Key Points
Question
What are the relative contributions of progression independent of relapse activity (PIRA) and relapse-associated worsening (RAW) to overall accumulating disability in patients with relapsing multiple sclerosis?
Findings
Applying a composite outcome measure to a typical population with active relapsing multiple sclerosis, this pooled analysis of 2 randomized clinical trials shows that the most part of confirmed disability accumulation occurs independently of relapse activity. Distinct prognostic factors were associated with PIRA vs RAW, and ocrelizumab had a beneficial outcome in both.
Meaning
These findings clearly demonstrate underlying progression in this relapsing multiple sclerosis population and challenge the current clinical distinction of relapsing and progressive forms of multiple sclerosis.
This secondary analysis of data from 2 randomized clinical trials examines the relative contribution of relapse-associated vs relapse-independent progression to overall confirmed disability accumulation in patients with multiple sclerosis and assesses respective baseline prognostic factors and outcomes of the 2 treatments.
Abstract
Importance
Accumulation of disability in multiple sclerosis may occur as relapse-associated worsening (RAW) or steady progression independent of relapse activity (PIRA), with PIRA regarded as a feature of primary and secondary progressive multiple sclerosis.
Objective
To investigate the contributions of relapse-associated worsening vs relapse-independent progression to overall confirmed disability accumulation (CDA) and assess respective baseline prognostic factors and outcomes of 2 treatments.
Design, Setting, and Participants
Analyses occurred from July 2015 to February 2020 on pooled data from the intention-to-treat population of 2 identical, phase 3, multicenter, double-blind, double-dummy, parallel-group randomized clinical trials (OPERA I and II) conducted between August 2011 and April 2015. In the trials, patients with relapsing multiple sclerosis (RMS), diagnosed using the 2010 revised McDonald criteria, were randomized from 307 trial sites in 56 countries; resulting data were analyzed in the pooled data set.
Interventions
Participants were randomized 1:1 to receive 600 mg of ocrelizumab by intravenous infusion every 24 weeks or subcutaneous interferon β-1a 3 times a week at a dose of 44 μg throughout a 96-week treatment period.
Main Outcomes and Measures
Confirmed disability accumulation was defined by an increase in 1 or more of 3 measures (Expanded Disability Status Scale, timed 25-ft walk, or 9-hole peg test), confirmed after 3 or 6 months, and classified per temporal association with confirmed clinical relapses (PIRA or RAW).
Results
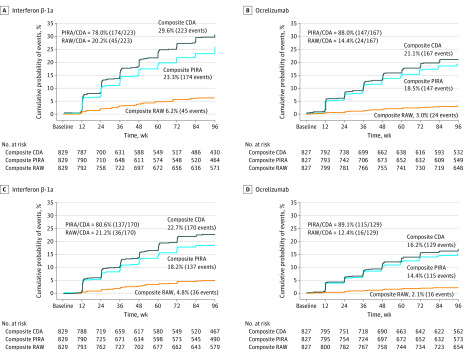
In the pooled OPERA I and II population (1656 of 2096 eligible participants), baseline demographics and disease characteristics were similar for patients randomized to interferon β-1a vs ocrelizumab (mean [SD] age, 37.2 [9.2] vs 37.1 [9.2] years; 552 [66.6%] vs 541 women [65.4%]). After 96 weeks, 12-week composite CDA had occurred in 223 (29.6% by Kaplan-Meier estimate) randomized to interferon β-1a and 167 (21.1%) randomized to ocrelizumab; 24-week composite CDA had occurred in 170 (22.7%) taking interferon β-1a and 129 (16.2%) taking ocrelizumab. The PIRA events were the main contributors to 12-week and 24-week composite CDA after 96 weeks in patients treated with interferon β-1a (174 of 223 [78.0%] and 137 of 170 [80.6%], respectively) and ocrelizumab (147 of 167 [88.0%] and 115 of 129 [89.1%], respectively); a minority had CDA explained by RAW events (69 of 390 [17.7%] and 52 of 299 [17.4%], respectively). Very few patients with composite CDA experienced both RAW and PIRA events (17 of 390 [4.4%] for 12-week and 15 of 299 [5.0%] for 24-week composite CDA). Ocrelizumab (vs interferon β-1a) was associated with reduced risk of composite CDA (hazard ratio [HR], 0.67) and confirmed PIRA (HR, 0.78) and RAW (HR, 0.47) events.
Conclusions and Relevance
Most disability accumulation in RMS is not associated with overt relapses. This indicates an underlying progression in this typical RMS population and challenges the current clinical distinction of relapsing and progressive forms of multiple sclerosis. Ocrelizumab was superior to interferon β-1a in preventing both RAW and PIRA.
Trial Registration
ClinicalTrials.gov Identifiers: OPERA I (NCT01247324) and OPERA II (NCT01412333).
Introduction
Multiple sclerosis (MS) is characterized by relapses with or without residual worsening and/or steady progression independent of relapses.1,2,3,4 A consensus statement suggested using the term disability worsening to describe a stepwise increase in disability in patients with relapsing MS (RMS) while reserving the term disability progression for patients in the progressive phase of MS, when disability accumulation occurs more continuously and independently of relapse activity.1 Most clinicians would not consider patients with RMS with a low level of disability to have secondary progressive MS (SPMS), in which accumulation of disability occurs independently of relapse activity,1 despite mounting data that patients with RMS frequently worsen over time, even when relapse activity appears well controlled.5,6,7
Typically, disability progression is measured using the Expanded Disability Status Scale (EDSS), where persistent increases in EDSS score are confirmed at 12 or 24 weeks or later points.1 An observational, single-arm study in patients receiving natalizumab, using a roving EDSS reference to more sensitively capture progressive events and potentially ascertain their independence of relapse occurrence, identified a considerable number of confirmed EDSS progression events that were independent of relapse activity (PIRA),6 suggesting that these events may constitute an important driver of disability accumulation in patients with RMS.7
Assessing composite confirmed disability accumulation (composite CDA), which captures overall (EDSS), upper (9-hole peg test [9HPT]), and lower (timed 25-ft walk [T25FW]) extremity function, better characterizes aspects of disease progression potentially missed with EDSS alone,8,9 leading to improved sensitivity for progression.10 Disability worsening because of incomplete recovery following relapse was previously defined as the onset of confirmed worsening by 1.0 point or more in EDSS score within 180 days of a relapse.11 A more stringent definition of relapse-associated worsening (RAW) could be CDA events in which the initial increase of disability (IID) is preceded by any protocol-defined relapse in the last 90 days.
