Abstract
Objectives
The rate of severe maternal morbidity in the United States increased approximately 200% during 1993–2014. Few studies have reported on the health of the entire pregnant population, including women at low risk for maternal morbidity. This information might be useful for interventions aimed at primary prevention of pregnancy complications. To better understand this, we sought to describe the distribution of comorbid risk among all delivery hospitalizations in Massachusetts and its association with the distribution of severe maternal morbidity.
Methods
Using an existing algorithm, we assigned an obstetric comorbidity index (OCI) score to delivery hospitalizations contained in the Massachusetts pregnancy to early life longitudinal (PELL) data system during 1998–2013. We identified which hospitalizations included severe maternal morbidity and calculated the rate and frequency of these hospitalizations by OCI score.
Results
During 1998–2013, PELL contained 1,185,182 delivery hospitalizations; of these 5325 included severe maternal morbidity. Fifty-eight percent of delivery hospitalizations had an OCI score of zero. The mean OCI score increased from 0.60 in 1998 to 0.82 in 2013. Hospitalizations with an OCI score of zero comprised approximately one-third of all deliveries complicated by severe maternal morbidity, but had the lowest rate of severe maternal morbidity (22.8/10,000 delivery hospitalizations).
Conclusions
The mean OCI score increased during the study period, suggesting that an overall increase in risk factors has occurred in the pregnant population in Massachusetts. Interventions that can make small decreases to the mean OCI score could have a substantial impact on the number of deliveries complicated by severe maternal morbidity. Additionally, all delivery facilities should be prepared for severe complications during low-risk deliveries.
Keywords: Severe maternal morbidity, Obstetric comorbidity index, Population health, Massachusetts
Introduction
For every maternal death that occurs in the United States, approximately 50 women suffer from severe morbidity that is directly or indirectly related to pregnancy (Callaghan et al. 2012). The rate of these severe maternal morbidities in the United States increased approximately 200% during 1993–2014 (Severe Maternal Morbidity 2017). Research has focused on identifying women and pregnancies at high risk for severe maternal morbidity and other adverse pregnancy outcomes (Mhyre et al. 2011). This has led to coordinated efforts aimed at secondary prevention of severe morbidity and mortality among pregnant women at high risk, such as the American College of Obstetrics and Gynecology (ACOG) guidelines for maternal levels of care, the Council on Patient Safety in Women’s Health patient safety bundles, and the work of collaboratives to improve perinatal quality (Preeclampsia and Hypertension, n.d.; D’Alton et al. 2014; Obstetric Care Consensus 2015; Henderson et al. 2014). With this focus on pregnant women at high risk, less attention has been given to the health profile of the entire pregnant population, which includes women at low risk for maternal morbidity. Such population level information could be important for the development of future public health interventions designed to improve maternal health, especially when it comes to primary versus secondary prevention efforts.
At the population level, a small to average individual risk for disease can account for a large portion of disease burden (Rose 1985, 2008). This is because people at low risk often account for a larger segment of the impacted population, compared with people at high risk. For a population of pregnant women, a significant portion of severe maternal morbidity might occur among pregnant women with low risk for adverse maternal health outcomes simply because the absolute numbers are higher, compared with the number of women at high risk. A study of severe maternal morbidity using 1998–2011 data from the National Inpatient Sample reported that the biggest increase in relative risk for severe maternal morbidity during the study period occurred among women with few risk factors for adverse maternal health outcomes, compared with women with more risk factors (Hehir et al. 2017).
A better understanding of the distribution of severe maternal morbidity along a continuum of risk might help to prioritize interventions aimed at reducing the greatest possible number of deliveries with severe complications. Thus, we sought to describe the distribution of comorbid risk among all delivery hospitalizations in Massachusetts and its association with severe maternal morbidity.
