Abstract
MicroRNAs (miRNAs/miRs) serve a key role in regulating the cell cycle and inducing tumorigenesis. Subgroup J of the avian leukosis virus (ALV-J) belongs to the family Retroviridae, subfamily Orthoretrovirinae and genus Alpharetrovirus that causes tumors in susceptible chickens. gga-miR-375 is downregulated and Yes-associated protein 1 (YAP1) is upregulated in ALV-J-induced tumors in the livers of chickens, and it has been further identified that YAP1 is the direct target gene of gga-miR-375. In the present study, it was found that ALV-J infection promoted the cell cycle and proliferation in DF-1 cells. As the cell cycle and cell proliferation are closely associated with tumorigenesis, further experiments were performed to determine whether gga-miR-375 and YAP1 were involved in these cellular processes. It was demonstrated that gga-miR-375 significantly inhibited the cell cycle by inhibiting G1 to S/G2 stage transition and decreasing cell proliferation, while YAP1 significantly promoted the cell cycle and proliferation. Furthermore, these cellular processes in DF-1 cells were affected by gga-miR-375 through the targeting of YAP1. Collectively, the present results suggested that gga-miR-375, downregulated by ALV-J infection, negatively regulated the cell cycle and proliferation via the targeting of YAP1.
Keywords: gga-microRNA-375, Yes-associated protein 1, cell cycle, cell proliferation, avian leukosis virus
Introduction
MicroRNAs (miRNAs/miRs), which are composed of 19-23 nucleotides, are a class of small, non-coding RNAs that serve a key role in regulating various biological processes, including tumor formation. miRNAs also modulate a variety of cellular processes, including the cell cycle and proliferation by attaching to the corresponding target binding sites at the 3' untranslated region on their respective mRNA (1-4). Moreover, miR-375 can regulate insulin secretion and is regarded as a pancreatic islet-specific miRNA (5). Previous studies have also demonstrated that the expression of miR-375 is enriched in different organs, while its expression is downregulated in numerous malignant cancer types, including gastric and esophageal cancer, hepatocellular carcinoma and head and neck cancer (6-9); hence, miR-375 is known to be an important cancer-related miRNA (10,11).
Avian leukemia is a general term used to describe various types of tumor diseases in poultry that are caused by the avian leukemia virus (ALV) and avian sarcoma virus (12). Avian leukemia was first identified in 1998 and can cause several infectious benign and malignant tumors, thus leading to large economic loss in the poultry industry (13-15). Until recently, there has been no effective vaccine to treat the disease. ALVs are classified into seven subgroups, referred to as A-E, J and K (16). Among these seven subgroups, ALV-J is the most prevalent and causes more damage to chickens compared with the other subgroups (17). Furthermore, ALV-J infection causes immunosuppression and various forms of myeloid leukosis and, to a lesser extent, solid tumors, such as hemangiomas and histiocytic sarcomas (12). Tumor hepatocyte gene chip screening in a previous study revealed that the expression of gga-miR-375 was significantly decreased in ALV-J-induced liver tumors in chickens (18).
The Hippo signaling pathway is a tumor suppressor pathway that regulates cell cycle distribution, cell proliferation, stem cell functions and organ size (18,19). The effectors of the Hippo signaling pathway, Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) serve a key role in tumorigenesis, and recent preclinical data support the development of novel Hippo pathway-based targeted therapies to prevent and treat malignancies using pharmacological inhibition of YAP and TAZ activity (20,21). YAP1 was originally identified as a factor that can control tissue growth and organ size in Drosophila melanogaster, and it is a key component of the Hippo signaling pathway and functions as an important oncogene in mammals (22,23). Furthermore, YAP1, a 65 kDa proline rich phosphoprotein, is the primary downstream effector of the Hippo signaling pathway and acts as a transcriptional co-activator, which can bind to transcription- and cell cycle-associated genes, including cyclin D1 and cyclin E, and therefore regulates cell proliferation (24). A previous study revealed that overexpression of YAP1 can promote tumorigenesis in most, but not all, tumor types evaluated (25,26). For example, as the core protein in the Hippo pathway, YAP1 can induce malignant mesothelioma cell proliferation (27). In addition, the abnormal expression of YAP1 could promote cell proliferation in vitro (28). It has also been shown that gga-miR-375 is significantly downregulated, while YAP1 is upregulated in liver tumors in chickens infected with ALV-J, and also that YAP1 is the target gene of gga-miR-375(29). Furthermore, previous studies have revealed that the cell cycle and cell proliferation have a close association with tumor formation (30-33). Considering that gga-miR-375 and YAP1 play a key role in tumorigenesis in ALV-J-infected chickens (29), the present study aimed to investigate whether gga-miR-375 and YAP1 affected the cell cycle and proliferation in DF-1 cells to further determine the novel function of gga-miR-375 and YAP1.
The present results suggested that ALV-J infection may promote the cell cycle by promoting cell transition from G1 to S/G2 phase and may increase cell proliferation in DF-1 cells. Furthermore, it was identified that gga-miR-375 inhibited the cell cycle by maintaining DF-1 cells within the G1 phase and decreased cell proliferation, while YAP1 promoted DF-1 cell transition from G1 to S/G2 phase. It was further demonstrated that the knockdown of gga-miR-375 expression promoted the cell cycle and cell proliferation by targeting YAP1. Therefore, the present results provide novel insights on the cell cycle and cell proliferation regulated by gga-miR-375. Furthermore, the critical role of gga-miR-375 and YAP1 in the cell cycle and cell proliferation was identified in DF-1 cells.
Materials and methods
Cell culture
DF-1 cells, an immortalized chicken embryo fibroblast cell line, were purchased from the American Type Culture Collection. Cells were cultured in DMEM supplemented (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a humidified incubator at 37˚C with 5% CO2.The DF-1 cell line is a classic model for in vitro research (34-36).
