Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly throughout the world since the first cases of coronavirus disease 2019 (COVID-19) were observed in December 2019 in Wuhan, China. It has been suspected that infected persons who remain asymptomatic play a significant role in the ongoing pandemic, but their relative number and effect have been uncertain. The authors sought to review and synthesize the available evidence on asymptomatic SARS-CoV-2 infection. Asymptomatic persons seem to account for approximately 40% to 45% of SARS-CoV-2 infections, and they can transmit the virus to others for an extended period, perhaps longer than 14 days. Asymptomatic infection may be associated with subclinical lung abnormalities, as detected by computed tomography. Because of the high risk for silent spread by asymptomatic persons, it is imperative that testing programs include those without symptoms. To supplement conventional diagnostic testing, which is constrained by capacity, cost, and its one-off nature, innovative tactics for public health surveillance, such as crowdsourcing digital wearable data and monitoring sewage sludge, might be helpful.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly throughout the world. Infected persons who remain asymptomatic may play a role in the ongoing pandemic, but their relative number and effect have been uncertain. This article reviews the available evidence on asymptomatic SARS-CoV-2 infection.
Key Summary Points
The likelihood that approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic suggests that the virus might have greater potential than previously estimated to spread silently and deeply through human populations.
Asymptomatic persons can transmit SARS-CoV-2 to others for an extended period, perhaps longer than 14 days.
The absence of COVID-19 symptoms in persons infected with SARS-CoV-2 might not necessarily imply an absence of harm. More research is needed to determine the significance of subclinical lung changes visible on computed tomography scans.
The focus of testing programs for SARS-CoV-2 should be substantially broadened to include persons who do not have symptoms of COVID-19.
In the early months of the coronavirus disease 2019 (COVID-19) pandemic, an iconic image has been the “proned” patient in intensive care, gasping for breath, in imminent need of artificial ventilation. This is the deadly face of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which as of 26 May 2020 had claimed more than 348 000 lives worldwide (1). But it is not the only face, because SARS-CoV-2 now seems to have a dual nature: tragically lethal in some persons and surprisingly benign in others.
Since February 2020 (2, 3), there have been reports of persons who were infected with SARS-CoV-2 but did not develop symptoms of COVID-19. In some cases (4, 5), the viral load of such asymptomatic persons has been equal to that of symptomatic persons, suggesting similar potential for viral transmission. The prevalence of asymptomatic SARS-CoV-2 infection, however, has remained uncertain. We sought to review and synthesize the available evidence on testing for SARS-CoV-2 infection, carried out by real-time reverse transcriptase polymerase chain reaction using nasopharyngeal swabs in all studies that specified the method of testing.
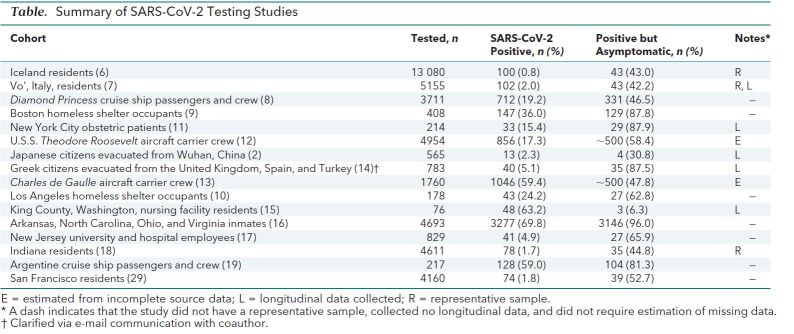
Most data from the 16 cohorts in this narrative review are not the output of large, carefully designed studies with randomly selected, representative samples. They do not generally purport to depict anything more than certain circumscribed cohorts at specific moments in time. We have not attempted to pool them for the purposes of statistical analysis. When viewed as a collection, though—as a kind of mosaic or patchwork—these data may offer potentially valuable insights into SARS-CoV-2 incidence and the highly variable effect of infection.