Methods
Study Sample
We included all delivery hospitalizations during 1998–2013 reported in the Massachusetts pregnancy to early life longitudinal data system (PELL). PELL is a relational data system that links infant birth and fetal death records to the corresponding maternal and infant hospital discharge administrative data for all delivery hospitalizations occurring in Massachusetts to resident women (Shapiro-Mendoza et al. 2008). PELL does not capture pregnancy terminations or pregnancies that end with miscarriage or stillbirth at ≤ 20 weeks gestation or ≤ 350 g because no fetal death records are produced for those events. For the purposes of analysis, multiple deliveries to the same woman during the study period were treated as separate observations. This study was approved by the Massachusetts Department of Public Health Human Subjects Review Board; the Centers for Disease Control and Prevention (CDC) reviewed this study for human subjects protection and deemed it non research.
Severe Maternal Morbidity
We defined the outcome of severe maternal morbidity using an algorithm previously published by Callaghan et al. (2012) and validated by others (Main et al. 2016; Sigakis et al. 2016). This definition uses a set of International Classification of Disease, Ninth Revision Clinical Modification (ICD-9-CM) codes to identify 25 lifesaving procedures or life-threatening events from delivery hospitalization administrative data (Online Resource Table). The severe maternal morbidity algorithm does not consider hospitalizations with implausibly short lengths of stay as hospitalizations in which severe morbidity occurred. This algorithm was applied to each of the delivery hospitalizations identified in the PELL data system. The rate of delivery hospitalizations complicated by severe maternal morbidity/10,000 delivery hospitalizations was calculated without the ICD-9-CM code for blood transfusion, because of concerns about its low specificity for severe morbidity relative to other ICD-9-CM codes in the algorithm (Main et al. 2016).
Maternal Risk
To operationalize a continuum of maternal risk for an adverse health outcome during delivery hospitalization, we used the obstetric comorbidity index (OCI) previously published by Bateman et al. (2013). OCI was developed and validated using Medicaid claims and enrollment data, and was externally validated for use in hospital discharge administrative data (Metcalfe et al. 2015). OCI assigns a weight of 1–5 to each of 20 conditions identified from ICD-9-CM codes at delivery and maternal age ≥ 35 years old (Online Resource Table). Conditions with higher weights were the more predictive of maternal end-organ injury or death during OCI development. Each delivery hospitalization could have multiple conditions, and the weights of each were summed with the age range score to assign a final score of 0–45 to each delivery hospitalization. Two modifications of the scoring algorithm were made to adapt OCI for the study context. ICD-9-CM codes related to two conditions, sickle cell anemia with crisis (282.6×) and eclampsia (642.6×), were omitted from the OCI algorithm because they are also used to define severe maternal morbidity, a study outcome. We have maintained the broader OCI algorithm categories that contained these codes, sickle cell disease and severe preeclampsia or eclampsia, in OCI because they contain diagnosis codes other than the two above that do not overlap with the severe maternal morbidity algorithm. Additionally, birth certificate data concerning method of delivery in PELL was used to supplement ICD-9-CM codes in OCI when determining previous cesarean delivery status.
Analysis
We applied both the OCI and the severe maternal morbidity algorithm to all PELL delivery hospitalizations during 1998–2013. Delivery hospitalizations were described using PELL demographic information, including maternal race/ethnicity, delivery payer source, and method of delivery. Age groups were < 20, 20–24, 25–29, 30–34, 35–39, 40–44, and ≥ 44 years. Rates of severe maternal morbidity were calculated overall, by age group, race/ethnicity, payer source, method of delivery, year, and by OCI score. The total number of delivery hospitalizations and the number complicated by severe maternal morbidity were tallied by OCI score. The mean population OCI score was calculated by year. All analyses performed in SAS 9.4.
Results
The PELL data system contained 1,185,182 delivery hospitalizations reported during 1998–2013. Twenty-two percent of deliveries were to women aged ≥ 35 years of age, 69% were to non-Hispanic white women, 61% were covered by private insurance, and 68% were vaginal births among women without a prior cesarean section (Table 1).