Ocrelizumab is a recombinant humanized monoclonal antibody that selectively depletes CD20-expressing B cells12,13 while preserving the capacity for B-cell reconstitution and maintaining preexisting humoral immunity.14,15 Ocrelizumab showed superior efficacy in both relapse activity and CDA vs interferon β-1a in the prospective, phase 3 OPERA I and OPERA II trials on RMS.16 Here, the OPERA study data sets were pooled to characterize the relative contributions of RAW vs PIRA events in a typical population of patients with a relapsing form of MS, assess the association of ocrelizumab vs interferon β-1a with composite PIRA and composite RAW, and analyze baseline prognostic factors for PIRA and RAW.
Methods
Trial Design and Patients
The pooled intention-to-treat (ITT) population of the 2 identical phase 3, multicenter, double-blind, double-dummy, parallel-group randomized clinical trials (OPERA I [NCT01247324] and OPERA II [NCT01412333]) in patients with RMS was used for the analyses. Study details, including Consolidated Standards of Reporting Trials (CONSORT) study flow, randomization, and blinding, have been reported previously.16 Key eligibility criteria included an age of 18 to 55 years, a diagnosis of RMS (per the 2010 revised McDonald criteria),17 and an EDSS score of 0 to 5.5 points at screening. Patients were randomized (1:1) to receive either 600 mg of ocrelizumab by intravenous infusion every 24 weeks or subcutaneous interferon β-1a 3 times per week at a dose of 44 μg throughout the 96-week treatment period (including a per-label incremental titration scheme in the first 4 weeks).
The relevant institutional review boards and ethics committees approved the trial protocols. All patients provided written informed consent.
Definitions and Analyses
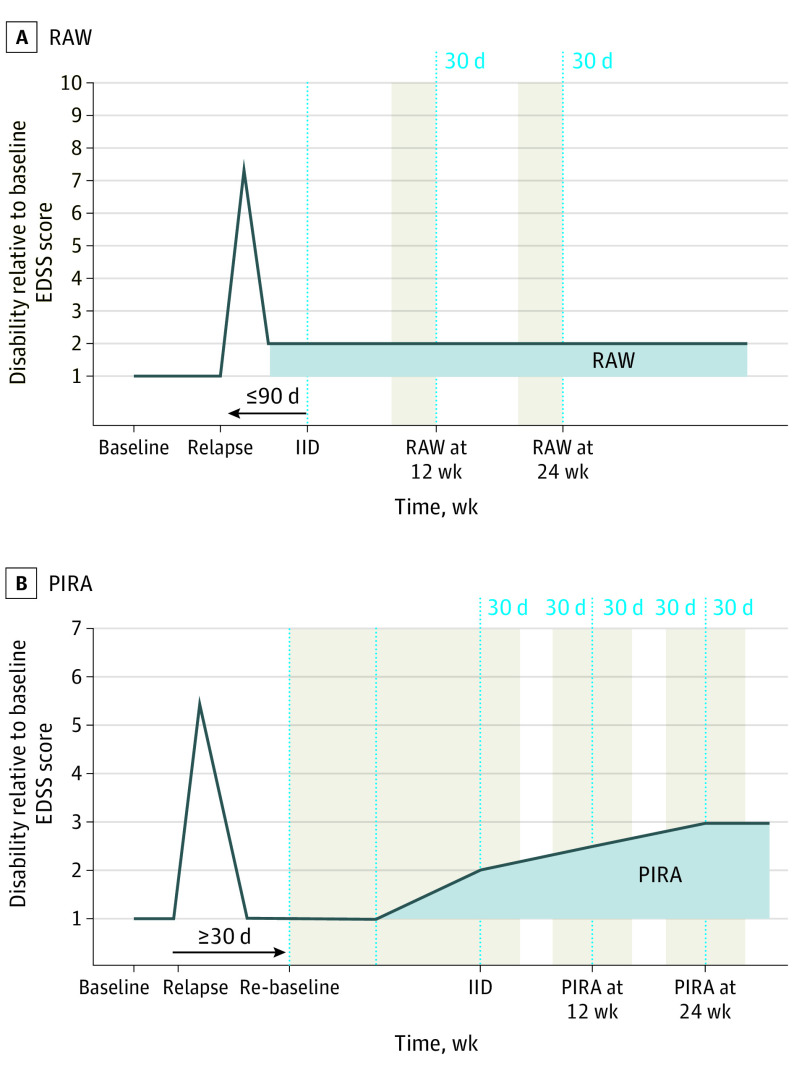
Composite CDA was defined as disability increase from study baseline, measured by EDSS (increase of ≥1.0 points if baseline EDSS was ≤5.5 points or an ≥0.5-point increase if baseline EDSS was >5.5 points) or an increase of 20% of more in T25FW or an increase of 20% or more in 9HPT confirmed after 12 or more or 24 or more weeks. Composite RAW events were defined as a subset of composite CDA events. In these, the initial disability increase from study baseline occurred 90 or fewer days after the onset of a protocol-defined relapse (Figure 1A).
Figure 1. Schematic Representations of Composite Relapse-Associated Worsening (RAW) and Composite Progression Independent of Relapse Activity (PIRA) Definitions.