The difficulty of distinguishing asymptomatic persons from those who are merely presymptomatic is a stumbling block. To be clear, the asymptomatic individual is infected with SARS-CoV-2 but will never develop symptoms of COVID-19. In contrast, the presymptomatic individual is similarly infected but eventually will develop symptoms. The simple solution to this conundrum is longitudinal testing—that is, repeated observations of the individual over time. Unfortunately, only 5 of our cohorts include longitudinal data. We must therefore acknowledge the possibility that some of the proportions of asymptomatic persons are lower than reported.
Methods
From 19 April through 26 May 2020, using the keywords COVID-19, SARS-CoV-2, symptoms, and asymptomatic, we periodically searched the published medical literature using the PubMed service maintained by the U.S. National Library of Medicine of the National Institutes of Health. We also searched for unpublished manuscripts using the bioRxiv and medRxiv services operated by Cold Spring Harbor Laboratory. In addition, we searched for news reports using Google and monitored relevant information shared on Twitter.
Cohorts
Iceland
In the largest cohort in our set (6), researchers in Iceland used the following 2 methods to screen the general population for SARS-CoV-2 infection: an open invitation for interested parties to register online then provide biosamples at a Reykjavik location, and a text message sent to “randomly chosen Icelanders between the ages 20 and 70 years” inviting them to participate in the same manner as the first group (Table)<(7–19)>. In all, 13 080 persons volunteered for the screening, 100 (0.8%) of whom tested positive for SARS-CoV-2. All who tested positive were aged 10 years or older. None of the 848 children younger than 10 years in the sample tested positive. Among those with positive results, 43 (43%) had no symptoms of COVID-19 at the time of testing. As the researchers note, though, “symptoms almost certainly developed later in some of them” (6).
Table. Summary of SARS-CoV-2 Testing Studies.
Vo', Italy
At the beginning and end of a 14-day lockdown imposed by authorities in the northern Italian town of Vo' (7), researchers collected nasopharyngeal swabs from 2812 residents during the first sampling effort and 2343 during the second; this represented 85.9% and 71.5%, respectively, of the entire population. In the first group, 30 (41.1%) of 73 persons who tested positive for SARS-CoV-2 had no symptoms. In the second, 13 (44.8%) of 29 who tested positive were asymptomatic. According to the researchers, in the roughly 2-week period between the sampling efforts, none of the asymptomatic persons developed any symptoms of COVID-19. In addition, through contact tracing, they confirmed that several new cases of SARS-CoV-2 infection that appeared during the second sampling had been caused by exposure to asymptomatic persons. In Vo' during the 14-day period studied, young children seemed to play no role in the transmission of SARS-CoV-2: “No infections were detected in either survey in 234 tested children ranging from 0 to 10 years, despite some of them living in the same household as infected people” (7).
Diamond Princess
On 3 February 2020, the Diamond Princess cruise ship returned to Yokohama, Japan, for quarantine (8), having transferred an ill passenger to shore in Hong Kong on 25 January who later tested positive for SARS-CoV-2. As of 16 March, 712 (19.2%) of 3711 passengers and crew had tested positive. At the time of testing, 331 (46.5%) of those with positive results were asymptomatic. Although the latter infected persons reported no symptoms, some actually had subclinical changes in their lungs. When computed tomography scans for 76 of these persons were examined, 54% showed lung opacities (20).
An independent statistical modeling analysis (21) based on data available as of 21 February claimed to estimate—with “a Bayesian framework using Hamiltonian Monte Carlo algorithm”—the proportion of asymptomatic persons on the Diamond Princess; it arrived at a figure of 17.9%. Considering, though, that data for asymptomatic persons were available only for 15 through 20 February and that the actual proportions of asymptomatic persons among those tested on these dates were 56.7%, 54.3%, 70.7%, 73.9%, 86.1%, and 46.2%, this estimate seems puzzling. In a separate news account (22), one of the coauthors of this analysis was reported to have estimated that “40% of the general population might be able to be infected [with SARS-CoV-2] without showing any signs.”
