Abstract
The knowledge surrounding the bovine vaginal microbiota and its implications on fertility and reproductive traits remains incomplete. The objective of the current study was to characterize the bovine vaginal bacterial community and estradiol concentrations at the time of artificial insemination (AI). Brangus heifers (n = 78) underwent a 7-d Co-Synch + controlled internal drug release estrus synchronization protocol. At AI, a double-guarded uterine culture swab was used to sample the anterior vaginal tract. Immediately after swabbing the vaginal tract, blood samples were collected by coccygeal venipuncture to determine concentrations of estradiol. Heifers were retrospectively classified as pregnant (n = 29) vs. nonpregnant (n = 49) between 41 and 57 d post-AI. Additionally, heifers were classified into low (1.1 to 2.5 pg/mL; n = 21), medium (2.6 to 6.7 pg/mL; n = 30), and high (7.2 to 17.6 pg/mL; n = 27) concentration of estradiol. The vaginal bacterial community composition was determined through sequencing of the V4 region from the 16S rRNA gene using the Illumina Miseq platform. Alpha diversity was compared via ANOVA and beta diversity was compared via PERMANOVA. There were no differences in the Shannon diversity index (alpha diversity; P = 0.336) or Bray–Curtis dissimilarity (beta diversity; P = 0.744) of pregnant vs. nonpregnant heifers. Overall, bacterial community composition in heifers with high, medium, or low concentrations of estradiol did not differ (P = 0.512). While no overall compositional differences were observed, species-level differences were present within pregnancy status and estradiol concentration groups. The implications of these species-level differences are unknown, but these differences could alter the vaginal environment thereby influencing fertility and vaginal health. Therefore, species-level changes could provide better insight rather than overall microbial composition in relation to an animal’s reproductive health.
Keywords: Brangus, estradiol, microbiome
Introduction
The role and importance of the vaginal microbiota in fertility and reproduction have been recently explored in sheep (Martinez-Roz et al., 2018), cattle (Clemmons et al., 2017), horses (Fraga et al., 2011), and humans (Chen et al., 2017). In general, the vaginal microbiota is dynamic and constantly changing in the aforementioned species. Furthermore, the vaginal microbiota composition differs between human and livestock species. Swartz et al. (2014) compared the vaginal microbiomes of bovine and ovine species to that of humans. They concluded that the microbiomes of ovine and bovine species are characteristically and compositionally different from humans and that these differences were attributed to varying physiological environments within the vaginal tract. Specifically, human vaginal microbiota has more Lactobacillus species that drive the acidic vaginal environment in humans. In comparison, the bovine and ovine species have a vaginal environment closer to neutral pH due to a lower abundance of Lactobacillus (Swartz et al., 2014). Therefore, previous research associating the human vaginal microbiome to fertility (Sirota et al., 2014) may not be directly applicable to livestock species.
Research in bovids analyzing the vaginal microbiome is limited. Previous studies have focused on the characterization of bovids with reproductive disorders (Moreno et al., 2016), pregnant and nonpregnant status (Laguardia-Nascimento et al., 2015; Ault et al., 2019), and the postpartum stage (Lima et al., 2019). However, inference from these studies is limited due to the small sample size with many including less than 25 animals.
The implication of hormones, specifically estrogen, on the vaginal microbiome has only been speculated. It is well known that reproductive hormones significantly affect the reproductive physiology overall. Specifically, estradiol has been shown to change the uterine pH in cattle (Perry and Perry, 2008). Additionally, in humans, it has been determined that estrogen increases the acidity of the vaginal environment (Gorodeski et al., 2005). Shifts in pH will greatly impact the ability of bacteria to survive within a certain environment. In humans, the acidity of the vaginal environment is considered a defense mechanism against many pathogens associated with sexually transmitted diseases (Miller et al., 2016). Therefore, estradiol could have significant impacts on the vaginal microbiome, and changes in the microbial composition could be dependent on circulating levels of estradiol.
There is a significant lack of research evaluating the vaginal microbiome’s role in bovine fertility and how the microbiome could be influenced by reproductive hormones. Therefore, the objectives of this study were to characterize the vaginal microbiota and to determine if vaginal microbiota composition differed across divergent concentrations of estradiol at the time of artificial insemination (AI) in 78 Brangus heifers. Additionally, we also aimed to compare the vaginal microbiota composition at the time of AI between heifers that were retrospectively classified as pregnant or nonpregnant. We hypothesized that different vaginal microbiota communities will either facilitate or inhibit pregnancy. Furthermore, we hypothesized that heifers with high, medium, and low concentrations of estradiol at the time of AI will differ in vaginal microbiota composition.
