Abstract
Crystalized deposits of monosodium urate activate the Nod-like receptor protein 3 (NLRP3) inflammasome, resulting in kidney damage. The present study investigated whether the NLRP3 inflammasome is associated with the progression of hyperuricaemia and gouty nephropathy. Adult male patients were recruited at the Affiliated Baoan Hospital of Shenzhen and divided into three groups of 15 patients each: The control group, the hyperuricaemia group and the gouty nephropathy group. General characteristics and organ function indicators were also measured for each patient. NLRP3, apoptosis-associated speck like protein (ASC) and caspase-1 mRNA and protein expressions in peripheral blood mononuclear cells were detected. The expression of certain downstream inflammatory factors, including interleukin (IL)-1β and IL-18 were also assessed in plasma. The results demonstrated that the concentration of uric acid and creatinine were increased in the hyperuricaemia and gouty nephropathy groups compared with the control group. NLRP3, ASC and caspase-1 mRNA and protein expression, and IL-1β and IL-18 expression were increased in the hyperuricaemia and gouty nephropathy groups compared with the control group. In addition, ASC and caspase-1 mRNA and protein expression, and IL-1β expression were higher in the gouty nephropathy group compared with the hyperuricaemia group. In conclusion, the present results supported the hypothesis that the NLRP3 inflammasome signalling pathway is associated with gouty nephropathy leading to initiation of the inflammatory response and causing renal damage.
Keywords: gouty nephropathy, hyperuricaemia, Nod-like receptor protein 3 inflammasome
Introduction
The role of uric acid in the causation and progression of chronic kidney disease (CKD) has been debated; however, uric acid has been reconsidered as a potential contributory risk factor for the development and progression of CKD over the last 15 years (1-2). Excessive uric acid, usually in the form of monosodium urate (MSU) crystals, precipitates in synovial cavities and other anatomic location to induce severe inflammation and debilitating pain (3-6). In particular, deposits of uric acid in the kidney causes gouty nephropathy, which is the most serious complication of hyperuricemia (3-6). It is considered that the activation of renin-angiotensin, inflammatory factors, endothelial dysfunction and cyclooxygenase-2 (COX-2) serve important roles in gouty nephropathy (5,6). The identified underlying mechanisms of gouty nephropathy have revealed that inflammation has a dominant role in its pathogenesis.
The Nod-like receptor protein 3 (NLRP3) inflammasome is comprised of NLRP3, apoptosis-associated speck like protein (ASC) and caspase-1 and is the most extensively studied inflammasome of recent years. It is involved in certain human inflammatory and autoimmune diseases, including cryopyrin-associated periodic syndrome, ischaemia reperfusion injury and atherosclerosis (7,8). Following detection of cellular stress, NLRP3 oligomerizes by homotypic interactions between NACHT domains. The pyrin domains (PYD) of NLRP3 then becomes exposed for ASC binding. The caspase activation and recruitment domains (CARD) of ASC in turn recruits pro-caspase-1 through CARD-CARD interactions (9). Following NLRP3 inflammasome formation, procaspase-1 is converted to active caspase-1, which regulates the maturation of proinflammatory cytokines, including interleukin (IL)-1β and IL-18(10), further aggravating renal damage.
MSU crystals cause the formation of inflammasomes in vivo (11). In addition, the inflammatory effect of MSU crystals is primarily mediated by NLRP3 inflammasomes driving the production of IL-1β and IL-18. IL-1β is likely the main agent that triggers systemic inflammation (3). Therefore, these observations prompted the present study to assess the role of the NLRP3 inflammasome in the mediation of the innate immune inflammatory response to MSU crystal deposition with regards to gouty nephropathy. The present study investigated the role of the NLRP3 inflammasome signalling pathway with the progression of hyperuricemia and gouty nephropathy, the results of which may provide a novel theoretical basis and therapeutic target for the early prevention and treatment of gouty nephropathy.
Materials and methods
Study subjects
A total of 45 male patients (18-70 years old) were recruited at the People's Hospital of Shenzhen Baoan between July 2016 and December 2017. According to the inclusion and exclusion criteria, these patients were divided into three groups (n=15): The control group, the hyperuricaemia group and the gouty nephropathy group. The present study was approved by the Ethics Committee of the Affiliated Bao'an Hospital of Shenzhen (approval no. BYL2016001). Written informed consent was obtained from all participants.
Inclusion criteria
Patients in the control group received a health examination. There were no abnormalities in the laboratory indicators of the selected subjects and patients had no history of cardiovascular disease or liver disease (including diabetes and gout). Patients also had no presence of infection or autoimmune disease. Hyperuricaemia was defined as levels of serum uric acid >6-7 mg/dl (12). The diagnosis of gouty nephropathy was based on the diagnosis of primary gout (13), with one or more of the following parameters: Urinary protein >150 mg/dl; urine white blood cells >5/high power field (HPF); urine red blood cells >3/high power field; serum creatinine >115 µmol/l; blood uric acid/creatinine ratio >2.5; ultrasound or ureterography revealing renal calculus and kidney shrinkage. All of the aforementioned cases excluded urinary tract infections and other diseases such as cancer.
Exclusion criteria
Exclusion criteria was based on previous literature (14) and was as follows: female; <18 years old or >70 years old; patients with secondary hyperuricaemia or stage 4-5 chronic kidney disease; acute hyperuricaemia and the presence of acute renal function deterioration factors; patients with severe cardiovascular disease, liver and kidney disease, lung disease, fractures, tumors, infectious and autoimmune disease, and mental illness; diseases that may affect NLRP3 inflammasome signalling pathways; patients who had been using uric acid drugs outside the hospital or had been treated with lipid-lowering drugs or anti-inflammatory and anti-oxidative drugs during the 4 weeks prior to admission.