Boston Homeless Shelter
After a cluster of 15 COVID-19 cases was identified over 5 days at a large homeless shelter in Boston, Massachusetts, the infected persons were removed from the shelter, and all occupants were subsequently tested over a 2-day period (9). Among 408 occupants, 147 (36.0%) tested positive for SARS-CoV-2, of whom 129 (87.8%) were asymptomatic (23). The researchers concluded that “front-door symptom screening in homeless shelter settings will likely miss a substantial number of COVID-19 cases in this high-risk population” (9).
Los Angeles Homeless Shelter
On 28 March, an initial case of COVID-19 was diagnosed with a positive test result at a homeless shelter in downtown Los Angeles, California (10). After a cluster of symptomatic persons was identified early in the week of 20 April, the shelter was closed to new occupants and testing was started for current occupants. As of 22 April, 43 (24.2%) of 178 completed tests were positive for SARS-CoV-2 and 27 (63.8%) of the persons who tested positive were asymptomatic.
New York City Obstetric Patients
Between 22 March and 4 April 2020, women who delivered infants at 2 New York City hospitals were tested for SARS-CoV-2 (11). Among 214 patients, 33 (15.4%) tested positive, 29 (87.9%) of whom were asymptomatic. The researchers note that “fever developed in 3 (10%) before postpartum discharge (median length of stay, 2 days)” (11). Two of those patients, though, were presumed to have endomyometritis, for which they were treated with antibiotics.
U.S.S. Theodore Roosevelt
The first case of SARS-CoV-2 infection aboard the American aircraft carrier U.S.S. Theodore Roosevelt was diagnosed on 22 March 2020 (24). As of 24 April, 4954 crew members had been tested for the virus; 856 (17.3%) tested positive (12). According to a news report, about 60% of those with positive results were asymptomatic (25). After an extended period of isolation, many of these asymptomatic persons continued to test positive for SARS-CoV-2. An internal U.S. Navy document stated, “Results of out-testing portions of the [Theodore Roosevelt] crew following 14 days of quarantine leads us to reevaluate our assessment of how the virus can remain active in an asymptomatic host” (26).
Charles de Gaulle Aircraft Carrier
On 8 April 2020, crew members aboard the French naval vessel Charles de Gaulle first began showing symptoms of COVID-19, 24 days after last having had contact with those outside the ship while docked on 15 March (27). On 10 April, 50 crew members received positive test results for SARS-CoV-2. The entire crew of 1760 was subsequently tested. As of 18 April, 1046 (59.4%) had tested positive, and of these, nearly 50% were asymptomatic (13).
Japanese Citizens Evacuated From Wuhan, China
As of 6 February 2020, a total of 565 Japanese citizens had been repatriated from Wuhan, China, on charter flights. Thirteen (2.3%) tested positive for SARS-CoV-2, of whom 4 (30.8%) were asymptomatic. As of 6 March, none of the latter persons had developed COVID-19 symptoms (2).
Greek Citizens Evacuated From Spain, Turkey, and the United Kingdom
From 20 through 25 March 2020, a total of 783 Greek citizens were repatriated from Spain, Turkey, and the United Kingdom on 7 flights. Forty (5.1%) tested positive for SARS-CoV-2 (14). At the time of testing, 39 (97.5%) were asymptomatic. At follow-up about 2 weeks later, 35 (87.5%) had remained asymptomatic (Lytras T. Personal communication.).
Nursing Facility Residents in King County, Washington
On 1 March 2020, a staff member who had worked at a 116-bed skilled-nursing facility in King County, Washington, on 26 and 28 February tested positive for SARS-CoV-2 (15). On 13 March, 76 (92.6%) of the facility's 82 current residents were tested; 23 (30.3%) tested positive. At the time of testing, 12 (52.2%) of the latter persons were asymptomatic. On 19 and 20 March, 49 residents were retested, including those who had previously received negative results and those who had tested positive but were asymptomatic or had atypical symptoms. In this second round of testing, 24 residents (49.0%) had positive results. Of these, 15 (63.5%) were asymptomatic. After a median of 4 days of follow-up, 24 (88.9%) of the 27 asymptomatic persons developed symptoms of COVID-19.
