency department; FTEs, full-time equivalents.
Patients were hard-matched on their sex and their physician’s sex.
Patients were matched on the logit of their propensity score, which was calculated by regressing exposure status on all baseline characteristics summarized in the table except for Charlson Comorbidity Index, frequency of hospitalizations in past year, frequency of ED visits in past year, and frequency of physician visits in past year.
Values suppressed to prevent back-calculation of small cells (ie, cells with <6 observations) within a column as per ICES guidelines.22
Subsequent Specialist Visits, Diagnostic Tests, and Procedures
Table 3 summarizes the probability of subsequent specialist visits, diagnostic tests, and procedures within 90 days for tested and untested patients within each recommendation-specific cohort after matching. Based on 21 766 matched pairs, low-value chest radiographs in low-risk patients were associated with an additional 0.14 (95% CI, 0.08-0.19), 0.87 (95% CI, 0.69-1.05), and 1.96 (95% CI, 1.71-2.22) patients having a bronchoscopy, an outpatient pulmonology visit, or an abdominal or thoracic CT scan within 90 days per 100 patients. Altering the CT scan end point to 180 days made no discernible difference in the direction or magnitude of absolute or relative risk differences between screened and unscreened patients in the matched chest radiograph cohort.
Table 3. Associations Between Screening and Subsequent Specialist Visits, Diagnostic Tests, and Procedures by Low-Value Screening Test.
| Outcome | Outcome observation window, d | Exposure | Risk, % | RD (95% CI)a | RR (95% CI)a | AFe, %b |
|---|---|---|---|---|---|---|
| Chest radiograph cohort (n = 21 766 matched pairs) | ||||||
| Outpatient pulmonology visit | 90 | Screened | 1.39 | 0.87 (0.69-1.05) | 2.67 (2.16-3.31) | 62.6 |
| Not screened | 0.52 | 1 [Reference] | 1 [Reference] | NA | ||
| Bronchoscopy | 90 | Screened | 0.16 | 0.14 (0.08-0.19) | 8.50 (3.02-23.95) | 87.5 |
| Not screened | 0.02 | 1 [Reference] | 1 [Reference] | NA | ||
| Abdominal or thoracic CT scan | 90 | Screened | 2.88 | 1.96 (1.71-2.22) | 3.14 (2.67-3.67) | 68.1 |
| Not screened | 0.92 | 1 [Reference] | 1 [Reference] | NA | ||
| ECG cohort (n = 122 843 matched pairs) | ||||||
| Outpatient cardiology visit | 90 | Screened | 2.70 | 1.92 (1.82-2.02) | 3.48 (3.24-3.74) | 71.1 |
| Not screened | 0.77 | 1 [Reference] | 1 [Reference] | NA | ||
| TTE | 90 | Screened | 7.12 | 5.49 (5.33-5.65) | 4.35 (4.15-4.57) | 77.1 |
| Not screened | 1.64 | 1 [Reference] | 1 [Reference] | NA | ||
| Cardiac stress test | 90 | Screened | 5.84 | 4.46 (4.31-4.61) | 4.24 (4.02-4.46) | 76.4 |
| Not screened | 1.38 | 1 [Reference] | 1 [Reference] | NA | ||
| Cardiac catheterization | 90 | Screened | 0.24 | 0.15 (0.12-0.18) | 2.64 (2.12-3.28) | 62.5 |
| Not screened | 0.09 | 1 [Reference] | 1 [Reference] | NA | ||
| Papanicolaou test cohort (n = 14 597 matched pairs) | ||||||
| Outpatient gynecology visit | 180 | Screened | 5.07 | 1.31 (0.84-1.78) | 1.35 (1.21-1.50) | 25.8 |
| Not screened | 3.76 | 1 [Reference] | 1 [Reference] | NA | ||
| Follow-up Papanicolaou test | 180 | Screened | 56.22 | 52.78 (51.92-53.63) | 16.35 (14.98-17.83) | 94.0 |
| Not screened | 3.44 | 1 [Reference] | 1 [Reference] | NA | ||
| Colposcopy | 180 | Screened | 1.03 | 0.84 (0.66-1.01) | 5.36 (3.58-8.02) | 81.6 |
| Not screened | 0.19 | 1 [Reference] | 1 [Reference] | NA |
Abbreviations: AFe, attributable fraction among the exposed; CT, computed tomography; ECG, electrocardiogram; NA, not applicable; RD, risk difference; RR, relative risk; TTE, transthoracic echocardiogram.