Detection of organ function indicators
Biochemical serum and urine samples were obtained following 8 h fasting. A total of 15 ml serum sample was collected from each patient and shipped to the Laboratory Services at the Affiliated Bao'an Hospital of Shenzhen (Guangdong, China) for biochemical analysis, which was obtained by centrifugation at 500 x g for 10 min at 4˚C. Urinary biochemical parameters were measured from the patients' first morning urine sample. Other standard parameters were measured including blood and urine analysis. The parameters tested for blood included white blood cell (WBC), red blood cell (RBC), hemoglobin (HB), platelets (PLT), total cholesterol (TC), total triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), total protein (TP), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (Cr), uric acid (UC), blood urea nitrogen (BUN), prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT) and fibrinogen (FIB). Other parameters tested for urine were urine leukocytes, urine erythrocytes and urine protein.
Isolation and culture of peripheral blood mononuclear cells
Centrifugation (speed, 1,600 x g; duration, 10 min; temperature, 4˚C) was performed on blood samples in an EDTA tube to obtain the buffy coat. Samples were stored at -80˚C for future use. Peripheral blood mononuclear cells were isolated with Ficoll-Paque Plus (GE Healthcare). Monocytes were isolated by magnetic bead negative selection according to the instructions of Dynabeads® Untouched™ Human Monocytes kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Isolated monocyte lysates were separated from which 5 µl was extracted for viability verification. Trypan Blue staining confirmed that the viability of the cells was >95%. Cell concentration was then adjusted to 5x105 cells/ml using a hemocytometer using the following formula: Number of cells/ml = number of cells counted in 100 small grids/100x400x10,000x dilution factor. The total number of monocytes was counted to be 34.311±19.912x105. A third of which was used for RNA extraction, whilst the rest were used for western blotting. The number of monocytes for RNA extraction and western blot analysis were divided 1:2. A total of 200 µl TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) was added to monocytes for RNA extraction, whereas 30-50 µl PBS (Invitrogen; Thermo Fisher Scientific, Inc.) was added for western blotting experiments.
Reverse transcription-quantitative PCR (RT-qPCR) to determine
NLRP3, ASC and caspase-1 mRNA expression in peripheral blood mononuclear cells. Total RNA was extracted from monocytes using TRIzol solution (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. RNA concentration and purity were assessed via electrophoresis and a UV spectrophotometer (Thermo Fisher Scientific, Inc.), respectively. RNA was reverse transcribed to cDNA following the protocol provided by the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). cDNA was stored at -20˚C prior to qPCR amplification using the TransStart® Tip Green qPCR Supermix 2X kit (TransGen Biotech Co., Ltd.), using the SYBR-Green I fluorophore, according to manufacturer's protocol. All qPCR reactions were performed in an Applied Biosystems 7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using the following thermocycling conditions: Initial denaturation at 95˚C for 30 sec, followed by 40 cycles of 95˚C for 5 sec and 60˚C for 30 sec. Finally, the melting curve was generated (dissociation, 60˚C at 30 sec and 95˚C at 15 sec) to determine normality. Gene expression was calculated using the 2-ΔΔCq method (15). β-actin was used as an internal reference for the mRNA levels of NLRP3, ASC and caspase-1 detected. The primer sequences used for qPCR are presented in Table I.
Table I.
Primer sequences.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| β-actin | 5'-AACCGCGAGAAGATGACCCAGAT-3' | 5'-GGATAGCACAGCCTGGATAGCA-3' |
| NLRP3 | 5'-ATGGGTTTACTGGAGTACCTTTC-3' | 5'-CTGTCTTCAATGCACTGGAATCTG-3' |
| ASC | 5'-GATGCTCTGTACGGGAAGGTC-3' | 5'-TCCAGTTCCAGGCTGGTGT-3' |
| Caspase-1 | 5'-GGAAGACTCATTGAACATATGCAAG-3' | 5'-CTTGTCAAAGTCACTCTTTCAGTG-3' |
NLRP3, nucleotide-binding domain and leucine-rich repeat protein 3; ASC, apoptosis-associated speck like protein.
Western blot analysis to determine
NLRP3, ASC and caspase-1 protein expression in peripheral blood mononuclear cells. Cells were lysed using ice-cold RIPA lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.) and protein concentration was quantified using Bicinchoninic Acid Assay kit (Thermo Fisher Scientific, Inc.). Protein samples (10 µg) were separated by 8, 10 or 12% SDS-PAGE before being transferred to PVDF membranes. The membranes were then blocked with 5% milk dissolved in TBS supplemented with 1% Tween-20 at room temperature for 1 h, prior to incubation with primary antibodies against NLRP (1:300; cat. no. sc-134306; Santa Cruz Biotechnology, Inc.), caspase-1 (1:300; cat. no. sc-622; Santa Cruz Biotechnology, Inc.), ASC (1:300; cat. no. sc-514414; Santa Cruz Biotechnology, Inc.) or GAPDH (1:1,000; cat. no. A01020; Abbkine, Inc.) diluted in PBS supplemented with 0.1% Tween-20 (PBST) and 1% bovine serum albumin [Sangon Biotech (Shanghai) Co., Ltd.] at 4˚C overnight. Subsequently, the PVDF membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse (1:1,000; cat.no. A0216; Beyotime Institute of Biotechnology) or goat anti-rabbit (1:10,000; cat. no. A21020-1; Abbkine, Inc.) secondary antibodies diluted in PBST supplemented with 5% milk at room temperature for 1 h with agitation. The proteins were then visualized using Pierce™ ECL Western Blotting Substrate (Thermo Fisher Scientific, Inc.) and all bands were analysed using Gel Doc™ XR+ Gel Documentation system (Bio-Rad Laboratories, Inc.) to calculate the densitometric values.