The researchers note, “More than half of residents with positive test results were asymptomatic at the time of testing and most likely contributed to transmission. Infection-control strategies focused solely on symptomatic residents were not sufficient to prevent transmission after SARS-CoV-2 introduction into this facility” (15).
Inmates in Arkansas, North Carolina, Ohio, and Virginia
Widespread outbreaks of COVID-19 in the correctional facilities of several states have led to large-scale screening programs. According to research by Reuters journalists (16), as of 25 April 2020, SARS-CoV-2 test results that include data on symptom status were available for 4693 inmates in the state prison systems of Arkansas, North Carolina, Ohio, and Virginia. Among these inmates, 3277 (69.8%) tested positive, of whom 3146 (96%) had no symptoms at the time of testing.
Rutgers University Students and Employees
From 24 March through 7 April 2020, researchers recruited 829 students and employees at Rutgers University and 2 affiliated hospitals for SARS-CoV-2 testing (17); 546 were health care workers. In total, 41 (4.9%) tested positive. Among health care workers, 40 (7.3%) tested positive, compared with 1 (0.4%) of those in other fields. Of all who tested positive, 27 (65.9%) reported no symptoms when they were tested.
Indiana Residents
From 25 April through 1 May 2020, the Indiana State Department of Health and the Indiana University Richard M. Fairbanks School of Public Health tested 4611 residents of Indiana for SARS-CoV-2 (18, 28). “This number includes more than 3,600 people who were randomly selected and an additional 900 volunteers recruited through outreach to the African American and Hispanic communities to more accurately represent state demographics” (28). In total, 78 (1.7%) tested positive; 35 (44.8%) of these persons were asymptomatic.
Argentine Cruise Ship Passengers and Crew
In mid-March 2020, a cruise ship departed Ushuaia, Argentina, for a planned 21-day expedition (19). After the emergence of a febrile passenger on the eighth day of the cruise, the ship's itinerary was altered, and it eventually docked at Montevideo, Uruguay, on the 13th day. All 217 passengers and crew members were tested; 128 (59.0%) tested positive, of whom 104 (81.3%) were asymptomatic.
San Francisco Residents
During 4 days in late April 2020, “4,160 adults and children, including more than half of the residents in the 16 square blocks that make up San Francisco Census Tract 229.01” in the Mission District, were tested (29). Seventy-four (1.8%) tested positive, of whom 39 (52.7%) were asymptomatic.
Discussion
Despite concerns about distinguishing asymptomatic from presymptomatic persons, data from 4 of 5 of the cohorts with longitudinal reporting suggest that a small fraction of asymptomatic persons may eventually develop symptoms. In the Italian and Japanese cohorts, 0% of asymptomatic persons became symptomatic. In the Greek and New York cohorts, 10.3% of asymptomatic persons became symptomatic. In the New York cohort, the figure might be as low as 3.4% because of the presumed diagnosis of endomyometritis in 2 of the 3 women who developed fevers. The observation period in this cohort, however, was extremely brief: a median of 2 days.
The King County cohort—in a skilled-nursing facility—is an outlier. Of 27 initially asymptomatic residents, 24 (88.9%) eventually developed symptoms and were therefore recategorized as having been presymptomatic. These persons were presumably much older and had more comorbid conditions than those in the other 4 longitudinal cohorts. In addition, they resided together in a single facility, which might have allowed for repeated exposures to infected persons. More research is needed to ascertain the effect of age and environmental factors on the natural history of COVID-19.
The Vo' cohort seems to confirm that asymptomatic persons can indeed transmit SARS-CoV-2 to others, and the experience aboard the U.S.S. Theodore Roosevelt suggests that they might be able to transmit the virus to others for longer than 14 days. These worrisome findings could explain, in part, the rapid spread of the virus around the globe. Persons who do not feel or look ill are likely to have far more interaction with others than those who have symptoms. If asymptomatic transmission is indeed common, testing only those with symptoms would seem to be folly.