A, Composite RAW and B, composite PIRA are the 2 nonmutually exclusive components (or drivers) of overall accumulation of disability, as measured by composite confirmed disability accumulation (CDA) in relapsing and progressive forms of MS. Study baseline is the reference point for disability changes measured over time; in the context of the studies, this is the time of randomization to study treatment, but in the context of the clinic, this would be the reference disability assessment visit from which subsequent changes are measured over time. The shaded areas represent the intervals around the neurological assessments that had to remain free of relapses to fulfill the criterion of independence from relapses (at initial event and confirmation points). Neurological assessments were scheduled to occur every 12 weeks, according to the protocol of the study; if a relapse occurred, there was 1 neurological assessment outside of the schedule, at a point corresponding to the leftmost point on the relapse triangle. EDSS indicates Expanded Disability Status Scale; IID indicates initial increase of disability; MS, multiple sclerosis.
Composite PIRA (Figure 1B) was defined as per the composite CDA, with the following modifications to ascertain event independence of relapse activity: the baseline reference assessment (EDSS, T25FW, or 9HPT values) was re-baselined 30 or more days after the onset of each relapse (with the first available assessment of respective scale ≥30 days after relapse onset). The re-baselined disability assessment could not be less than the original study baseline value. No protocol-defined relapse should occur between baseline reference assessment and within 30 days after the initial increase of disability (IID) and 30 days prior to and after the IID confirmation.
The following 6 sensitivity analyses for composite PIRA were also included: ones in which (1) there was an inclusion of events for which the re-baselined EDSS, T25FW, or 9HPT scores were less than the original study baseline, (2) IID occurred 90 or more days after the onset of a relapse, (3) a re-baseline was the first available assessment 60 or more days after the onset of last relapse, (4) no protocol-defined relapse was allowed between baseline or re-baseline and IID and between IID and 30 days after IID confirmation time, (5) a subgroup of patients without relapses during the study was included, and (6) patients were censored at the onset of the first protocol-defined relapse. Sensitivity analyses were also conducted for composite CDA, which involved censoring patients (1) before any new T2 lesions appeared or (2) before any new and enlarging T2 lesions or first relapse, whichever was earlier.
After completion of the 96-week controlled treatment period, most patients participated in the open-label extension phase of the OPERA studies; their EDSS assessments were used to confirm potential IID events that occurred at the end of the controlled period. In contrast with EDSS, the T25FW and 9HPT assessments were not collected during the open-label extension phase of OPERA I/II, and therefore the confirmation times for these 2 components were truncated at week 96.
In addition to analyses of the overall ITT population, a post hoc subgroup analysis was performed. This included only patients suggested to be at higher risk of SPMS according to a recent article18 (baseline EDSS ≥4.0 points and pyramidal Kurtzke functional systems score ≥2).
Statistical Analyses
All treatment outcome estimates are based on ITT analyses; statistical hypotheses were tested at the 5% significance level (α = .05) against 2-sided alternatives. Hazard ratios were calculated by Cox regression and P values by log-rank test, stratified by study, region (US vs rest of the world), and baseline EDSS score (<4.0 vs ≥4.0 points). Kaplan-Meier estimates of 12-week composite CDA, RAW, and PIRA, and consequently the relative contributions of composite RAW and PIRA to overall composite CDA, are based on first events.
Potential prognostic factors (eMethods in Supplement 1) for the different time-to-event outcomes (first protocol-defined relapse, composite CDA, PIRA, and RAW) were identified based on a univariate Cox regression analysis across treatment arms of all randomized patients in the pooled OPERA I/II population for each baseline covariate, which included the respective covariate, treatment, and covariate–treatment interaction. The P values are based on Wald statistics (eMethods in Supplement 1 and the study protocol in Supplement 2 for additional analysis details). Baseline covariates in the prognostic factor analysis included patient demographics, disease history, as well as clinical and imaging-based disease status collected in the OPERA studies (eMethods in Supplement 1). The analyses were performed using SAS version 9.4 (SAS Inc) and R version 3.5.3 (R Foundation for Statistical Computing) between July 2015 and February 2020 on pooled data collected between August 2011 and April 2015.
Results
Baseline Demographics and Characteristics
A total of 1656 of 2096 eligible patients were included in this study. Patient disposition and reasons for discontinuation are shown in eFigure 1 in Supplement 1 by treatment group. Key baseline demographics and disease characteristics were similar for patients randomized to interferon β-1a vs ocrelizumab in the pooled OPERA I/II population (mean [SD] values: age, 37.2 [9.2] vs 37.1 [9.2] years; relapses in the last year, 1.33 [0.69] vs 1.32 [0.67]; Expanded Disability Status Scale score, 2.8 [1.3] points vs 2.8 [1.3] points; 552 women [66.6%] vs 541 women [65.4%]) and the subgroup of patients at a higher risk of SPMS (mean [SD] values: age, 41.5 [8.4] vs 40.2 [9.3] years; relapses in the last year, 1.37 [0.81] vs 1.33 [0.66]; Expanded Disability Status Scale score, 4.7 [0.6] points vs 4.6 [0.6] points; 122 women [67.8%] vs 110 women [62.9%]) (Table 1). Patients in the subgroup at higher risk of SPMS, compared with the intention-to-treat population, were older (as above), had a longer MS disease duration (mean [SD] time, subgroup at higher risk of SPMS: interferon β-1a, 10.4 [7.3] years; ocrelizumab, 10.5 [7.0] years; intention-to-treat population: interferon β-1a, 6.5 [6.1] years; ocrelizumab, 6.7 [6.2] years), had a higher EDSS score (as above), and had a higher total brain T2 hyperintense lesion volume (subgroup at higher risk of SPMS: interferon β-1a, 13.8 [13.8] cm3 vs ocrelizumab, 16.6 [17.0] cm3; intention-to-treat population: interferon β-1a, 10.2 [11.8] cm3 vs ocrelizumab, 10.8 [14.1] cm3) but also had a similar rate of recent relapses and T1 gadolinium (Gd)–enhancing lesions.