Virus and plasmid
The GD1109 strain of ALV-J and the PRK5-Flag were gifted from Professor Wencheng Lin, South China Agricultural University. The PRK5-Flag-YAP1 plasmid has been previously constructed and preserved (29). The cDNA of DF-1 cells was used to amplify YAP1 gene which was cloned into the pRK5-FLAG vector. The primers for YAP1 amplification are as follows: 5'-ATGGATCCCGGGCAGCCTCA-3' and 5'-AGTTTTCTTGGTTATAG-3'. The groups of pRK5-FLAG vector and mock were the control groups. For each transfection, 2 µg of plasmid were respectively transfected in a 6-well plate for 48 h using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer's protocol. Western blot analysis was used to detect the efficiency of cell transfection. 48 h after cell transfection, subsequent experiments were performed.
RNA oligoribonucleotides and cell transfection
gga-miR-375 mimic and its negative control miRNA (gga-miR-NC) or anti-gga-miR-375 and anti-gga-miR-con inhibitor or siRNA-YAP1 and its negative control siRNA-scramble (siRNA-Scra) were purchased from Shanghai GenePharma Co., Ltd. The RNA oligoribonucleotides are listed in Table I. To prove YAP1 is a mediator of gga-miR-375, the following groups were set up: Anti-gga-miR-375; anti-gga-miR-375 + siRNA-YAP1; and anti-gga-miR-con + siRNA-YAP1. DF-1 cells (5x104 cells per well) were cultured in 6-well plates overnight at 37˚C prior to transfection. Transfection was performed using X-tremeGENE small interfering (si)RNA transfection reagent (Roche Diagnostics GmbH) in a 6-well plate according to the manufacturer's protocol, and used at the concentration of 40 nM for each transfection (29). Following 48 h of transfection, reverse transcription-quantitative PCR (RT-qPCR) and western blotting were conducted.
Table I.
RNA oligoribonucleotides used for cell transfection.
| Gene | Sequence (5'-3') |
|---|---|
| gga-miR-375 | Sense: UUUGUUCGUUCGGCUCGCGUUA |
| Antisense: UAACGCGAGCCGAACGAACAAA | |
| gga-miR-NC | Sense: UUGUACUACACAAAAGUACUG |
| Antisense: GUACUUUUGUGUAGUACAAUU | |
| Anti-gga-miR-con | Sense: CAGUACUUUUGUGUAGUACAA |
| Anti-gga-miR-375 | Sense: UAACGCGAGCCGAACGAACAAA |
| siRNA-YAP1 | Sense: GGACAGAGAUACUUCCUUATT |
| Antisense: UAAGGAAGUAUCUCUGUCCTT | |
| siRNA-Scra | Sense: UUCUCCGAACGUGUCACGUTT |
| Antisense: ACGUGACACGUUCGGAGAATT |
miR, microRNA; NC, negative control.
Infection experiments
ALV-J infection experiments included the experimental and the mock infected control group, which were performed in 6-well plates. 2 ml DMEM with 1.0x105 cells were added into each well. Once the density of the cells reached 70-80%, the experimental group was infected with a multiplicity of infection of 1 for ALV-J at 37˚C and viral infection was detected at 24, 48 and 72 h.
RNA isolation and RT-qPCR
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract total RNA from DF-1 cells. RNA was reverse transcribed into cDNA using the PrimeScript RT reagent kit (Takara Bio, Inc.) according to the manufacturer's protocol. The following RT conditions were used: Initial denaturation at 37˚C for 15 min, followed by 85˚C for 5 sec and 4˚C for 5 min. The relative expression levels of miRNAs were determined using TaqMan miRNA assay (Thermo Fisher Scientific, Inc.) on an CFX96 Touch™ RT PCR detection system (Bio-Rad Laboratories, Inc.). The following thermocycling conditions were used for this qPCR: Initial denaturation at 95˚C for 15 min, followed by 40 cycles at 95˚C for 10 sec and at 60˚C for 60 sec. The relative expression levels of mRNA were quantified using the SYBR Premix Ex Taq (Takara Bio, Inc.) according to the manufacturer's protocol. The following thermocycling conditions were used for this qPCR: Initial denaturation at 95˚C for 3 min, followed by 40 cycles at 95˚C for 5 sec and at 60˚C for 30 sec. β-actin and U6 were used for mRNA and miRNA normalization, respectively. Specific primers were purchased from Sangon Biotech Co., Ltd. and are listed in Table II. The relative mRNA expression was calculated using the 2-ΔΔCq method (37).
Table II.
Primers used for reverse transcription-quantitative PCR.
| Gene | Primers (5'-3') |
|---|---|
| YAP1 | F: GAACTCAGCATCAGCCATGA |
| R: CTACGGAGAGCCAATTCCTG | |
| gga-miR-375 | TGTTCGTTCGGCTCGCGTTA |
| β-actin | F: CTGGCACCTAGCACAATGAA |
| R: CTGCTTGCTGATCCACATCT | |
| U6 | F: GCCTGGACTGAGTAAGAGCG |
| R: GCCCCTTTTTGTCCGTTTCC |
F, forward; R, reverse; miR, microRNA; YAP1, Yes-associated protein 1.
Cell proliferation and the cell cycle assay
DF-1 cells (4,000 cells/well) were cultured in 96-well plates and when the cell density reached 70-80%, transfection was performed. A total of 10 µl Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) reagent was added to each well 24, 48 and 72 h after transfection and then incubated at 37˚C for 4 h according to the manufacturer's instructions. The absorbance was recorded at 450 nm.
The cell cycle assay was performed in 6-well plates and when the cell density reached 70-80%, transfection was performed. Flow cytometry was used to evaluate the distribution of cells in different phases of the cell cycle, basing the evaluation on the DNA content of 500 µl 1% propidium iodide (PI; Thermo Fisher Scientific, Inc.)-stained nuclei. After transfection for 48 h, cells were trypsinized and resuspended in 75% ethanol at a density of 1x106 cells/ml, overnight at 4˚C, resuspended in PBS and dyed using the cell cycle dye solution (Cell Cycle and Apoptosis Analysis kit; Nanjing KeyGen Biotech Co., Ltd.) for 30 min at 37˚C in the dark. A flow cytometry (BD FACScalibur; BD Bioscience) was used to evaluate the cell cycle. Each group contained three replicate wells and all of the experiments were repeated independently three times. The results were analyzed using CellQuest Pro 4.0.2 (BD Biosciences).
