Abstract
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults. Although UM and cutaneous melanoma are derived from melanocytes, UM differs clinically and biologically from its more common skin counterparts. More than half of primary UMs metastasize. However, there is currently no effective treatment for metastatic UM. Therefore, studying mutations related to the metastasis, growth, proliferation, and survival of UM can help researchers understand its pathogenesis and metastatic mechanism, thereby leading to a more effective treatment. In addition, we provide an overview of the recent basic and clinical studies to provide a strong foundation for developing novel anti-carcinogenesis targets for future interventions.
Keywords: pathogenesis, therapeutic strategy, uveal melanoma
Introduction
Uveal melanoma (UM) is a common primary malignant eye tumor. If diagnosed early and treated locally before tumor metastasis, the survival rate can reach up to 90%.1 Unfortunately, approximately 50% of patients develop systemic metastases at the time of diagnosis.2–4 The distant metastasis organs are most often the liver, followed by the lungs and bones. The average survival time of these patients is reported to be approximately 2–15 months.5,6 Traditional treatments include eyeball removal, local tumor resection, plaque brachytherapy, and laser photocoagulation.7,8 Eyeball removal causes visual deprivation and affects appearance. Metastasis rates are relatively high in tumor resection. Laser photocoagulation is not effective and is clinically less used. However, plaque brachytherapy is an effective treatment for UMs, including large melanomas or those near the optic nerve head. Plaque brachytherapy has been shown to provide good clinical outcomes with respect to long-term local control and prevention of distant metastasis. Recent improvements in plaque design not only allow higher effective radiation delivery rate, but also reduce radiation-induced damage to normal tissues. Therefore, lower radiation doses—less than the traditional radiation dose of 85 Gy—may be effective. Further, endoresection of melanomas combined with plaque brachytherapy can also be employed safely for primary local tumor control.9 At present, new therapeutic strategies, such as targeted therapy, immunotherapy, and radiation therapy, face challenges in improving the clinical survival rate with good functional outcomes.
Clinical feature of uveal melanoma
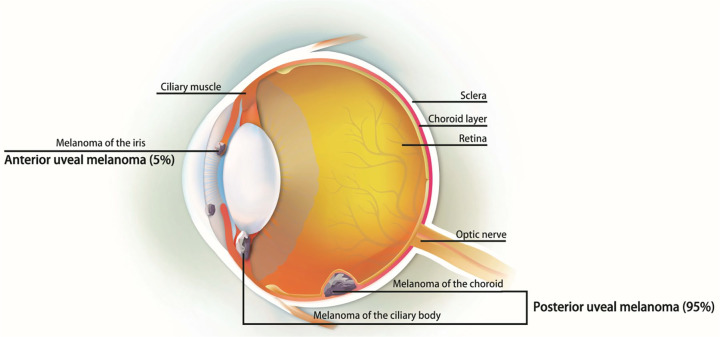
Ocular melanoma can originate from the uvea (83%), conjunctiva (5%), or other sites (10%) (Figure 1). UM may involve the iris, ciliary body, or choroid, which are collectively referred to as the uvea. The most common site of UM is the choroid, accounting for about 90%.10 Choroid melanoma is the most frequently found UM, and presents as dome- or mushroom-shape due to a rupture of the Bruch’s membrane. The mean size of the basal diameter is 11.3 mm, with a thickness of 5.5 mm.10 The lesions present as pigmented, non-pigmented, or mixed. Pigmented color melanoma should be differentiated from benign nevi. In iris melanoma, a majority of the tumor was round, and less was diffused.11,12 Changes in iris color (heterochromia), distortion of pupil margin, corectopia, and hyphema were found upon clinical examinations.13 In addition, secondary glaucoma was noted, especially due to tumor compression into the angle.14 Ciliary body melanoma is usually diagnosed late, after the lesion is too large to be hidden by the iris. Most patients express metamorphopsia or painless vision loss. Other symptoms include floater, photopsia, visual field loss, or pain.15 In general, clinical features predicting growth include increased tumor thickness, retinal detachment, or the presence of subretinal fluid. Features indicating non-neoplastic tumors include the presence of drusen over the tumor surface, retinal pigment epithelial atrophy, and intraretinal migration of the pigment epithelium.16,17
Figure 1.
The ocular melanoma originated from the iris, choroid, and ciliary body, which divided into anterior UM (5%) and posterior UM (95%).
UM, uveal melanoma.
Gene mutation in uveal melanoma
GNAQ and GNA11
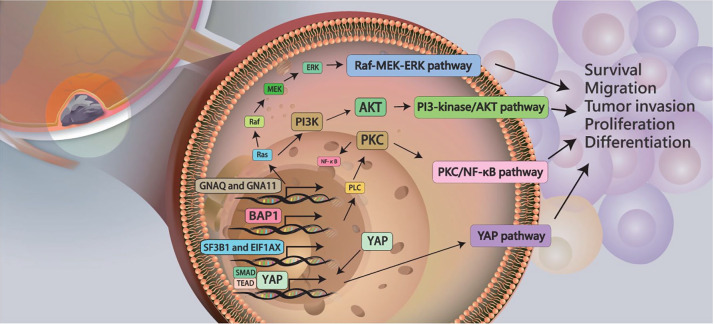
Mutations of oncogenes GNAQ and GNA11 were detected in 83% of UMs, including primary or metastatic UM.18 Somatic mutations in exon 5 (Q209) and exon 4 (R183) of GNA11 and GNAQ are present in a mutually exclusive pattern.18 The distribution of these mutation sites is different. GNAQ (209) mutations are more common in UM, and GNA11 (Q209) mutations are more common in metastatic UM. GNAQ (R183) and GNA11 (R183) mutation frequencies are low in both primary and metastatic tumors.18 GNAQ or GNA11 mutations in primary UM are not associated with clinical demographic characteristics, such as sex, age, overall survival (OS), metastasis-free survival, tumor thickness, diameter, pigment, extracellular matrix, cytogenetic, or molecular signal differences. Analysis of mutation frequency of GNAQ and GNA11 genes in UM revealed a 51.9% mutation frequency for the GNAQ gene, and the most common mutation site as Q209. The mutation rate for the GNA11 gene was 25.9%.19 Multiple downstream signaling pathways of GNAQ or GNA11 gene mutations, including the RAF (v-raf murine sarcoma viral oncogene homologue)/MEK [mitogen-activated protein kinase (MAPK) extracellular signal regulated kinase]/ERK (extracellular signal regulated kinase) pathway, PI3 (phosphatidylinositol 3)-kinase/AKT (v-akt murine thymoma viral oncogene homolog), protein kinase C, and YAP (yes-associate protein) pathways, have been investigated.20 Mutations in GNAQ or GNA11 lead to YAP over-activation. Activation of YAP induces uncontrolled cell growth, inhibits cell death, and leads to the formation of malignant tumors.21 In addition, GNA11 mutation might induce the MAPK pathway to promote spontaneously metastasizing tumors (Figure 2).22,23
Figure 2.