Table 1. Baseline Demographics and Disease Characteristics.
| Variable | Mean (SD) | |||
|---|---|---|---|---|
| Intention-to-treat population | Subgroup at higher risk of SPMS11,a | |||
| Interferon β-1a (n = 829) | Ocrelizumab (n = 827) | Interferon β-1a (n = 180) | Ocrelizumab (n = 175) | |
| Age, y | 37.2 (9.2) | 37.1 (9.2) | 41.5 (8.4) | 40.2 (9.3) |
| Female, No. (%) | 552 (66.6) | 541 (65.4) | 122 (67.8) | 110 (62.9) |
| Time since MS symptom onset, y | 6.5 (6.1) | 6.7 (6.2) | 10.4 (7.3) | 10.5 (7.0) |
| Time since MS diagnosis, y | 3.9 (4.9) | 4.0 (4.9) | 6.5 (6.1) | 6.3 (5.7) |
| Naive to MS disease-modifying treatment, No. (%) | 606 (73.4)b,c | 605 (73.3)b,d | 123 (68.3) | 118 (67.4) |
| Expanded Disability Status Scale score | 2.8 (1.3)e | 2.8 (1.3) | 4.7 (0.6) | 4.6 (0.6) |
| No. of relapses | ||||
| In the last y | 1.33 (0.69)f | 1.32 (0.67)c | 1.37 (0.81)g | 1.33 (0.66)h |
| In the last 2 y | 1.76 (0.92)f | 1.79 (0.91)c | 1.93 (1.05)g | 1.87 (0.94)h |
| Magnetic resonance imaging | ||||
| Patients with T1 gadolinium-enhancing lesions, No. (%) | 327 (39.8)i | 333 (40.7)j | 61 (34.1)g | 65 (37.6)k |
| Brain T2 hyperintense lesion volume, cm3 | 10.2 (11.8)l | 10.8 (14.1)m | 13.8 (13.8)n | 16.6 (17.0)k |
Abbreviations: MS, multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
Defined by baseline Expanded Disability Status Scale scores of 4.0 or more and a pyramidal Kurtzke functional systems score of 2 or more.11
Data include patients who were untreated with any disease-modifying therapy in the 2 years before screening. The inclusion criteria did not select for untreated patients.
n = 826.
n = 825.
n = 828.
n = 827.
n = 179.
n = 175.
n = 822.
n = 818.
n = 173.
n = 824.
n = 822.
n = 180.
Composite CDA, RAW, and PIRA: ITT population
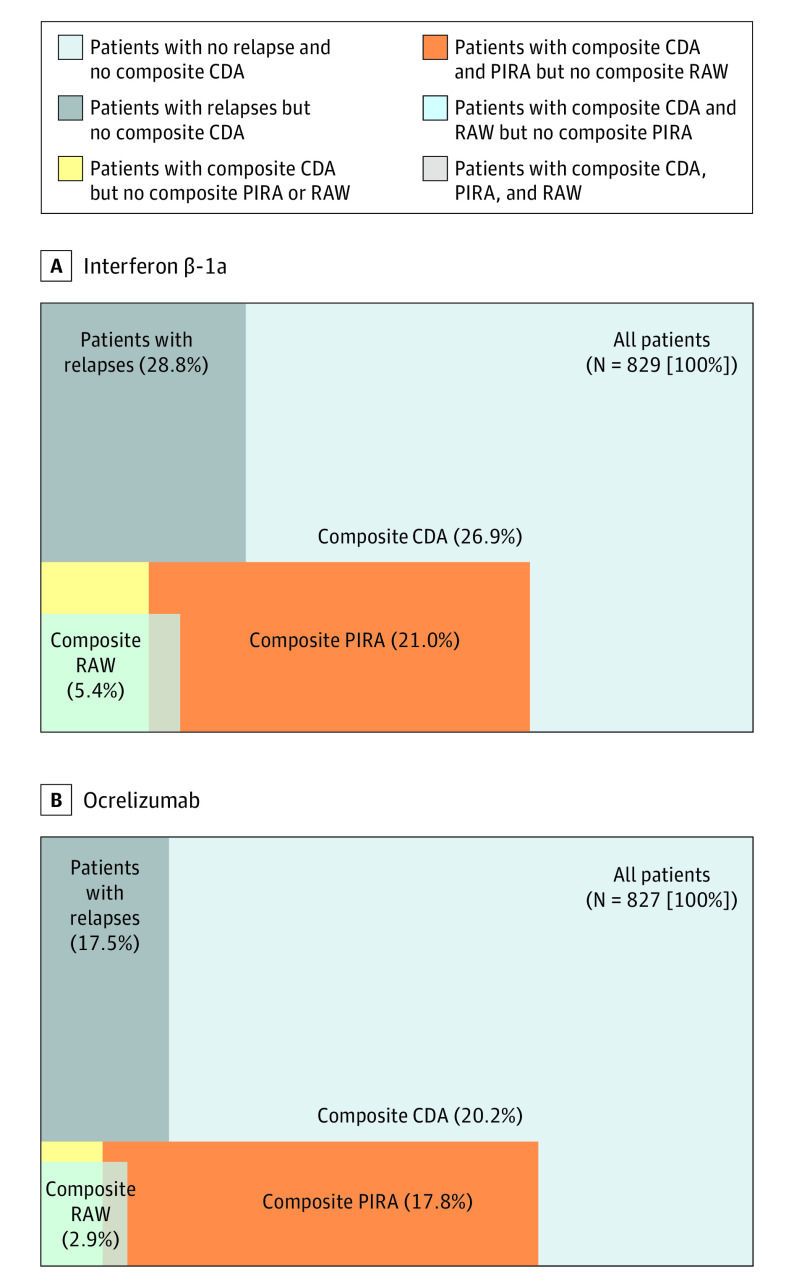
Most first events of composite CDA corresponded to composite PIRA events, both in patients treated with interferon β-1a (174 of 223 [78.0%] of 12-week and 137 of 170 [80.6%] of 24-week composite CDA events) and ocrelizumab (147 of 167 [88.0%] of 12-week and 115 of 129 [89.1%] of 24-week composite CDA events) (Figure 2). The populations of patients experiencing composite RAW and composite PIRA events appeared to be mostly nonoverlapping across both treatment groups; only 17 of 390 patients (4.4%) with 12-week composite CDA events and 15 of 299 patients (5.0%) with 24-week composite CDA events experienced both RAW and PIRA events (Figure 3 and eFigure 2 in Supplement 1). The few patients in each treatment group who experienced 12-week or 24-week composite CDA without fulfilling either definition of composite PIRA or composite RAW were more frequent with interferon β-1a treatment (14 of 829 [1.7%] and 8 of 829 [1.0%], respectively) compared with ocrelizumab treatment (3 of 827 [0.4%] and 2 of 827 [0.2%], respectively).