ELISA for the determination of IL-1β and IL-18 levels in plasma
IL-1β (cat. no. 70-EK101B2) and IL-18 (cat. no. 70-EK1182) ELISA kits [Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.] were used to determine the concentration of IL-1β and IL-18 in 2 ml total plasma, according to manufacturer's protocol.
Statistical analysis
Each experiment was repeated three times. All data were analysed using SPSS v.17.0 (SPSS, Inc.) software and expressed as the mean ± standard deviation. One-way analysis of variance was performed to compare the mean of multiple groups and a least significant difference post hoc test was used. P<0.05 was considered to indicate a statistically significant difference.
Results
Kidney function is damaged in hyperuricaemia and gouty nephropathy groups
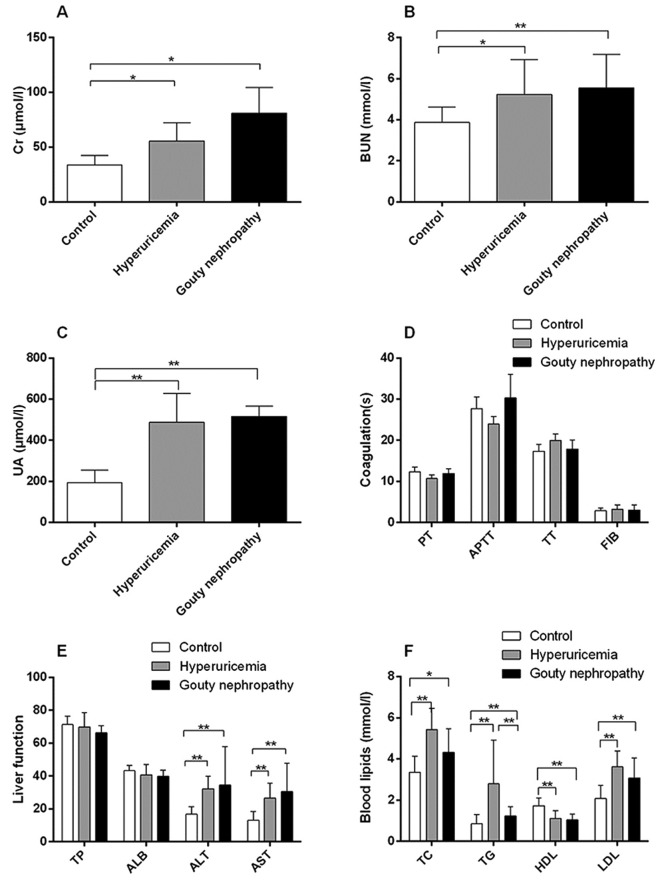
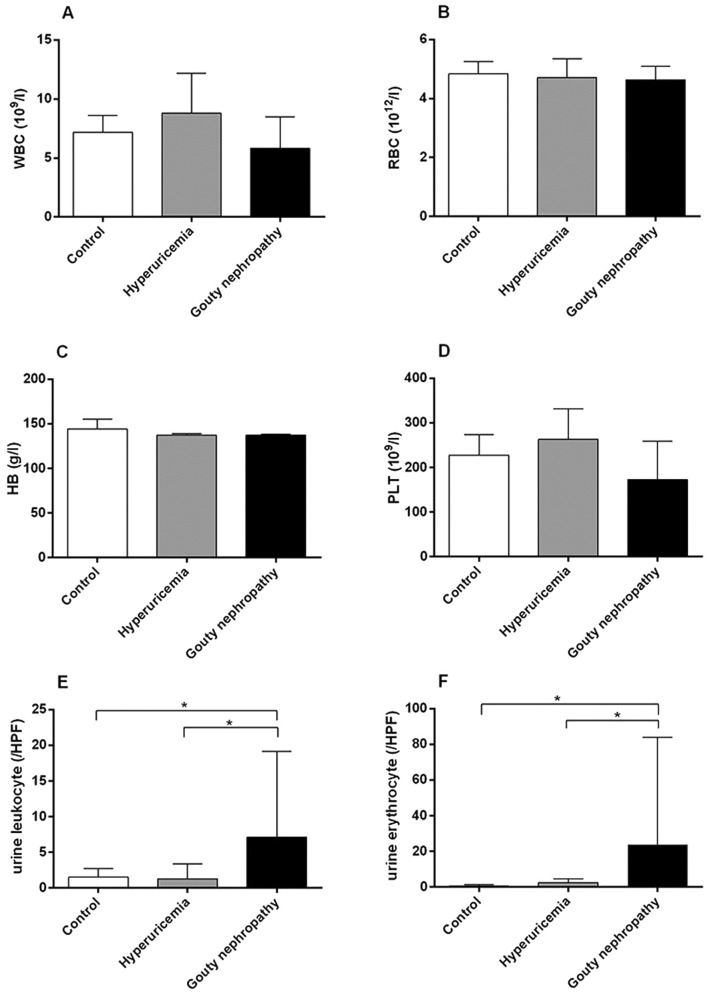
Patient general characteristics are provided in Table II, Figs. 1 and 2. The expression of uric acid (Fig. 1C), blood urea nitrogen (Fig. 1B) and blood lipids (Fig. 1F) including total cholesterol, total triglycerides and low-density lipoprotein was higher in the hyperuricaemia and gouty nephropathy groups compared with the control group, but the level of high-density lipoprotein exhibited the opposite trend. Levels of urine leukocytes and erythrocytes were higher in the gouty nephropathy group compared with both the control and hyperuricemia groups (Fig. 2E-F). Serum creatinine (Fig. 1A), uric acid (Fig. 1C), blood urea nitrogen (Fig. 1B) were greater in the gouty nephropathy group and hyperuricaemia group compared with the control group. Serum creatinine and urine protein are common indicators of renal function and used to monitor the progression of chronic kidney disease (16). These results demonstrated that the kidney had been further damaged in the progression from hyperuricaemia to gouty nephropathy.