Table 1.
Characteristics of women with live birth delivery hospitalizations—Massachusetts 1998–2013
| Massachusetts delivery hospitalizations |
Delivery hospitalizations with severe maternal morbidity No. |
Rate of severe maternal morbidity among delivery hospitalizations Per 10,000 | ||
|---|---|---|---|---|
| No. | % | |||
| Age (years) | ||||
| < 20 | 71,795 | 6.1 | 271 | 37.7 |
| 20–24 | 183,292 | 15.5 | 606 | 33.1 |
| 25–29 | 288,534 | 24.3 | 1110 | 38.5 |
| 30–34 | 379,855 | 32.1 | 1556 | 41.0 |
| 35–39 | 213,222 | 18.0 | 1299 | 60.9 |
| 40–44 | 45,840 | 3.9 | 434 | 94.7 |
| > 44 | 2623 | 0.2 | 49 | 186.8 |
| Missing | 21 | 0 | 0 | 0 |
| Race/ethnicity | ||||
| non-Hispanic white | 812,646 | 68.6 | 3068 | 37.8 |
| non-Hispanic black | 98,958 | 8.3 | 881 | 89.0 |
| Hispanic | 164,640 | 13.9 | 858 | 52.1 |
| Asian or Pacific islander | 84,435 | 7.1 | 390 | 46.2 |
| American Indian or other | 21,484 | 1.8 | 102 | 47.5 |
| Missing or unknown | 3019 | 0.3 | 26 | 86.1 |
| Delivery payer source | ||||
| Private | 718,834 | 60.7 | 2900 | 40.3 |
| Public | 437,742 | 36.9 | 2259 | 51.6 |
| Self-pay | 13,760 | 1.2 | 100 | 72.7 |
| Free care | 14,797 | 1.2 | 66 | 44.6 |
| Missing or unknown | 49 | 0 | 0 | 0 |
| Method of delivery | ||||
| Vaginal | 809,249 | 68.3 | 1633 | 20.2 |
| VBAC | 31,516 | 2.7 | 127 | 40.3 |
| Primary cesarean | 205,570 | 17.3 | 2236 | 108.8 |
| Repeat cesarean | 138,112 | 11.7 | 1320 | 95.6 |
| Missing or unknown | 725 | 0.1 | 9 | 124.1 |
| Total | 1,185,182 | - | 5325 | 44.9 |
VBAC vaginal birth after cesarean
The overall rate of severe maternal morbidity among all delivery hospitalizations during 1998–2013 was 44.9/10,000 delivery hospitalizations (Table 1). Severe maternal morbidity rates were highest among the oldest age groups, non-Hispanic black women, deliveries classified as self-pay or billed to public insurance, and deliveries by cesarean section.
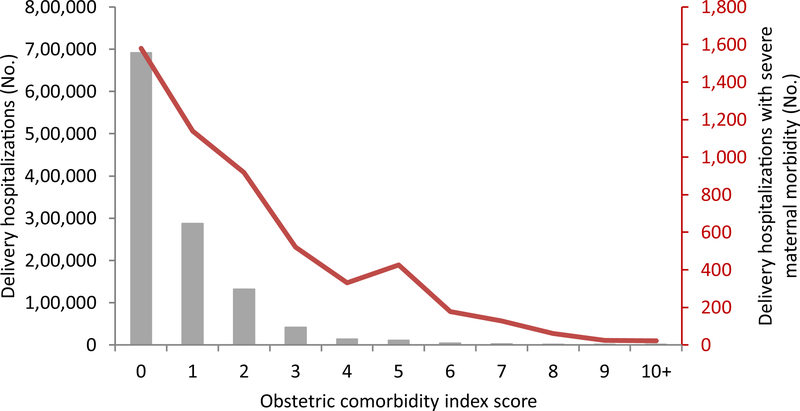
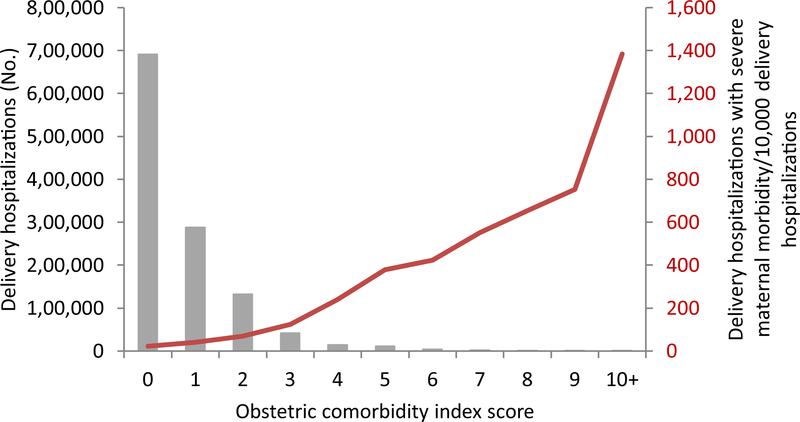
