Abstract
Introduction: The activity of butyrylcholinesterase (BChE) in blood reflects liver function and has recently been associated with systemic inflammatory response and tumor cachexia. As these conditions have been previously linked with pancreatic cancer (PC), the purpose of the present study was to evaluate the prognostic impact of plasma BChE in PC. Methods: Data from 574 consecutive PC patients, treated between 2004 and 2018 at a single academic center, was evaluated. The primary endpoint was cancer-specific survival (CSS), analyzed by Kaplan–Meier curve, and both univariate and multivariate Cox proportional models. Results: BChE activity negatively correlated with other liver parameters (bilirubin, gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and C-reactive protein (CRP)), and positively correlated with albumin levels, respectively (p < 0.01). In univariate analysis, a low plasma BChE activity was a factor of poor CSS (hazard ratio: 1.4, 95% confidence interval: 1.129–1.754, p = 0.002). In multivariate analysis, tumor stage, tumor grade, administration of chemotherapy, bilirubin levels and a low BChE activity (hazard ratio: 1.42, 95% confidence interval: 1.10–1.82; p = 0.006) were identified as independent prognostic factors. Conclusion: Decreased activity of BChE in blood plasma predicts shorter survival time in PC patients. Therefore, BChE might be helpful in additional stratification of patients into different prognostic risk groups.
Keywords: butyrylcholinesterase, prognostic factor, pancreatic cancer
1. Introduction
Pancreatic cancer (PC) ranges among the most aggressive tumor types with a five-year survival rate varying between two to nine percent worldwide [1]. In the United States, the five-year-survival rate determined for the period between 2008 and 2014 was 34 percent when diagnosed at early local tumor stages, while it was reduced to 12 percent in locally advanced stages. When distant metastases are already present, the five-year-survival is only three percent [2]. In 2019, PC was the fourth leading cause of cancer related mortality despite the fact that PC was the 10th most common cancer type in men and the 9th most common in women [2]. Typically, at the time of diagnosis only a small number of patients are identified at a stage that allows curative resection. Prognostic biomarkers are helpful in identifying patients with poor clinical outcome, and may help to stratify patients into clinical trials and more intense treatment plans [3].
Currently applied prognostic variables include histological grading, assessment of lymph node metastases, measurement of tumor size and determination of intrapancreatic perineural invasion [4,5,6,7]. However, the majority of these established clinico-pathological prognosticators are only available for assessment after surgery, making treatment decisions based on prognostic markers in metastatic PC more difficult. Measurement of novel molecular biomarkers are associated with high costs, time-consuming procedures and laboratory efforts [8,9]. Therefore, the search and establishment of easily determinable and available pre-treatment prognostic biomarkers is warranted and intensively studied [10,11,12].
Cholinesterases are enzymes that catalyze the hydrolysation of acetylcholine and other choline esters with high activity. There are two types of cholinesterases: Firstly, the acetylcholinesterase (AChE), which is mainly located in the nervous system, muscles and in erythrocytes and shows particularly high affinity to acetylcholine [13]. Secondly, the butyrylcholinesterase (BChE), also called pseudocholinesterase, plasma cholinesterase or serum cholinesterase, which is an α-glycoprotein with lower affinity to acetylcholine and which is located in the nervous system, liver and various other tissues [13]. Circulating BChE activity is an established marker for measuring liver synthesis and the nutritional status [14,15]. Moreover, there is evidence for its indicative role in systemic inflammation [16,17].
As PC frequently affects the liver´s functional state and nutritional status through the formation of liver metastases, portal vein thrombosis and systemic inflammatory responses, alterations in the BChE activity may reflect the aggressiveness of the disease. Therefore, we aimed at assessing the potentially prognostic value of BChE together with several other liver parameters in PC patients.