Materials and Methods
Animal management and treatments
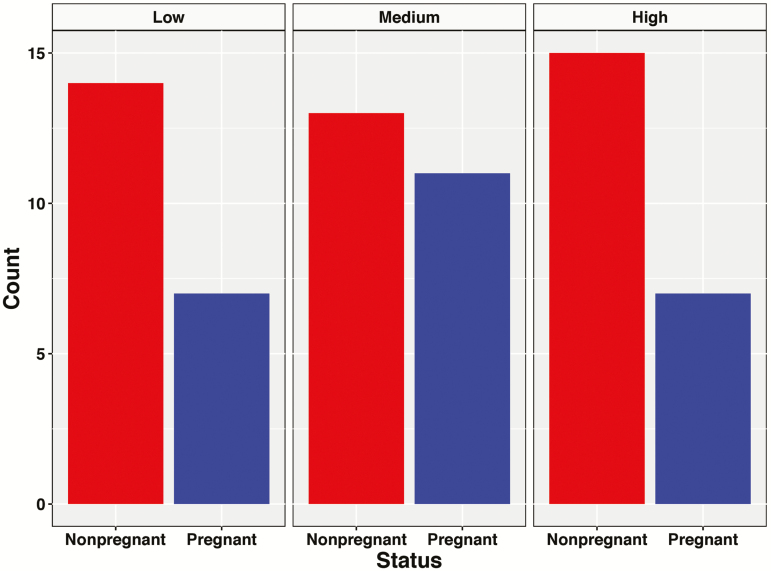
Animal care and use were approved by the Mississippi State University Institutional Animal Care and Use Committee (#18-386). Brangus heifers (n = 78) were housed at the H.H. Leveck Animal Research Center (Mississippi State, MS) in a 25-acre pasture grazing seasonal grasses. All heifers were fed the same concentrate diet twice daily with ad libitum water and minerals. Heifers were approximately 18 mo old at the time of breeding with an average weight of 405 ± 38 kg. All heifers underwent a 7-d Co-Synch + controlled internal drug release (CIDR) estrous synchronization protocol. Heifers were synchronized and artificially inseminated in six groups over a 3-wk period. All heifers were housed in the same pasture throughout the study. All heifers were artificially inseminated with commercially available semen from a single Angus sire. Transrectal ultrasonography was used to determine pregnancy status between days 41 and 57 post-AI dependent on the breeding group (Figure 1).
Figure 1.
A bar graph representing the number of pregnant (blue bar) and nonpregnant (red bar) heifers in each group (high, medium, or low) of estradiol concentration.
Vaginal swab collection
A double-guarded equine uterine culture swab (Minitube Ref. 17214/2950) was utilized to sample the anterior vaginal tract of each heifer immediately before AI. Heifers were restrained in a hydraulic chute and the vulva was cleaned, by wiping with a paper towel, to prevent swab contamination. The double-guarded unit containing the swab was removed from sterile packaging and immediately inserted through the vulva into the vaginal tract. The swab was angled upward, over the pelvic shelf, and toward the anterior vagina. Once the swab would not move forward with pressure, the cotton swab was exposed from the sterile guarding to make direct contact with the anterior vagina. The swab was rotated for approximately 30 s then retracted back into the sterile guarding. The entire double-guarded swab unit was removed from the heifer’s vaginal tract. After removal, the unit was broken down, by removing the external layer to expose the swab in the sterile tubing. The sterile tube containing the swab was snapped at a pre-determined length, then capped with sterile caps to prevent airborne contamination. The swab was closely examined in the sterile guarding for any urine (yellow staining) or feces. If contamination looked possible, the heifer was re-swabbed. Two uncontaminated swabs were collected per animal. The swabs were stored at −80 °C with the swab end upright in a sterile tube until further analysis.
Blood collection and estradiol assay
At the time of AI, blood was collected via coccygeal venipuncture. Blood was allowed to clot at room temperature and placed on ice until transported to the laboratory for processing. Approximately 2 h after collection, blood tubes were centrifuged at 2,000 × g at 4 °C for 10 min. Serum was immediately collected and transferred into sterile 1.5 mL tubes and then stored at −80 °C until further analysis. Circulating serum concentrations of estradiol were analyzed via radioimmunoassay with the methodology previously published by Perry et al. (2004). The intra- and inter-assay coefficient of variation for estradiol assays were 4.7% and 6.1%, respectively. Heifers were classified into low (1.1 to 2.5 pg/mL; n = 21), medium (2.6 to 6.7 pg/mL; n = 30), and high (7.2 to 17.6 pg/mL; n = 27) concentration of estradiol determined from previous literature (Jinks et al., 2013) (Figure 1).
Bacterial community analysis
The bacterial community analysis was performed by Microbiome Insights Co. located in Vancouver, Canada. Genomic DNA was extracted using the MoBio PowerMag Soil DNA Isolation Bead Plate (Mo Bio Laboratories, Inc., Carlsbad, CA, USA) on a KingFisher robot following the manufacturer’s protocol. Blank control swabs (n = 2) were included in the analysis for contamination checks. Bacterial amplicons from the V4 region of the 16S rRNA gene were amplified using the 515F 5′-GTGCCAGCMGCCGCGGTAA-3′ and the 806R 5′-GGACTACHVGGGTWTCTAAT-3′ primers as described by Kozich et al. (2013). Sequencing was done with an Illumina MiSeq (Illumina, San Diego, CA, USA) using the 300-bp paired-end kit (v.3). Quality filtering, taxonomic classification using the Greengenes (v. 13_8) database, and clustering into 97%-similarity operational taxonomic units (OTUs) were done using the mothur software package (v. 1.39.5) (Schloss et al., 2009).
Statistical analysis
The vaginal bacterial community comparisons of interest were between pregnant and nonpregnant heifers and across the low, medium, and high estradiol concentrations. The R software program (R Core Team, 2013) was used to conduct the statistical analyses. Alpha diversity was calculated using the Shannon index and significance was tested using ANOVA. Beta diversity was computed using the Bray–Curtis dissimilarity and visualized using the principal coordinate analysis (PCoA) plot. Differences in community structure were assessed using the permutational multivariate analysis of variance (PERMANOVA) with pregnant vs. nonpregnant or low, medium, or high estradiol group as the main fixed factor and using 9,999 permutations for significance testing in R (adonis function from the Vegan package). After the estradiol classification, the PROC Mixed procedure in SAS 9.4 Software was utilized to determine the significance between classification groups.
Results
Sequencing information
A total of 80 swabs were analyzed including two blank control swabs. Vaginal swabs from heifers grouped retrospectively as pregnant (n = 29) or nonpregnant (n = 49). A total of 1,156,788 quality-filtered reads were obtained with an average of 14,459 quality-filtered reads per sample that were assigned to 8,358 OTUs. Control samples had minimum quality-filtered reads (80 and 218), thus contamination was considered absent. Samples with less than 1,000 quality-filtered reads were removed from the analyses (three nonpregnant and two pregnant samples).