Several mutations of oncogenes, including GNAQ, GNA11, BAP1, SF3B1, and EIF1AX, may induced cell survival, migration, invasion, proliferation, and differentiation in UM via signaling pathways, including Raf-MEK-ERK pathway, PI3-kinase/Akt, protein kinase C/NF-κB, and YAP pathways.
Akt, v-akt murine thymoma viral oncogene homolog; BAP1, breast cancer susceptibility gene 1 (BRCA1)-associated protein 1; EIF1AX ; GNA11, G protein subunit alpha 11; GNAQ, G protein subunit alpha Q; NF-κB, nuclear factor kappa B; PI3, phosphatidylinositol 3; Raf-MEK-ERK, v-raf murine sarcoma viral oncogene homologue)/mitogen-activated protein kinase (MAPK) extracellular signal regulated kinase/extracellular signal regulated kinase; UM, uveal melanoma; YAP, yes-associate protein.
BAP1
Comparative analysis of genes on chromosome 3 in class 1 and class 2 tumors revealed that 85% of the class 2 tumors had mutations in BAP1 [breast cancer susceptibility gene 1 (BRCA1)-associated protein 1], while no mutations were detected in class 1 tumors.24 BAP1 is located at 3p21.1, and class 2 tumor cells have only one copy of the BAP1 gene on chromosome 3. BAP1 plays a role as a tumor suppressor gene in UM, and its loss makes tumor cells more prone to metastasis. The BAP1 molecule is a deubiquitinating enzyme that regulates the function of target proteins through the removal of ubiquitin molecules. For example, BAP1 can remove ubiquitin molecules on histone H2A, thereby altering the expression of downstream genes that are regulated by histone H2A. BAP1-regulated genes play an important role in melanocyte differentiation. Further, BAP1 deletion de-differentiates UM cells, exhibiting stem cell-like morphology and promoting tumor metastasis.25 In a retrospective cohort study by Gupta et al. that included 507 UM patients, germline BAP1 mutations were found to be associated with tumor diameter, ciliary body involvement, and metastases.26 These data suggest that BAP1 mutations are involved in aggressive tumor progression and associated with larger tumors, higher rates of ciliary body involvement, and metastases.27
SF3B1 and EIFlAX
SF3B1 (the splicing factor 3b1) is involved in pre-mRNA splicing. A mutation in SF3B1 is found in 19% of UM cases, and is significantly associated with prognosis.28 SF3B1 mutation results in selective splicing of a range of mRNAs; however, it is unclear how these mutations contribute to tumorigenesis. EIF1AX (eukaryotic translation initiation factor 1A, X-linked) is a protein encoded by EIFlAX that is involved in protein translation. EIFlAX mutation in UM patients is associated with a good prognosis;29 however, the carcinogenic mechanism of this mutation is still unclear. Interestingly, the appearance of BAP1, SF3B1, and EIFlAX mutations is almost mutually exclusive, suggesting that development of a mutation in one of the genes will not necessarily lead to another mutation in patients.
Pathogenesis of uveal melanoma
Multiple downstream signaling pathways, such as MEK, PI3K/AKT, and protein kinase, have been investigated in UM. MEK/MAPK and P13K/AKT signaling pathways are activated in UM.30,31 High activation of the P13K/AKT signaling pathway is attributed to GNAQ mutations and PTEN (phosphatase and tensin homolog) deletions.32,33 Mutant GNAQ and GNA11 are considered to be upstream molecules of the MEK/MAPK signaling pathway. Initially, GTP-bound GNAQ leads to phospholipase C (PLC) β activation, generating the second messenger diacylglycerol (DAG), which promotes protein kinase C (PKC) δ and ε to bind the C1 domains. RAS (rat sarcoma viral oncogene homolog) plays an important role in linking GNAQ to the RAS/RAF/MEK/ERK signaling pathway.34 Exogenously expressed mutant GNAQ upregulates MAPK phosphorylation, whereas knockdown of the GNAQ mutant reduces MAPK phosphorylation and increases G0/G1 phase cell population.18,35 In previous studies, PKC inhibition alone in UM could not completely suppress MAPK signaling.21,36 The data suggested that PKC-independent effectors may regulate MAPK signaling in UM.33 In addition, mutant GNAQ/11 promoted UM tumorigenesis via YAP, independent of PLC β.21,36 The tumor suppressor gene, PTEN, was also found to be lost in 75% of primary UM, and PTEN mutation occurred in 25% of these losses.37
The downstream signaling of mutant Gαq and Gα11 was investigated, especially in RAF-MEK1/2-ERK1/2 signaling. MEK1/2 small molecule inhibitors with trametinib or selumetinib inhibit the growth of a variety of UM cells. In metastatic UM patients, the resistance to MEK inhibitors was commonly reported and several studies found that hepatocyte growth factor (HGF) signaling may elicit resistance to MEK inhibitors.38,39 HGF expressed by the liver at the secondary site plays an important role for UM to preferentially metastasize to the liver. After binding HGF, activation of c-MET triggered several cellular signaling pathways involved in proliferation, migration, and invasion.40 In UM population, the expression of c-Met is significantly associated with mortality.41 As such, hepatic metastases are more likely to develop when the primary UM expresses high levels of c-Met.42 Some researchers have compared c-Met expression in UM primary tumors and metastases, and detected c-Met expression in both primary tumors and metastases; however, c-Met expression was higher in metastatic tumors than in primary tumors.43 Another growth factor secreted by the liver is insulin-like growth factor 1 (IGF-1). IGF-1 regulates cell proliferation, survival, and migration by binding to its receptor, IGF-1R, which is expressed in primary UM tissues.41 Multivariate analysis of immunohistochemistry showed that IGF-1R expression was associated with UM lethality. Immunohistochemical analysis of tissue samples from 24 patients with UM liver metastases revealed exogenous and endogenous IGF-1 and their interaction with IGF-1R in hepatic metastasis of UM.44 In addition, previous studies have demonstrated that hepatic stellate cells provide innate resistance to MEK inhibitors in metastatic UM through HGF-cMET signaling. After inhibition of HGF signaling, the cMET-targeting agent in UM cells overcomes the resistance to MEK inhibitors mediated by stellate cells or exogenous hepatocyte growth factors. The mechanism affecting MEK, in turn, includes growth factors NRG1 and HGF-induced metastatic UM cells.38,45 However, the molecular mechanisms by which HGF/c-Met and IGF-1/IGF-1R promote UM liver metastasis have not been elucidated.