2. Results
The patient cohort in this study comprised a total of 574 patients, with 268 (46.7%) females and 306 (53.3%) males, all of which had histologically confirmed pancreatic adenocarcinoma. Median age in the cohort was 66 years (interquartile range: 58–72 years). Of this cohort, 25% had stage I or II disease, 5.4% had stage III disease and 69.6% of the patients, representing the largest sub-group, were diagnosed with stage IV metastatic disease, respectively. The tumor grading was G1 or G2 in 59.8% of patients, and G3 or G4 in 40.2%. In our cohort, 411 patients (71.4%) received chemotherapy and 173 (30.1%) of all 574 patients underwent surgical resection. Overall, the median survival was seven months (range 0–112 months) in our cohort. A summary of these patient characteristics is delineated in Table 1.
Table 1.
Clinico-pathological characteristics of patients comprising the study cohort (n = 574).
| Characteristics | No. Pancreatic Cancer (%) |
|---|---|
|
Gender female male |
268 (46.7) 306 (53.3) |
|
Tumor stage I + II III IV |
143 (25) 31 (5.4) 399 (69.6) |
|
Tumor grade 1 + 2 3 + 4 |
343 (59.8) 231 (40.2) |
|
Surgical resection yes no |
173 (30.1) 401 (69.9) |
|
Chemotherapy missing cases yes no |
1 (0.2) 410 (71.4) 163 (28.4) |
|
Karfnosky Index missing cases ≤80 90–100 |
5 (9) 337 (58.7) 232 (40.4) |
|
Cancer specific survival alive dead |
53 (9.2) 521 (90.8) |
The median value of the liver parameters determined in the patient cohort was the following: total serum bilirubin, 0.8 mg/dL (normal range: 0.10–1.2 mg/dL; interquartile range: 0.48–2.07); GGT (gamma-glutamyl transferase), 157.50 U/L (upper normal limit: 55 U/L; interquartile range: 47.75–429.00); AST (aspartate aminotransferase), 34 U/L (upper normal limit: 45 U/L; interquartile range: 23.00–68.00); ALT (alanine aminotransferase), 45 U/L (upper normal limit: 35 U/L; interquartile range: 24.00–103); ALP (alkaline phosphatase), 145.5 U/L (normal range: 40–130 U/L; interquartile range: 84.25–145.00); PT (prothrombin time), 98% (normal range: 70–120%; interquartile range: 88–105.5); BChE, 6565 U/L (normal range: 3900–11,000 U/L; interquartile range: 5457–7937); CA 19-9, 809.5 U/L (upper normal limit: 37 U/L; interquartile range: 116–6237).
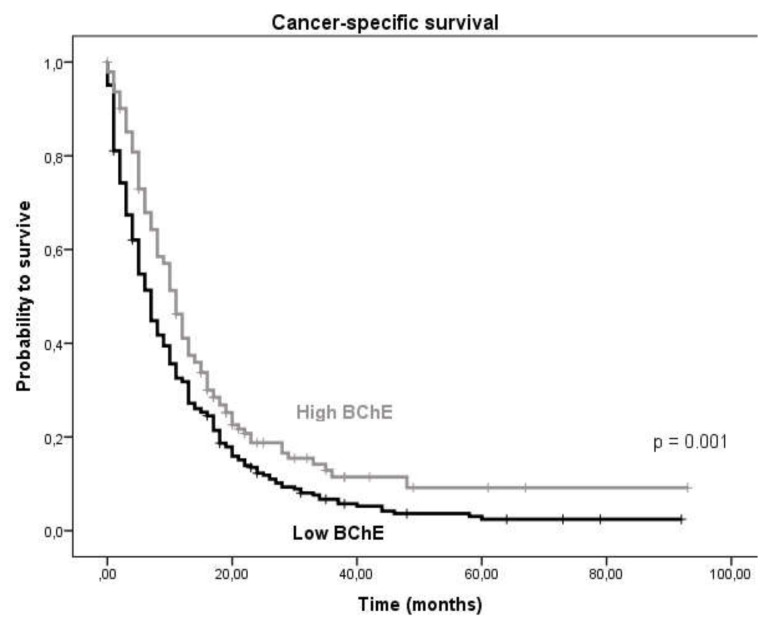
By applying ROC (receiver operating curve) analysis, the optimal cut-off to differentiate between survival in our patient cohort for BChE was identified to be ≤7272 U/L. Dividing the cohort into two groups according to the cut-off value we observed a significant association between low BChE activity and poor patient survival (Figure 1). The median survival time was 11 months (95% confidence interval: 9.44–12.56) vs. 7 months (95% confidence interval: 5.95–8.05) in the high vs. low BChE group, respectively (p-value = 0.001, log-rank test).