Taxonomic composition
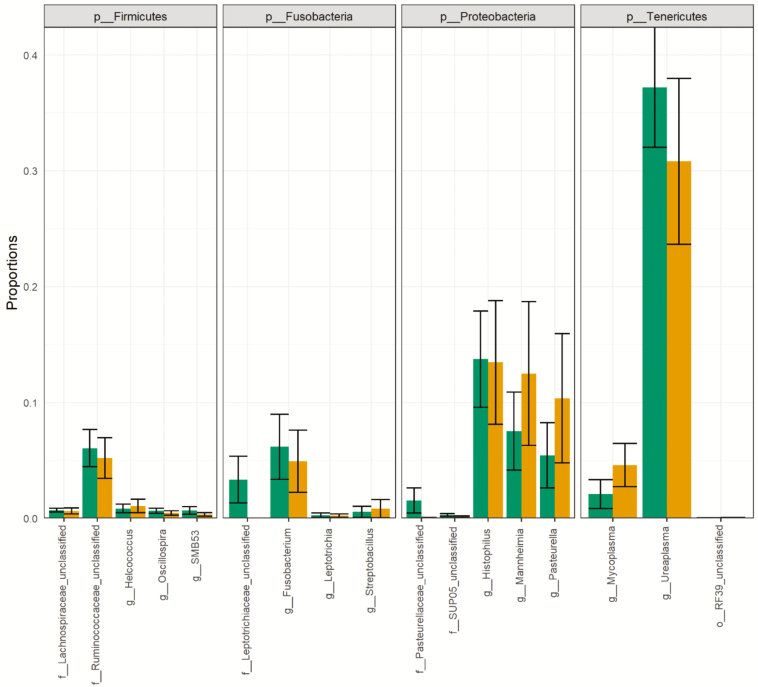
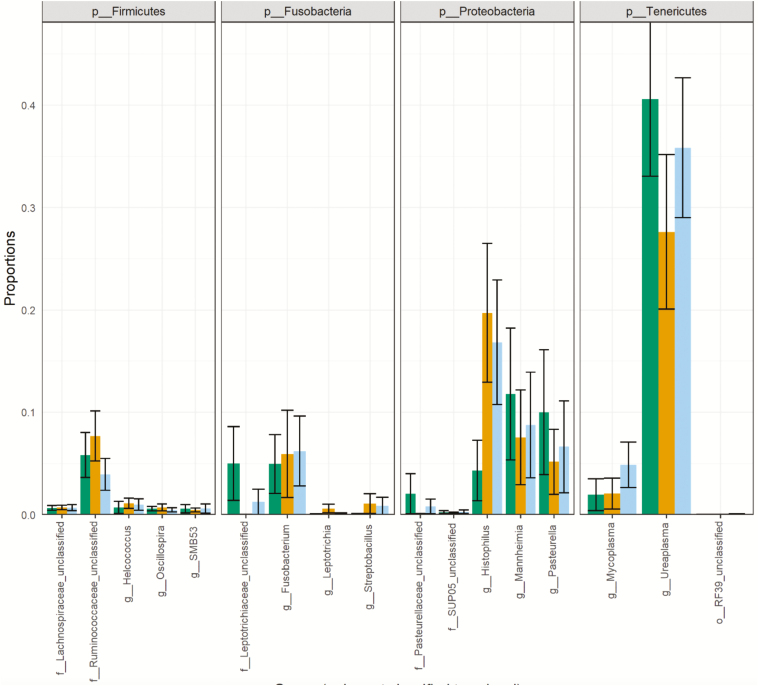
The four most abundant phyla were Tenericutes (36%), Proteobacteria (30%), Fusobacteria (7.6%), and Firmicutes (1.8%), respectively. There were no compositional differences (Figure 2) or proportional differences (Supplementary Figure S1) between nonpregnant and pregnant heifers. Additionally, no compositional differences (Figure 3) or proportional differences (Supplementary Figure S2) were seen across heifers with high, medium, or low estradiol concentrations
Figure 2.
Relative abundance of the five most abundant genus-level taxa within the four most abundant phyla. The orange bars represent nonpregnant heifers and the green bars represent pregnant heifers.
Figure 3.
Relative abundance of the five most abundant genus-level taxa within the four most abundant phyla. The bars represent heifers with high (green), medium (blue), and low (orange) estradiol concentrations.
Comparing vaginal microbiomes of pregnant vs. nonpregnant heifers
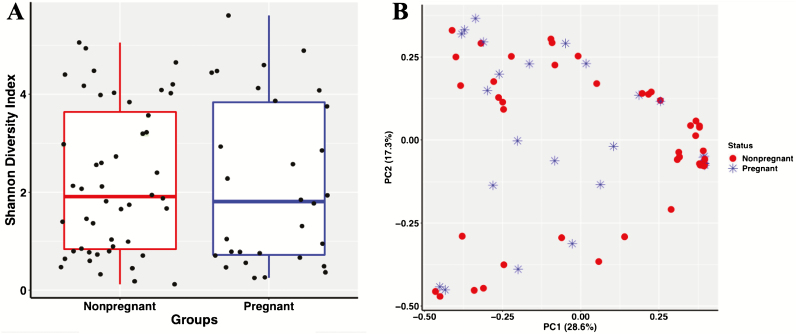
There were no differences in alpha diversity between pregnant and nonpregnant heifers (Figure 4A, P = 0.366). The Shannon diversity index showed pregnant heifers with a mean of 2.18 ± 1.68 and nonpregnant heifers with a mean of 2.22 ± 1.48. The PCoA displayed no clustering of samples by pregnancy status (Figure 4B). Furthermore, the PERMANOVA showed similar (P = 0.744) overall bacterial profiles between pregnant and nonpregnant heifers. There were three OTUs that were different between pregnant and nonpregnant heifers which included Pasteurella multocida (P < 0.001), Pasteurellaceae unclassified (P < 0.001), and Fusobacterium unclassified (P = 0.001).Pasteurellaceae unclassified and Fusobacterium unclassified were more abundant in nonpregnant heifers, whereas P. multocida was more abundant in pregnant heifers. Noteworthy, only three animals lacked the presence of at least one of these species within their reproductive tract.