Risks (cumulative incidences expressed as percentages) were not rounded before calculating these values.
AFe, % = (RD/risk in No. screened) × 100. The value was calculated based on rounding the numerator and denominator values in this table. AFe was not reported if 95% CIs for RD contain the null value (ie, zero).
Based on 122 843 matched pairs, low-value ECGs in low-risk patients were associated with an additional 1.92 (95% CI, 1.82-2.02), 5.49 (95% CI, 5.33-5.65), and 4.46 (95% CI, 4.31-4.61) patients having an outpatient cardiology visit, a TTE, or a cardiac stress test within 90 days per 100 patients. In addition, ECG screening was statistically significantly associated with an additional 0.15 (95% CI, 0.12-0.18) patients receiving a cardiac catheterization per 100 patients.
Papanicolaou test cohort results were based on 14 597 matched pairs. Low-value Papanicolaou tests in low-risk female patients were associated with an additional 1.31 (95% CI, 0.84-1.78), 52.8 (95% CI, 51.92-53.63), and 0.84 (95% CI, 0.66-1.01) patients having an outpatient gynecology visit, a follow-up Papanicolaou test, or colposcopy within 180 days per 100 patients.
Secondary Outcomes
The marginal probability of secondary outcomes, which are listed in Table 4 as percentages, was low for most events regardless of screening status. In particular, 19 (<0.1%) patients in the chest radiograph cohort had a pneumonectomy or lobectomy procedure, 512 (0.2%) patients in the ECG cohort had a coronary revascularization procedure, and 55 (0.2%) female patients in the Papanicolaou test cohort had a hysterectomy. Patients who had a chest radiograph were 433% more likely to have a pneumonectomy or lobectomy procedure vs untested patients, and patients who had an ECG were 55% more likely to have a coronary revascularization vs untested patients; however, absolute risk differences were minimal. The proportion of patients having a hysterectomy at 1 year was similar between patient groups.
Table 4. Associations Between Screening and Adverse Clinical Outcomes at 1 Year by Low-Value Screening Test.
| Outcome | Exposure | Risk, % | RD (95% CI)a | RR (95% CI)a | AFe, %b |
|---|---|---|---|---|---|
| Chest radiograph cohort (n = 21 766 matched pairs) | |||||
| Pneumonectomy or lobectomy procedure | Screened | 0.07 | 0.06 (0.02 to 0.10) | 5.33 (1.55 to 18.30) | 85.7 |
| Not screened | 0.01 | 1 [Reference] | 1 [Reference] | NA | |
| Hospitalization | Screened | 3.15 | 1.93 (−0.00 to 0.01) | 1.07 (0.96 to 1.18) | NR |
| Not screened | 2.96 | 1 [Reference] | 1 [Reference] | NA | |
| ED visit | Screened | 14.41 | 0.73 (0.08 to 1.38) | 1.05 (1.01 to 1.10) | 5.1 |
| Not screened | 13.68 | 1 [Reference] | 1 [Reference] | NA | |
| Death | Screened | 0.21 | 0.10 (0.03 to 1.81) | 2.00 (1.21 to 3.30) | 47.6 |
| Not screened | 0.11 | 1 [Reference] | 1 [Reference] | NA | |
| ECG cohort (n = 122 843 matched pairs) | |||||
| Coronary revascularization procedure | Screened | 0.25 | 0.09 (0.05 to 0.13) | 1.55 (1.30 to 1.85) | 36.0 |
| Not screened | 0.16 | 1 [Reference] | 1 [Reference] | NA | |
| Hospitalization | Screened | 3.45 | −0.05 (−0.19 to 0.01) | 0.99 (0.95 to 1.03) | NR |
| Not screened | 3.50 | 1 [Reference] | 1 [Reference] | NA | |
| ED visit | Screened | 15.55 | 0.00 (−0.36 to 0.21) | 1.00 (0.98 to 1.01) | NR |
| Not screened | 15.63 | 1 [Reference] | 1 [Reference] | NA | |
| Death | Screened | 0.14 | −0.01 (−0.05 to 0.01) | 0.89 (0.72 to 1.09) | NR |
| Not screened | 0.15 | 1 [Reference] | 1 [Reference] | NA | |
| Papanicolaou test cohort (n = 14 597 matched pairs) | |||||
| Hysterectomy | Screened | 0.21 | 0.05 (−0.05 to 0.15) | 1.29 (0.76 to 2.20) | NR |
| Not screened | 0.16 | 1 [Reference] | 1 [Reference] | NA | |
| Hospitalization | Screened | 4.82 | −0.24 (−0.07 to 0.03) | 0.95 (0.86 to 1.05) | NR |
| Not screened | 5.06 | 1 [Reference] | 1 [Reference] | NA | |