Targeted therapy in uveal melanoma
Targeted therapy refers to the use of standardized biomarkers to identify the presence of a disease-specific gene or gene profile that controls tumor growth and to determine a treatment for a specific target. The main target sites discovered in UM include: BAP1 (BRCA1 associated protein-1), GNAQ/GNA11, IGF-1, TGF (transforming growth factor), VEGF (vascular endothelial growth factor), and c-Kit genes.46,47
BAP1 gene targeted therapy
BAP1 is a tumor suppressor gene that encodes a histone H2A ubiquitin hydrolase that regulates cell differentiation, cell cycle arrest, and DNA repair.48–50 Inactivation or gene mutations in BAP1 are considered to be an important feature of advanced UM metastasis, resulting in lower survival rate.24,49,51 Histone H2A ubiquitin hydrolase inhibitors can reverse the downstream effects of BAP1 deletions, and therefore have therapeutic value. One of the representative drugs is a histone deacetylase (HDAC) inhibitor that was found to inhibit tumor growth in UM cell lines in vitro and in vivo.25,52
GNAQ gene targeted therapy
GNAQ and GNA11 are homologous genes. Both are believed to have a positive effect on the proliferation and metastasis of UM.19,53 The mechanism is related to the PKC and MAPK cell signaling pathways.32,33,54 Novel PKC antagonists, enzastaurin, AEB071, and AHT956, inhibit GNAQ/GNA11 mutations by reducing PKC and MAPK signaling, thereby causing tumor cell apoptosis.32,33,54 In addition, studies have shown that a combination of PKC antagonist, AEB071, and MEK antagonist, PD0325901, inhibits GNAQ/GNA11 and increases apoptosis through different pathways. Therefore, combination therapy is much more effective than monotherapy.55 In addition, selumetinib indirectly inhibits the mutant GNAQ/GNA11 by antagonizing MAPK, which can increase the drug response rate by 14% compared with that of the traditional chemotherapy drugs, temozolomide and dacarbazine.56,57 In the selumetinib (AZD6244: ARRY-142886) (Hyd-Sulfate) in Metastatic Uveal Melanoma (SUMIT) study, a phase III double-blind trial (ClinicalTrial.gov identifier: NCT01974752) reported by Carvajal et al.,58 129 patients with metastatic UM were divided randomly into two groups, selumetinib (75 mg twice daily) plus dacarbazine (n = 97) or placebo plus dacarbazine (n = 32), for which progression-free survival (PFS) was not significantly different compared with the placebo group [85% versus 75%, 2.8 versus 1.8 months, hazard ratio (HR): 0.78, 95% confidence interval (CI): 0.48–1.27].
c-Kit gene targeted therapy
The c-Kit gene is a class III receptor tyrosine kinase transmembrane receptor family member and plays a role in differentiation, proliferation, and programmed cell death. Further, it is involved in intracellular signaling and plays a key role in hematopoiesis. It also shows overexpression in UM and is a potential carcinogenic driver in this tumor type.59,60 c-Kit protein is overexpressed in non-metastatic tumors, yet its expression is significantly reduced after metastasis61; however, the specific reasons are still unknown. Imatinib is a specific tyrosine kinase inhibitor targeting the c-Kit gene. It has been found that imatinib has significant therapeutic effects in patients with c-Kit mutations, especially in patients with exon 11 and 13 mutations.62 Currently, a phase II clinical study on imatinib is underway.63 Imatinib is a c-kit inhibitor that has been shown to reduce the proliferation and invasion rates of UM cells.64 C-kit is usually overexpressed in UM cells and metastatic lesions. In a study by Pereira et al.,64 a statistical significant decrease was found in the proliferation and invasion rates of all five cell lines tested, including four human UM cell lines (92.1, SP6.5, MKT-BR, OCM-1) and one human uveal transformed melanocyte cell line (UW-1). However, the efficacy of Imatinib in UM has yet to be addressed.65–67 In addition, Sunitinib, an oral multitargeted tyrosine kinase inhibitor blocking c-kit signaling, has been used as an adjuvant treatment for high-risk UM. In an analysis by Valsecchi et al., UM patients received adjuvant sunitinib for 6 months had longer OS, with HR of 0.53 (95% CI: 0.29–0.99) in univariate analysis.68 In the UM population, sunitinib was associated with better OS.
Role of IGF-1
Liver metastasis often occurs in UM, and the cause of its occurrence is still unknown. In recent years, many studies have demonstrated that growth hormone secretion by the liver facilitates tumor metastasis to the liver.5,41,69,70 One of the growth factors is IGF-1, which promotes tumor cell proliferation and tumorigenesis in UM and many other malignant tumors.71,72 A combination of IGF-1 and epidermal growth factor (EGF) signaling induces UM cell migration and invasion, thereby increasing the risk of metastasis.69,73 In addition, differences in expression levels of the IGF-1 receptor, IGF-1R, have been found in many UM tumors. It has become an important clinical parameter for UM assessment of prognosis.41,69 Studies on human UM stem cells have found that the IGF-1R inhibitor, cyclo-lignan picropodophyllin (PPP), can inhibit cell survival, growth, invasion, and migration.70,74 In an UM mouse model study, the use of PPP in mice subjected to xenograft liver transplantation effectively induced tumor regression and reduced liver metastasis.74 In addition, inhibition using a monoclonal antibody, IMC-A12 (cixutumumab), reduced IGF-1 activity and migration of UM cells. However, the result of the clinical study using IMC-A12 for metastatic UM was negative. A phase II trial by Patel et al. (ClinicalTrials.gov Identifier: NCT01413191), investigated the administration of cixutumumab 10 mg/kg on days 1 and 15 for a 4-week course in patients with metastatic UM; the trial found complete response in 0/17 patients, partial response in 0/17 patients, progressive disease in 8/17 patients, and stable disease in 9/17 patients.