The finding that 54% of the 76 asymptomatic persons on the Diamond Princess who were examined by computed tomography appeared to have significant subclinical abnormalities in their lungs is disturbing. Further research will be required to confirm this potentially important finding, taking into account possible confounding factors, including the age of passengers aboard the Diamond Princess. If confirmed, this finding suggests that the absence of symptoms might not necessarily mean the absence of harm. The subclinical nature of the finding raises the possibility that SARS-CoV-2 infection causes subtle deficits in lung function that might not be immediately apparent.
Does the relatively high proportion (60.5%) of asymptomatic cases on the U.S.S. Theodore Roosevelt—whose crew members, presumably, are mostly in their 20s and 30s—suggest that asymptomatic infection is more likely in younger persons? Perhaps, but it must be noted that the proportion of asymptomatic infection (47.8%) on the Charles de Gaulle aircraft carrier seems to be only marginally higher than average. A case series from Wuhan, China, from 24 December 2019 to 24 February 2020 included data for “78 patients from 26 cluster cases of exposure to the Hunan seafood market or close contact with other patients with COVID-19” (30). Asymptomatic patients “were younger (median [interquartile range] age, 37 [26-45] years vs 56 [34-63] years; P < .001), and had a higher proportion of women (22 [66.7%] women vs 14 [31.%] [sic] women; P = .002).”
As noted earlier, the data and studies reviewed here are imperfect in many ways. The ideal study of asymptomatic SARS-CoV-2 infection has yet to be done. What might that study look like? Most important, it must include a large, representative sample of the general population, similar to the U.S. serosurvey for which the National Institutes of Health is currently recruiting (31). In contrast to the narrowly defined cohorts here, it will be illuminating to have data that accurately reflect the population at large. In addition, longitudinal data must be collected over a sufficiently long time to distinguish between asymptomatic and presymptomatic cases.
Closed cohorts, such as cruise ships, aircraft carriers, and correctional facilities, offer both advantages and disadvantages. Because the likelihood of viral exposure is so much greater than in other settings, the “treatment” that participants receive may be close to uniform. As a result, we may learn more about the average incidence of asymptomatic infection. But the confined environment—which ensures frequent, overlapping interaction between participants—makes it challenging to accurately trace contacts and elucidate the chain of viral transmission.
On the basis of the 3 cohorts with representative samples—Iceland and Indiana, with data gathered through random selection of participants, and Vo', with data for nearly all residents—the asymptomatic infection rate may be as high as 40% to 45%. A conservative estimate would be 30% or higher to account for the presymptomatic admixture that has thus far not been adequately quantified. In any case, these high rates are not aligned with current testing programs that have predominantly focused on symptomatic cases. Beyond expanding testing to those without symptoms or known exposure, our inability to recognize carriers might make necessary the broad adoption of preventive strategies, such as masks.
The 96% rate of asymptomatic infection among thousands of inmates in 4 state prison systems is remarkable. Without any longitudinal data, we cannot estimate the number of presymptomatic cases. If the missing data prove to be similar to the Italian, Japanese, Greek, and New York cohorts, though, the vast majority of these persons will remain asymptomatic. Why, then, might the asymptomatic infection rate in this setting be so anomalously high?
One plausible factor could be cross-immunity imparted by the betacoronaviruses HCoV-OC43 and HCoV-HKU1, which has been proposed as a mitigating factor in the spread of SARS-CoV-2 (32). According to the U.S. Centers for Disease Control and Prevention, HCoV-HKU1 was active across the United States from late November 2019 through mid-February 2020 (33). In a locked-down congregate setting like a prison, it seems possible that contagious respiratory viruses could spread rapidly, so it would be interesting to do a serosurvey for antibodies to these betacoronaviruses. Still, 96% is very high. It would be prudent to review the source data carefully for errors.
What individual differences might account for why 2 persons of the same age, sex, and health status, for example, have idiosyncratic responses to SARS-CoV-2 infection? Why does one come through with nary a symptom, while the other lies near death in intensive care? At the moment, we simply do not know. If ever there were a need for precision medicine—for deeply and thoroughly understanding the multitudinous “-omics” that shape each of us—this is it. Perhaps there will be not just 1 therapy or vaccine for SARS-CoV-2 but versions that are individualized to maximize their efficacy.