| ED visit | Screened | 26.05 | 4.08 (3.12 to 5.04) | 1.19 (1.14 to 1.23) | 15.7 |
| Not screened | 21.96 | 1 [Reference] | 1 [Reference] | NA | |
| Death | Screened | 0.26 | −0.19 (−0.32 to −0.06) | 0.58 (0.39 to 0.86) | NA |
| Not screened | 0.45 | 1 [Reference] | 1 [Reference] | 42.2c |
Abbreviations: AFe, attributable fraction among the exposed; ECG, electrocardiogram; ED, emergency department; NA, not applicable; NR, not reported; RD, risk difference; RR, relative risk.
Risks (cumulative incidences expressed as percentages) were not rounded before calculating these values.
AFe, % = (RD/risk in No. screened) × 100. The value was calculated based on rounding the numerator and denominator values in this table. AFe was not reported if 95% CIs for RD contain the null value (ie, zero).
Where exposure is “not screened” because we observed a protective association between screening and death. The value was calculated as (RD/risk in No. not screened) × 100, where RD = 0.19 (ie, RD between not screened vs screened), based on rounded values in this table.
Risk differences between patient groups were comparably low for hospitalization and death at 1 year (528 deaths [69 in the chest radiograph cohort, 355 in the ECG cohort, and 104 in the Papanicolaou test cohort]). Compared with untested patients, patients who received a chest radiograph had a higher risk of 1-year mortality (risk difference [RD], 0.10; 95% CI, 0.03-1.81), and patients who had a Papanicolaou test had a lower risk of death (RD, −0.19; 95% CI, −0.32 to −0.06). There was no difference in the risk of death between patients who had an ECG vs those who did not. There was also no difference in hospitalization rates between patient groups across all 3 cohorts. Finally, patients who had a chest radiograph or Papanicolaou test had a higher risk of having at least 1 ED visit within 1 year vs those who did not.
Discussion
In this large, population-based retrospective cohort study of patients eligible to receive a low-value screening test as part of an AHE, patients who received such tests had higher odds of downstream clinical service use, including specialty consultations, repeat testing, or other imaging tests compared with patients who did not. The absolute risk of secondary outcomes, such as hospitalization or death, was low among both screened and unscreened patients. To our knowledge, this work is the largest cohort study to demonstrate the association of low-value screening tests with downstream health care use, or care cascades, in primary care.
Our finding that low-value care is associated with increased downstream clinical service use is supported by prior literature. One study7 reported that routine use of ECGs in low-risk patients was associated with a higher risk of subsequent cardiac testing. Although that was a Canadian study, prior research suggests these findings are generalizable to the United States as well. A 2013 study by Kale et al23 found that rates of overuse of ECGs and chest radiographs in primary care were 11.3% and 7.0%, respectively, consistent with our findings. Qin et al24 reported that 19% of US women younger than 21 years had a Papanicolaou test in the past 12 months, with more than 70% of the tests being possibly unnecessary. Ganguli et al6 observed that receipt of an ECG before cataract surgery was associated with 5 to 11 cascade events per 100 patients, which included specialist visits, tests, or new medications. Those authors estimated that the additional cost of downstream testing was $565 per beneficiary. Further research has shown that most clinicians have participated in a care cascade associated with a number of health care system factors, demonstrating how common this practice is.25 The present study adds to the literature by reporting the presence of care cascades across multiple low-value screening tests (ie, chest radiographs, ECGs, and Papanicolaou tests) around the AHE.