Figure 2. Relative Contributions of Composite Relapse-Associated Worsening (RAW) and Progression Independent of Relapse Activity (PIRA) vs Overall Composite Confirmed Disability Accumulation (CDA) in Treatment Groups.
The graphs compare the 12-week confirmed (A and B) and 24-week confirmed (C and D) overall composite CDA. All percentages indicate Kaplan-Meier proportions of patients with confirmed disability accumulation at week 96 in the pooled OPERA I and OPERA II intention-to-treat population.
Figure 3. Proportions of Patients With All Respective Combinations for 12-Week Composite Confirmed Disability Accumulation (CDA), Composite Relapse-Associated Worsening (RAW), and Composite Progression Independent of Relapse Activity (PIRA).
The graphs compare the pooled OPERA I and OPERA II population, analyzed by intention to treat. Surface-proportional Venn diagrams for patients receiving interferon β-1a (A) and ocrelizumab (B). For patients with protocol-defined relapses, composite CDA, composite RAW, and composite PIRA, all numbers embedded in these surface-proportional Venn diagrams represent the percentage of patients who experienced these specific types of events, alone or in combination. All values weremeasured by an increase in Expanded Disability Status Scale score (≥1.0 if the baseline score was ≤5.5, or ≥0.5 if the baseline score was >5.5) or an increase of 20% or more in the timed 25-ft walk or 20% or more in the 9-hole peg test. The segment indicating patients with relapses corresponds to the triangular shapes indicating relapse on each panel in Figure 1; the composite RAW and composite PIRA segments correspond to the shaded segments labeled RAW and PIRA in Figure 1A and B, respectively.
In both treatment groups, most patients experiencing 12-week or 24-week composite PIRA events were free from protocol-defined relapses during the complete study duration (Figure 3 and eFigure 2 in Supplement 1). The proportion of patients with at least 1 protocol-defined relapse in the OPERA studies was lower with ocrelizumab than interferon β-1a.16,19 Of the patients with relapses, 93 of 239 patients (38.9%) and 43 of 145 patients (29.7%) experienced 12-week composite CDA in the interferon β-1a and ocrelizumab groups, respectively. Of those same patients with relapses, 45 of 239 patients (18.8%) and 24 of 145 patients (16.6%) experienced 12-week composite RAW in the interferon β-1a and ocrelizumab groups, respectively.
Relative Contributions of EDSS, T25FW, and 9HPT Components to Composite RAW and PIRA Events
Analyses of the relative proportion of composite PIRA and composite RAW events driven by EDSS, T25FW, and 9HPT show that an increase in EDSS accounted for most of the 12-week (41 of 69 [59.4%]) and 24-week (36 of 52 [69.2%]) composite RAW events. In contrast, most of the 12-week composite PIRA events (148 of 321 [46.1%]) and 24-week composite PIRA events (122 of 251 [48.6%]) were driven by T25FW, with EDSS contributing 29.3% (94 of 321) and 29.5% (74 of 251), respectively (eTable 1 in Supplement 1).
Time at Risk of Composite PIRA and RAW
Based on the proposed method of re-baselining at each relapse, patients were temporarily not at risk of composite PIRA from the time of onset of each relapse until the time of the first available disability assessment (EDSS, T25FW, or 9HPT) was measured 30 or more days later. The mean cumulative time not at risk of a composite PIRA event per patient (ie, between relapse onset and re-baselining ≥30 days later) for all patients (ie, including those with no relapses) was a mean (SD) of 26.7 (52.7) days for patients treated with interferon β-1a (n = 829) and 14.9 (39.6) days for patients treated with ocrelizumab (n = 827).
Prognostic Factors for Time to First Event of Composite CDA, RAW, and PIRA and Protocol-Defined Relapse
Based on significance level and effect size, the baseline factors associated with 12-week composite CDA and 12-week composite PIRA were essentially identical (eTable 2 in Supplement 1). A higher risk of 12-week composite PIRA was based on a false discovery rate of 10% and significantly associated with a higher T1 hypointense lesion volume (hazard ratio [HR], 1.56) and T2 hyperintense lesion volume (HR, 1.51), a lower whole-brain volume (HR, 0.66) and cortical gray matter volume (HR, 0.57), a longer disease duration (HR, 1.80), male sex (HR, 0.67), lower perceived health-related quality of life (as measured by the 36-Item Short Form Survey; HR, 0.67), and higher disability burden (as measured by the EDSS score [HR, 1.48], multiple functional system scores, the 9HPT [HR, 1.50], Paced Auditory Serial Addition Test [HR, 0.69], or Multiple Sclerosis Functional Composite Score [HR, 0.62]20) at baseline. In contrast, only the presence of acute baseline magnetic resonance imaging (MRI) lesion activity as measured by T1 Gd-enhancing lesions was associated with a higher risk of protocol-defined relapses (HR, 1.47) and 12-week composite RAW (HR, 2.38), although it should be noted that the RAW analyses are based on fewer events.
Composite CDA, RAW, and PIRA: Treatment Outcomes
In the pooled ITT cohort (N = 1656), compared with interferon β-1a, ocrelizumab was associated with a reduction in the risk of 12-week overall composite CDA by 33% (HR, 0.67 [95% CI, 0.55-0.82]; P < .001; Figure 2A and B and Table 2). The corresponding risk reduction for the 24-week overall composite CDA was 30% (HR, 0.70 [95% CI, 0.55–0.88]; P = .002; Figure 2C and D and eTable 3 in Supplement 1). The risk reductions associated with 12-week and 24-week composite PIRA were 22% (HR, 0.78 [95% CI, 0.63-0.98]; P = .03) and 22% (HR, 0.78 [95% CI, 0.61-1.00]; P = .05), respectively, and those associated with 12-week and 24-week composite RAW were 53% (HR, 0.47 [95% CI, 0.29-0.78]; P = .003) and 59% (HR, 0.41 [95% CI, 0.23-0.75]; P = .003), respectively (Figure 2, Table 2, and eTable 3 in Supplement 1).