Figure 1.
CSS (cancer-specific survival) in patients with low BChE activity vs. high BChE activity.
The BChE activity was significantly negatively correlated with bilirubin levels (R = −0.295), GGT (R= −0.195), AST levels (R = −0.126), ALP (R = −0.221), C-reactive protein (CRP) levels (R = −0.361) and age (R = −0.134), whereas it was positively correlated with albumin levels (R = 0.499) and PT (R = 0.125) (p-values < 0.01 for all tested variables, Spearman correlation). No correlations were observed for CEA levels, CA 19-9 and neutrophil-lymphocyte ratio (p > 0.05). A significant correlation was identified for body mass index (BMI) (R = 0.113, p = 0.023). No significant association of BChE activity and tumor grade, stage or Karnofsky performance status was observed (p > 0.05).
Univariate and multivariate Cox analyses were also applied to examine the prognostic value of BChE in relation to other clinico-pathological parameters. Univariate analysis demonstrated the prognostic value of surgical resection (hazard ratio:0.339, 95% confidence interval: 0.775–0.418, p < 0.001), tumor grading (hazard ratio:1.269, 95% confidence interval: 1.065–1.512, p = 0.008), high tumor stage (hazard ratio: 3.789, 95% confidence interval: 2.995–4.794, p < 0.001), chemotherapy (hazard ratio: 0.412, 95% confidence interval: 0.339–0.501, p < 0.001) and CA 19-9 levels (hazard ratio: 1.872, 95% confidence interval: 1.554–2.256, p < 0.001).
By using the calculated optimized cut off values for all laboratory variables, we found bilirubin (hazard ratio: 0.746, 95% confidence interval: 0.610–0.913, p = 0.004), GGT (hazard ratio: 1.443, 95% confidence interval: 1.093–1.905, p = 0.010), ALT (hazard ratio: 0.791, 95% confidence interval: 0.658–0.951, p = 0.013), ALP (hazard ratio: 1.440, 95% confidence interval: 1.101–1.884, p = 0.008) and BChE (hazard ratio: 1.406, 95% confidence interval: 1.129–1.754, p = 0.002) to show a significant association with CSS (cancer-specific survival) in univariate analysis (Table 2).
Table 2.
Univariate and multivariate Cox proportional analysis regarding CSS in pancreatic cancer patients.
| Variable | Subset | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR 1 (95% CIl) 2 | p | HR 1 (95% CI) 2 | p | ||
| Gender | Female/Male | 1.159 (0.975–1.377) | 0.94 | 1.003 (0.794–1.266) | 0.98 |
| Grading | G3+4/G1+2 | 1.269 (1.065–1.512) | 0.008 | 1.699 (1.342–2.15) | <0.001 |
| Staging | Stage III/I+II | 3.161 (2.099–4.761) | <0.001 | 2.254 (1.367–3.717) | 0.001 |
| Stage IV/I+II | 3.789 (2.995–4.794) | <0.001 | 3.001 (2.178–4.136) | <0.001 | |
| Chemotherapy | Yes/No | 0.412 (0.339–0.501) | <0.001 | 0.329 (0.251–0.432) | <0.001 |
| Surgical resection | Yes/No | 0.339 (0.775–0.418) | <0.001 | not included | |
| CA 19-9 | >1191.7/≤1191.7 U/mL | 1.872 (1.554–2.256) | <0.001 | 1.288 (1.015–1.635) | 0.037 |
| Bilirubin | >1.9/≤1.9 mg/dL | 0.746 (0.610–0.913) | 0.004 | 0.694 (0.502–0.96) | 0.027 |
| GGT | >25/≤25 U/L | 1.443 (1.093–1.905) | 0.010 | 1.1 (0.711.686) | 0.663 |
| AST | >42/≤42 U/L | 0.880 (0.737–1.052) | 0.160 | 1.017 (0.713–1.45) | 0.925 |
| ALT | >64/≤64 U/L | 0.791 (0.658–0.951) | 0.013 | 0.876 (0.623–1.231) | 0.446 |
| ALP | >70/≤70 U/L | 1.440 (1.101–1.884) | 0.008 | 1.406 (0.937.11) | 0.1 |
| BChE | ≤7272/>7272 U/L | 1.406 (1.129–1.754) | 0.002 | 1.416 (1.10–1.818) | 0.006 |
| PT | >70/≤70% | 0.706 (0.485–1.028) | 0.069 | 0.777 (0.466–1.293) | 0.331 |
Bold values indicate significance (p ≤ 0.05). 1 HR, hazard ratio; 2 CI, confidence interval.