In countries like the United States that have been hardest hit by the SARS-CoV-2 pandemic, it has been apparent for some time that the amount of testing must be significantly and rapidly increased—perhaps by an order of magnitude or more. With this new knowledge that a large proportion of those infected with SARS-CoV-2 have no symptoms, the urgency for more testing becomes even greater.
In a perfect world, perhaps using simple, accurate, inexpensive technology that is still on the drawing board (34), we would test each person every day for SARS-CoV-2. Until that is possible, innovative surveillance tactics might provide useful data for public health officials. Self-monitoring with internet-connected thermometers and smart watches that monitor heart rate, then crowdsourcing the resulting data, has been shown to accurately predict the incidence of influenza-like illness as reported by the California Department of Public Health and the Centers for Disease Control and Prevention (35–37). Similarly, monitoring sewage sludge provided “SARS-CoV-2 RNA concentrations [that] were a seven-day leading indicator ahead of compiled COVID-19 testing data and led local hospital admissions data by three days” (38).
The early data that we have assembled on the prevalence of asymptomatic SARS-CoV-2 infection suggest that this is a significant factor in the rapid progression of the COVID-19 pandemic. Medical practice and public health measures should be modified to address this challenge.
Biography
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M20-3012.
Corresponding Author: Eric J. Topol, MD, Scripps Research Translational Institute, 3344 North Torrey Pines Court, 3rd Floor, La Jolla, CA 92037; e-mail, etopol@scripps.edu.
Current Author Addresses: Mr. Oran and Dr. Topol: Scripps Research Translational Institute, 3344 North Torrey Pines Court, 3rd Floor, La Jolla, CA 92037.
Author Contributions: Conception and design: D.P. Oran, E.J. Topol.
Analysis and interpretation of the data: D.P. Oran, E.J. Topol.
Drafting of the article: D.P. Oran, E.J. Topol.
Critical revision of the article for important intellectual content: E.J. Topol.
Final approval of the article: D.P. Oran, E.J. Topol.
Statistical expertise: E.J. Topol.
Administrative, technical, or logistic support: E.J. Topol.
Collection and assembly of data: D.P. Oran, E.J. Topol.
Correction: This article was corrected on 17 June 2020 to update the publication and access dates for reference 12.
Footnotes
This article was published at Annals.org on 3 June 2020.
References
- 1. COVID-19 Case Tracker. Johns Hopkins Coronavirus Resource Center. 2020. Accessed at https://coronavirus.jhu.edu. on 26 May 2020.
- 2. doi: 10.1016/j.ijid.2020.03.020. Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) [Letter]. Int J Infect Dis. 2020;94:154-155. [PMID: 32179137] doi:10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed]
- 3. doi: 10.1001/jama.2020.2565. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020. [PMID: 32083643] doi:10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed]
- 4. doi: 10.1056/NEJMc2001737. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients [Letter]. N Engl J Med. 2020;382:1177-1179. [PMID: 32074444] doi:10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed]
- 5. doi: 10.15585/mmwr.mm6913e1. Kimball A, Hatfield KM, Arons M, et al; Public Health – Seattle & King County. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility — King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377-381. [PMID: 32240128] doi:10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed]
- 6. doi: 10.1056/NEJMoa2006100. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020. [PMID: 32289214] doi:10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed]
- 7. Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of COVID-19 outbreak in the municipality of Vo, Italy. Preprint. Posted online 18 April 2020. medRxiv. doi:10.1101/2020.04.17.20053157.
- 8. doi: 10.15585/mmwr.mm6912e3. Moriarty LF, Plucinski MM, Marston BJ, et al; CDC Cruise Ship Response Team. Public health responses to COVID-19 outbreaks on cruise ships — worldwide, February–March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:347-352. [PMID: 32214086] doi:10.15585/mmwr.mm6912e3. [DOI] [PMC free article] [PubMed]
- 9. Baggett TP, Keyes H, Sporn N, et al. COVID-19 outbreak at a large homeless shelter in Boston: implications for universal testing. Preprint. Posted online 15 April 2020. medRxiv. doi:10.1101/2020.04.12.20059618.