The frequency distribution of OCI scores is shown as bars in Figs. 1 and 2. Fifty-eight percent of delivery hospitalizations (n = 691,044) were assigned an OCI score of zero and the number of hospitalizations assigned to each score decreased with increasing score. Among the 5325 deliveries complicated by severe maternal morbidity, 1579 (30%) were among deliveries with an OCI score of zero (Fig. 1, Table 2). Deliveries with an OCI score of zero had the lowest rates of severe morbidity, with a rate of 22.8/10,000 delivery hospitalizations (Fig. 2, Table 2). Deliveries with OCI scores of ≥ 10 had the highest rates of severe morbidity at 1,383.6/10,000 delivery hospitalizations.
Fig. 1.
Count of all delivery hospitalizations and those with severe maternal morbidity by obstetric comorbidity index score—Massachusetts 1998–2013
Fig. 2.
Count of all delivery hospitalizations and rate of severe maternal morbidity/10,000 delivery hospitalizations by obstetric comorbidity index score—Massachusetts 1998–2013
Table 2.
Distribution of delivery hospitalizations with and without severe maternal morbidity and rate of severe maternal morbidity by obstetric comorbidity index score—Massachusetts, 1998–2013
| Obstetric comorbidity index score | Delivery hospitalizations |
Rate of delivery hospitalizations with severe maternal morbidity | ||
|---|---|---|---|---|
| No. without severe morbidity | No. with severe morbidity | Total | Per 10,000 | |
| 0 | 689,465 | 1579 | 691,044 | 22.8 |
| 1 | 286,512 | 1139 | 287,651 | 39.6 |
| 2 | 130,842 | 919 | 131,761 | 69.7 |
| 3 | 41,257 | 520 | 41,777 | 124.5 |
| 4 | 13,468 | 330 | 13,798 | 239.2 |
| 5 | 10,799 | 425 | 11,224 | 378.7 |
| 6 | 4023 | 178 | 4201 | 423.7 |
| 7 | 2174 | 127 | 2301 | 551.9 |
| 8 | 873 | 61 | 934 | 653.1 |
| 9 | 307 | 25 | 332 | 753.0 |
| 10+ | 137 | 22 | 159 | 1383.6 |
| Total | 1,179,857 | 5325 | 1,185,182 | 44.9 |
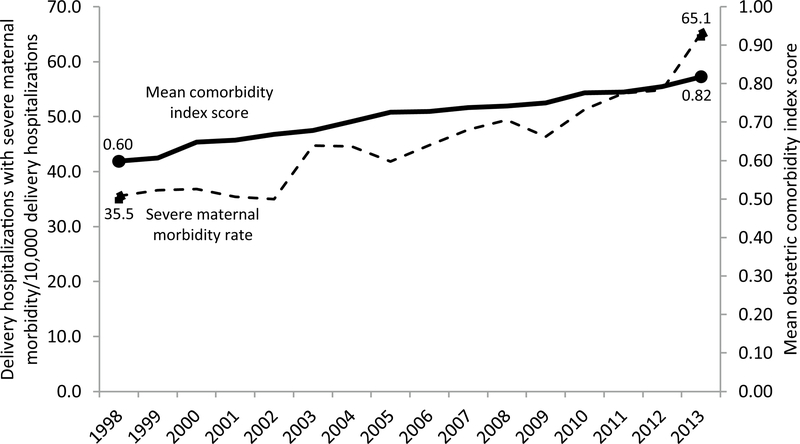
The population wide rate of severe maternal morbidity among delivery hospitalizations increased steadily during 1998–2013 (Fig. 3). The yearly mean OCI score among delivery hospitalizations also rose steadily during the study period, from 0.60 in 1998 to 0.82 in 2013 (Fig. 3). The rise in mean OCI score coincided with a 14.1% decrease in the proportion of deliveries with an OCI score of 0 and a 24.1% increase in the proportion of deliveries with an OCI score of ≥ 1.