The absolute number of clinical events at 1 year was low, an unsurprising finding given the healthy patient population studied. Slightly higher risks of pneumonectomy or lobectomy procedure and coronary revascularization procedure were observed among those who had chest radiographs and ECGs, respectively, vs patients who did not. This observation may have been because of detection of substantial disease (either incidentally through screening or in response to clinical findings during the physical examination); however, the increased procedure risk did not translate to clinically meaningful reductions in 1-year mortality risk. Although there were some differences in mortality in the chest radiograph and Papanicolaou test cohorts, the absolute mortality rate across all cohorts was low, making the clinical importance of these marginal differences in outcome questionable.
The founding principle of the Choosing Wisely campaigns has been to encourage patients and physicians to have conversations about the risks and benefits of tests, treatments, and procedures.2,26 Many low-value screening tests are known to have limited benefit because of the low prevalence of disease among young and healthy populations; however, the risks associated with these screening tests have largely not been quantified to date.13,27 The results of the present study support the premise that seemingly low-risk screening tests may lead to physician visits or tests that could inconvenience the patient and, in some instances, expose the patient to potential harm. False-positive screening test results can lead to more invasive procedures, such as bronchoscopy, cardiac catheterization, or colposcopy. Rarely, low-value screening tests may lead to major surgery, with the potential for life-limiting complications. Therefore, in discussing the risks and benefits of screening tests with low-risk patients, physicians should help patients weigh the potential for harm against uncertain benefit.
Limitations
The findings of this study are subject to several limitations. First, administrative data lack the clinical granularity (including clinical history and physical examination and laboratory results) to confirm the intentions of the ordered health care services. Therefore, it is possible that some of these low-value tests were ordered because of undocumented clinical concerns rather than for preventive health or screening. Second, we cannot definitively attribute subsequent downstream tests to the results of the index screening test. Although we controlled for several measured characteristics at the patient, physician, and billing group levels through propensity score matching, we acknowledge that there may be unmeasured confounders (most likely in the chest radiograph cohort and the Papanicolaou test cohort, where there were substantial but small outcome differences) that could have biased our results.18 Third, we did not have the results of the index chest radiograph, ECG, or Papanicolaou test. Any abnormalities detected through testing may have influenced the decision to order downstream testing, which may be appropriate. Despite these limitations, we believe that our analysis presents novel data regarding the downstream burden of care attributable to 3 low-value screening tests that should be of interest to patients, clinicians, and policy makers.
Conclusions
In this large, population-based retrospective cohort study, low-risk patients who received 1 of 3 low-value screening tests (chest radiograph, ECG, or Papanicolaou test) as part of an AHE were more likely to have further downstream health care services compared with untested patients. Specifically, their likelihood of subsequent specialist visits, diagnostic tests, and procedures was increased.
eTable 1. Eligibility Criteria to Identify Annual Health Examinations Involving Patients Eligible for Low-Value Screening Tests of Interest
eTable 2. Low-Value Screening Test (Exposure) Definitions
eTable 3. Study Outcome Definitions
eReferences.
References
- 1.Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. Published online October 7, 2019. doi: 10.1001/jama.2019.13978 [DOI] [PubMed] [Google Scholar]
- 2.Levinson W, Kallewaard M, Bhatia RS, Wolfson D, Shortt S, Kerr EA; Choosing Wisely International Working Group . “Choosing Wisely”: a growing international campaign. BMJ Qual Saf. 2015;24(2):167-174. doi: 10.1136/bmjqs-2014-003821 [DOI] [PubMed] [Google Scholar]
- 3.Pendrith C, Bhatia M, Ivers NM, et al. Frequency of and variation in low-value care in primary care: a retrospective cohort study. CMAJ Open. 2017;5(1):E45-E51. doi: 10.9778/cmajo.20160095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouck Z, Ferguson J, Ivers NM, et al. Physician characteristics associated with ordering 4 low-value screening tests in primary care. JAMA Netw Open. 2018;1(6):e183506-e183506. doi: 10.1001/jamanetworkopen.2018.3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr EA, Kullgren JT, Saini SD. Choosing Wisely: how to fulfill the promise in the next 5 years. Health Aff (Millwood). 2017;36(11):2012-2018. doi: 10.1377/hlthaff.2017.0953 [DOI] [PubMed] [Google Scholar]