In multivariate analysis only bilirubin (hazard ratio: 0.694, 95% confidence interval: 0.502–0.96, p = 0.027) and BChE (hazard ratio: 1.416, 95% confidence interval: 1.10–1.181, p = 0.006) remained as independent prognostic markers (Table 2). In addition, CA 19-9 remained as a significant predictor of CSS in multivariate analysis (hazard ratio: 1.288, 95% confidence interval: 1.015–1.635, p = 0.037) (Table 2).
Furthermore, as shown in Table 2, our analysis demonstrates that tumor grading (hazard ratio: 1.699, 95% confidence interval: 1.342–2.15, p < 0.001), high tumor stage (hazard ratio: 3.001, 95% confidence interval: 2.178–4.136, p < 0.001) and chemotherapy (hazard ratio: 0.329, 95% confidence interval: 0.251–0.432), p < 0.001) were significant independent prognostic markers of CSS when analyzed by multivariate Cox proportional analysis (Table 2).
3. Discussion
In our study, we identified for the first time an association between low activity of BChE in plasma samples at time of diagnosis and poor CSS in a large cohort of PC patients. In general, BChE is well known as a marker for liver function and serves as an indicator of the nutritional status evaluated in daily routine [14,15]. Given the fact that PC tends to frequently metastasize in the liver, low BChE activity may reflect the decreased liver function due to the presence of multiple metastases. In our study, we observed a significant correlation of BChE and BMI, which might also reflect the association with the nutritional status. In addition, bilirubin appeared to carry potential as an additional independent prognostic marker in multivariate analysis. Bilirubin was also positively correlated to the localization of tumors in the pancreatic head, which may indicate that these cancers might have been diagnosed in earlier disease stages due to jaundice provoked by cholestasis, and may therefore have a better prognosis. In contrast, our study did not reveal an association between GGT, ALT, AST and prothrombin time in CSS in multivariate analysis, although these parameters were negatively correlated with the BChE activity.
To the best of our knowledge, this is the largest study investigating the usefulness of BChE as a prognostic biomarker in a cohort of PC patients. In a previously published study including 75 PC patients at disease recurrence after local therapy, BChE activity below 300 U/L was identified as an independent prognostic marker in multivariate analysis in patients without peritoneal dissemination. Nevertheless, there was no association with the presence of liver metastases. Furthermore, low BChE activity was associated with histologically confirmed nerve plexus invasion at the time of curative resection as well with anemia, poor performance status, cachexia, hypoalbuminemia, hypocholesterinemia and ascites, all signs of exceedingly advanced disease [18]. However, this retrospective study only included PC patients at recurrence following curative resection, whereas our study explored a cohort consisting of PC patients at primary diagnosis across all tumor stages, establishing the BChE activity as a prognostic marker in a broader patient spectrum. This data is in line with reports for other types of tumors. For instance, low BChE level was revealed as an independent marker of shorter survival time in colorectal carcinoma, upper tract urothelial carcinoma, clear cell renal cell carcinoma, prostate cancer and cervical cancer [19,20,21,22,23,24]. In gastric cancer patients, a lower activity of BChE when compared to healthy controls has been described [25]. Santarpia et al. found lower levels of BChE in terminal cancer patients receiving parenteral nutrition who had shorter survival time [26]. However, a study by Prabhu et al. including a small cohort of oral squamous cell carcinoma patients (n = 39) showed an increase in BChE levels in cancer patients compared to healthy controls [27]. In a retrospective study by Pavo et al. aiming to find independent pre-treatment prognostic liver parameters in various cancer entities for the prediction of all-cause mortality, both low levels of BChE and low levels of albumin were identified as independent prognostic parameters. They were independent of primary and secondary hepatic involvement at the time of diagnosis [28]. Interestingly, different liver parameters such as bilirubin, ALT, AST and GGT did not turn out as prognostic parameters in this analysis, a similar finding to our study [28].