- 10. Chou E. Dozens positive for coronavirus at LA's Skid Row homeless shelter, after all residents tested. Los Angeles Daily News. 21 April 2020. Updated 22 April 2020. Accessed at www.dailynews.com/2020/04/21/dozens-positive-for-coronavirus-at-las-skid-row-homeless-shelter-after-all-residents-tested. on 23 April 2020.
- 11. doi: 10.1056/NEJMc2009316. Sutton D, Fuchs K, D'Alton M, et al. Universal screening for SARS-CoV-2 in women admitted for delivery [Letter]. N Engl J Med. 2020;382:2163-2164. [PMID: 32283004] doi:10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed]
- 12. U.S. Navy COVID-19 updates. Daily Update: April 24, 2020. Navy Live. 24 April 2020. Accessed at https://navylive.dodlive.mil/2020/03/15/u-s-navy-covid-19-updates. on 24 April 2020.
- 13. Coronavirus: bilan définitif de 1046 cas sur le Charles de Gaulle. Le Figaro. 18 April 2020. Accessed at www.lefigaro.fr/international/coronavirus-bilan-definitif-de-1046-cas-sur-le-charles-de-gaulle-20200418. on 21 April 2020.
- 14. doi: 10.1093/jtm/taaa054. Lytras T, Dellis G, Flountzi A, et al. High prevalence of SARS-CoV-2 infection in repatriation flights to Greece from three European countries. J Travel Med. 2020;27. [PMID: 32297940] doi:10.1093/jtm/taaa054. [DOI] [PMC free article] [PubMed]
- 15. doi: 10.1056/NEJMoa2008457. Arons MM, Hatfield KM, Reddy SC, et al; Public Health–Seattle and King County and CDC COVID-19 Investigation Team. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081-2090. [PMID: 32329971] doi:10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed]
- 16. So L, Smith G. In four U.S. state prisons, nearly 3,300 inmates test positive for coronavirus — 96% without symptoms. Reuters. 25 April 2020. Accessed at www.reuters.com/article/us-health-coronavirus-prisons-testing-in-idUSKCN2270RX. on 26 April 2020.
- 17. Barrett ES, Horton DB, Roy J, et al. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers at the onset of the U.S. COVID-19 epidemic. Preprint. Posted online 24 April 2020. medRxiv. doi:10.1101/2020.04.20.20072470.
- 18. Ratliff V. About 45% of COVID-19-positive Hoosiers don't know it. News and Tribune. 14 May 2020. Accessed at www.newsandtribune.com/about-45-of-covid-19-positive-hoosiers-don-t-know-it/article_1f424a76-9570-11ea-ae01-037620a46735.html. on 14 May 2020.
- 19. doi: 10.1136/thoraxjnl-2020-215091. Ing AJ, Cocks C, Green JP. COVID-19: in the footsteps of Ernest Shackleton. Thorax. 2020. [PMID: 32461231] doi:10.1136/thoraxjnl-2020-215091. [DOI] [PMC free article] [PubMed]
- 20. doi: 10.1148/ryct.2020204002. Inui S, Fujikawa A, Jitsu M, et al. Chest CT findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19). Radiol Cardiothorac Imaging. 2020;2. doi:10.1148/ryct.2020200110. [DOI] [PMC free article] [PubMed]
- 21. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25. doi:10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed]
- 22. USS Roosevelt's asymptomatic cases helping scientists understand virus. NBC 7 San Diego. 20 April 2020. Accessed at www.nbcsandiego.com/videos/uss-roosevelts-asymptomatic-cases-could-help-scientists-understand-virus/2309131. on 21 April 2020.
- 23. doi: 10.1001/jama.2020.6887. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020. [PMID: 32338732] doi:10.1001/jama.2020.6887. [DOI] [PMC free article] [PubMed]
- 24. Peniston B. The battle of USS Theodore Roosevelt: a timeline. Defense One. 7 April 2020. Updated 13 April 2020. Accessed at www.defenseone.com/threats/2020/04/timeline-battle-uss-theodore-roosevelt/164408. on 20 April 2020.