Western blot analysis
gga-miR-375 mimic, pRK5-Flag-YAP1, small interfering (si)RNA-YAP1 and gga-miR-375 inhibitor were transfected into DF-1 cells and subsequently subjected to western blot analysis. Proteins were homogenized or lysed in ice-cold RIPA lysis buffer (Santa Cruz Biotechnology, Inc.). The concentration of the protein was examined using Bradford protein assay kit (Bio-Rad Laboratories, Inc.). Protein samples (40 µg/lane) were separated by 12% SDS-PAGE and transferred to PVDF membranes (EMD Millipore). The membranes were blocked in Tris-buffered saline with 5% non-fat milk and 0.5% BSA for 1 h at room temperature. Membranes with corresponding proteins were incubated with the polyclonal rabbit anti-YAP1 primary antibody (cat. no. 13584-1-AP; 1:1,000; ProteinTech Group, Inc.), polyclonal rabbit anti-phospho-YAP1 primary antibody (cat. no. 13008; 1:11,000; Cell Signaling Technology, Inc.), polyclonal rabbit anti-cyclin D1 primary antibody (cat. no. 55506; 1:1,000; Cell Signaling Technology, Inc.), polyclonal mouse anti-p53 primary antibody (cat. no. 2524; 1:11,000; Cell Signaling Technology, Inc.), polyclonal rabbit anti-cyclin E primary antibody (cat. no. 4129; 1:1,000; Cell Signaling Technology, Inc.), monoclonal mouse anti-p27 primary antibody (the antibody was generated and generously provided by Professor Wencheng Lin; 1:1,000), polyclonal rabbit anti-MST1 primary antibody (cat. no. 3682; 1:1,000; Cell Signaling Technology, Inc.), polyclonal rabbit anti-SAV1 primary antibody (cat. no. 3507; 1:1,000; Cell Signaling Technology, Inc.), polyclonal rabbit anti-MOB1 primary antibody (cat. no. 3863; 1:1,000; Cell Signaling Technology, Inc.), polyclonal rabbit anti-LATS1 primary antibody (cat. no. 9153; 1:1,000; Cell Signaling Technology, Inc.), monoclonal rabbit anti-GAPDH primary antibody (cat. no. 2118; 1:1,000; Cell Signaling Technology, Inc.) and polyclonal mouse anti-β-actin primary antibody (cat. no. 33308; 1:1,000; Bioss Antibodies, Inc.) overnight at 4˚C. The membranes were then incubated with a horseradish peroxidase (HRP)-conjugated mouse polyclonal anti-rabbit IgG (H+L)-(cat. no. 0293R; 1:500; Bioss Antibodies, Inc) and goat polyclonal anti-mouse IgG-HRP (cat. no. 7076; 1:11,000; Cell Signaling Technology, Inc.) for 1.5 h at RT and washed four times using 0.1% Tween-20 solution. Blots were visualized with SuperSignal™ West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Inc.) and bands were quantified using Image-Pro Plus v6.0 (Media Cybernetics, Inc.).
Luciferase reporter assay
The putative target genes of gga-miR-375 were predicted using public available algorithms, including TargetScan (http://targetscan.org) and miRDB (http://www.mirdb.org). The predicted gga-miR-375 binding sites in the wild-type (WT) 3'UTR of YAP1 and the corresponding mutant type (MUT) gga-miR-375 binding sites were cloned into a pGL3 vector (Promega Corporation). For the luciferase reporter assay, DF-1 cells (1x105 cells/well) were seeded in 96-well plates and co-transfected with 300 ng WT-YAP1 or MUT-YAP1, and 100 nM of gga-miR-375 mimic or gga-miR-NC using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer's protocol. After 48 h transfection, the relative luciferase activities were measured using the dual-luciferase reporter assay system (Promega Corporation). Renilla luciferase activity was used for normalization.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 statistical software (SPSS, Inc.). A one-way or two-way ANOVA followed by the Tukey's post hoc tests were used to compare differences between two groups. A Student's t-test was used to compare differences between two groups. P<0.05 was considered to indicate a statistically significant difference. For each separate set of experiments, three independent replicates were evaluated. Data are presented as the mean ± SD.
Results
Infection with ALV-J promotes the cell cycle and cell proliferation in DF-1 cells
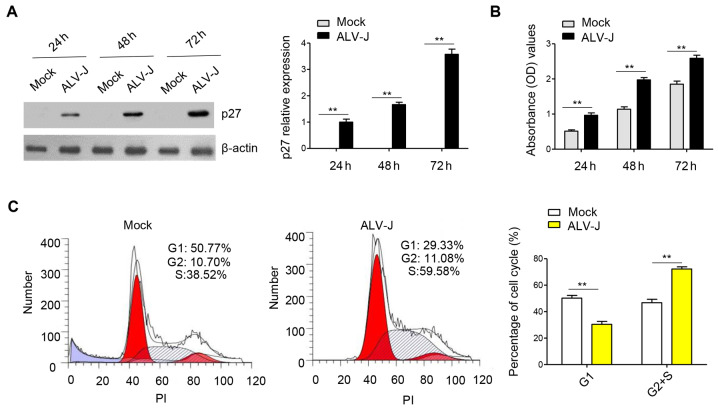
In order to investigate the effect of ALV-J infection on cell proliferation, DF-1 cells were infected with ALV-J and the capsid protein p27 of ALV-J was used to track the viral replication (Fig. 1A). CCK-8 is a widely used method for cell proliferation based on water soluble tetrazolium-8; therefore, a CCK-8 assay was used to determine proliferation of DF-1 cells. It was identified that ALV-J infection could significantly promote cell proliferation compared with the mock group (Fig. 1B). Furthermore, the cell cycle was assessed using flow cytometry and the results indicated that the percentage of cells in G1 phase was decreased, while the percentage of cells in S and G2 phase was significantly increased in the ALV-J infected group compared with the mock group (Fig. 1C). Thus, the results indicated that ALV-J infection in vitro promoted proliferation and the cell cycle by accelerating the transition from G1 phase to S/G2 phase.
Figure 1.