Similar to our findings, a study by Lampón et al. found a negative correlation between CRP levels and BChE activity in patients with chronic systemic inflammation [16]. In their study, they suggest that BChE may be a negative inflammatory reactant [16]. Acetylcholine suppresses the production of pro-inflammatory cytokines such as TNF, IL-1 β, IL-6 and IL-18 through the cholinergic anti-inflammatory pathway and as a result regulates immune reactions [29,30]. It is supposed that an enhanced activity of cholinesterases such as AChE and BChE play a role in systemic inflammation through suppression of the cholinergic anti-inflammatory pathway by hydrolytic destruction of acetylcholine [17]. Furthermore, Pavo et al. showed a significant inverse correlation between BChE levels and inflammatory markers such as CRP, IL-6 and serum amyloid A in cancer patients [28]. Another study showed a significant association between cholinesterase activities including BChE with IL-6 and TNF-α but not with CRP in frail elderly patients without cancer [31]. Possibly, low BChE activity may be an indicator of systemic inflammatory reaction.
Our study has some limitations, mainly due to its retrospective nature. BChE activity is known to be a marker of impaired liver synthesis, meaning that other non-cancer related pre-existing liver diseases might influence the activity and contribute as co-morbidities and confounders to the prognostic significance of the marker. Furthermore, we did not determine the genetic variants of BChE in single patients before blood sampling, although these genetic variants can bear different activities of BChE in plasma [32,33].
Nevertheless, to the best of our knowledge, our study represents to date the largest one validating the prognostic value of BChE activity in PC patients.
4. Materials and Methods
In our study, we included data of 574 patients with a histologically verified adenocarcinoma of the pancreas. All patients were treated at the Division of Clinical Oncology, Medical University of Graz, between 2004 and 2018. Data regarding clinico-pathological variables and laboratory values were retrieved from medical records at the Division of Clinical Oncology and pathological records from the Institute of Pathology. Staging was performed in accordance with the 7th edition TNM classification system [34]. For analysis we evaluated selected laboratory values obtained within 7 days up to two weeks before the date of diagnosis or treatment, including the following parameters: bilirubin, ALP, GGT, ALT, AST, PT and BChE. Liver enzymes and BChE activity were measured using a Cobas automatic biochemical analyzer with matched reagent kits (Roche, Basel, Switzerland). Plasma BChE activity was determined using Cobas cholinesterase kit and butyrylthiocholine served as the substrate. Patient clinical and radiological conditions were evaluated every three months during the first three years, every six months over the following five years, and finally every year for curative resected tumor stages. Date of death was retrieved from the central registry of the Austrian Bureau of Statistics. There were no drop-outs due to a lack in proper follow-up. Our study was approved by the local ethics committee of the Medical University of Graz (Ethics Committee of the Medical University of Graz, Austria; document number No. 26-196 ex 13/14). Because of the retrospective data collection, there was no requirement for a written informed consent by the individual patients. Instead, we had a “waiver of consent” granted by the local ethics committee.
