Abstract
Role of Paroxysmal Depolarization in Focal Seizure Activity
Tryba AK, Merricks EM, Lee S, et al. J Neuroph. 2019;122(5):1861-1873. doi.org/10.1152/jn.00392.2019
We analyze the role of inhibition in sustaining focal epileptic seizure activity. We review ongoing seizure activity at the mesoscopic scale that can be observed with microelectrode arrays as well as at the macroscale of standard clinical electroencephalogram. We provide clinical, experimental, and modeling data to support the hypothesis that paroxysmal depolarization (PD) is a critical component of the ictal machinery. We present dual-patch recordings in cortical cultures showing reduced synaptic transmission associated with presynaptic occurrence of PD, and we find that the PD threshold is cell size related. We further find evidence that optically evoked PD activity in parvalbumin neurons can promote propagation of neuronal excitation in neocortical networks in vitro. Spike sorting results from microelectrode array measurements around ictal wave propagation in human focal seizures demonstrate a strong increase in putative inhibitory firing with an approaching excitatory wave, followed by a sudden reduction of firing at passage. At the macroscopic level, we summarize evidence that this excitatory ictal wave activity is strongly correlated with oscillatory activity across a centimeter-sized cortical network. We summarize Wilson–Cowan-type modeling showing how inhibitory function is crucial for this behavior. Our findings motivated us to develop a network motif of neurons in silico, governed by a reduced version of the Hodgkin–Huxley formalism, to show how feedforward, feedback, PD, and local failure of inhibition contribute to observed dynamics across network scales. The presented multidisciplinary evidence suggests that the PD not only is a cellular marker or epiphenomenon but actively contributes to seizure activity. NEW & NOTEWORTHY: We present mechanisms of ongoing focal seizures across meso- and macroscales of microelectrode array and standard clinical recordings, respectively. We find modeling, experimental, and clinical evidence for a dual role of inhibition across these scales: local failure of inhibition allows propagation of a mesoscopic ictal wave, whereas inhibition elsewhere remains intact and sustains macroscopic oscillatory activity. We present evidence for PD as a mechanism behind this dual role of inhibition in shaping ictal activity.
Commentary
A dynamic imbalance of inhibition and excitation has long been implicated in ictogenesis. Most antiepileptic treatments have been developed under the dogma that an epileptic brain has too little inhibition (eg, barbiturates and benzodiazepines enhance GABAergic neurotransmission) or too much excitation (eg, parampanel, topiramate, and levetiracetam reduce glutamatergic transmission). However, tweaking the excitatory–inhibitory (E-I) balance in the brain is a less-than-perfect treatment, often ineffective or producing substantial side effects. In the worst cases, antiepileptic drugs can have paradoxical effects, worsening seizures.1
Experimental studies aimed at dissecting the interplay between excitation and inhibition suggest that transiently dysfunctional inhibition, in which GABA synapses temporarily become ineffective or even excitatory, may be responsible for the paradoxical drug effects. For example, in human and animal studies, interneurons fire earlier and/or harder than principal cells during the onset of epileptiform activity,2,3 before failing by either entering depolarization block4,5 or causing postsynaptic chloride to accumulate such that the GABAA reversal potential becomes depolarized.6 In some models, optogenetic activation of interneurons alone is sufficient to initiate seizures.7
In the highlighted study, Tryba et al8 use data ranging in scale from cell culture to human electroencephalogram (EEG) to develop a model aimed at describing sometimes the dichotomous role of GABA in an epileptic brain. They form initial hypotheses based largely on simultaneous recordings of neuronal spiking, local field potential (LFP), and EEG from patients with epilepsy. Two key observations from these data motivate the work in this article: (1) There is a dramatic difference in propagation speed of the ictal spiking wave front and LFP oscillations (see https://media.nature.com/original/nature-assets/ncomms/journal/v3/n9/extref/ncomms2056-s6.mov),9 and (2) the ictal wave front is able to synchronize activity across a scale of many centimeters.10 The authors characterize these observations with 2 colloquialisms often loosely used to describe neural activity during a seizure: “hyperexcitation” is used to describe the slowly propagating high frequency spiking activity in the ictal core while “hypersynchrony” is assigned to the widespread Eastern Cooperative Oncology Group oscillations, which are synchronized by the ictal core.
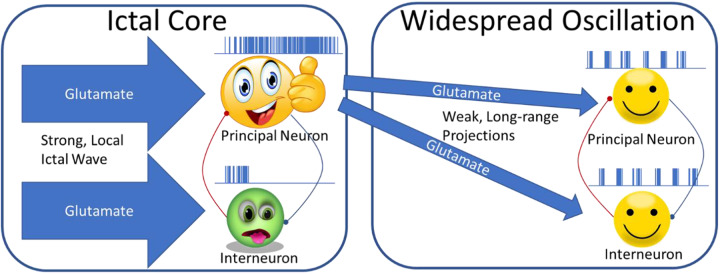
The model in the highlighted article (summarized in Figure 1 cartoon) proposes that hyperactivity at the ictal core drives interneurons in the ictal core into a state of depolarization block (aka paroxysmal depolarization shift). In silico and in vitro experiments support this model by demonstrating that interneurons are more readily driven into depolarization block than principal neurons. Importantly, they use paired recordings from cultured neurons to confirm that neither principal neurons nor interneurons elicit postsynaptic currents during depolarization block. Together, these findings support the idea that interneurons are preferentially driven into depolarization block, which causes them to stop releasing GABA, effectively disinhibiting the local network.