VEGF treatment
Although current systemic anti-angiogenic therapy does not produce significant clinical response in UM patients,75,76 VEGF blockers have produced partial positive results in animal models. Anti-VEGF monoclonal antibody, ranibizumab, has been investigated as a single drug for phase II clinical trials of UM patients.77 In the Neoadjuvant IntraviTreal Ranibizumab treatment in high risk Ocular melanoma patients trial (NITRO Trial, EudraCT Number: 2011-000961-10), a two-stage, single-center, Phase II single-arm study, progressive disease was found in all treated patients (n = 7), and there was no survival benefit with intravitreal ranibizumab administration. Bevacizumab combined with the chemotherapy drug, temozolomide, has been used in patients with CMM (cutaneous malignant melanoma), and is entering phase II clinical trials. Sorafenib is an anti-tumor drug that inhibits angiogenesis. It has a dual anti-tumor effect, a RAF kinase inhibitor effect, which can result in inhibition of tumor proliferation by blocking the RAS/RAF/MEK/ERK signaling pathway, and the effect of inhibiting the tyrosine kinase activity of VEGF receptor (VEGFR), and platelet-derived VEGFR, thereby blocking the tumor angiogenesis and indirectly inhibiting tumor growth and metastasis.78 It also significantly inhibits tumor growth and metastasis to the lungs.79 In clinical trials, sorafenib extended survival in 31.2% of the patients to 6 months, and an OS rate of 6 months was reached 62.5% of the time. When combined with sorafenib and fotemustine (FTMU), 37.5% of patients with stage IV metastatic UM were locally controlled or stabilized, resulting in an average median survival of 15.9 months, for those with metastasis.80
Immunotherapy
Immunotherapy is the application of immunological principles and methods to enhance the patient’s active or passive immunity through exogenous injection of tumor vaccines, monoclonal antibodies, adoptive immune cells, and effector molecules, thereby stimulating the body’s anti-tumor response.81 The immune response ultimately kills tumor cells and inhibits tumor growth.82 Immunotherapy with checkpoint inhibition, including anti-CTLA-4 and PD- 1/PD-L1 blockade, in patients with advanced UM has garnered great interest. Other agents may include IMCgp100, glembatumumab, and MEK inhibitors. Because of better responses and fewer treatment-related deaths, checkpoint inhibitors serve as second-line therapy for locally advanced or metastatic UM. UM is generally insensitive to traditional chemotherapeutic drugs; therefore, researchers have adopted an emerging treatment, immunotherapy, for UM clinical trials.
Interferon in UM
interferon (IFN) is a cytokine with a wide range of biological activities. It can exert anti-tumor effects through various mechanisms, including inhibition of tumor cell proliferation and tumor angiogenesis, and apoptotic induction, to enhance the body’s immune function to kill tumor cells and inhibit the expression of multiple oncogenes.83 At present, IFN is used widely as a clinical treatment for various tumors, especially for tumors caused by some infectious agents. In UM patients, IFN served as an adjuvant treatment for primary UM, but not for treatment of metastatic UM. However, IFN has shown a poorer therapeutic effect in UM than in other tumors. Treatment with IFN-α-2b does not improve patient survival, with a 5-year mortality rate of 15–17%.84 In a study in which 39 UM patients were injected subcutaneously with the same dose of IFN-α-2b, 46% of the patients had to reduce the therapeutic dose due to severe side effects, including decreased white blood cell counts, thrombocytopenia, abnormal heart and liver function, etc. Further, IFN did not extend survival for UM patients, even though IFN showed a positive effect in the treatment of cutaneous melanoma. A phase II trial by Nathan et al. investigated a regimen of bleomycin + vincristine + lomustine + dacarbazine (BOLD) with intercycle alpha interferon-2b in 20 patients with metastatic uveal melanoma, and observed four objective responses [response rate (RR) = 20%].85 However, in a study by Buder et al. study, BOLD with INF-α2b was used in four phase II trials with metastatic UM.85–89 The estimated overall response rate (ORR) was 10.3% (substantial heterogeneity, p = 0.16; 95% CI: 4.8–18.7%). Therefore, IFN has been employed as an adjuvant treatment in UM.
TGF-β in UM
The eyes of UM patients are in an unusual microenvironment for immune remission. UM escapes immune system surveillance, which promotes tumor growth, increasing cell survival and metastasis. The mechanism whereby UM avoids the immune response is still unclear; however, TGF-β has shown immunosuppressive effects in UM. In clinical studies, TGF-β1 and TGF-β2 immunohistochemical examinations were performed on 13 surgically resected UM specimens. All tissue specimens showed positive results for TGF-β, and confirmed the immunosuppressive properties of TGF-β. It is also believed that TGF-β causes immunosuppression in the eye of UM patients, thereby inhibiting the host’s immune response against the tumor. An increase in the level of TGF-β may suggest an enhanced ability of the tumor to evade host immune surveillance.90 Other studies have confirmed that TGF-β is negatively correlated with major histocompatibility complex I (MHC-I) in UM. Specifically, when cultured UM cells were treated with TGF-β, MHC-I expression decreased by about 30%, thereby increasing the sensitivity of UM cells to natural killer cell-mediated cell autolysis.91
Immune checkpoint blockades
In recent years, immunological checkpoint inhibitors have achieved favorable results in skin management due to targeting of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) ipilimumab and programmed cell death-1 (PD-1) pembrolizumab in the management of cutaneous melanoma.92 Immune checkpoint inhibitors are the new revolutionary first-line treatment for the management of advanced or metastatic UM.93 Immune checkpoint inhibitors target checkpoints to promote T cell activation, leading to tumor lysis and degradation.93
Based on the mechanism of action of monoclonal antibodies on tumor cells, currently used antibodies can be roughly classified into the following categories: direct anti-tumor monoclonal antibody and specific monoclonal antibody that can be directed to the surface of a tumor cell. Antigen binding, which blocks the transduction of certain signaling pathways in tumor cells, can inhibit tumor cell proliferation or induce apoptosis. Anti-tumor monoclonal antibody conjugates, including chemical compounds, radionuclides, and toxins, can be transported to tumor cells in a targeted manner by the specificity of monoclonal antibodies without killing normal cells, thereby improving efficacy and reducing side effects. There is also an immune-mediated tumor cell. Killing substances, such as monoclonal antibodies, injected into the human body can induce complement activation, activate antibody-dependent cytotoxicity, and participate in the regulation of T lymphocyte function, which enhances the autoimmune response to kill tumor cells.94