Table 2. Kaplan-Meier Estimates of 12-Week Composite Confirmed Disability Accumulation, Composite Relapse-Associated Worsening, and Composite Progression Independent of Relapse Activity Events, by Treatment Group and Component.
| 12-wk Confirmed value | Kaplan-Meier estimates at week 96, No. of events (%)a | Hazard ratio (95% CI)b | P value | |
|---|---|---|---|---|
| Interferon β-1a (n = 829) | Ocrelizumab (n = 827) | |||
| Composite confirmed disability accumulation | 223 (29.6) | 167 (21.1) | 0.67 (0.55-0.82) | <.001 |
| Expanded Disability Status Scale | 113 (15.2) | 75 (9.8) | 0.60 (0.45-0.81) | <.001 |
| Timed 25-ft walk | 127 (18.5)c | 103 (14.4)d | 0.74 (0.57-0.96) | .02 |
| 9-Hole peg test | 31 (4.6)d | 26 (3.6)e | 0.80 (0.47-1.34) | .39 |
| Relapse-associated worsening | ||||
| Composite | 45 (6.2) | 24 (2.9) | 0.47 (0.29-0.78) | .003 |
| Expanded Disability Status Scale | 34 (4.7) | 16 (1.9) | 0.41 (0.22-0.76) | .003 |
| Timed 25-ft walk | 13 (1.9)c | 10 (1.4)d | 0.71 (0.31-1.62) | .41 |
| 9-Hole peg test | 3 (0.5)d | 3 (0.4)e | 0.96 (0.19-4.75) | .96 |
| Progression independent of relapse activity | ||||
| Composite | 174 (23.3) | 147 (18.5) | 0.78 (0.63-0.98) | .03 |
| Expanded Disability Status Scale | 72 (9.5) | 58 (7.0) | 0.75 (0.53-1.07) | .11 |
| Timed 25-ft walk | 107 (15.5)c | 90 (12.6)d | 0.77 (0.58-1.03) | .07 |
| 9-Hole peg test | 27 (4.0)d | 22 (3.1)e | 0.78 (0.44-1.37) | .38 |
Kaplan-Meier proportion of patients with confirmed accumulation at week 96.
Based on Cox proportional hazards model adjusted by baseline Expanded Disability Status Scale score (<4.0 vs ≥4.0), region (US vs rest of world), and study (OPERA I vs OPERA II).
770 Patients included in analysis.
775 Patients included in analysis.
773 Patients included in analysis.
Consistent trends in the benefits of ocrelizumab treatment on the components of composite PIRA were observed for 12-week, 24-week, and 48-week confirmation periods (Table 2 and eTable 3 in Supplement 1). Composite RAW was mostly driven by EDSS, and consistent results were observed for treatment outcomes on RAW in EDSS scores using 12-week, 24-week, and 48-week confirmation periods. The event rate was too low to reliably evaluate any treatment outcomes on T25FW-defined and 9HPT-defined RAW.
Sensitivity Analyses of Different Definitions of PIRA
As shown in eTable 4 in Supplement 1, risk reductions associated with ocrelizumab vs interferon β-1a were consistently observed across the wide range of sensitivity analyses for 12-week and 24-week composite PIRA on re-baselining, timing of IID, and relapses. Similar findings were obtained for composite CDA associated with new and enlarging T2 lesions and relapses.
Composite RAW and PIRA in a Subgroup of Patients at Potentially Higher Risk of SPMS Disease Course Based on Defined Baseline Characteristics
In the subgroup of patients at higher risk of SPMS (baseline EDSS of ≥4.0 points and pyramidal Kurtzke functional systems score ≥2), the risk of 12-week and 24-week composite PIRA was lower with ocrelizumab compared with interferon β-1a by 39% (HR, 0.61 [95% CI, 0.39-0.98]; P = .04) and 36% (HR, 0.64 [95% CI, 0.39-1.06]; P = .08), respectively. The corresponding 12-week and 24-week risk reductions for composite RAW were 54% (HR, 0.46 [95% CI, 0.17-1.24]; P = .12) and 33% (HR, 0.67 [95% CI, 0.21–2.13]; P = .50), respectively (eTable 5 in Supplement 1). Consistent reductions were observed across all components of the composite PIRA and for RAW defined by EDSS in this specific subgroup (eTable 5 in Supplement 1), while the event rates for RAW-T25FW and RAW-9HPT were too low in the comparator arm to evaluate any treatment outcome on these components separately.
Discussion
In this study, we provide evidence that, in a typical population with relapsing MS, 80% to 90% of overall disability accumulation occurred independently of relapses. This observation, obtained in the setting of 2 state-of-the-art prospective phase 3 trials, challenges the current paradigm of a dichotomy between relapsing and progressive disease courses. Together with findings previously obtained in less well-controlled observational settings,5,6,7 our study strongly supports that MS may be a single disease continuum with an underlying progressive disease course and a highly variable superimposed accumulation of disability resulting from relapses with incomplete recovery. The more sensitive and comprehensive assessment criteria applied in this study, together with the suppression of relapse activity achieved by both interferon β-1a and ocrelizumab treatments, uncovered this continuous progression in a phase of the disease that is usually dominated by relapses.