Table II.
General characteristics of enrolled patients.
| Characteristics | Control (n=15) | Hyperuricemia (n=15) | Gouty nephropathy (n=15) |
|---|---|---|---|
| Age (years) | 45.20±10.19 | 48.27±14.91 | 48.3±11.5 |
| Height (m) | 1.72±0.06 | 1.70±0.05 | 1.66±0.05 |
| Body weight (kg) | 72.25±6.97 | 69.98±12.08 | 61.39±6.83 |
| BMI (kg/m2) | 24.28±1.53 | 24.38±4.53 | 22.19±2.43 |
| SBP (mmHg) | 116.00±7.61 | 115.93±9.83 | 121.33±10.87 |
| DBP (mmHg) | 79.27±7.98 | 76.47±7.60 | 74.80±8.78 |
| FBG (mmol/l) | 4.73±0.45 | 5.17±0.70 | 4.77±0.39 |
Values are presented as the mean ± standard deviation. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic pressure; FBG, fasting blood-glucose.
Figure 1.
Organ function indicators of enrolled patients, including renal and liver function, coagulation and blood lipids. (A) Levels of Cr, (B) BUN, (C) UA, (D) coagulation functions, (E) liver function and (F) blood lipids of patients in the control, hyperuricemia and gouty nephropathy groups. *P<0.05 and **P<0.01, with comparisons indicated by lines. Cr, creatinine; BUN, blood urea nitrogen; UA, uric acid; PT, prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time; FIB, fibrinogen; TP, total protein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TC, total cholesterol; TG, total triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein.
Figure 2.
Organ function indicators of enrolled patients, including blood and urine analysis. (A) WBC, (B) RBC, (C) HB, (D) PLT, (E) urine leukocytes and (F) urine erythrocytes levels of patients in the control, hyperuricemia and gouty nephropathy groups. *P<0.05, with comparisons indicated by lines. WBC, white blood cell; RBC, red blood cell; HB, hemoglobin; PLT, platelets; HPF, high power field.
Successful peripheral blood mononuclear cell isolation
Peripheral blood mononuclear cells were isolated from the peripheral blood of 45 patients for in vitro experiments. Trypan blue staining (Fig. 3) confirmed that >95% were viable cells (Table III).
Figure 3.
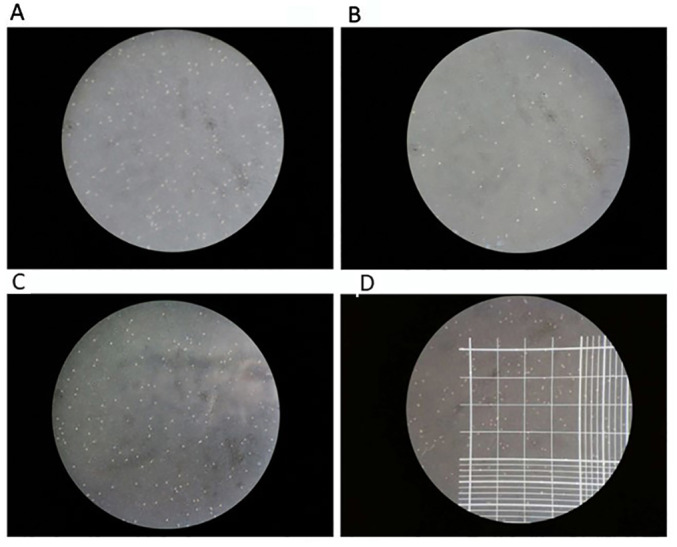
Representative images of trypan blue staining of peripheral blood mononuclear cells (magnification, x10). Trypan Blue staining test was used to confirmed the cell viability, followed by the counting of cells. Trypan blue staining can stain dead cells blue, whilst the live cells are not colored. (A) Densely distributed colorless transparent particles under the microscope, representing live cells. (B) Scattered distribution of colorless transparent particles, showing fewer living cells. (C) Densely distributed colorless transparent particles, representing live cells. (D) Cell counting schematic. The area of every grid is 1.0 mm2 and the volume is 0.1 µl. Cells in the double-lined area were counted as red blood cells, whereas cells in the single-lined area counted as white blood cells. Magnification, x10.
Table III.
Peripheral blood mononuclear cells count.
| Group | n | Count (105) |
|---|---|---|
| Control | 15 | 33.047±22.755 |
| Hyperuricemia | 15 | 43.627±18.317 |
| Gouty nephropathy | 15 | 26.567±13.506 |
NLRP3, ASC and caspase-1 mRNA expression are increased in the hyperuricaemia and gouty nephropathy groups
NLRP3, ASC and caspase-1 mRNA levels were significantly increased in the hyperuricaemia and gouty nephropathy groups compared with the control group (Table IV). Compared with the hyperuricaemia group, the gouty nephropathy group had a significantly higher expression of ASC and caspase-1 mRNA (Table IV). The mRNA expression in peripheral blood mononuclear cells demonstrated that the NLRP3 inflammasome may serve a pivotal role in gouty nephropathy.