Anti-CTLA-4 therapy
Ipilimumab is a specific CTLA-4 (cytotoxic T lymphocyte antigen 4) inhibitor that was approved by the Food and Drug Administration (FDA) in 2011 for the treatment of advanced malignant cutaneous melanoma. Its main mechanism of action is through enhanced T lymphocyte cell-mediated immune responses that exert an anti-tumor effect. CTLA-4 is a transmembrane receptor on the surface of T lymphocytes, which can compete with cluster of differentiation 28 (CD28) for binding to ligand B7 and negatively regulate the activity of T lymphocytes. Studies have found that CTLA-4 binds to B7 and can limit the signal transduction involved in CD28, resulting in the inability of T lymphocytes to respond to immune responses. T lymphocytes can also be produced by inhibiting the production of IL-2 (interleukin 2) and its receptors. Remaining in the G1 phase, inhibition of proliferation can occur and induce apoptosis of T lymphocytes. Injectable ipilimumab can specifically bind to CTLA-4, block CTLA-4 binding to B7, restore T lymphocyte activity, and enhance the body’s own immune response to kill cancer cells.95 A study by Heppt et al. reported that, at a dose of 10 mg/kg, ipilimumab improved the median OS to 5.2–9.8 months.96
Anti-PD-1 therapy
Anti-PD-1 antibodies, pembrolizumab or nivolumab, have been shown to target PD-1 in tumor cells. Pembrolizumab (2 mg/kg), working as an anti-PD-1 immunotherapy, demonstrated ORRs of 30%. Nivolumab (3 mg/kg), a monoclonal antibody against PD-1, showed 6% ORRs. However, there are few studies investigating immune checkpoint inhibitors, especially in combination treatment. Therefore, additional randomized clinical trials are necessary to understand the efficacy of immune checkpoint inhibitors and provide strong evidence in UM.81,97 Regarding the RR of ipilimumab in metastatic UM, the immune-related best ORR was 5% (4 patients), and 29% (24 patients) had stable disease for more than 3 months with 34% disease control rate. The median OS and PFS were 6.0 months (1-year OS rate: 31%) and 3.6 months (1-year PFS rate: 11%).98 Anti-PD-1 antibodies boost the host immune system as anti-tumorigenesis treatment. Cancer cells have been shown to over-express PD-L1 with immunosuppressive function to inhibit the interaction between PD-1 and PD-L1, leading to decreased T cell activity and antitumor function. In a study by Bender et al., patients with metastatic UM were administered 2 mg/kg pembrolizumab q3w and 3 mg/kg nivolumab Q2W at two German university hospitals (ClinicalTrials.gov identifier: NCT02083484), and the median PFS and OS were 3 months (range 0.75–6.75 months) and 5 months (range 1–16 months), respectively.99 In a study by van der Kooij et al.,100 patients with unresectable metastatic UM were treated with 2 mg/kg pembrolizumab Q3W or 3 mg/kg nivolumab Q2W. The clinical outcome demonstrated improved OS (median 9.6 months) and PFS (median 2.3 months). Results from several phase II trials are forthcoming, including administration of pembrolizumab in metastasized UM (ClinicalTrials.gov identifier: NCT02359851) and the use of combination of ipilimumab and nivolumab in UM patients (ClinicalTrials.gov identifiers: NCT02626962 and NCT01585194). These results may provide a strong evidence supporting the use of anti-PD-1 antibodies in UM patients.
Combination therapy with anti-PD-1 and anti-CTLA4 therapeutics has emerged as a novel anti-tumor treatment and has been reported to be more effective than single antibody treatment. Anti-CTLA4 antibodies may promote activity of the T cell immune response. Further, anti-PD-1 can induce reactivation of CD8+ T cells to lyse cancer cells. In a study by Algazi et al., the partial RR to treatment with PD-1 and PD-1 Ligand antibodies was 3.6%.101 Stable disease more than 6 months was reported in five patients (8.9%). Disease progression was 85.7%. There was no association between clinical outcome, including OS and PFS, and prior response or exposure to ipilimumab. In patients with metastatic UM, the durable remissions rate of PD-1 and PD-L1 antibodies was low. In addition, a newly developed fusion molecule, IMCgp100, showed potential prolongation of OS in metastatic UM. In a study by Middleton et al., 84 HLA-A2+ advanced melanoma patients received IMCgp100 (ClinicalTrials.gov identifier: NCT01211262), which induced a transient increase in IFN-inducible cytokines.102 These patients presented with longer OS (p = 0.0002) and tumor shrinkage (p = 0.003). In a study by Sato et al., 19 metastatic UM patients with HLA-A*0201+ were investigated and treated with IMCgp100.103 Similar results were reported, in which the IMCgp100 group demonstrated longer OS. Further prospective studies are needed to confirm the low RR.
Conclusion
This review presents treatment trends for UM, including genetic mutations, molecular prognosis, new advances in targeted therapies, and adjuvant immunotherapy. For patients, due to the high mortality rates and high frequency of metastasis associated with UM, there is an immediate requirement to explore new treatments that inhibit melanoma growth before metastasis to distant tissues. Established or new treatments including targeted therapy or immunotherapies provide better treatment choices and benefits at an early time-point in tumor development.
Acknowledgments
Giou-Teng Yiang, Po-Chen Lin, Chien-Sheng Chen and Andy Po-Yi Tsai participated in discussions, provided critical intellectual input.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The present study was funded by grants SRD-108014 and SRD-108015 from Show Chwan Memorial Hospital, Taiwan.
Contributor Information
Meng-Yu Wu, Department of Emergency Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei Department of Emergency Medicine, School of Medicine, Tzu Chi University, Hualien.
Tzu-Ting Lai, Department of Ophthalmology, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei, Taiwan.
Wan-Ting Liao, Institute of Medicine, Chung Shan Medical University, Taichung Chinese Medicine Department, Show Chwan Memorial Hospital, Changhua.
Chia-Jung Li, Department of Obstetrics and Gynecology, Kaohsiung Veterans General Hospital, No.386, Dazhong 1st Road, Zuoying District, Kaohsiung City 81362 Institute of BioPharmaceutical sciences, National Sun Yat-sen University, Kaohsiung.