- 25. Stewart P, Ali I. U.S. to start antibody testing of sailors on coronavirus-hit aircraft carrier. Reuters. 17 April 2020. Accessed at www.nytimes.com/reuters/2020/04/17/us/17reuters-health-coronavirus-usa-military.html. on 20 April 2020.
- 26. Seligman L. Sailors keep testing positive on aircraft carrier, despite 2-week isolation. Politico. 21 April 2020. Accessed at www.politico.com/news/2020/04/21/navy-extends-isolation-for-uss-theodore-roosevelt-sailors-may-delay-ship-departure-198081. on 22 April 2020.
- 27. Coronavirus: 50 cas de contamination à bord du “Charles-de-Gaulle”, trois marins évacués. Le Parisien. 10 April 2020. Accessed at www.leparisien.fr/politique/coronavirus-50-cas-de-contamination-a-bord-du-charles-de-gaulle-trois-marins-evacues-10-04-2020-8297511.php. on 21 April 2020.
- 28. IU, ISDH release preliminary findings about impact of COVID-19 in Indiana. Indiana University. 13 May 2020. Accessed at https://news.iu.edu/stories/2020/05/iupui/releases/13-preliminary-findings-impact-covid-19-indiana-coronavirus.html. on 13 May 2020.
- 29. McFarling UL. When hard data are ‘heartbreaking': testing blitz in San Francisco shows Covid-19 struck mostly low-wage workers. STAT. 28 May 2020. Accessed at www.statnews.com/2020/05/28/sobering-finding-covid19-struck-mostly-low-wage-essential-workers-san-francisco. on 30 May 2020.
- 30. doi: 10.1001/jamanetworkopen.2020.10182. Yang R, Gui X, Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Netw Open. 2020;3:e2010182. [PMID: 32459353] doi:10.1001/jamanetworkopen.2020.10182. [DOI] [PMC free article] [PubMed]
- 31. National Institutes of Health. NIH begins study to quantify undetected cases of coronavirus infection. 10 April 2020. Accessed at www.nih.gov/news-events/news-releases/nih-begins-study-quantify-undetected-cases-coronavirus-infection. on 22 April 2020.
- 32. doi: 10.1126/science.abb5793. Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860-868. [PMID: 32291278] doi:10.1126/science.abb5793. [DOI] [PMC free article] [PubMed]
- 33. Centers for Disease Control and Prevention. National trends for common human coronaviruses. The National Respiratory and Enteric Virus Surveillance System. 2020. Accessed at www.cdc.gov/surveillance/nrevss/coronavirus/natl-trends.html. on 27 April 2020.
- 34. Zhang F, Abudayyeh OO, Gootenberg JS. A protocol for detection of COVID-19 using CRISPR diagnostics. 21 March 2020. Accessed at www.broadinstitute.org/files/publications/special/COVID-19. detection (updated).pdf on 7 May 2020.
- 35. doi: 10.1093/cid/ciy073. Miller AC, Singh I, Koehler E, et al. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis. 2018;67:388-397. [PMID: 29432526] doi:10.1093/cid/ciy073. [DOI] [PubMed]
- 36. doi: 10.1177/1460458219897152. Ackley SF, Pilewski S, Petrovic VS, et al. Assessing the utility of a smart thermometer and mobile application as a surveillance tool for influenza and influenza-like illness. Health Informatics J. 2020:1460458219897152. [PMID: 31969046] doi:10.1177/1460458219897152. [DOI] [PubMed]
- 37. doi: 10.1016/S2589-7500(19)30222-5. Radin JM, Wineinger NE, Topol EJ, et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digit Health. 2020;2:e85-93. doi:10.1016/S2589-7500(19)30222-5. [DOI] [PMC free article] [PubMed]
- 38. Peccia J, Zulli A, Brackney DE, et al. SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. Preprint. Posted online 22 May 2020. medRxiv. doi:10.1101/2020.05.19.20105999.