Statistical Analyses
We determined CSS as the time (in months) between date of diagnosis and cancer-related death. Statistical analyses were performed using Statistical Package for Social Sciences version 20.0 (SPSS Inc., Chicago, IL, USA) and MedCalc (Windows version 18.5, MedCalc Software bvba, Ostend, Belgium). For analysis of the relation between clinico-pathological parameters and plasma laboratory values we utilized non-parametric tests (Mann-Whitney U and χ2 test). To define an optimized cut-off value, ROC analysis was performed [35,36]. Kaplan–Meier analysis was applied to estimate patient survival status in test versus comparison group, which was done by using log-rank test. To define independent clinico-pathological factors influencing CCS we used backward stepwise multivariate Cox analysis. Estimated hazard ratios derived from Cox analyses were defined as relative risks with 95% confidence intervals. We considered a two-sided p < 0.05 as statistically significant.
5. Conclusions
In conclusion, in the present study, lower activity of BChE in plasma was demonstrated to represent a prognostic factor in PC patients. This simple, highly reproducible, inexpensive and easily available marker shows a potential to select patients at high risk for poor clinical outcome for appropriate treatment strategies.
Author Contributions
Conceiving and design of the study: E.V.K. and M.P.; performing statistical analyses: M.P.; interpretation of the results: E.V.K. and M.P.; writing of the first draft of the manuscript: E.V.K.; contribution to the writing of the manuscript: E.V.K., D.A.B., J.M.R., F.P., J.S., K.S., P.K., K.L., J.L, H.S., M.S., A.G. and M.P.; agreed with the manuscript’s results and conclusions: E.V.K., D.A.B., J.M.R., F.P., K.S., P.K., K.L., J.L., H.S., M.S., A.G. and M.P.; criteria for authorship read and met E.V.K., D.A.B., J.M.R., F.P., K.S., P.K., K.L., H.S., M.S., A.G. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Austrian Science Fund (FWF) (DK-MCD W1226, to MP); Open Access Funding by the Austrian Science Fund (FWF).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Ilic M., Ilic I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016;22:9694–9705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA. Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Golan T., Hammel P., Reni M., Van Cutsem E., Macarulla T., Hall M.J., Park J.O., Hochhauser D., Arnold D., Oh D.Y., et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N. Engl. J. Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fesinmeyer M.D., Austin M.A., Li C.I., De Roos A.J., Bowen D.J. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 2005;14:1766–1773. doi: 10.1158/1055-9965.EPI-05-0120. [DOI] [PubMed] [Google Scholar]
- 5.Fortner J.G., Klimstra D.S., Senie R.T., Maclean B.J. Tumor size is the primary prognosticator for pancreatic cancer after regional pancreatectomy. Ann. Surg. 1996;223:147–153. doi: 10.1097/00000658-199602000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raut C.P., Tseng J.F., Sun C.C., Wang H., Wolff R.A., Crane C.H., Hwang R., Vauthey J.N., Abdalla E.K., Lee J.E., et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann. Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozaki H., Hiraoka T., Mizumoto R., Matsuno S., Matsumoto Y., Nakayama T., Tsunoda T., Suzuki T., Monden M., Saitoh Y., et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg. Today. 1999;29:16–22. doi: 10.1007/BF02482964. [DOI] [PubMed] [Google Scholar]