References
- 1. Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, et al. Ocular melanoma: an overview of the current status. Int J Clin Exp Pathol 2013; 6: 1230–1244. [PMC free article] [PubMed] [Google Scholar]
- 2. Violanti SS, Bononi I, Gallenga CE, et al. New insights into molecular oncogenesis and therapy of uveal melanoma. Cancers (Basel) 2019; 11: pii: E694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diener-West M, Reynolds SM, Agugliaro DJ, et al. Screening for metastasis from choroidal melanoma: the collaborative ocular melanoma study group report 23. J Clin Oncol 2004; 22: 2438–2444. [DOI] [PubMed] [Google Scholar]
- 4. Finger PT, Kurli M, Reddy S, et al. Whole body PET/CT for initial staging of choroidal melanoma. Br J Ophthalmol 2005; 89: 1270–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coupland SE, Lake SL, Zeschnigk M, et al. Molecular pathology of uveal melanoma. Eye 2013; 27: 230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van den Bosch T, Kilic E, Paridaens D, et al. Genetics of uveal melanoma and cutaneous melanoma: two of a kind? Dermatol Res Pract 2010; 2010: 360136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krantz BA, Dave N, Komatsubara KM, et al. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol 2017; 11: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carvajal RD, Schwartz GK, Tezel T, et al. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol 2017; 101: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reichstein D, Karan K. Plaque brachytherapy for posterior uveal melanoma in 2018: improved techniques and expanded indications. Curr Opin Ophthalmol 2018; 29: 191–198. [DOI] [PubMed] [Google Scholar]
- 10. Shields CL, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol 2009; 127: 989–998. [DOI] [PubMed] [Google Scholar]
- 11. Goto H. A. review 36. Strategy for clinical diagnosis of intraocular tumor. Nippon Ganka Gakkai Zasshi 2008; 112: 919–935. [PubMed] [Google Scholar]
- 12. Henderson E, Margo CE. Iris melanoma. Arch Pathol Lab Med 2008; 132: 268–272. [DOI] [PubMed] [Google Scholar]
- 13. Shields CL, Kaliki S, Shah SU, et al. Iris melanoma: features and prognosis in 317 children and adults. J AAPOS 2012; 16: 10–16. [DOI] [PubMed] [Google Scholar]
- 14. Shields CL, Shields JA, Shields MB, et al. Prevalence and mechanisms of secondary intraocular pressure elevation in eyes with intraocular tumors. Ophthalmology 1987; 94: 839–846. [DOI] [PubMed] [Google Scholar]
- 15. Damato EM, Damato BE. Detection and time to treatment of uveal melanoma in the United Kingdom: an evaluation of 2,384 patients. Ophthalmology 2012; 119: 1582–1589. [DOI] [PubMed] [Google Scholar]
- 16. The Collaborative Ocular Melanoma Study Group. Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. Arch Ophthalmol 1997; 115, 1537–1544. [DOI] [PubMed] [Google Scholar]
- 17. Butler P, Char DH, Zarbin M, et al. Natural history of indeterminate pigmented choroidal tumors. Ophthalmology 1994; 101: 710–716; discussion 717. [DOI] [PubMed] [Google Scholar]
- 18. Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med 2010; 363: 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Onken MD, Worley LA, Long MD, et al. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci 2008; 49: 5230–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shoushtari AN, Carvajal RD. GNAQ and GNA11 mutations in uveal melanoma. Melanoma Res 2014; 24: 525–534. [DOI] [PubMed] [Google Scholar]
- 21. Yu FX, Luo J, Mo JS, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 2014; 25: 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Metz CH, Scheulen M, Bornfeld N, et al. Ultradeep sequencing detects GNAQ and GNA11 mutations in cell-free DNA from plasma of patients with uveal melanoma. Cancer Med 2013; 2: 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murali R, Wiesner T, Rosenblum, et al. GNAQ and GNA11 mutations in melanocytomas of the central nervous system. Acta Neuropathol 2012; 123: 457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010; 330: 1410–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landreville S, Agapova OA, Matatall KA, et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res 2012; 18: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta MP, Lane AM, DeAngelis MM, et al. Clinical characteristics of uveal melanoma in patients with germline BAP1 mutations. JAMA Ophthalmol 2015; 133: 881–887. [DOI] [PubMed] [Google Scholar]
- 27. Koopmans AE, Verdijk RM, Brouwer RW, et al. Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Mod Pathol 2014; 27: 1321–1330. [DOI] [PubMed] [Google Scholar]
- 28. Harbour JW, Roberson ED, Anbunathan H, et al. Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nat Genet 2013; 45: 133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin M, Maßhöfer L, Temming P, et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat Genet 2013; 45: 933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zuidervaart W, Van Nieuwpoort F, Stark M, et al. Activation of the MAPK pathway is a common event in uveal melanomas although it rarely occurs through mutation of BRAF or RAS. Br J Cancer 2005; 92: 2032–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Populo H, Soares P, Rocha AS, et al. Evaluation of the mTOR pathway in ocular (uvea and conjunctiva) melanoma. Melanoma Res 2010; 20: 107–117. [DOI] [PubMed] [Google Scholar]
- 32. Sagoo MS, Harbour JW, Stebbing J, et al. Combined PKC and MEK inhibition for treating metastatic uveal melanoma. Oncogene 2014; 33: 4722–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene 2014; 33: 4724–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen X, Wu Q, Depeille P, et al. RasGRP3 mediates MAPK pathway activation in GNAQ mutant uveal melanoma. Cancer Cell 2017; 31: 685–696e686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009; 457: 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feng X, Degese MS, Iglesias-Bartolome R, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 2014; 25: 831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abdel-Rahman MH, Yang Y, Zhou XP, et al. High frequency of submicroscopic hemizygous deletion is a major mechanism of loss of expression of PTEN in uveal melanoma. J Clin Oncol 2006; 24: 288–295. [DOI] [PubMed] [Google Scholar]
- 38. Cheng H, Terai M, Kageyama K, et al. Paracrine Effect of NRG1 and HGF drives resistance to MEK inhibitors in metastatic uveal melanoma. Cancer Res 2015; 75: 2737–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hendrix MJ, Seftor EA, Seftor RE, et al. Regulation of uveal melanoma interconverted phenotype by hepatocyte growth factor/scatter factor (HGF/SF). Am J Pathol 1998; 152: 855–863. [PMC free article] [PubMed] [Google Scholar]