ALV-J infection in DF-1 cells promotes proliferation and the cell cycle. (A) DF-1 cells were infected with ALV-J GD1109 strain with a multiplicity of injection of 1. Cells were harvested at 24, 48 and 72 h for western blot analysis with antibodies against p27 and β-actin. p27, the capsid protein of ALV-J, was used to measure the viral replication in DF-1 cells. Densitometry analysis was conducted to compare the viral replication in ALV-J-infected group compared with the mock group. (B) Cell proliferation analysis was performed using the Cell Counting Kit-8 assay in DF-1 cells infected with ALV-J, which were examined at 24, 48 and 72 h after transfection. The mock group is the control group. (C) Cell cycle assay was performed in DF-1 cells infected with ALV-J for 48 h, stained with PI and evaluated with a FACSCalibur flow cytometer compared with the mock group. Data are presented as the mean ± SD of three independent experiments. **P<0.01. ALV-J, avian leukosis virus; PI, propidium iodide; OD, optical density.
Overexpression of gga-miR-375 inhibits the cell cycle and proliferation of DF-1 cells
A previous study showed that ALV-J infection could downregulate gga-miR-375, while upregulating YAP1 expression (18). In addition, YAP1 was confirmed to be a target gene of gga-miR-375 in a previous study (29). In the present study, the effect of ALV-J infection on gga-miR-375 and YAP1 expression levels was analyzed, and a luciferase reporter gene assay experiment was performed to identify whether YAP1 is a target gene of gga-miR-375 (Fig. S1).
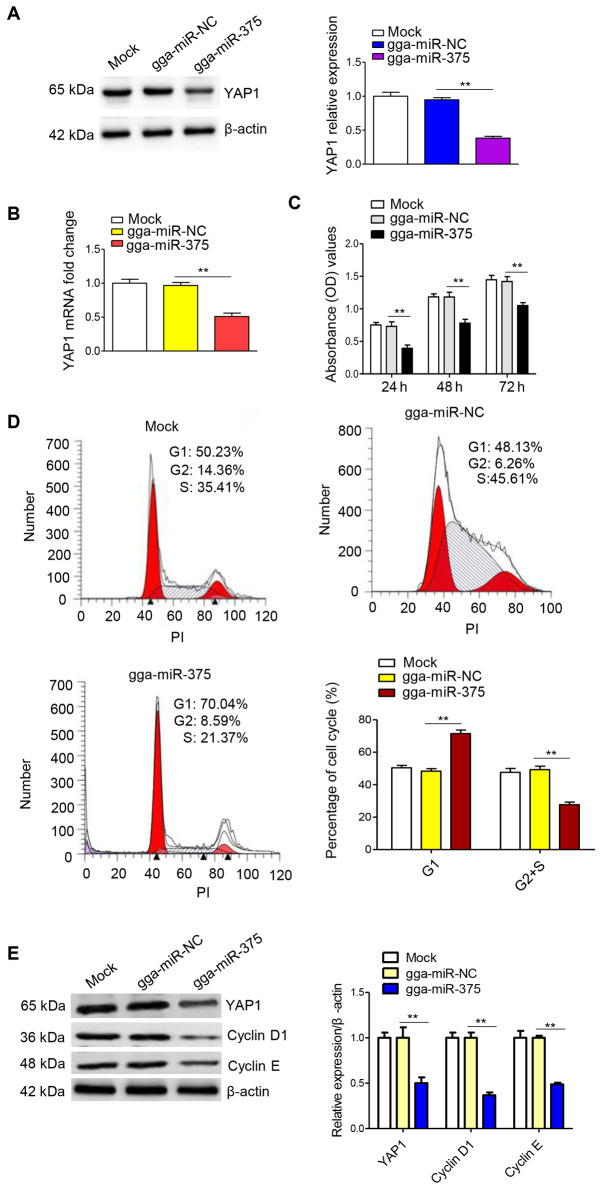
Next, the effect of gga-miR-375 overexpression on YAP1, cell proliferation and the cell cycle was assessed. Western blot analysis and RT-qPCR results suggested that gga-miR-375 overexpression significantly decreased YAP1 protein (Fig. 2A) and mRNA expression (Fig. 2B) levels. To determine the effect of gga-miR-375 overexpression on cell proliferation, a CCK-8 assay was performed and the results indicated that overexpression of gga-miR-375 could significantly inhibit cell proliferation compared with the NC group (Fig. 2C). In addition, flow cytometry was conducted to investigate the effect of gga-miR-375 on the cell cycle, 48 h after transfection in DF-1 cells. It was identified that the overexpression of gga-miR-375 could significantly increase the percentage of cells in G1 phase, while the percentage of cells in S/G2 phase was significantly decreased compared with the NC group (Fig. 2D), which was consistent with the cell proliferation results. In addition, overexpression of gga-miR-375 downregulated the protein expression levels of YAP1, cyclin D1 and cyclin E (Fig. 2E). Furthermore, overexpression of gga-miR-375 upregulated the protein expression of p53 (Fig. S2A). Collectively, the results demonstrated that overexpression of gga-miR-375 downregulated cell proliferation and the cell cycle by sequestering the cells in G1 phase.
Figure 2.
Overexpression of gga-miR-375 inhibits proliferation and the cell cycle in DF-1 cells. DF-1 cells transfected with gga-miR-375 mimic decreases YAP1 (A) protein and (B) mRNA expression levels after 48 h of transfection. gga-miR-NC and mock are the control groups. (C) Overexpression of gga-miR-375 inhibits cell proliferation as indicated by Cell Counting Kit-8 assay results. (D) Overexpression of gga-miR-375 inhibits cell cycle progression as measured with a FACSCalibur flow cytometer. (E) Overexpression of gga-miR-375 downregulates the expression levels of YAP1, cyclin D1 and cyclin E. Data are presented as the mean ± SD of three independent experiments. **P<0.01. NC, negative control; miR, microRNA; YAP1, Yes-associated protein 1; OD, optical density; PI, propidium iodide.