Figure 4.
Alpha diversity boxplot of the vaginal bacterial microbiota in Brangus heifers measured by the Shannon diversity index (panel A). The red box represents nonpregnant heifers and the blue box represents pregnant heifers. Black dots represent values for individual samples. Alpha diversity did not differ (P = 0.366) between pregnant vs. nonpregnant heifers. PCoA depicting Bray–Curtis dissimilarities across samples (panel B). Nonpregnant heifers are represented by red circles and pregnant heifers are represented by blue stars. There were no differences in beta diversity between pregnancy status (P = 0.74). PC, principal coordinate.
Estradiol concentrations
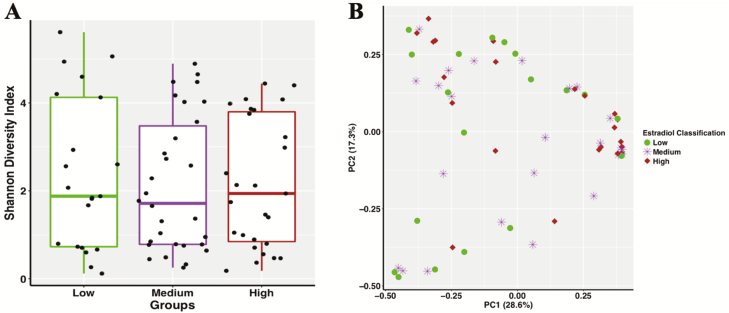
There were significant differences between heifer group classifications of serum estradiol concentrations; heifers in the low, medium, or high serum estradiol group had significantly different (P < 0.05) serum estradiol concentrations. Alpha diversity was measured using the Shannon index to evaluate differences between the vaginal microbiomes of heifers with high, medium, and low serum estrogen concentrations (Figure 5A). No differences were found across the high (2.16 ± 1.46), medium (2.13 ± 1.53), and low (2.37 ± 1.74) samples (Figure 5A, P = 0.512). There were no differences in beta diversity across these samples either (P = 0.48). A principal component analysis was utilized to visualize the relationship across samples and no clusters were observed (Figure 5B). There were eight statistically different OTUs observed in animals with varying estradiol concentrations. Leptotrichiaceae unclassified (P < 0.0001), P. multocida (P < 0.001), and Pasteurellaceae unclassified (P < 0.001) were more abundant in heifers with high concentrations of estradiol compared with heifers with low and medium concentrations of estradiol. Histophilus somni (P < 0.001), Actinobacillus seminis (P < 0.001), and Fusobacterium unclassified (P = 0.001) were more abundant in the vaginal microbiome of heifers with medium and low estradiol concentrations compared with heifers with high concentrations of estradiol. Bacteroidetes unclassified (P < 0.001) were more abundant in the vaginal microbiome of heifers with low estradiol concentrations compared with heifers with medium and high estradiol concentrations.
Figure 5.
Alpha diversity boxplot of vaginal bacterial microbiota in Brangus heifers measured by the Shannon diversity index (panel A). The green box represents heifers with low estrogen concentration (1.1 to 2.5 pg/mL), the purple box represents heifers with medium estrogen concentration (2.6 to 6.7 pg/mL), and the brown represents heifers with high estrogen concentration (7.2 to 17.6 pg/mL). Black dots represent values for individual samples. Alpha diversity did not differ across groups (P = 0.51). PCoA depicting Bray–Curtis dissimilarities across samples (panel B). Heifers with high (maroon diamond; 7.2 to 17.6 pg/mL), medium (purple star; 2.6 to 6.7 pg/mL), and low (green circle; 1.1 to 2.5 pg/mL) estradiol concentrations are represented. There were no differences in beta diversity between estradiol groups (P = 0.48). PC, principal coordinate.
Discussion
There were no differences in the overall bacterial community composition between heifers that became pregnant vs. nonpregnant. However, the taxonomic profiles within our study are different compared with the current literature. Other studies commonly report Proteobacteria, Firmicutes, Bacteriodetes, and Actinobacteria as the most abundant phyla within the vaginal tract of the bovids (Laguardia-Nascimento et al., 2015; Lima et al., 2019). In contrast, the phyla Tenericutes and Fusobacterium were more abundant, than Bacteriodetes and Actinobacteria, in our heifers. The implications of this difference are not yet known. Differences could be attributed to environmental factors, age, sexual maturity, or fertility issues within the sample groups, but more research is needed to confirm these speculations.