Fig. 3.
Rate of delivery hospitalizations with severe maternal morbidity and mean obstetric comorbidity index score by year—Massachusetts 1998–2013
Discussion
We have taken a population-level approach to describe the distribution of comorbid risk factors among Massachusetts deliveries using hospital administrative discharge data linked to infant birth certificates and fetal death records. An increase in the burden of comorbidities complicating deliveries, as illustrated by the increase in mean OCI score, accompanied the rising population wide rate of severe maternal morbidity. We found that deliveries with no identified comorbid risk factors had the lowest rate of severe maternal morbidity, but that they comprised one-third of those deliveries complicated by severe maternal morbidity. This finding is consistent with a growing body of research that reports a significant portion of adverse maternal outcomes occur among women with few diagnosed risk factors (Danilack et al. 2015; Friedman et al. 2016; Hehir et al. 2017).
Our findings are consistent with the idea that a small, individual risk among a large number of persons can account for a large portion of total disease (Rose 1985, 2008; Keyes and Galea 2016). The overall comorbid risk among deliveries in Massachusetts remained small during 1998–2013, with a yearly mean delivery OCI score of < 1, but increased steadily. This finding suggests that there has been an overall increase in risk factors among the pregnant population in Massachusetts, which might in part explain the rising rate of severe maternal morbidity among deliveries. The small increase in average OCI could be secondary to relatively small increases in the burden of high-scoring conditions (such as severe preeclampsia), but could also stem from larger increases in low-scoring conditions (such as previous cesarean delivery). More research is needed to investigate the changes in OCI distribution over time. Interventions that can make decreases to the mean population score, while not readily noticeable at the individual or facility level might in turn have a substantial impact on the total number of deliveries complicated by severe maternal morbidity (Rose 1985, 2008). In the future, identifying OCI components that are most closely associated with the rising average OCI score may help to prioritize public health interventions to decrease maternal morbidity in Massachusetts.
An example of an intervention that might address the average OCI score is an effort by the Massachusetts perinatal quality collaborative (MPQC) to decrease the overall state cesarean section rate. Having had a previous cesarean delivery is one of the comorbidities included in OCI and, because cesarean deliveries are relatively common across the Massachusetts pregnant population, one possible driver of the increasing average OCI score (Massachusetts Births 2014). MPQC began its work in 2007 with a goal of decreasing the number of cesarean deliveries in participating hospitals among women with no indication for requiring a cesarean delivery. Although MPQC’s work is probably not the sole driver, the overall and primary cesarean rate has decreased every year since peaking in 2008 (Massachusetts Births 2014). A Health care level interventions, such as the MPQC’s efforts, with components targeted to women at low risk, could impact the maternal population risk and potentially decrease the burden of maternal morbidity.