Knockdown of gga-miR-375 promotes the cell cycle and cell proliferation in DF-1 cells
The expression of gga-miR-375 was knocked down using a gga-miR-375 inhibitor, and the effect on cell proliferation and cell cycle was subsequently investigated. DF-1 cells were transfected with either gga-miR-375 inhibitor or an inhibitor control, and a mock group was used as the control group. The RT-qPCR results demonstrated that the gga-miR-375 inhibitor could significantly downregulate the mRNA expression of gga-miR-375 compared with the control group (Fig. 3A). In addition, western blot analysis and RT-qPCR results suggested that knockdown of gga-miR-375 significantly upregulated the protein and mRNA expression levels of YAP1 (Fig. 3B and C). Cell proliferation was determined using a CCK-8 assay, and the results indicated that knockdown of gga-miR-375 could significantly promote proliferation compared with the control group (Fig. 3D). Furthermore, flow cytometry was performed to assess the effect of gga-miR-375 knockdown on the cell cycle, 48 h after transfection in DF-1 cells, and it was identified that knockdown of gga-miR-375 significantly decreased the number of cells in G1 phase, but increased the number of cells in S/G2 phase (Fig. 3E). In addition, knockdown of gga-miR-375 in DF-1 cells upregulated the total protein expression levels of YAP1, cyclin D1 and cyclin E (Fig. 3F). It was also identified that the knockdown of gga-miR-375 downregulated the protein expression of p53 (Fig. S2B). Therefore, the results suggested that knockdown of gga-miR-375 could promote cell proliferation and cell cycle progression by increasing the number of DF-1 cells from G1 to S/G2 phase.
Figure 3.
Knockdown of gga-miR-375 promotes proliferation and the cell cycle in DF-1 cells. (A) DF-1 cells transfected with anti-gga-miR-375 had decreased gga-miR-375 mRNA expression at 48 h following transcription. Anti-gga-miR-con group and mock group were used as the controls. DF-1 cells transfected with anti-gga-miR-375 exhibited increased (B) YAP1 protein and (C) mRNA expression levels compared with the control groups. (D) Overexpression of anti-gga-miR-375 promotes cell proliferation as indicated by Cell Counting Kit-8 assay results 24, 48 and 72 h after transfection. (E) Overexpression of anti-gga-miR-375 promotes the cell cycle as demonstrated by flow cytometry results. (F) DF-1 cells transfected with anti-gga-miR-375 had upregulated expression levels of YAP1, cyclin D1 and cyclin E. Anti-gga-miR-con and mock groups were used as the control groups. Data are presented as the mean ± SD of three independent experiments. **P<0.01 vs. anti-miR-con group. miR, microRNA; YAP1, Yes-associated protein 1; NC, negative control; con, control; OD, optical density.
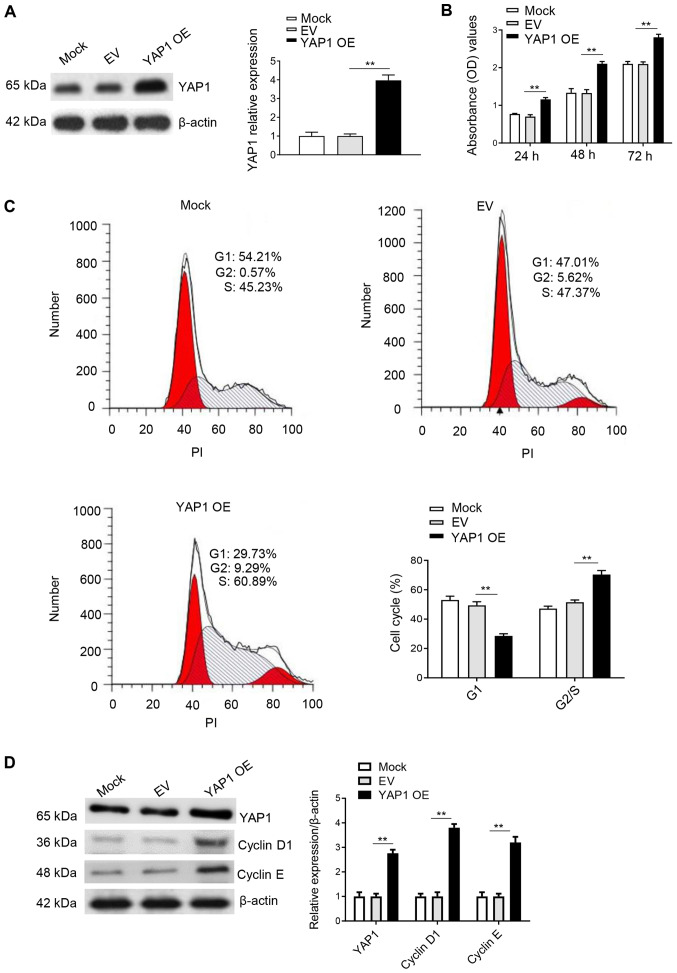
Overexpression of YAP1 promotes the cell cycle progression and proliferation in DF-1 cells
DF-1 cells were cultured in 6-well plates, transfected with pRK5-Flag-YAP1 overexpression plasmid and collected 48 h after transfection. Western blot analysis results indicated that YAP1 was successfully overexpressed in DF-1 cells (Fig. 4A). Moreover, the CCK-8 assay results identified that the transfection of YAP1 for 24, 48 and 72 h could significantly promote cell proliferation compared with the control groups (Fig. 4B). Furthermore, the effect of YAP1 on the cell cycle was determined using flow cytometry 48 h after transfection. It was identified that overexpression of YAP1 in DF-1 cells significantly decreased the percentage of cells in G1 phase, while the percentage of cells in S and G2 phase was significantly increased compared with the control group (Fig. 4C). In addition, overexpression of YAP1 increased the protein expression levels of cyclin D1 and cyclin E (Fig. 4D). Thus, it was speculated that the overexpression of YAP1 promoted cell proliferation and the cell cycle by accelerating the transition of DF-1 cells from G1 to G2/S phase.
Figure 4.
OE of YAP1 promotes proliferation and cell cycle in DF-1 cells. (A) DF-1 cells transfected with pRK5-Flag-YAP1 were harvested after 48 h for western blot analysis with antibodies against YAP1 and β-actin. (B) OE of YAP1 promotes cell proliferation as identified by Cell Counting Kit-8 assay results 24, 48 and 72 h after transfection. (C) OE of YAP1 promotes the cell cycle, as demonstrated by flow cytometry. (D) OE of YAP1 promotes the protein expression levels of cyclin D1 and cyclin E after 48 h of transfection. Data are presented as the mean ± SD of three independent experiments. **P<0.01 vs. EV group. EV, empty vector; YAP1, Yes-associated protein 1; OD, optical density; OE, overexpression; PI, propidium iodide.