Nevertheless, species-level differences in this study between pregnant and nonpregnant heifers could have implications surrounding fertility and reproductive performance. Specifically, the presence of Fusobacterium spp. and P. multocida in nonpregnant heifers. Fusobacterium species have been found to be causative agents in morbidity and mortality in both humans and animals (Citron, 2002; Afra et al., 2013). Fusobacterium spp. are anaerobic, gram-negative rods that are considered pathogenic in humans (Huggan and Murdoch, 2008). Specifically, Fusobacterium necrophorum is a known causative agent of puerperal metritis in postpartum cows (Machado et al., 2014). Therefore, nonpregnant heifers in this study with colonization of Fusobacterium spp. could have had vaginal infections inhibiting pregnancy success. Next, P. multocida is an opportunistic pathogenic, gram-negative rod that has previously been associated with bovine respiratory disease in cattle (Dabo et al., 2007). It is debatable whether the colonization of P. multocida has negative or positive impacts on fertility. It is still undetermined if P. multocida is pathogenic in the reproductive tract. Lima et al. (2019) found that calves inoculated with upper respiratory tract potential pathogens, such as P. multocida, at birth were less likely to develop disease later in life. It could be speculated that P. multocida is considered pathogenic in nonpregnant animals, but during gestation, it increases for inoculation of the calf at parturition. Further studies following the vaginal microbiome throughout gestation are necessary to provide evidence to support this hypothesis.
There were no differences in the microbial composition of heifers with high, medium, and low concentrations of estradiol. These results are in agreement with a study published by Ault et al. (2019) in which they compared progesterone levels to the microbiome; however, they found no differences in the microbiome with varying progesterone concentrations. These conclusions are quite shocking considering the significant impacts hormones have on the vaginal tract throughout the estrous cycle (Jinks et al., 2013). Therefore, while we may not have seen any direct differences at the time of AI, we cannot rule out with complete certainty that hormones have no impacts on the vaginal microbiome throughout gestation.
In the current study, all heifers had undergone an estrus synchronization protocol with a CIDR. All heifers had a new CIDR inserted for 7 d according to the protocol. The CIDR applicator was cleaned with Nolvasan solution between heifers to prevent contamination. To date, there is a lack of knowledge surrounding how an estrus synchronization protocol, especially using CIDRs, could influence the vaginal microbiome. In microbiology, it is widely accepted that specific bacteria require a certain pH range to grow regardless of their environment. In cattle, the pH of the vaginal tract fluctuates during the estrous cycle; the pH of the vaginal tract is the most acidic during ovulation when estradiol is peaking (Lewis and Newman, 1984). Estradiol causes a change in vaginal secretions and causes a more acidic vaginal environment compared with a vaginal environment under the influence of progesterone (Gorodeski et al., 2005). A limitation to this study is that vaginal pH was not measured in these heifers. Moreover, there has also been speculation that the immunosuppressive role of progesterone in the reproductive tract environment could allow for the easy colonization of pathogenic bacteria (Padula and Macmillan, 2006). Studies in humans have eluded to this concept where they have found women in the luteal phase of their menstrual cycle are more susceptible to sexually transmitted diseases vs. women in the follicular phase (Wessels et al., 2018). It is not clear the influence that exogenous progestins have in bovine species, but Martinez-Ros et al. (2018) showed that CIDRs altered the vaginal pH in ewes.
In the majority of bovine estrus synchronization protocols, there is a very short window between the removal of the CIDR and AI (Beef Reproduction Task Force, 2019). Therefore, a CIDR insert could alter the vaginal pH by decreasing the acidity which inhibits the growth of commensal bacteria. Commensal bacteria often inhibit the growth of pathogens in environments by competing for nutrients and attachment sites (Abt and Pamer, 2014). Due to insufficient time before AI, recolonization of commensal bacteria that may be conducive to pregnancy by preventing the growth of pathogens is not possible and, therefore, decreased fertility results. Additionally, studies have shown that CIDRs can cause vaginosis and improper handling could introduce bacteria into the vaginal tract (McDougall, 2018). Dias et al. (2019) found that vaginosis caused by CIDR usage does not affect fertility in beef cattle; it is possible that the symptoms of vaginosis commonly found after CIDR insertion could be an immune response to the foreign object itself. Nevertheless, an immune response in the vaginal tract during CIDR insertion could result in a decrease in commensal bacteria translating to an environment that is more susceptible to colonization of pathogens. Therefore, more research focused on CIDR usage and its relationship with bacterial colonization of the reproductive tract is needed.
Similarly, to the pregnant and nonpregnant heifers, there were differences in specific OTUs between heifers with different estradiol concentrations. P. multocida, Leptotrichiaceae unclassified, and H. somni have all been associated with bovine respiratory disease by being found in the lungs of infected animals postmortem (Hellenbrand et al., 2013; Johnston et al., 2017). The significance of these bacteria present in the vaginal microbiota at the time of AI is not clear. These bacteria were more commonly present in heifers with medium to high concentrations of estradiol compared with heifers with low concentrations of estradiol. In theory, these heifers should have been more receptive to breeding and pregnancy, but the pregnancy rate in the current study was very poor (36%); it is possible that these bacteria were acting as opportunistic pathogens at timed artificial insemination (TAI) in these Brangus heifers. Furthermore, these bacteria commonly grow in a pH that is near neutral; heifers with high or medium estradiol should have had a slightly acidic vaginal pH to inhibit the growth of these opportunistic pathogens (Shah et al., 2008; Cho et al., 2017; Bandara et al., 2018). The presence of these bacteria makes it more plausible that vaginal pH was not physiologically decreased enough at the time of AI to inhibit the growth of these opportunistic pathogens which eludes to the heifers with high estradiol not having more pregnancies (Figure 1). A potential mechanism for these heifers having a pH closer to neutral is CIDR usage with inadequate time for estradiol to alter the vaginal environment before AI. More directly in humans, estradiol acidifies the vaginal tract by active proton secretion through the apical vaginal membrane by utilizing tight junctions to maintain an acidic environment (Gorodeski et al., 2005). Progesterone, the hormone on CIDRs, inhibits the insertion of tight junction proteins in the reproductive tract (Garfield et al., 1978). Therefore, it is plausible that CIDR insertion prevents normal physiological processes from occurring before ovulation leading to an increased vaginal pH at AI accompanied by increased opportunistic pathogens in the vaginal microbiota.