Table IV.
Expression of NLRP3, ASC and caspase-1 mRNA in peripheral blood mononuclear cells.
| Group | n | NLRP3 | ASC | Caspase-1 |
|---|---|---|---|---|
| Control | 15 | 23.54±1.19 | 25.88±1.18 | 26.99±1.43 |
| Hyperuricemia | 15 | 26.05±1.89a | 27.49±3.56a | 28.05±2.41a |
| Gouty nephropathy | 15 | 25.43±1.66a | 32.21±3.01a,c | 30.80±2.80a,b |
Values are presented as the mean ± standard deviation.
aP<0.01 vs. the control group;
bP<0.05 vs. the hyperuricemia group;
cP<0.01 vs. the hyperuricemia group; NLRP3, nucleotide-binding domain and leucine-rich repeat protein 3; ASC, apoptosis-associated speck like protein.
Expression of NLRP3, ASC, caspase-1 protein increases in the hyperuricaemia and gouty nephropathy group
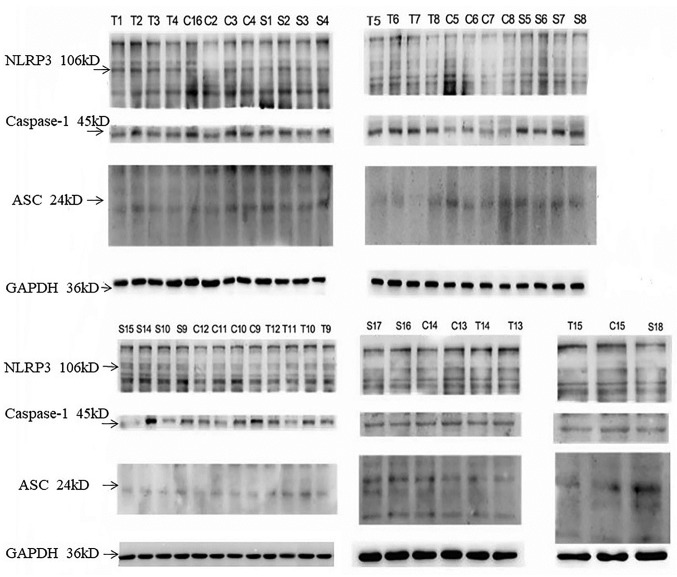
Gouty nephropathy group had a significantly higher expression of NLRP3, ASC and caspase-1 protein compared with the control group (Table V). The expression of ASC and caspase-1 protein was higher in the gouty nephropathy group compared with the hyperuricaemia group (Table V; Fig. 4). Peripheral blood mononuclear cell protein expression (Fig. 4; Table V) also corresponded with mRNA expression (Table IV), indicating the important role of the NLRP3 inflammasome in gouty nephropathy.
Table V.
Expression of NLRP3, ASC, caspase-1 protein in peripheral blood mononuclear cells.
| Group | n | NLRP3/GAPDH | ASC/GAPDH | Caspase-1/GAPDH |
|---|---|---|---|---|
| Control | 15 | 0.45±0.18 | 0.22±0.08 | 0.57±0.16 |
| Hyperuricemia | 15 | 0.55±0.12 | 0.26±0.11a | 0.57±0.16 |
| Gouty nephropathy | 15 | 0.60±0.16b | 0.47±0.19a,c | 0.78±0.15b,c |
Values are presented as the mean ± standard deviation, GAPDH was used as an internal reference.
aP<0.05 vs. the control group;
bP<0.01 vs. the control group;
cP<0.05 vs. the hyperuricemia group. NLRP3, nucleotide-binding domain and leucine-rich repeat protein 3; ASC, apoptosis-associated speck like protein.
Figure 4.
Expression of NLRP3, ASC and caspase-1 protein in peripheral blood mononuclear cells. Representative western blots of peripheral blood mononuclear cells isolated from control, hyperuricemia and gouty nephropathy patients. NLRP3, Nod-like receptor protein 3; ASC, apoptosis-associated speck like protein; C, control group; T, hyperuricemia group; S, gouty nephropathy group.
Expression of IL-1β and IL-18 is increased in the hyperuricaemia and gouty nephropathy group
IL-1β and IL-18 levels were significantly higher in the hyperuricaemia and gouty nephropathy groups compared with the control group (Table VI). IL-1β expression was significantly elevated in the gouty nephropathy group compared with the hyperuricaemia group (Table VI). The increased expression of inflammatory factors demonstrated that the NLRP3 inflammasome was associated with gouty nephropathy.
Table VI.
Expression of IL-1β and IL-18 in plasma of patients.
| Group | n | IL-1β (pg/ml) | IL-18 (pg/ml) |
|---|---|---|---|
| Control | 15 | 28.41±14.05 | 323.35±96.16 |
| Hyperuricemia | 15 | 55.19±16.79a | 718.16±211.42a |
| Gouty nephropathy | 15 | 81.20±17.63a,b | 870.67±371.13a |
Values are mean ± standard deviation.
aP<0.01 vs. control group;
bP<0.01 vs. hyperuricemia group. IL-1β, interleukin-1β; IL-18, interleukin-18.