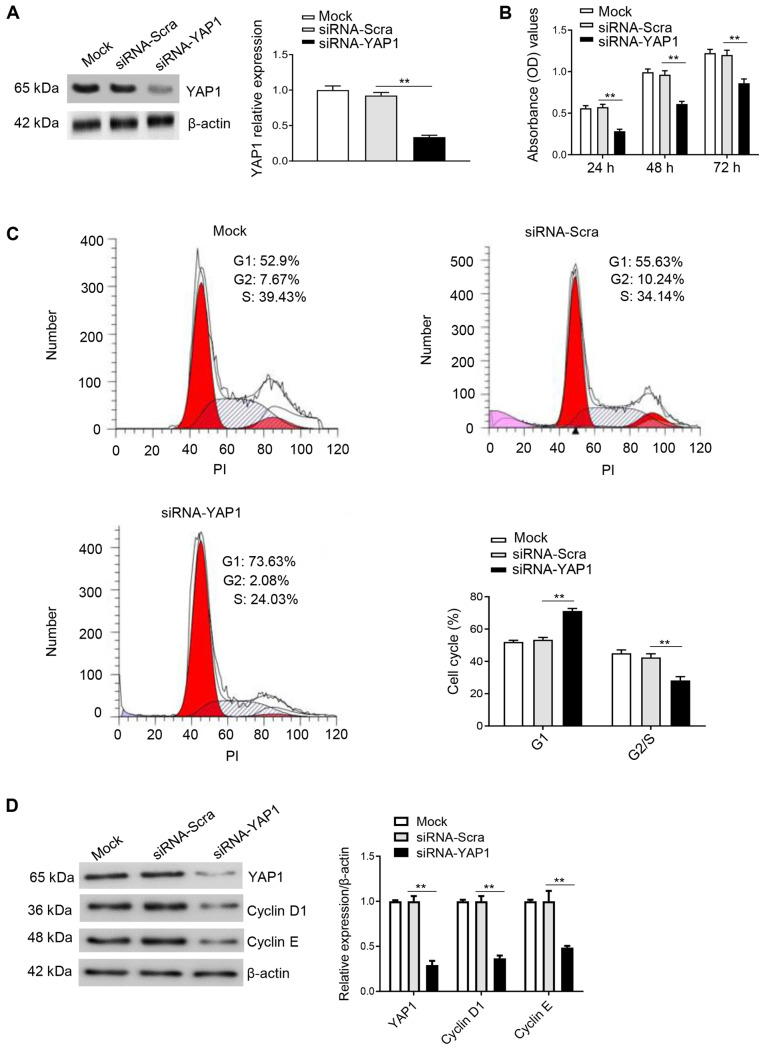
Knockdown of YAP1 inhibits cell cycle progression and proliferation in DF-1 cells
YAP1 was knocked down using siRNA, and its effect on cell proliferation and the cell cycle was evaluated. Either siRNA-YAP1 or siRNA-Scra control were transfected into DF-1 cells. Western blot analysis results indicated that YAP1 expression was significantly decreased in the siRNA-YAP1 transfection group (Fig. 5A). Moreover, the CCK8 assay results identified that knockdown of YAP1 could significantly inhibit cell proliferation compared with the control group (Fig. 5B). Furthermore, the effect of YAP1 knockdown on the cell cycle was observed using flow cytometry 48 h after transfection in DF-1 cells. The results suggested that knockdown of YAP1 in DF-1 cells significantly upregulated the number of cells in G1 phase and downregulated the number of cells in S/G2 phase (Fig. 5C). Furthermore, knockdown of YAP1 inhibited the protein expression levels of cyclin D1 and cyclin E (Fig. 5D). Collectively, the results suggested that knockdown of YAP1 inhibited proliferation and the cell cycle by maintaining DF-1 cells in the G1 phase.
Figure 5.
Knockdown of YAP1 inhibits proliferation and the cell cycle in DF-1 cells. (A) DF-1 cells were transfected with siRNA-YAP1 and harvested 48 h later for western blot analysis with antibodies against YAP1 and β-actin. (B) Knockdown of YAP1 inhibits cell proliferation as demonstrated by Cell Counting Kit-8 assay results 24, 48 and 72 h after transfection. (C) Flow cytometry results indicated that knockdown of YAP1 inhibited the cell cycle. (D) Knockdown of YAP1 inhibits the protein expression levels of cyclin D1 and cyclin E after 48 h transfection. Data are presented as the mean ± SD of three independent experiments. **P<0.01 vs. siRNA-Scra group. YAP1, Yes-associated protein 1; siRNA, small interfering RNA; Scra, scramble; PI, propidium iodide; OD, optical density.