Additionally, A. seminis was significantly increased in heifers with low to medium estradiol concentrations at TAI compared with heifers with high concentrations of estradiol. In sheep and goats, A. seminis causes infections in both the male and female reproductive tracts that leads to decreased fertility and abortions (Cebra and Cebra, 2012). Therefore, this bacterium could be negatively affecting the reproductive tract by decreasing the cyclicity and fertility of heifers.
The differences in OTUs could indicate that vaginal dysbiosis was occurring in some groups of heifers. However, the microbiome of infected or normal bovine vaginal tracts throughout the estrous cycle has yet to be fully characterized. Therefore, dysbiosis cannot be confirmed because there is no standard healthy vaginal composition for comparison to our heifer’s vaginal microbiota. Normally, dysbiosis occurs when the commensal bacteria die due to unfavorable environmental conditions. These conditions include changes in pH, decreased nutrient availability, introduction of chemicals, stress, antibiotic usage, dietary changes, and many other factors (Levy et al., 2017). The introduction of CIDRs, stress due to animal handling, and other environmental factors could have led to vaginal dysbiosis in the heifers used in the current study. Africa et al. (2014) evaluated the effects of bacterial vaginosis in women, finding that this infection altered the equilibrium of the normal vaginal microbiota and caused dysbiosis; furthermore, bacterial vaginosis has been reported to cause pelvic inflammatory disease, adverse pregnancy outcomes, increased susceptibility to reproductive tract infections, and infertility. Therefore, the decreased fertility observed in this study could be a result of vaginal dysbiosis, but conformation of this requires more characterization research specifically evaluating healthy heifers at TAI.
Lastly, it is important to note that in all heifers, the phyla Tenericutes were the most abundant within the vaginal microbiome, specifically Mycoplasma and Ureaplasma species. These bacteria are small pleomorphic obligate intracellular cocci that lack a cell wall (Combaz-Sohnchun and Kuhn, 2017). Therefore, the identification of these bacteria using a microscope is extremely difficult. Due to the difficulty to culture and identify, these species could be easily overlooked in the analysis of fertility issues. Both species, Mycoplasma and Ureaplasma, are commensals of the urogenital tract. Ureaplasma urealyticum has been found to cause spontaneous abortions in women between 10 and 20 wk of gestation (Ahmadi et al., 2014). Mycoplasma species have been associated with spontaneous abortion, polyarthritis, and mastitis in cattle (Hum et al., 2008). Normally, these species are more abundant in certain environments and inoculate the vaginal tracts of animals in those environments; therefore, certain farms are more susceptible to negative reproductive effects from these bacteria than others based on location (Murai and Higuchi, 2019). As previously mentioned, the pregnancy rates of the heifers utilized in this research were surprisingly low. The decreased fertility of these heifers may be attributed to the accidental introduction of Mycoplasma and Ureaplasma species to the uterine body by the AI gun; (Crane and Hughes 2018) found a potential for Ureaplasma species to infect embryos resulting in abortion, gestational losses, and pathologies in calves after birth. Therefore, many heifers on this project could have had successful conception, but once the embryo migrated to the uterine body, these opportunistic pathogens caused early embryonic death leading to low pregnancy rates at 41 to 57 d post-AI.
In conclusion, no differences were found in the vaginal bacterial community profiles of heifers with different pregnancy status or estradiol concentrations. Overall, it can be concluded that the vaginal microbiome is diverse and finding significant differences in the composition as a whole has proven difficult. However, species-level differences can provide insights into the innerworkings of the vaginal microbiome to determine if an animal is more susceptible to infection, spontaneous abortion, or successful conception. To fully understand the vaginal microbiome, more research is needed, especially research focused on the species-level changes in the vaginal microbiome throughout the estrus cycle, gestation, immediately following parturition, and any other significant reproductive events.
Supplementary Material
Acknowledgments
This publication is a contribution of the Mississippi Agricultural and Forestry Experiment Station. This material is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project under accession number 1011100. Additional funding was provided by the U.S. Department of Agriculture, Agricultural Research Service, Biophotonic Initiative number 58-6402-3-018.