Our findings also have implications for individual level risk reduction and secondary prevention of severe maternal morbidity. First, our results support ACOG’s existing recommendation that all birth facilities, including those facilities caring predominantly for pregnant women at low risk, be capable of stabilizing and transferring patients to tertiary care hospitals (Obstetric Care Consensus 2015). Although our study does not aim to explain why women with no identifiable comorbid risk factors go on to develop severe maternal morbidity, further research is needed to identify new and relevant nonbiomedical and biomedical risk factors that can be used to ensure women deliver in risk-appropriate facilities as outlined by ACOG. This research might require comparisons across different populations of women at low risk, such as those groups residing in different states or nations, to identify ubiquitous risk factors within a these populations that women are exposed to prior to or during pregnancy (Keyes and Galea 2016).
Our study has several strengths. First, severe maternal morbidity is a more frequent occurrence than maternal mortality, allowing sufficient sample size for analytical exploration of adverse delivery outcomes. Severe maternal morbidity also has a definition based in a clearly defined algorithm. Second, we used OCI to convert disparate categorical risk factors for severe maternal morbidity into a single measure of risk and applied it to every delivery hospitalization, a novel population-level approach to maternal health. This approach can be used in the future to investigate maternal risk burden among subpopulations, such as by race/ethnicity and by socioeconomic factors (e.g. neighborhood and income level). Finally, both measures have been previously validated using large, population-based samples, and severe maternal morbidity rates can be compared across states and with national estimates.
Our study also has limitations. First, although PELL is a robust, population-level data system, it includes only delivery hospitalizations in Massachusetts and our results might not be generalizable to other states with differing maternal characteristics. Similarly, PELL does not capture pregnancy terminations or pregnancies that end with miscarriage or stillbirth at ≤ 20 weeks gestation or ≤ 350 g, because fetal death records are not completed for these deliveries. Second, both OCI and the severe maternal morbidity algorithm rely on hospital administrative data. Research has shown that some billing codes in the severe maternal morbidity algorithm have low sensitivity for their respective clinical conditions (Main et al. 2016). Future analysis using the OCI and severe maternal morbidity algorithms will likely be affected be the transition from ICD-9-CM to ICD-10-CM, though it is too early to tell what the affects will be. Third, OCI captures only biomedical comorbid risk factors, and does not take into account environmental risk factors and the social determinants of health. Finally, OCI does not include all known medical risk factors for adverse maternal health outcomes (e.g. obesity).
The population health approach offers a framework for research and interventions to facilitate improvements in maternal health outcomes, and we have taken the first step in applying it to deliveries in Massachusetts. Future analyses using OCI should focus on identification of specific comorbid risk factors that are both amenable to intervention and significant contributors to the population risk burden. Efforts to identify ubiquitous exposures and adapt OCI to nontraditional biomedical risk factors for adverse maternal health outcomes may also be explored. A population health framework can also guide efforts such as state perinatal quality collaboratives in identifying health care processes that are relevant to decreasing the maternal population’s overall burden of severe maternal morbidity to complement interventions tailored toward only the highest risk pregnancies. Finally, use of a tool such as OCI gives public health leaders the ability to track maternal health status, compared with maternal health outcomes in their jurisdictions.
Supplementary Material
Significance.
What is already known on this subject? The rate of severe maternal morbidity in the United States has steadily increased. Women with more comorbid risk factors have a higher risk for adverse maternal health outcomes.
What this study adds? Women with few to no comorbid risk factors comprised a substantial proportion of all severe maternal morbidities occurring among delivery hospitalizations in Massachusetts. The mean obstetric comorbidity index score among delivery hospitalizations increased during 1998–2013, suggesting an overall increase in risk factors among pregnant women in Massachusetts.
Acknowledgements
Dave Goodman, Michael Gronostaj, Centers for Disease Control and Prevention; Xiaohui Cui, Massachusetts Department of Public Health.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10995-019-02796-3) contains supplementary material, which is available to authorized users.