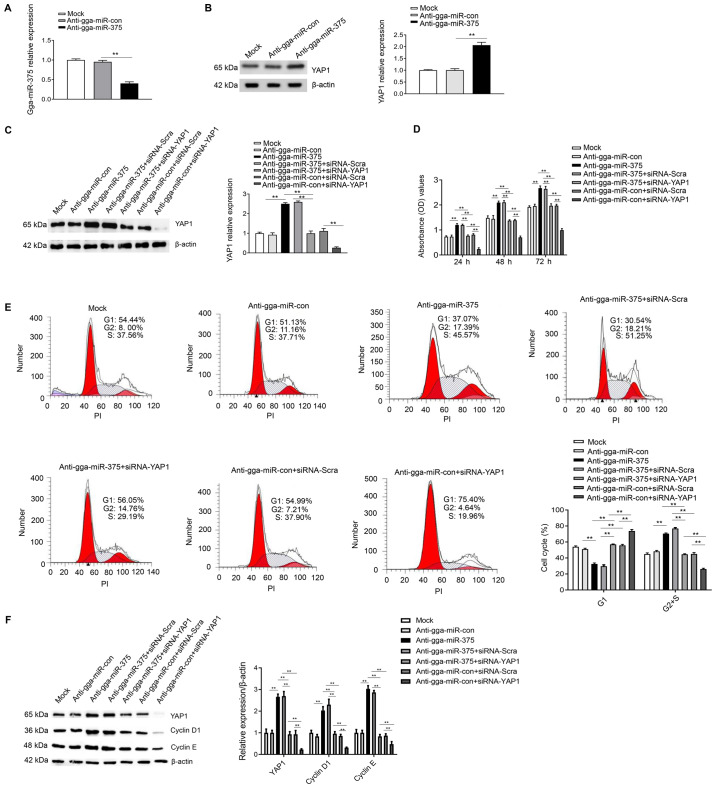
Knockdown of gga-miR-375 expression promotes the cell cycle and cell proliferation by targeting YAP1
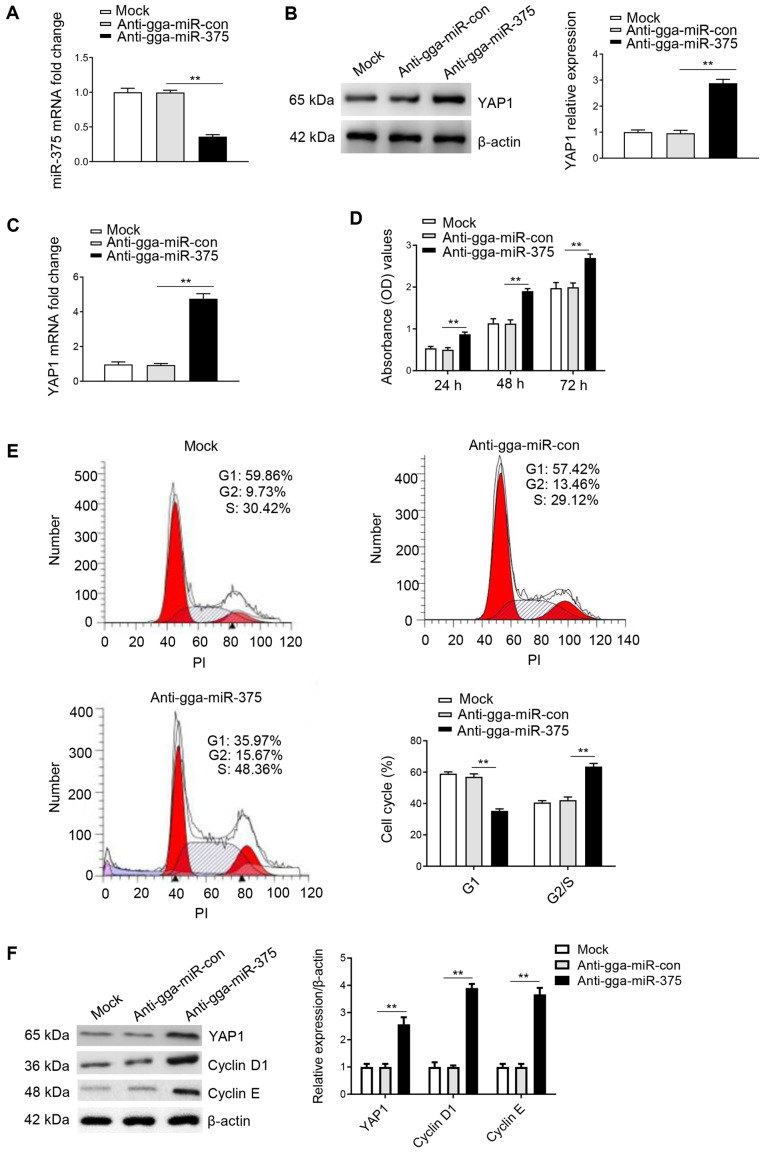
To further assess whether YAP1 is a mediator of gga-miR-375, the following groups were used: Anti-gga-miR-375; anti-gga-miR-375 + siRNA-YAP1; and anti-gga-miR-con + siRNA-YAP1. In addition, anti-gga-miR-con, anti-gga-miR-375 + siRNA-Scra and anti-gga-miR-con + siRNA-Scra were used as the control groups, while the mock group was used as a negative control. It was identified that gga-miR-375 was successfully silenced by transfection of anti-gga-miR-375 (Fig. 6A), and western blot analysis results indicated that inhibiting the expression of gga-miR-375 through transfection of anti-gga-miR-375 could significantly upregulate the protein expression of YAP1 compared with the anti-gga-miR-con group (Fig. 6B). The results also indicated that transfection of anti-gga-miR-375 could significantly increase the protein expression of YAP1 compared with the anti-gga-miR-con group (Fig. 6C). Moreover, the CCK-8 assay results identified that the anti-gga-miR-375 only group or anti-gga-miR-375 + siRNA-Scra group could significantly promote cell proliferation from 24-72 h (Fig. 6D). However, proliferation was decreased after co-transfection with anti-gga-miR-375 + siRNA-YAP1 compared with the anti-gga-miR-375 + siRNA-Scra group (Fig. 6D), thus indicating that YAP1 may be a mediator of gga-miR-375 in regulating cell proliferation.
Figure 6.
Knockdown of gga-miR-375 expression promotes the cell cycle and cell proliferation by targeting YAP1. (A) DF-1 cells transfected with anti-gga-miR-375 had decreased gga-miR-375 mRNA expression at 48 h as assessed by reverse transcription-quantitative PCR. Anti-gga-miR-con and mock groups are the controls. (B) Protein expression of YAP1 was measure after DF-1 cells were transfected for 48 h with anti-gga-miR-375. (C) Protein expression levels of YAP1 after 48 h transfection with anti-gga-miR-375 or co-transfected with anti-gga-miR-375 + siRNA-YAP1. (D) Knockdown of gga-miR-375 promoted cell proliferation by targeting YAP1, as identified by Cell Counting Kit-8 assay results after 24, 48 and 72 h. (E) Flow cytometry results indicated that knockdown of gga-miR-375 expression promoted the cell cycle by targeting YAP1. The cell cycle assay was performed after knockdown of gga-miR-375 or gga-miR-375 + YAP1 in DF-1 cells and evaluated with a FACSCalibur flow cytometer 48 h after transfection. (F) DF-1 cells transfected with anti-gga-miR-375 or anti-gga-miR-375 + siRNA-YAP1 were harvested 48 h after transfection for western blot analysis with antibodies against YAP1, cyclin D1, cyclin E and β-actin. Data are presented as the mean ± SD of three independent experiments. **P<0.01. YAP1, yes-associated protein 1; miR, microRNA; siRNA, small interfering RNA; PI, propidium iodide; OD, optical density; Scra, scramble.