Discussion
Uric acid is involved in the pathogenesis of kidney disease. Increasing evidence supports uric acid as a cause or exacerbating factor for kidney fibrosis and progressive CKD with an elevated serum uric acid level independently predicting the development of CKD (17,18). Elevated uric acid levels induce oxidative stress and endothelial dysfunction, resulting in the development of both systemic and glomerular hypertension (19,20). It is well established that uric acid forms urate crystals in sufficient concentrations to block renal collecting tubes, which causes interstitial nephritis, gouty nephropathy and interstitial fibrosis (21). Furthermore, the kidney damage caused by uric acid is the result of this sort of obstruction and crucially, it can be exacerbated by the inflammatory response that it initiates. Uric acid activates cytoplasmic phospholipase A2 and inflammatory transcription factor NF-κB, which increases the production of various systemic cytokines, including tumour necrosis factor-α, kidney monocyte chemotactic protein 1 and blood vessel COX-2 (22,23). In addition, hyperuricaemia aggravates kidney injury by recruiting IL-1β-secreting macrophages, activating NLRP3 inflammasomes in macrophages and promoting chemokine secretion in proximal tubular cells (24). Uric acid, in its soluble form, is responsible for increasing the production of IL-1β in an NLRP3-dependent manner and is associated with kidney damage (25).
The inflammatory response is closely associated with the innate immune system. Intracellular nucleotide-binding oligomerization domain-like receptors form a group of pattern recognition receptors that are involved in a wide variety of innate host immune responses when stimulated by pathogen-associated molecular patterns dangerous-associated molecular patterns (DAMPs) (26). One of the most thoroughly investigated members is the intracellular pattern recognition receptor NLRP3 inflammasome complex consisting of NLRP3, ASC and caspase-1(27). Upon activation, NLRP3 oligomerizes via homotypic interactions between NACHT domains and raises ASC and caspase to form an inflammatory complex, resulting in activated caspase-1. Activated caspase-1 cleaves intracellular IL-1β and IL-18 precursors to form mature IL-1β and IL-18, which are secreted into the extracellular matrix (Fig. 5) (28). The inflammasome is understood to have a fundamental role in the progression of autoinflammatory diseases and to have additional roles in infection control, the progression of immune pathologies and the recognition of tissue damage. NLRP3 inflammasome is activated in response to a variety of infectious stimuli or by cellular stress caused by various sterile danger signals, including high concentrations of extracellular ATP, a decrease in extracellular osmolality or pH, crystals of MSU or cholesterol and the degradation of extracellular matrix components (27).
Figure 5.
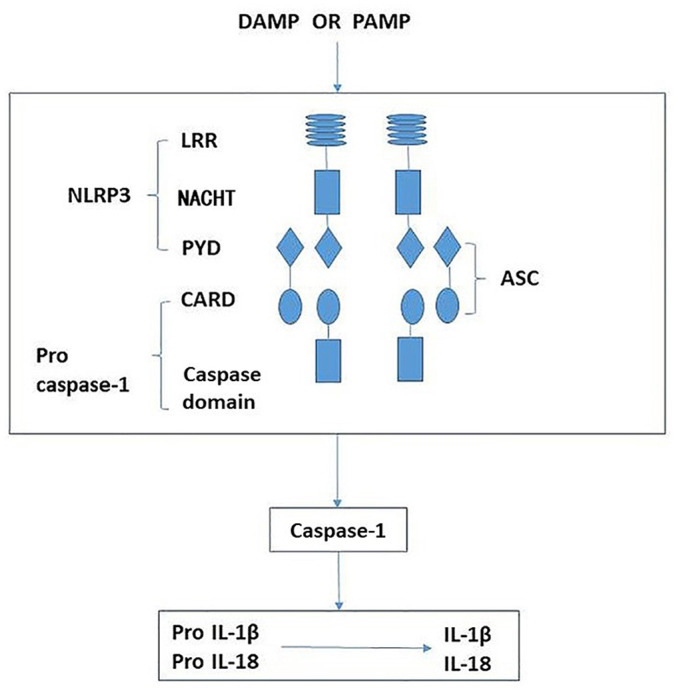
Schematic demonstrating the activation of NLRP3 leading to release of proinflammatory factors. The NLRP3 inflammasome is comprised of NLRP3, ASC and caspase-1. When sensing danger signals, including PAMP and DAMP, NLRP3 oligomerizes via homotypic interactions between NACHT domains. The PYD of NLRP3 is then exposed for interaction with the PYD of ASC. The CARD of ASC in turn recruits pro-caspase-1 through CARD-CARD interactions, resulting in activated caspase-1. Activated caspase-1 then cleaves intracellular IL-1β and IL-18 precursors to form mature IL-1β and IL-18, which are secreted into the extracellular matrix. NLRP3, nucleotide-binding domain and leucine-rich repeat protein 3; ASC, apoptosis-associated speck like protein; LRR, leucine-rich repeat; NACHT, nucleotide-binding and oligomerization domain; PYD, pyrin domain; CARD, caspase recruitment domain; IL-1β, interleukin-1β; IL-18, interleukin-18; PAMP, pattern-associated molecular pattern; DAMP, danger-associated molecular pattern.
In the present study, the concentration of uric acid and creatinine as well as the expression of mRNA and protein in NLRP3 inflammasomes including NLRP3 mRNA, ASC mRNA, caspase-1 mRNA and ASC protein levels increased in the hyperuricaemia and gouty nephropathy groups compared with the control group. Importantly, the elevated levels of IL-1β and IL-18 indicated that NLRP3 inflammasomes were activated in hyperuricaemia and gouty nephropathy. The expression of ASC and caspase-1 mRNA and protein was higher in the gouty nephropathy group than in the hyperuricaemia group with the level of IL-1β also being significantly increased. The results indicated that the NLRP3 inflammasome was activated and induced the polymerization of the adaptor molecule ASC, leading to the production of IL-1β. Taken together, these results indirectly indicated that the NLRP3 inflammasome has a pivotal role in the progression of gouty nephropathy.