Our observation provides the clinical counterpart to accumulating evidence from neuropathological, imaging, and biomarker studies that have also suggested a more continuous destructive process across all clinically defined stages of MS. Neuropathological studies have shown higher rates of axonal loss (the hallmark of permanent deficits) in early and relapsing MS than in more advanced and progressive stages.21,22 A meta-analysis performed by the Magnetic Resonance Imaging in MS (MAGNIMS) group demonstrated similar rates of annualized cerebral volume loss across all clinically defined MS courses, from clinically isolated syndromes to primary progressive MS,23 and recent studies measuring neurofilament light chain, a specific marker of neuroaxonal damage, showed similar blood levels of this protein in relapsing and progressive clinical phenotypes of MS.24,25
Ocrelizumab was superior to interferon β-1a in preventing composite CDA as well as both incomplete recovery of relapses (RAW) and PIRA. Similar trends as for composite CDA were observed across the individual components (EDSS, T25FW, and 9HPT) of the composite for PIRA and RAW. The association of ocrelizumab with disability accumulation was also evident in the subgroup of patients defined by a higher risk of SPMS (EDSS ≥4.0 points and pyramidal Kurtzke functional systems score ≥2),18 with a more pronounced reduction in the risk of 12-week and 24-week composite PIRA in this specific subpopulation.6 The effect of ocrelizumab on progression independent of relapses is further supported in principle by an effect in a primary progressive population.26
The proposed primary definition of RAW and PIRA is based on the assumptions that relapse-associated change resolves to a large extent within 30 days and further change after 90 days is unlikely. Although to some extent arbitrary, these numbers reflect clinical experience, are used for established definitions of relapses and, in this study, are supported by multiple sensitivity analyses of composite PIRA in the overall ITT-analyzed population. An analysis that disregarded all post-relapse data and an analysis of composite CDA in the subgroup of patients without any relapses during the 2-year study follow-up were consistent with the main analysis in the overall ITT-analyzed population in terms of composite PIRA event frequency and the benefit of ocrelizumab.
Limitations
As an intrinsic limitation of the PIRA analysis, we cannot exclude that milder relapses that patients may not have recalled or that would not have fulfilled the per-protocol relapse definition may have contributed to PIRA events across all tested definitions. A connection of PIRA with acute focal inflammatory activity is unlikely at least on the ocrelizumab arm because of the near-complete elimination of MRI activity. In principle, it would be possible to determine progression independent of MRI activity by re-baselining after the detection of a lesion; however, in practice, the limited frequency of MRI assessments in phase 3 MS trials (in our case at weeks 24, 48, and 96) does not provide sufficient granularity in time. Nevertheless, we performed a sensitivity analysis of patients with composite CDA and no new or enlarging T2 lesions. The results obtained were consistent with those of the primary analysis of composite PIRA events (eTable 4 in Supplement 1).
The large-scale univariate analysis of the prognostic value of baseline covariates for clinical disability outcomes identified only baseline MRI acute lesion activity as a factor associated with a composite RAW. In contrast, composite PIRA was associated with T1 and T2 lesion burden, whole-brain and cortical gray matter atrophy, disease duration, male sex, lower perceived health status, and disability at baseline. That composite PIRA and composite RAW events were associated with different baseline disease characteristics suggests distinct underlying pathobiological mechanisms. Along the same lines, events of PIRA and RAW appeared to be largely nonoverlapping and occurred in essentially distinct patients. We also observed that composite PIRA was mostly driven by T25FW-measured and 9HPT-measured deterioration, while composite RAW was mainly driven by EDSS. The higher frequency of EDSS-based detection of composite RAW may in part reflect the protocol definition of a relapse in these studies, which included EDSS change.
Currently, the detection and diagnosis of a progressive disease course in patients with relapsing-onset MS is grossly subjective, based solely on clinical judgment, and without a consensus clinical metric for its onset.1 Composite PIRA might serve as a criterion to mark, on measurable clinical grounds, the putative onset of the progressive phase in patients with RMS. However, a non–clinically obvious progressive MS course can be present in patients with RMS and remain unnoticed because of the lack of granularity of our clinical measures, but also, and perhaps more importantly, as a result of compensation and reorganization within the central nervous system.
The prominent role of PIRA vs RAW events with respect to overall CDA indicates that optimal prevention or delay of long-term accrual of irreversible disability in patients with RMS will depend on the control of the underlying progressive disease course, as measured by PIRA events.
Conclusions
Our results show that most overall disability accumulation in RMS is attributable to an underlying progressive disease course independent of relapse activity, challenging the current phenotypical distinction of relapsing and progressive forms of MS. In this study, ocrelizumab was superior to interferon β-1a in preventing confirmed disability accumulation, irrespective of its association with relapses.
eTable 1. Relative Contribution of EDSS, T25FW, and 9HPT to Composite RAW and Composite PIRA Events
eTable 2. Prognostic Baseline Covariates for Clinical Endpoints in the Pooled Population of the OPERA I and OPERA II Trials (N = 1656)
eTable 3. Summary of 24-Week Composite Confirmed Disability Accumulation (CDA), Composite Relapse-Associated Worsening (RAW), and Composite Progression Independent of Relapse Activity (PIRA) by Component (OPERA I and OPERA II Pooled ITT Population)
eTable 4. Sensitivity Analyses of Composite Progression Independent of Relapse Activity (PIRA)
eTable 5. Composite 12- and 24-Week Confirmed Relapse-Associated Worsening (RAW) and Composite Progression Independent of Relapse Activity (PIRA) in Patients at Higher Risk of SPMSa
eFigure 1. Patient disposition: Pooled OPERA I and OPERA II Studies
eFigure 2. Proportions of patients with all respective combinations for 24-week composite Confirmed Disability Accumulation (CDA), composite Relapse-Associated Worsening (RAW), and composite Progress Independent of Relapse Activity (PIRA) (OPERA I and OPERA II pooled ITT population)
eMethods.
Study Protocol and SAP.
Data Sharing Statement.