- 8.Al-Zoughbi W., Schauer S., Pichler M., Hoefler G. Early Loss of Forkhead Transcription Factor, O Subgroup, Member 1 Protein in the Development of Pancreatic Ductal Adenocarcinoma. Pathobiology. 2018;85:342–347. doi: 10.1159/000492433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Zoughbi W., Pichler M., Gorkiewicz G., Guertl-Lackner B., Haybaeck J., Jahn S.W., Lackner C., Liegl-Atzwanger B., Popper H., Schauer S., et al. Loss of adipose triglyceride lipase is associated with human cancer and induces mouse pulmonary neoplasia. Oncotarget. 2016;7:33832–33840. doi: 10.18632/oncotarget.9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stotz M., Szkandera J., Stojakovic T., Seidel J., Samonigg H., Kornprat P., Schaberl-Moser R., Seggewies F., Hoefler G., Gerger A., et al. The lymphocyte to monocyte ratio in peripheral blood represents a novel prognostic marker in patients with pancreatic cancer. Clin. Chem. Lab. Med. 2015;53:499–506. doi: 10.1515/cclm-2014-0447. [DOI] [PubMed] [Google Scholar]
- 11.Stotz M., Szkandera J., Seidel J., Stojakovic T., Samonigg H., Reitz D., Gary T., Kornprat P., Schaberl-Moser R., Hoefler G., et al. Evaluation of uric acid as a prognostic blood-based marker in a large cohort of pancreatic cancer patients. PLoS ONE. 2014;9:e104730. doi: 10.1371/journal.pone.0104730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szkandera J., Stotz M., Eisner F., Absenger G., Stojakovic T., Samonigg H., Kornprat P., Schaberl-Moser R., AlZoughbi W., Ress A.L., et al. External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PLoS ONE. 2013;8:e78225. doi: 10.1371/journal.pone.0078225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis L., Britten J.J., Morgan M. Cholinesterase. Its significance in anaesthetic practice. Anaesthesia. 1997;52:244–260. doi: 10.1111/j.1365-2044.1997.084-az0080.x. [DOI] [PubMed] [Google Scholar]
- 14.Donini L.M., Savina C., Ricciardi L.M., Coletti C., Paolini M., Scavone L., De Felice M.R., Laviano A., Fanelli F.R., Cannella C. Predicting the outcome of artificial nutrition by clinical and functional indices. Nutrition. 2009;25:11–19. doi: 10.1016/j.nut.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Santarpia L., Grandone I., Contaldo F., Pasanisi F. Butyrylcholinesterase as a prognostic marker: A review of the literature. J. Cachexia. Sarcopenia Muscle. 2013;4:31–39. doi: 10.1007/s13539-012-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lampón N., Hermida-Cadahia E.F., Riveiro A., Tutor J.C. Association between butyrylcholinesterase activity and low-grade systemic inflammation. Ann. Hepatol. 2012;11:356–363. doi: 10.1016/S1665-2681(19)30932-9. [DOI] [PubMed] [Google Scholar]
- 17.Das U.N. Acetylcholinesterase and butyrylcholinesterase as markers of low-grade systemic inflammation. Ann. Hepatol. 2012;11:409–411. doi: 10.1016/S1665-2681(19)30940-8. [DOI] [PubMed] [Google Scholar]
- 18.Mitsunaga S., Kinoshita T., Hasebe T., Nakagohri T., Konishi M., Takahashi S., Gotohda N., Ochiai A. Low serum level of cholinesterase at recurrence of pancreatic cancer is a poor prognostic factor and relates to systemic disorder and nerve plexus invasion. Pancreas. 2008;36:241–248. doi: 10.1097/MPA.0b013e31815b6b2b. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M., Saito H., Uejima C., Tanio A., Tada Y., Matsunaga T., Sakamoto T., Honjo S., Ashida K., Fujiwara Y. Combination of Serum Albumin and Cholinesterase Levels as Prognostic Indicator in Patients ith Colorectal Cancer. Anticancer Res. 2019;39:1085–1090. doi: 10.21873/anticanres.13217. [DOI] [PubMed] [Google Scholar]
- 20.Koie T., Ohyama C., Yamamoto H., Hatakeyama S., Imai A., Yoneyama T., Hashimoto Y., Kitayam M., Hirota K. Significance of preoperative butyrylcholinesterase as an independent predictor of survival in patients with muscle-invasive bladder cancer treated with radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2014;32:820–825. doi: 10.1016/j.urolonc.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B., Shen C., Jin J., Song Y., Zhao Z., Zhang X., Wang G., Fan Y., Mi Y., Hu S., et al. Pretreatment serum pseudocholinesterase level as a novel prognostic biomarker for upper tract urothelial carcinoma. Int. Urol. Nephrol. 2016;48:1993–1999. doi: 10.1007/s11255-016-1401-1. [DOI] [PubMed] [Google Scholar]