Although the specific activation mechanism of the NLRP3 inflammasome is unclear, recent studies have proposed three hypotheses regarding the activation of NLRP3 inflammasomes: Firstly, all bacteria, viruses, particles or crystals can stimulate cells to produce reactive oxygen species, which can activate the NLRP3 inflammasome (29). In the course of oxidative stress, thioredoxin, following its own oxidation, releases thioredoxin-interacting protein, activating the NLRP3 inflammasome (30). Secondly, crystals or particles, such as MSU, silicon dioxide, amyloid and cholesterol crystals, are phagocytosed by macrophages into lysosomes. This accumulation causes lysosomal rupture and the release of cathepsin B and other proteases, inducing the activation of the NLRP3 inflammasome (31,32) Finally, stimulation induces cells to release ATP on the cell surface of the P2X7 purine receptor, triggering the potassium ion outflow mechanism and calcium wave transport mechanism leading to mitochondrial damage. This releases mitochondrial DNA (mtDNA) and activates the NLRP3 inflammasome (33,34). Previous studies have demonstrated that NLRP3 can identify uric acid crystals and other dangerous signals (3,35). Uric acid crystals trigger IL-1β-mediated inflammation by activating the NLRP3 inflammasome via the aforementioned three mechanisms (36).
The present study hypothesized that MSU acts as an endogenous DAMP, contributing to the progression of the NLRP3 inflammasome in hyperuricaemia and gouty nephropathy. When high levels of uric acid in the body cause uric acid crystals to be deposited in tissues, macrophages directly phagocytose urate crystals, which are too large to be efficiently cleared and are likely to induce the production of ROS on their way to becoming lysosomes (9). Following the phagocytosis of MSU, macrophages form phagocytic bodies and the subsequent intracellular rupture of these bodies releases cathepsin B, which activates the NLRP3 inflammasome (37). MSU also activates the NLRP3 inflammasome by causing intracellular potassium efflux (38), leading to mitochondrial damage. Furthermore, by releasing mtDNA, the maturation and release of IL-1β and IL-18 is stimulated. A previous study determined that uric acid activates NLRP3 inflammasomes via mitochondrial ROS production and their results demonstrated the direct role of hyperuricaemia in activating NLRP3 inflammasomes in macrophages (39). Berberine may influence the NLRP3 inflammasome and be involved in MSU crystal-induced innate immune responses, attenuating the expression of NLRP3, further inhibiting the downstream signalling of molecular IL-1β and eventually having a role in the treatment of gouty arthritis (40). The present study observed that gouty nephropathy is associated with elevated levels of uric acid in serum and high expression of NLRP3, ASC and caspase-1 mRNA and protein, as well as IL-1β and IL-18 expression. The present results indicated that uric acid-induced NLRP3 inflammasomes are associated with gouty nephropathy with the underlying mechanism potentially involving one of the aforementioned three hypotheses.
The present study has limitations as the exact association between NLRP3 inflammasomes and gouty nephropathy was not elucidated. Further studies are therefore required to explore the role of the NLRP3 inflammasome in humans and in vivo. In addition to the need for large clinical trials, more studies are required to better understand the biology of uric acid. To the best of our knowledge, clinical study into the regulation mechanism of the NLRP3 inflammasome signalling pathway with regards to gouty nephropathy has not been performed.
In conclusion, the present study demonstrated that the NLRP3 inflammasome was associated with the progression of hyperuricaemia and gouty nephropathy, leading to the inflammatory response and renal damage. Although the precise mechanism of the NLRP3 inflammasome signalling pathway and the means by which MSU triggers the inflammasome remains a matter of debate, the importance of the NLRP3 inflammasome in gouty nephropathy has been established. The present study proposes that future therapeutic strategies for gouty nephropathy should be based on blocking uric acid or inhibiting its activation of the NLRP3 inflammasome. Further studies will be required to explore the mechanism of how uric acid activates the NLRP3 inflammasome to allow for the early diagnosis of gouty nephropathy.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the Shenzhen Science and Technology Research and Development Fundamental Research Project (grant no. JCYJ20160427191440905).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YZZ and XLS designed the experiments, analyzed the data and wrote the manuscript. YPX and YZZ performed the experiments. FJG and ASZ analyzed and interpreted the data. JHC contributed to the design of the experimental methods, wrote the manuscript and approved the version to be published and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Affiliated Bao'an Hospital of Shenzhen (approval no. BYL2016001) and written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, Jo I, Johnson RJ, Kang DH. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol. 2013;304(F471-F480) doi: 10.1152/ajprenal.00560.2012. [DOI] [PubMed] [Google Scholar]
- 2.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Ghaemi-Oskouie F, Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr Rheumatol Rep. 2011;13:160–166. doi: 10.1007/s11926-011-0162-1. [J] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol. 2007;3:443–449. doi: 10.1038/ncprheum0556. [DOI] [PubMed] [Google Scholar]