- 40. Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol 2011; 3: S7–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Economou MA, All-Ericsson C, Bykov V, et al. Receptors for the liver synthesized growth factors IGF-1 and HGF/SF in uveal melanoma: intercorrelation and prognostic implications. Acta Ophthalmol 2008; 86 Thesis 4: 20–25. [DOI] [PubMed] [Google Scholar]
- 42. Mallikarjuna K, Pushparaj V, Biswas J, et al. Expression of epidermal growth factor receptor, ezrin, hepatocyte growth factor, and c-Met in uveal melanoma: an immunohistochemical study. Curr Eye Res 2007; 32: 281–290. [DOI] [PubMed] [Google Scholar]
- 43. Gardner FP, Serie DJ, Salomao DR, et al. c-MET expression in primary and liver metastases in uveal melanoma. Melanoma Res 2014; 24: 617–620. [DOI] [PubMed] [Google Scholar]
- 44. Yoshida M, Selvan S, McCue PA, et al. Expression of insulin-like growth factor-1 receptor in metastatic uveal melanoma and implications for potential autocrine and paracrine tumor cell growth. Pigment Cell Melanoma Res 2014; 27: 297–308. [DOI] [PubMed] [Google Scholar]
- 45. Cheng H, Chua V, Liao C, et al. Co-targeting HGF/cMET signaling with MEK inhibitors in metastatic uveal melanoma. Mol Cancer Ther 2017; 16: 516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goh AY, Layton CJ. Evolving systemic targeted therapy strategies in uveal melanoma and implications for ophthalmic management: a review. Clin Exp Ophthalmol 2016; 44: 509–519. [DOI] [PubMed] [Google Scholar]
- 47. Brouwer NJ, Gezgin G, Wierenga AP, et al. Tumour angiogenesis in uveal melanoma is related to genetic evolution. Cancers (Basel) 2019; 11: pii: E979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Field MG, Kuznetsov JN, Bussies PL, et al. BAP1 loss is associated with DNA methylomic repatterning in highly aggressive class 2 uveal melanomas. Clin Cancer Res 2019; 25: 5663–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Br J Cancer 2014; 111: 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Di Pompo G, Salerno M, Rotili D, et al. Novel histone deacetylase inhibitors induce growth arrest, apoptosis, and differentiation in sarcoma cancer stem cells. J Med Chem 2015; 58: 4073–4079. [DOI] [PubMed] [Google Scholar]
- 51. Ewens KG, Kanetsky PA, Richards-Yutz J, et al. Chromosome 3 status combined with BAP1 and EIF1AX mutation profiles are associated with metastasis in uveal melanoma. Invest Ophthalmol Vis Sci 2014; 55: 5160–5167. [DOI] [PubMed] [Google Scholar]
- 52. Onken MD, Worley LA, Ehlers JP, et al. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res 2004; 64: 7205–7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Musi E, Schwartz GK, Yoo JH, et al. Tris DBA palladium is an orally available inhibitor of GNAQ mutant uveal melanoma in vivo. Oncotarget 2019; 10: 4424–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu X, Zhu M, Fletcher JA, et al. The protein kinase C inhibitor enzastaurin exhibits antitumor activity against uveal melanoma. PLoS One 2012; 7: e29622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. von Euw E, Atefi M, Attar N, et al. Antitumor effects of the investigational selective MEK inhibitor TAK733 against cutaneous and uveal melanoma cell lines. Mol Cancer 2012; 11: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA 2014; 311: 2397–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boru G, Cebulla CM, Sample KM, et al. Heterogeneity in mitogen-activated protein kinase (MAPK) pathway activation in uveal melanoma with somatic GNAQ and GNA11 Mutations. Invest Ophthalmol Vis Sci 2019; 60: 2474–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carvajal RD, Piperno-Neumann S, Kapiteijn E, et al. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: a phase III, multicenter, randomized trial (SUMIT). J Clin Oncol 2018; 36: 1232–1239. [DOI] [PubMed] [Google Scholar]
- 59. Mohamed A, Gonzalez RS, Lawson D, et al. Tumor stem cells (CD271, c-kit, SOX10) in melanomas: prognostic and outcome implications. Appl Immunohistochem Mol Morphol 2014; 22: 142–145. [DOI] [PubMed] [Google Scholar]
- 60. Papaspyrou G, Garbe C, Schadendorf D, et al. Mucosal melanomas of the head and neck: new aspects of the clinical outcome, molecular pathology, and treatment with c-kit inhibitors. Melanoma Res 2011; 21: 475–482. [DOI] [PubMed] [Google Scholar]
- 61. Wallander ML, Layfield LJ, Emerson LL, et al. KIT mutations in ocular melanoma: frequency and anatomic distribution. Mod Pathol 2011; 24: 1031–1035. [DOI] [PubMed] [Google Scholar]
- 62. All-Ericsson C, Girnita L, Müller-Brunotte A, et al. c-Kit-dependent growth of uveal melanoma cells: a potential therapeutic target? Invest Ophthalmol Vis Sci 2004; 45: 2075–2082. [DOI] [PubMed] [Google Scholar]
- 63. Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011; 305: 2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pereira PR, Odashiro AN, Marshall JC, et al. The role of c-kit and imatinib mesylate in uveal melanoma. J Carcinog 2005; 4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pereira PR, Odashiro AN, Lim LA, et al. Current and emerging treatment options for uveal melanoma. Clin Ophthalmol 2013; 7: 1669–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Blum ES, Yang J, Komatsubara KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park) 2016; 30: 29–43. [PubMed] [Google Scholar]
- 67. Souto EB, Zielinska A, Luis M, et al. Uveal melanoma: physiopathology and new in situ-specific therapies. Cancer Chemother Pharmacol 2019; 84: 15–32. [DOI] [PubMed] [Google Scholar]
- 68. Valsecchi ME, Orloff M, Sato R, et al. Adjuvant sunitinib in high-risk patients with uveal melanoma: comparison with institutional controls. Ophthalmology 2018; 125: 210–217. [DOI] [PubMed] [Google Scholar]
- 69. All-Ericsson C, Girnita L, Seregard S, et al. Insulin-like growth factor-1 receptor in uveal melanoma: a predictor for metastatic disease and a potential therapeutic target. Invest Ophthalmol Vis Sci 2002; 43: 1–8. [PubMed] [Google Scholar]
- 70. Wu X, Zhou J, Rogers AM, et al. c-Met, epidermal growth factor receptor, and insulin-like growth factor-1 receptor are important for growth in uveal melanoma and independently contribute to migration and metastatic potential. Melanoma Res 2012; 22: 123–132. [DOI] [PubMed] [Google Scholar]
- 71. Yan F, Liao R, Farhan M, et al. Elucidating the role of the FoxO3a transcription factor in the IGF-1-induced migration and invasion of uveal melanoma cancer cells. Biomed Pharmacother 2016; 84: 1538–1550. [DOI] [PubMed] [Google Scholar]