- 6.Ganguli I, Lupo C, Mainor AJ, et al. Prevalence and cost of care cascades after low-value preoperative electrocardiogram for cataract surgery in fee-for-service Medicare beneficiaries. JAMA Intern Med. Published online June 3, 2019. doi: 10.1001/jamainternmed.2019.1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia RS, Bouck Z, Ivers NM, et al. Electrocardiograms in low-risk patients undergoing an annual health examination. JAMA Intern Med. 2017;177(9):1326-1333. doi: 10.1001/jamainternmed.2017.2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choosing Wisely Canada. College of Family Physicians of Canada Thirteen things physicians and patients should question. Published 2019. Accessed August 28, 2019. https://choosingwiselycanada.org/family-medicine/
- 9.Choosing Wisely Canada. Canadian Cardiovascular Society Five things physicians and patients should question. Published 2017. Accessed August 28, 2019. https://choosingwiselycanada.org/cardiology/
- 10.Government of Ontario Personal health visit. Published 2013. Accessed August 28, 2019. http://www.health.gov.on.ca/en/pro/programs/phys_services/docs/periodic_health_visit_is_ea_en.pdf
- 11.Bouck Z, Mecredy G, Ivers NM, et al. Routine use of chest x-ray for low-risk patients undergoing a periodic health examination: a retrospective cohort study. CMAJ Open. 2018;6(3):E322-E329. doi: 10.9778/cmajo.20170138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health Quality Ontario. System performance: wait times for diagnostic imaging. Accessed January 27, 2020. https://www.hqontario.ca/System-Performance/Wait-Times-for-Diagnostic-Imaging?utm_source=Ontario.ca&utm_medium=Referral&utm_campaign=WT%20Referral
- 13.US Preventive Services Task Force Final recommendation statement: cervical cancer: screening. Published 2019. Accessed August 28, 2019. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/cervical-cancer-screening
- 14.Statistics Canada. Postal CodeOM Conversion File (PCCF), Reference Guide. Published June 2017. Accessed September 20, 2019. https://www150.statcan.gc.ca/n1/en/catalogue/92-154-G
- 15.Chan B. Supply of Physicians’ Services in Ontario. Institute for Clinical Evaluative Sciences; 1999. [Google Scholar]
- 16.Glazier RH, Hutchison B, Kopp A. Comparison of Family Health Teams to Other Primary Care Models, 2004/05 to 2011/12. Institute for Clinical Evaluative Sciences; 2015. [Google Scholar]
- 17.Scholle SH, Roski J, Adams JL, et al. Benchmarking physician performance: reliability of individual and composite measures. Am J Manag Care. 2008;14(12):833-838. [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150-161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirji KF, Fagerland MW. Calculating unreported confidence intervals for paired data. BMC Med Res Methodol. 2011;11:66. doi: 10.1186/1471-2288-11-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ICES Working with ICES data. Accessed April 15, 2020. https://www.ices.on.ca/Data-and-Privacy/ICES-data/Working-with-ICES-Data
- 23.Kale MS, Bishop TF, Federman AD, Keyhani S. Trends in the overuse of ambulatory health care services in the United States. JAMA Intern Med. 2013;173(2):142-148. doi: 10.1001/2013.jamainternmed.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin J, Saraiya M, Martinez G, Sawaya GF. Prevalence of potentially unnecessary bimanual pelvic examinations and Papanicolaou tests among adolescent girls and young women aged 15-20 years in the United States. JAMA Intern Med. Published online January 6, 2020. doi: 10.1001/jamainternmed.2019.5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganguli I, Simpkin AL, Lupo C, et al. Cascades of care after incidental findings in a US national survey of physicians [published correction appears in JAMA Netw Open. 2019;2(11):e1916768]. JAMA Netw Open. 2019;2(10):e1913325. doi: 10.1001/jamanetworkopen.2019.13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choosing Wisely. Promoting conversations between patients and clinicians. Published 2019. Accessed August 28, 2019. https://www.choosingwisely.org/
- 27.Tigges S, Roberts DL, Vydareny KH, Schulman DA. Routine chest radiography in a primary care setting. Radiology. 2004;233(2):575-578. doi: 10.1148/radiol.2332031796 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Eligibility Criteria to Identify Annual Health Examinations Involving Patients Eligible for Low-Value Screening Tests of Interest
eTable 2. Low-Value Screening Test (Exposure) Definitions
eTable 3. Study Outcome Definitions
eReferences.