References
- 1.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286. doi: 10.1212/WNL.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodin DS, Traboulsee A, Knappertz V, et al. ; 16-Year Long Term Follow-up Study Investigators . Relationship between early clinical characteristics and long term disability outcomes: 16 year cohort study (follow-up) of the pivotal interferon β-1b trial in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(3):282-287. doi: 10.1136/jnnp-2011-301178 [DOI] [PubMed] [Google Scholar]
- 3.Jokubaitis VG, Spelman T, Kalincik T, et al. ; MSBase Study Group . Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann Neurol. 2016;80(1):89-100. doi: 10.1002/ana.24682 [DOI] [PubMed] [Google Scholar]
- 4.Koch-Henriksen N, Thygesen LC, Sørensen PS, Magyari M. Worsening of disability caused by relapses in multiple sclerosis: a different approach. Mult Scler Relat Disord. 2019;32:1-8. doi: 10.1016/j.msard.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 5.Cree BA, Gourraud PA, Oksenberg JR, et al. ; University of California, San Francisco MS-EPIC Team . Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80(4):499-510. doi: 10.1002/ana.24747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kappos L, Butzkueven H, Wiendl H, et al. ; Tysabri® Observational Program (TOP) Investigators . Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult Scler. 2018;24(7):963-973. doi: 10.1177/1352458517709619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cree BAC, Hollenbach JA, Bove R, et al. ; University of California, San Francisco MS-EPIC Team . Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653-666. doi: 10.1002/ana.25463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lublin F, Miller DH, Freedman MS, et al. ; INFORMS study investigators . Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10023):1075-1084. doi: 10.1016/S0140-6736(15)01314-8 [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Waubant E, Cutter G, Wolinsky J, Leppert D. Composite end points to assess delay of disability progression by MS treatments. Mult Scler. 2014;20(11):1494-1501. doi: 10.1177/1352458514527180 [DOI] [PubMed] [Google Scholar]
- 10.Cadavid D, Cohen JA, Freedman MS, et al. The EDSS-Plus, an improved endpoint for disability progression in secondary progressive multiple sclerosis. Mult Scler. 2017;23(1):94-105. doi: 10.1177/1352458516638941 [DOI] [PubMed] [Google Scholar]
- 11.Chan A, Phillips JT, Fox RJ, Zhang A, Okwuokenye M, Kurukulasuriya NC. Differential recovery from relapse between treatment groups in the CONFIRM study of delayed-release dimethyl fumarate. Mult Scler J. 2014;20(suppl 1):67-284. [Google Scholar]
- 12.Genovese MC, Kaine JL, Lowenstein MB, et al. ; ACTION Study Group . Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58(9):2652-2661. doi: 10.1002/art.23732 [DOI] [PubMed] [Google Scholar]
- 13.Klein C, Lammens A, Schäfer W, et al. Response to: monoclonal antibodies targeting CD20. MAbs. 2013;5(3):337-338. doi: 10.4161/mabs.24108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiLillo DJ, Hamaguchi Y, Ueda Y, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180(1):361-371. doi: 10.4049/jimmunol.180.1.361 [DOI] [PubMed] [Google Scholar]
- 15.Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol. 2006;24:467-496. doi: 10.1146/annurev.immunol.24.021605.090517 [DOI] [PubMed] [Google Scholar]
- 16.Hauser SL, Bar-Or A, Comi G, et al. ; OPERA I and OPERA II Clinical Investigators . Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221-234. doi: 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 17.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorscheider J, Buzzard K, Jokubaitis V, et al. ; MSBase Study Group . Defining secondary progressive multiple sclerosis. Brain. 2016;139(Pt 9):2395-2405. doi: 10.1093/brain/aww173 [DOI] [PubMed] [Google Scholar]
- 19.Havrdová E, Arnold DL, Bar-Or A, et al. No evidence of disease activity (NEDA) analysis by epochs in patients with relapsing multiple sclerosis treated with ocrelizumab vs interferon beta-1a. Mult Scler J Exp Transl Clin. 2018;4(1):2055217318760642. doi: 10.1177/2055217318760642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Multiple Sclerosis Society . Multiple sclerosis functional composite—administration and SCORING manual. Published 2001. Accessed October 2, 2018. http://main.nationalmssociety.org/docs/HOM/MSFC_Manual_and_Forms.pdf
- 21.Pfeifenbring S, Bunyan RF, Metz I, et al. Extensive acute axonal damage in pediatric multiple sclerosis lesions. Ann Neurol. 2015;77(4):655-667. doi: 10.1002/ana.24364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278-285. doi: 10.1056/NEJM199801293380502 [DOI] [PubMed] [Google Scholar]
- 23.De Stefano N, Giorgio A, Battaglini M, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74(23):1868-1876. doi: 10.1212/WNL.0b013e3181e24136 [DOI] [PubMed] [Google Scholar]
- 24.Disanto G, Barro C, Benkert P, et al. ; Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382-2391. doi: 10.1093/brain/awy154 [DOI] [PubMed] [Google Scholar]
- 26.Montalban X, Hauser SL, Kappos L, et al. ; ORATORIO Clinical Investigators . Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209-220. doi: 10.1056/NEJMoa1606468 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Relative Contribution of EDSS, T25FW, and 9HPT to Composite RAW and Composite PIRA Events
eTable 2. Prognostic Baseline Covariates for Clinical Endpoints in the Pooled Population of the OPERA I and OPERA II Trials (N = 1656)
eTable 3. Summary of 24-Week Composite Confirmed Disability Accumulation (CDA), Composite Relapse-Associated Worsening (RAW), and Composite Progression Independent of Relapse Activity (PIRA) by Component (OPERA I and OPERA II Pooled ITT Population)
eTable 4. Sensitivity Analyses of Composite Progression Independent of Relapse Activity (PIRA)
eTable 5. Composite 12- and 24-Week Confirmed Relapse-Associated Worsening (RAW) and Composite Progression Independent of Relapse Activity (PIRA) in Patients at Higher Risk of SPMSa
eFigure 1. Patient disposition: Pooled OPERA I and OPERA II Studies
eFigure 2. Proportions of patients with all respective combinations for 24-week composite Confirmed Disability Accumulation (CDA), composite Relapse-Associated Worsening (RAW), and composite Progress Independent of Relapse Activity (PIRA) (OPERA I and OPERA II pooled ITT population)
eMethods.
Study Protocol and SAP.
Data Sharing Statement.