Figure 1.
Neurons in the ictal core receive strong glutamatergic input from the locally propagating seizure. This drives interneurons into depolarization block, while principal neurons fire at a high rate. Excitatory neurons in the ictal core provide synchronous weak drive to distant principal cells and interneurons. Feedback inhibition in these distal networks generates an oscillation that is synchronized with spiking in the ictal core.
In the proposed model, neurons distant from the ictal core receive relatively weaker excitatory input via long-range projections from the ictal core. In this distal region, GABAergic inhibition remains intact and reciprocal local E-I connectivity generates low frequency oscillations. The authors reproduce this scenario in vitro using electrophysiological recordings and optogenetic manipulation in neocortical rodent brain slices. First, they stimulate the slice with extracellular current and record a negligible LFP response 300 µm away. Next, using an optical fiber placed between the stimulus and recording electrodes, they optically stimulate channel rhodopsin-expressing interneurons with a light intensity that drives them into depolarization block. Under these conditions of optically induced disinhibition, the extracellular current injection produces a sustained low-frequency oscillation in the LFP electrode.
The authors simulate both the hyperexcited ictal core and the hypersynchronous global oscillation using phenomenological models of point neurons (ie, models which reproduce the spiking dynamics of simulated neurons but not by comprehensively simulating detailed physiology). Given the lack of anatomical detail available for the neuronal circuits involved, particularly for the human data, such a reduced model seems appropriate. Another strength of such a reduced model is that it leaves open the possibility that alternative mechanisms may underlie the observed dynamics. For example, elevated spiking in the ictal core relies in part on hyperactivity leading to disinhibition. Experiments in the highlighted article show that depolarization block could be the mechanism of this, but it does not preclude the possibility that other mechanisms such as postsynaptic chloride accumulation, extracellular potassium elevation, or rebound excitation following a volley of interneuron spiking could produce a similar effect.
Together these multiscale data sets are unified into a model that could explain seizure core propagation and inform development of novel antiepileptic therapies. Ultimately, it remains unclear whether locally disrupting the ictal spiking wave front is sufficient to terminate a seizure. The human microelectrode recordings highlighted here capture a fraction of a slowly propagating wave front that initiated elsewhere, perhaps by a different mechanism. That being said, regardless of the underlying molecular mechanism, the proposed model provides a testable hypothesis that overly activating interneurons help to sustain the ictal core. It reasonably follows that inhibiting said interneurons would bring them back into a functional dynamic range, which may, in turn, have anticonvulsant effects. While it is currently not possible to test this hypothesis in humans, it could certainly be tested in silico and it would even be feasible to perform closed loop optogenetic inhibition of interneurons in animal models of epilepsy. If preclinical results are promising, translating such a finding to an antiepileptic therapy would require developing an electrical stimulation protocol that preferentially inhibits interneurons in the ictal core to truncate seizures or perhaps developing a pharmacological intervention that reduces the propensity of interneurons to enter depolarization block. The experimental evidence and computational modeling in this study provide an elegant foundation on which to explore such novel therapies.
By Kyle P. Lillis
Footnotes
ORCID iD: Kyle P. Lillis  https://orcid.org/0000-0003-0219-8113
https://orcid.org/0000-0003-0219-8113
References
- 1. Morris G, Leite M, Kullmann DM, Pavlov I, Schorge S, Lignani G. Activity clamp provides insights into paradoxical effects of the anti-seizure drug carbamazepine. J Neurosci. 2017;37(22):5484–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Muldoon SF, Villette V, Tressard T, et al. GABAergic inhibition shapes interictal dynamics in awake epileptic mice. Brain J Neurol. 2015;138(1):2875–2890. [DOI] [PubMed] [Google Scholar]
- 3. Khoshkhoo S, Vogt D, Sohal VS. Dynamic, cell-type-specific roles for GABAergic interneurons in a mouse model of optogenetically inducible seizures. Neuron. 2017;93:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95(2):3948–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmed OJ, Kramer MA, Truccolo W, et al. Inhibitory single neuron control of seizures and epileptic traveling waves in humans. BMC Neurosci. 2014;15:F3. [Google Scholar]
- 6. Magloire V, Cornford J, Lieb A, Kullmann DM, Pavlov I. KCC2 overexpression prevents the paradoxical seizure-promoting action of somatic inhibition. Nat Commun. 2019;10(1):1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bohannon AS, Hablitz JJ. Optogenetic dissection of roles of specific cortical interneuron subtypes in GABAergic network synchronization. J Physiol. 2018;596(5):901–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tryba AK, Merricks EM, Lee S, et al. Role of paroxysmal depolarization in focal seizure activity. J Neurophysiol. 2019;122(5):1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schevon CA, Weiss SA, McKhann G, Jr, et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun. 2012;3:1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eissa TL, Dijkstra K, Brune C, et al. Cross-scale effects of neural interactions during human neocortical seizure activity. Proc Natl Acad Sci U S A. 2017;114(40):10761–10766. [DOI] [PMC free article] [PubMed] [Google Scholar]