- 22.Koie T., Ohyama C., Mikami J., Iwamura H., Fujita N., Sato T., Kojima Y., Fukushi K., Yamamoto H., Imai A., et al. Preoperative butyrylcholinesterase level as an independent predictor of overall survival in clear cell renal cell carcinoma patients treated with nephrectomy. Sci. World J. 2014;2014:e948305. doi: 10.1155/2014/948305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koie T., Ohyama C., Hatakeyama S., Imai A., Yoneyama T., Hashimoto Y., Yoneyama T., Tobisawa Y., Hosogoe S., Yamamoto H., et al. Significance of preoperative butyrylcholinesterase as an independent predictor of biochemical recurrence-free survival in patients with prostate cancer treated with radical prostatectomy. Int. J. Clin. Oncol. 2016;21:379–383. doi: 10.1007/s10147-015-0880-x. [DOI] [PubMed] [Google Scholar]
- 24.Poetsch N., Sturdza A., Aust S., Polterauer S., Grimm C., Schwameis R., Pötter R., Koelbl H., Reinthaller A., Seebacher V. The value of pretreatment serum butyrylcholinesterase level as a novel prognostic biomarker in patients with cervical cancer treated with primary (chemo-)radiation therapy. Strahlenther. Onkol. 2019;195:430–440. doi: 10.1007/s00066-019-01430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu S.Z., Zhao X.H., Ping Q., Li S.B., Pan B.R. Alterations of serum cholinesterase in patients with gastric cancer. World J. Gastroenterol. 2005;11:4604–4606. doi: 10.3748/wjg.v11.i29.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santarpia L., Alfonsi L., Pasanisi F., De Caprio C., Scalfi L., Contaldo F. Predictive factors of survival in patients with peritoneal carcinomatosis on home parenteral nutrition. Nutrition. 2006;22:355–360. doi: 10.1016/j.nut.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Prabhu K., Naik D., Ray S. Significance of serum butyrylcholinesterase levels in oral cancer. Australas. Med. J. 2011;4:374–378. doi: 10.4066/AMJ.2011.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavo N., Goliasch G., Wurm R., Cho A., Novak J.F., Pacher R., Hülsmann M., Raderer M., Gisslinger H., Steger G.G., et al. Subclinical involvement of the liver is associated with prognosis in treatment naïve cancer patients. Oncotarget. 2017;8:81250–81260. doi: 10.18632/oncotarget.17131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosas-Ballina M., Tracey K.J. Cholinergic control of inflammation. J. Intern. Med. 2009;265:663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard R.E., O’Mahony M.S., Calver B.L., Woodhouse K.W. Plasma esterases and inflammation in ageing and frailty. Eur. J. Clin. Pharmacol. 2008;64:895–900. doi: 10.1007/s00228-008-0499-1. [DOI] [PubMed] [Google Scholar]
- 32.Lockridge O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol. Ther. 2015;148:34–46. doi: 10.1016/j.pharmthera.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Lockridge O., Norgren R.B., Johnson R.C., Blake T.A. Naturally Occurring Genetic Variants of Human Acetylcholinesterase and Butyrylcholinesterase and Their Potential Impact on the Risk of Toxicity from Cholinesterase Inhibitors. Chem. Res. Toxicol. 2016;29:1381–1392. doi: 10.1021/acs.chemrestox.6b00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edge S.B., Compton C.C. The american joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 35.Szkandera J., Stotz M., Absenger G., Stojakovic T., Samonigg H., Kornprat P., Schaberl-Moser R., Alzoughbi W., Lackner C., Ress A.L., et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br. J. Cancer. 2014;110:183–188. doi: 10.1038/bjc.2013.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Absenger G., Szkandera J., Pichler M., Stotz M., Arminger F., Weissmueller M., Schaberl-Moser R., Samonigg H., Stojakovic T., Gerger A. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br. J. Cancer. 2013;109:395–400. doi: 10.1038/bjc.2013.346. [DOI] [PMC free article] [PubMed] [Google Scholar]