- 72. Seccareccia E, Brodt P. The role of the insulin-like growth factor-I receptor in malignancy: an update. Growth Horm IGF Res 2012; 22: 193–199. [DOI] [PubMed] [Google Scholar]
- 73. Oba J, Esmaeli B, Ellerhorst JA, et al. Trends in hepatocyte growth factor, insulin-like growth factor 1, thyroid-stimulating hormone, and leptin expression levels in uveal melanoma patient serum and tumor tissues: correlation to disease progression. Melanoma Res 2017; 27: 126–133. [DOI] [PubMed] [Google Scholar]
- 74. Girnita A, All-Ericsson C, Economou MA, et al. The insulin-like growth factor-I receptor inhibitor picropodophyllin causes tumor regression and attenuates mechanisms involved in invasion of uveal melanoma cells. Acta Ophthalmol 2006; 12: 1383–1391. [DOI] [PubMed] [Google Scholar]
- 75. Velho TR, Kapiteijn E, Jager MJ. New therapeutic agents in uveal melanoma. Anticancer Res 2012; 32: 2591–2598. [PubMed] [Google Scholar]
- 76. Moser JC, Pulido JS, Dronca RS, et al. The Mayo clinic experience with the use of kinase inhibitors, ipilimumab, bevacizumab, and local therapies in the treatment of metastatic uveal melanoma. Melanoma Res 2015; 25: 59–63. [DOI] [PubMed] [Google Scholar]
- 77. Sharma RK, Balaiya S, Chalam KV. Bevacizumab suppression of establishment of micrometastases in experimental ocular melanoma. Invest Ophthalmol Vis Sci 2010; 51: 6906–6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ibrahim N, Yu Y, Walsh WR, et al. Molecular targeted therapies for cancer: sorafenib mono-therapy and its combination with other therapies (review). Oncol Rep 2012; 27: 1303–1311. [DOI] [PubMed] [Google Scholar]
- 79. Huang S, Shao K, Liu Y, et al. Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis. ACS Nano 2013; 7: 2860–2871. [DOI] [PubMed] [Google Scholar]
- 80. Niederkorn A, Wackernagel W, Artl M, et al. Response of patients with metastatic uveal melanoma to combined treatment with fotemustine and sorafenib. Acta Ophthalmol 2014; 92: e696–e697. [DOI] [PubMed] [Google Scholar]
- 81. Javed A, Arguello D, Johnston C, et al. PD-L1 expression in tumor metastasis is different between uveal melanoma and cutaneous melanoma. Immunotherapy 2017; 9: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 82. Schuler-Thurner B, Bartz-Schmidt KU, Bornfeld N, et al. Immunotherapy of uveal melanoma: vaccination against cancer. Multicenter adjuvant phase 3 vaccination study using dendritic cells laden with tumor RNA for large newly diagnosed uveal melanoma. Ophthalmologe 2015; 112: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 84. Minn AJ. Interferons and the immunogenic effects of cancer therapy. Trends Immunol 2015; 36: 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lane AM, Egan KM, Harmon D, et al. Adjuvant interferon therapy for patients with uveal melanoma at high risk of metastasis. Ophthalmology 2009; 116: 2206–2212. [DOI] [PubMed] [Google Scholar]
- 85. Nathan FE, Berd D, Sato T, et al. BOLD+interferon in the treatment of metastatic uveal melanoma: first report of active systemic therapy. J Exp Clin Cancer Res 1997; 16: 201–208. [PubMed] [Google Scholar]
- 86. Buder K, Gesierich A, Gelbrich G, et al. Systemic treatment of metastatic uveal melanoma: review of literature and future perspectives. Cancer Med 2013; 2: 674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pyrhonen S, Hahka-Kemppinen M, Muhonen T, et al. Chemoimmunotherapy with bleomycin, vincristine, lomustine, dacarbazine (BOLD), and human leukocyte interferon for metastatic uveal melanoma. Cancer 2002; 95: 2366–2372. [DOI] [PubMed] [Google Scholar]
- 88. Kivela T, Suciu S, Hansson J, et al. Bleomycin, vincristine, lomustine and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. Eur J Cancer 2003; 39: 1115–1120. [DOI] [PubMed] [Google Scholar]
- 89. Becker JC, Terheyden P, Kämpgen E, et al. Treatment of disseminated ocular melanoma with sequential fotemustine, interferon alpha, and interleukin 2. Br J Cancer 2002; 87: 840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Esser P, Grisanti S, Bartz-Schmidt K. TGF-beta in uveal melanoma. Microsc Res Tech 2001; 52: 396–400. [DOI] [PubMed] [Google Scholar]
- 91. Ma D, Niederkorn JY. Transforming growth factor-beta down-regulates major histocompatibility complex class I antigen expression and increases the susceptibility of uveal melanoma cells to natural killer cell-mediated cytolysis. Immunology 1995; 86: 263–269. [PMC free article] [PubMed] [Google Scholar]
- 92. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jindal V. Role of immune checkpoint inhibitors and novel immunotherapies in uveal melanoma. Chin Clin Oncol 2018; 7: 8. [DOI] [PubMed] [Google Scholar]
- 94. Coulson A, Levy A, Gossell-Williams M. Monoclonal antibodies in cancer therapy: mechanisms, successes and limitations. West Indian Med J 2014; 63: 650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Graziani G, Tentori L, Navarra P. Ipilimumab: a novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol Res 2012; 65: 9–22. [DOI] [PubMed] [Google Scholar]
- 96. Heppt MV, Steeb T, Schlager JG, et al. Immune checkpoint blockade for unresectable or metastatic uveal melanoma: a systematic review. Cancer Treat Rev 60: 44–52. [DOI] [PubMed] [Google Scholar]
- 97. Namikawa K, Mori T, Muto Y, et al. PD-L1 expression and clinical outcome after nivolumab monotherapy in various subtypes of melanoma: a single-institutional retrospective study. Ann Oncol 2018; 29(Suppl. 9): ix105–ix108. [Google Scholar]
- 98. Maio M, Danielli R, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann Oncol 2013; 24: 2911–2915. [DOI] [PubMed] [Google Scholar]
- 99. Bender C, Enk A, Gutzmer R, et al. Anti-PD-1 antibodies in metastatic uveal melanoma: a treatment option? Cancer Med 2017; 6: 1581–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van der Kooij MK, Joosse A, Speetjens FM, et al. Anti-PD1 treatment in metastatic uveal melanoma in the Netherlands. Acta Oncol 2017; 56: 101–103. [DOI] [PubMed] [Google Scholar]
- 101. Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016; 122: 3344–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Middleton MR, Steven NM, Evans TJ, et al. Pharmacodynamic effect of IMCgp100 (TCR–CD3 bispecific) on peripheral cytokines and association with overall survival in patients with advanced melanoma. J Clin Oncol 2019; 37: 9523. [Google Scholar]
- 103. Sato T, Nathan PD, Hernandez-Aya L, et al. Redirected T cell lysis in patients with metastatic uveal melanoma with gp100-directed TCR IMCgp100: Overall survival findings. J Clin Oncol 2018; 36: 9521. [Google Scholar]