- 5.Kim IY, Lee DW, Lee SB, Kwak IS. The role of uric acid in kidney fibrosis: Experimental evidences for the causal relationship. BioMed Res Int. 2014;2014(638732) doi: 10.1155/2014/638732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito I, Saruta T, Kondo K, Nakamura R, Oguro T, Yamagami K, Ozawa Y, Kato E. Serum uric acid and the renin-angiotensin system in hypertension. J Am Geriatr Soc. 1978;26:241–247. doi: 10.1111/j.1532-5415.1978.tb02396.x. [DOI] [PubMed] [Google Scholar]
- 7.Gao L, Shen F, Lii YS. Effects of NLRP3 inflammasome on cerebral ischemia reperfusion injury. J Shanghai Jiaotong University Medical Science. 2015;35:1896–1899. doi: 10.1155/2018/9163521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 10.Zheng SC, Zhu XX, Xue Y, Zhang LH, Zou HJ, Qiu JH, Liu Q. Role of the NLRP3 inflammasome in the transient release of IL-1β induced by monosodium urate crystals in human fibroblast-like synoviocytes. J Inflamm (Lond) 2015;12(30) doi: 10.1186/s12950-015-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiltz C, Lioté F, Prudhommeaux F, Meunier A, Champy R, Callebert J, Bardin T. Monosodium urate monohydrate crystal-induced inflammation in vivo: Quantitative histomorphometric analysis of cellular events. Arthritis Rheum. 2002;46:1643–1650. doi: 10.1002/art.10326. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Hu J, Song N, Chen R, Zhang T, Ding X. Hyperuricemia increases the risk of acute kidney injury: A systematic review and meta-analysis. BMC Nephrol. 2017;18:27–41. doi: 10.1186/s12882-016-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XX, Sun WF, Hou Y, Liu YW, He Y. Clinical observation on fufang tufuling keli treating 40 cases of hyperuricemia: a randomize-controlled study. J Tradit Chin Med. 2016;57:41–45. [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 17.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric Acid and incident kidney disease in thy community. J Am soc Nephriol. 2008;19:1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, Franco M, Rodríguez-Iturbe B, Johnson RJ. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol. 2008;295(F1134-F1141) doi: 10.1152/ajprenal.00104.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 21.Liu LQ. Pathogenesis of hyperuric acid nephropathy. J Clin Nephrol. 2011;11:53–55. [Google Scholar]
- 22.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [J] [DOI] [PubMed] [Google Scholar]
- 23.Prasad Sah OS, Qing YX. Associations between hyperuricemia and chronic kidney disease: A Review. Nephrourol Mon. 2015;7(e27233) doi: 10.5812/numonthly.7(3)2015.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gul A, Zager P. Does altered uric acid metabolism contribute to diabetic kidney disease pathophysiology? Curr Diab Rep. 2018;18(18) doi: 10.1007/s11892-018-0985-5. [DOI] [PubMed] [Google Scholar]
- 25.Braga TT, Forni MF, Correa-Costa M, Ramos RN, Barbuto JA, Branco P, Castoldi A, Hiyane MI, Davanso MR, Latz E, et al. Soluble uric acid activates the NLRP3 inflammasome. Sci Rep. 2017;7(39884) doi: 10.1038/srep39884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isaka Y, Takabatake Y, Takahashi A, Saitoh T, Yoshimori T. Hyperuricemia-induced inflammasome and kidney diseases. Nephrol Dial Transplant. 2016;31:890–896. doi: 10.1093/ndt/gfv024. [DOI] [PubMed] [Google Scholar]
- 27.Baroja-Mazo A, Martín-Sánchez F, Gomez AI, Martínez CM, Amores-Iniesta J, Compan V, Barberà-Cremades M, Yagüe J, Ruiz-Ortiz E, Antón J, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014;15:738–748. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- 28.Xia M, Boini KM, Abais JM, Xu M, Zhang Y, Li PL. Endothelial NLRP3 inflammasome activation and enhanced neointima formation in mice by adipokine visfatin. Am J Pathol. 2014;184:1617–1628. doi: 10.1016/j.ajpath.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 30.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 31.Gasse P, Riteau N, Charron S, Girre S, Fick L, Pétrilli V, Tschopp J, Lagente V, Quesniaux VF, Ryffel B, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 32.Jiang H, Yan YQ, Jiang W, Zhou RB. NLRP3 inflammasome: Activation, regulation, and role in diseases. Sci Sin Vitae. 2017;47:125–131. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1016/j.molimm.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Jin-Feng LI, Xie D, Ping-Ping HE, Tang YY, Yu-Lin TU, Yin K. Research advances of the NLRP3 inflammasome and metabolic disease. Prog Biochem Biophys. 2014;41:425–434. doi: 10.3724/SP.J.1206.2013.00166. [DOI] [Google Scholar]
- 35.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 36.Xu LL. NLRP3 inflammasomes and kidney diseases. J Nephrol Dialy Transpl. 2011;20:554–558. doi: 10.1111/nep.12785. [DOI] [PubMed] [Google Scholar]
- 37.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stutz A, Golenbock DT, Latz E. Inflammasomes: Too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SM, Lee SH, Kim YG, Kim S-Y, Seo JW, Choi YW, Kim DJ, Jeong KH, Lee TW, Ihm CG, et al. Hyperuricemia-induced NLRP3 activation of macrophage contributes to the progression of diabetic nephropathy. Am J Physiol-renal. 2015;308:993–1003. doi: 10.1152/ajprenal.00637.2014. [DOI] [PubMed] [Google Scholar]
- 40.Liu YF, Wen CY, Chen Z, Wang Y, Huang Y, Tu SH. Effects of berberine on NLRP3 and IL-1β expressions in monocytic THP-1 cells with monosodium urate crystals-induced inflammation. Biomed Res Int. 2016;2016(2503703) doi: 10.1155/2016/2503703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.