Glossary
Abbreviations
- AI
artificial insemination
- CIDR
controlled internal drug release
- OTU
operational taxonomic unit
- PCoA
principal coordinate analysis
- PERMANOVA
permutational multivariate analysis of variance
- TAI
timed artificial insemination
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Abt, M. C., and Pamer E. G.. . 2014. Commensal bacteria mediated defenses against pathogens. Curr. Opin. Immunol. 29:16–22. doi: 10.1016/j.coi.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afra, K., Laupland K., Leal J., Lloyd T., and Gregson D.. . 2013. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect. Dis. 13:264. doi: 10.1186/1471-2334-13-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Africa, C. W., Nel J., and Stemmet M.. . 2014. Anaerobes and bacterial vaginosis in pregnancy: virulence factors contributing to vaginal colonisation. Int. J. Environ. Res. Public Health. 11:6979–7000. doi: 10.3390/ijerph110706979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi, A., Khodabandehloo M., Ramazanzadeh R., Farhadifar F., Nikkhoo B., Soofizade N., and Rezaii M.. . 2014. Association between Ureaplasma urealyticum endocervical infection and spontaneous abortion. Iran J. Microbiol. 6:392–397. [PMC free article] [PubMed] [Google Scholar]
- Ault, T. B., Clemmons B. A., Reese S. T., Dantas F. G., Franco G. A., Smith T. P. L., Edwards J. L., Myer P. R., and Pohler K. G.. . 2019. Uterine and vaginal bacterial community diversity prior to artificial insemination between pregnant and non-pregnant postpartum cows. Amer Soc of Anim. Sci. J. Anim. Sci. 97(10):4298–4304. doi: 10.1093/jas/skz210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara, A. B., Zuo Z., McCutcheon K., Ramachandran S., Heflin J. R., and Inzana T. J.. . 2018. Identification of Histophilus somni by a nanomaterial optical fiber biosensor assay. J. Vet. Diagn. Invest. 30:821–829. doi: 10.1177/1040638718803665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beef Reproduction Task Force . 2019. Beef heifer protocols. DeForest (Wisconsin): ABS-USA. [Google Scholar]
- Cebra, C., and Cebra M.. . 2012. Diseases of the hematologic, immunologic, and lympatic systems (multisystem disease). In: D. G. Pugh, editor. Sheep and Goat Medicine. 2nd ed. Maryland Heights (MO): Saunders. [Google Scholar]
- Chen, C., Song X., Wei W., Zhong H., Dai J., Lan Z., Li F., Yu X., Feng Q., and Wang Z., . et al. 2017. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 8:875. doi: 10.1038/s41467-017-00901-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, E. H., Park K. S., Yang M., Song D. J., Huh H. J., Ki C. S., and Lee N. Y.. . 2017. Laboratory identification of Leptotrichia species isolated from bacteremia patients at a single institution. Ann. Lab. Med. 37:272–276. doi: 10.3343/alm.2017.37.3.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron, D. M. 2002. Update on the taxonomy and clinical aspects of the genus Fusobacterium. Clin. Infect. Dis. 35(Suppl 1):S22–S27. doi: 10.1086/341916 [DOI] [PubMed] [Google Scholar]
- Clemmons, B. A., Reese S. T., Dantas F. G., Franco G. A., Smith T. P. L., Adeyosoye O. I., Pohler K. G., and Myer P. R.. . 2017. Vaginal and uterine bacterial communities in postpartum lactating cows. Front. Microbiol. 8:1047. doi: 10.3389/fmicb.2017.01047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combaz-Sohnchen, N., and Kuhn A.. . 2017. A systematic review of Mycoplasma and Ureaplasma in urogynaecology. Geburtshilife Frauenheilkd. 77(12): 1299–1303. doi: 10.1055/s-0043-119687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane, M. B., and Hughes C. A.. . 2018. Can Ureaplasma diversum be transmitted from donor to recipient through the embryo? Two case reports outlining U. diversum losses in bovine embryo pregnancies. Can. Vet. J. 59:43–46. [PMC free article] [PubMed] [Google Scholar]
- Dabo, S. M., Taylor J. D., and Confer A. W.. . 2007. Pasteurella multocida and bovine respiratory disease. Anim. Health Res. Rev. 8:129–150. doi: 10.1017/S1466252307001399 [DOI] [PubMed] [Google Scholar]
- Dias, N. W., Sales A., Timlin C., Pancini S., Currin J., and Mercadante V. R. G.. . 2019. PSII- 21 Vaginitis incidence and effects on fertility of beef females enrolled on estrus synchronization protocols using a controlled internal drug (CIDR) device. J. Anim. Sci. 97(3):242. doi: 10.1093/jas/skz258.491 [DOI] [Google Scholar]
- Fraga, M., Perelmuter K., Delucchi L., and Zununino P.. . 2011. Equine native microbiota as a source of beneficial microbes. In: J. E. Leffhalm, editor. Horses: biology, domestication, and human interactions. Hauppauge (NY): Nova Science Publishers; p. 111–119. [Google Scholar]
- Garfield, R. E., Sims S. M., Kannan M. S., and Daniel E. E.. . 1978. Possible role of gap junctions in activation of myometrium during parturition. Am. J. Physiol. 235:C168–C179. doi: 10.1152/ajpcell.1978.235.5.C168 [DOI] [PubMed] [Google Scholar]
- Gorodeski, G. I., Hopfer U., Liu C. C., and Margles E.. . 2005. Estrogen acidifies vaginal pH by up-regulation of proton secretion via the apical membrane of vaginal-ectocervical epithelial cells. Endocrinology 146:816–824. doi: 10.1210/en.2004-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellenbrand, K. M., Forsythe K. M., Rivera-Rivas J. J., Czuprynski C. J., and Aulik N. A.. . 2013. Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages. Microb. Pathog. 54:67–75. doi: 10.1016/j.micpath.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggan, P. J., and Murdoch D. R.. . 2008. Fusobacterial infections: clinical spectrum and incidence of invasive disease. J. Infect. 57:283–289. doi: 10.1016/j.jinf.2008.07.016 [DOI] [PubMed] [Google Scholar]
- Hum, S., Kessell A., Djordjevic S., Rheinberger R., Hornitzky M., Forbes W., and Gonsalves J.. . 2008. Mastitis, polyarthritis, and abortion caused by Mycoplasma species bovine group 7 in dairy cattle. Aust. Vet. J. 78(11):744–750. doi: 10.1111/j.1751-0813.2000.tb10444.x [DOI] [PubMed] [Google Scholar]