Extended author information available on the last page of the article
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. (2013). Development of a comorbidity index for use in obstetric patients. Obstetrics and Gynecology, 122(5), 957–965. 10.1097/AOG.0b013e3182a603bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan WM, Creanga AA, & Kuklina EV (2012). Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstetrics and Gynecology, 120(5), 1029–1036. 10.1097/A0G.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- D’Alton ME, Main EK, Menard MK, et al. (2014). The national partnership for maternal safety. Obstetrics and Gynecology, 123(5), 973–977. 10.1097/A0G.0000000000000219. [DOI] [PubMed] [Google Scholar]
- Danilack VA, Nunes AP, & Phipps MG (2015). Unexpected complications of low-risk pregnancies in the United States. American Journal of Obstetrics and Gynecology. 10.1016/j.ajog.2015.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AM, Wright JD, Ananth CV, et al. (2016). Population-based risk for peripartum hysterectomy during low- and moderate-risk delivery hospitalizations. American Journal of Obstetrics and Gynecology. 10.1016/j.ajog.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehir MP, Ananth CV, Wright JD, et al. (2017). Severe maternal morbidity and comorbid risk in hospitals performing < 1000 deliveries per year. American Journal of Obstetrics and Gynecology. 10.1016/j.ajog.2016.10.029. [DOI] [PubMed] [Google Scholar]
- Henderson ZT, Suchdev DB, Abe K, et al. (2014). Perinatal quality collaboratives: improving care for mothers and infants. Journal of Women’s Health (Larchmt), 23(5), 368–372. 10.1089/jwh.2014.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes K, & Galea S. (2016). Population health science. New York: Oxford University Press. [Google Scholar]
- Main EK, Abreo A, McNulty J, et al. (2016). Measuring severe maternal morbidity: validation of potential measures. American Journal of Obstetrics and Gynecology. 10.1016/j.ajog.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Massachusetts Births 2013 (2014). Massachusetts Department of Public Health; Retrieved November 27, 2017, from https://www.mass.gov/files/documents/2016/07/nz/birth-report-2013.pdf [Google Scholar]
- Metcalfe A, Lix LM, Johnson JA, et al. (2015). Validation of an obstetric comorbidity index in an external population. BJOG, 122(13), 1748–1755. 10.1111/1471-0528.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhyre JM, Bateman BT, & Leffert LR (2011). Influence of patient comorbidities on the risk of near-miss maternal morbidity or mortality. Anesthesiology, 115(5), 963–972. 10.1097/ALN.0b013e318233042d. [DOI] [PubMed] [Google Scholar]
- Obstetric Care Consensus No. 2: Levels of maternal care (2015). Obstetrics and Gynecology, 125(2), 502–515. 10.1097/01.aog.0000460770.99574.9f [DOI] [PubMed] [Google Scholar]
- Rose G. (1985). Sick individuals and sick populations. International Journal of Epidemiology, 14(1), 32–38. [DOI] [PubMed] [Google Scholar]
- Rose G. (2008). Rose’s strategy of preventive medicine. New York: Oxford University Press. [Google Scholar]
- Severe Maternal Morbidity in the United States (2017). Retrieved November 27, 2017, from https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html
- Shapiro-Mendoza CK, Tomashek KM, Kotelchuck M, et al. (2008). Effect of late-preterm birth and maternal medical conditions on newborn morbidity risk. Pediatrics, 121(2), e223–e232. 10.1542/peds.2006-3629. [DOI] [PubMed] [Google Scholar]
- Sigakis MJ, Leffert LR, Mirzakhani H, et al. (2016). The validity of discharge billing codes reflecting severe maternal morbidity. Anesthesia and Analgesia, 123(3), 731–738. 10.1213/ANE.0000000000001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preeclampsia and hypertension in pregnancy: Resource overview. Retrieved November 27, 2017, from https://www.acog.org/Womens-Health/Preeclampsia-and-Hypertension-in-Pregnancy
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.