- Jinks, E. M., Smith M. F., Atkins J. A., Pohler K. G., Perry G. A., Macneil M. D., Roberts A. J., Waterman R. C., Alexander L. J., and Geary T. W.. . 2013. Preovulatory estradiol and the establishment and maintenance of pregnancy in suckled beef cows. J. Anim. Sci. 91:1176–1185. doi: 10.2527/jas.2012-5611 [DOI] [PubMed] [Google Scholar]
- Johnston, D., Earley B., Cormican P., Murray G., Kenny D. A., Waters S. M., McGee M., Kelly A. K., and McCabe M. S.. . 2017. Illumina MiSeq 16S amplicon sequence analysis of bovine respiratory disease associated bacteria in lung and mediastinal lymph node tissue. BMC Vet. Res. 13:118. doi: 10.1186/s12917-017-1035-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich, J. J., Westcott S. L., Baxter N. T., Highlander S. K., and Schloss P. D.. . 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79:5112–5120. doi: 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguardia-Nascimento M., Branco K. M. G. R., Gasparini M. R., Giannattasio-Ferraz S., Leite L. R., Araujo F. M. G., Nicoli J. R., Oliveria G. C., and Barbosa-Stancioli E. D.. 2015. Vaginal microbiome characterization of Nellore cattle using metagenomic analysis. PLoS ONE 10(11):e0143294. doi: 10.1371/journal.pone.0143294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, M., Kolodziejczyk A. A., Thaiss C. A., and Elinav E.. . 2017. Dysbiosis and the immune system. Nat. Rev. Immunol. 17:219–232. doi: 10.1038/nri.2017.7 [DOI] [PubMed] [Google Scholar]
- Lewis, G. S., and Newman S. K.. . 1984. Changes throughout estrous cycles of variables that might indicate estrus in dairy cows. J. Dairy Sci. 67:146–152. doi: 10.3168/jds.S0022-0302(84)81278-3 [DOI] [PubMed] [Google Scholar]
- Lima, S. F., Bicalho M. L. S., and Bicalho R. C.. . 2019. The Bos taurus maternal microbiome: role in determining the progeny early-life upper respiratory tract microbiome and health. PLoS One 14:e0208014. doi: 10.1371/journal.pone.0208014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, V. S., Bicalho M. L., Meira Junior E. B., Rossi R., Ribeiro B. L., Lima S., Santos T., Kussler A., Foditsch C., Ganda E. K., . et al. 2014. Subcutaneous immunization with inactivated bacterial components and purified protein of Escherichia coli, Fusobacterium necrophorum and Trueperella pyogenes prevents puerperal metritis in Holstein dairy cows. PLoS One. 9:e91734. doi: 10.1371/journal.pone.0091734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Ros, P., Lozano M., Hernandex F., Tirado A., Rios-Abellan A., Lopez Mendoza M. C., and Gonzalez-Bulnes A.. . 2018. Intravaginal device-type and treatment-length for ovine estrus synchronization modify vaginal mucus and microbiota and affect fertility. Animals (Basel). 8:226. doi: 10.3390/ani8120226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall, S. 2018. Comparing purulence and vaginitis associated with progesterone releasing agents. New Zealand: AgriHealth. [Google Scholar]
- Miller, E. A., Beasley D. E., Dunn R. R., and Archie E. A.. . 2016. Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique. Front. Microbiol. 7:1936. doi: 10.3389/fmicb.2016.01936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, G. C., Fontana C., Cocconcelli P. S., Callegari M. L., and Otero M. C.. . 2016. Vaginal microbial communities from synchronized heifers and cows with reproductive disorders. J. Appl. Microbiol. 121:1232–1241. doi: 10.1111/jam.13239 [DOI] [PubMed] [Google Scholar]
- Murai, K., and Higuchi H.. . 2019. Prevalence and risk factors of Mycoplasma bovis infection in dairy farms in northern Japan. Res. Vet. Sci. 123:29–31. doi: 10.1016/j.rvsc.2018.12.006 [DOI] [PubMed] [Google Scholar]
- Padula, A. M., and Macmillan K. L.. . 2006. Effect of treatment with two intravaginal inserts on the uterine and vaginal microflora of early postpartum beef cows. Aust. Vet. J. 84:204–208. doi: 10.1111/j.1751-0813.2006.tb12800.x [DOI] [PubMed] [Google Scholar]
- Perry, G. A., and Perry B. L.. . 2008. Effect of preovulatory concentrations of estradiol and initiation of standing estrus on uterine pH in beef cows. Domest. Anim. Endocrinol. 34:333–338. doi: 10.1016/j.domaniend.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Perry, G. A., Smith M. F., and Geary T. W.. . 2004. Ability of intravaginal progesterone inserts and melengestrol acetate to induce estrous cycles in postpartum beef cows. J. Anim. Sci. 82:695–704. doi: 10.2527/2004.823695x [DOI] [PubMed] [Google Scholar]
- R Core Team . 2013. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. [Google Scholar]
- Schloss, P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., Lesniewski R. A., Oakley B. B., Parks D. H., Robinson C. J. et al. 2009. Introducing mothur: open-source, platform independent, community supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75(23):7537-7541. doi: 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, A. H., Kamboh A. A., Rajput N., and Korejo N. A.. . 2008. A study on the optimization of physico-chemical conditions for the growth of Pasteurella multocida under in vitro. J. Agri. Soc. Sci. 4:176–179. [Google Scholar]
- Sirota, I., Zarek S. M., and Segars J. H.. . 2014. Potential influence of the microbiome on infertility and assisted reproductive technology. Semin. Reprod. Med. 32:35–42. doi: 10.1055/s-0033-1361821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz, J. D., Lachman M., Westveer K., O’Neill T., Geary T., Kott R. W., Berardinelli J. G., Hatfield P. G., Thomson J. M., Roberts A., . et al. 2014. Characterization of the vaginal microbiota of ewes and cows reveals a unique microbiota with low levels of lactobacilli and near neutral pH. Front. Vet. Sci. 1:19. doi: 10.3389/fvets.2014.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels, J. M., Felker A. M., Dupont H. A., and Kaushic C.. . 2018. The relationship between sex hormones, the vaginal microbiome, and immunity in HIV-1 susceptibility in women. Dis. Model. Mech. 11(9):dmm035147. doi: 10.1242/dmm.035147 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.