The cell cycle analysis results demonstrated that co-transfection of anti-gga-miR-375 + siRNA-YAP1 could inhibit the cell cycle by maintaining cells in G1 phase compared with the anti-gga-miR-375 + siRNA-Scra group (Fig. 6E), therefore suggesting that YAP1 is a mediator of gga-miR-375 in regulating cell cycle. Furthermore, western blot analysis results identified that co-transfection of anti-gga-miR-375 + siRNA-YAP1 could decrease the protein expression levels of YAP1, cyclin D1 and cyclin E compared with the anti-gga-miR-375 + siRNA-Scra group (Fig. 6F). Collectively, the results indicated that knockdown of gga-miR-375 expression promoted the cell cycle and cell proliferation by targeting YAP1.
It has been shown that serine phosphorylation of YAP1 leads to YAP1 downregulation by cytoplasm sequestration and/or degradation, while non-phosphorylated (p)-YAP1 is activated and enters the nucleus (38). Moreover, YAP1 acts as a transcriptional activator and can bind to numerous transcription factors to regulate the expression levels of various genes (23,39). Therefore, the present study detected p-YAP1 expression after ALV-J infection, knockdown of gga-miR-375 or overexpression of gga-miR-375. It was found that ALV-J infection or knockdown of gga-miR-375 could decrease p-YAP1 expression, while the overexpression of gga-miR-375 could increase p-YAP1 expression (Fig. S3). The results also demonstrated that after ALV-J infection or knockdown of gga-miR-375, p-YAP1 expression was downregulated, thus indicating that YAP1 is not degraded in the cytoplasm and this leads to an increase of YAP1. Since YAP1 is a member of the Hippo signaling pathway, the present study detected the expression levels of other members of this signaling pathway, including macrophage stimulating 1 (MST1), MOB kinase activator 1 (MOB1) and large tumor suppressor kinase 1 (LATS1), after overexpression gga-miR-375. It was demonstrated that the overexpression of gga-miR-375 had no effect on the protein expression levels of MST1, salvador family WW domain containing protein 1, MOB1 and LATS1 (Fig. S4). Therefore, the results suggested that gga-miR-375 directly affected YAP1, which in turn affected the cell cycle and cell proliferation, and ultimately tumor formation.
Discussion
ALV-J is a retrovirus that commonly causes natural infection in chickens, and possibly neoplastic disease and other production problems (40). The cell cycle is regulated by a large number of transcription factors and signaling molecules, and affects cell proliferation (41); endless proliferation of cells is a hallmark of cancer (42). Furthermore, tumors are a type of disease characterized by uncontrolled cell proliferation. The cell cycle is a continuous and precise process, and it serves a key role in monitoring and regulating the development of tumor formation. Moreover, abnormality of cell cycle regulation is closely associated with the occurrence and development of tumors (43). Therefore, an in-depth study of cell cycle regulation would help to further the understanding of the mechanism involved in tumor formation. As ALV-J infection causes tumors in chickens, it was hypothesized that ALV-J infection could affect cell proliferation and the cell cycle. Therefore, the present study investigated the effect of ALV-J infection on these cellular processes in the DF-1 cell line. The results demonstrated that ALV-J infection promoted cell proliferation and the cell cycle by downregulating G1 phase, but upregulating S/G2 phase.
miRNAs serve an important role in numerous biological processes, including cell development, differentiation and proliferation, and may target oncogenes or acts as a tumor suppressor in the cell cycle (44). In the process of cell proliferation, miRNA can serve as a tumor suppressor or oncogene regulator, depending on the tissue and cellular environment (45). A previous study revealed that gga-miR-375 is significantly downregulated in ALV-J-induced tumors in the livers of chickens (29). Considering that in the present study, ALV-J infection was identified to promote cell proliferation and the cell cycle, it was speculated that gga-miR-375 may affect these cellular processes and could regulate the target gene YAP1, which may serve a key role in controlling this effect. Moreover, p53 is a tumor suppressor gene and its primary role is to arrest the cell cycle (46). In the present study, overexpression of gga-miR-375 was also identified to increase p53 protein expression, while knockdown of gga-miR-375 decreased p53 protein expression. Therefore, the results indicated that the downregulation of gga-miR-375 by ALV-J infection decreased the protein expression of p53, which affected the arrest of the cell cycle.
The present study also examined the effect of gga-miR-375 and YAP1 on cell proliferation and the cell cycle. It was identified that overexpression of gga-miR-375 inhibited cell proliferation and the cell cycle by reducing cell transformation from G1 phase to S/G2 phase, while knockdown of gga-miR-375 promoted these cellular processes by increasing cellular transition from G1 phase to S/G2 phase. YAP1 is a target gene of gga-miR-375, and gga-miR-375 serves a suppressive role in the regulation of YAP1 (18,47). Furthermore, YAP1 has been reported to be a core protein in the Hippo pathway that affects the cell cycle (48-50). In relation to the target gene of gga-miR-375, YAP1, the flow cytometry cell cycle analysis results demonstrated that YAP1 overexpression could increase the transition of DF-1 cells from G1 to S/G2 phase. Moreover, the CCK-8 assay results suggested that the overexpression of YAP1 could increase cell proliferation from 24-72 h. However, knockdown of YAP1 decreased cell proliferation and inhibited the cell cycle by maintaining DF-1 cells at G1 phase.
Cyclin D1 and cyclin E play a key role in regulating the cell cycle, and the downregulation of these factors results in the arrest of the cell cycle (51,52). The present results suggested that gga-miR-375 overexpression in DF-1 cells downregulated the total protein expression levels of YAP1, cyclin D1 and cyclin E. However, knockdown of gga-miR-375 in DF-1 cells upregulated the protein expression of YAP1, and also increased the protein expression levels of cyclin D1 and cyclin E. Moreover, overexpression of YAP1 increased the expression levels of cyclin D1 and cyclin E, while knockdown of YAP1 inhibited the expression levels of these factors. Therefore, the present results suggested that downregulation of gga-miR-375 and upregulation of YAP1 by ALV-J infection affected the cell cycle by altering the protein expression levels of cyclin D1 and cyclin E. Numerous studies have shown that cyclin D1 and cyclin E can mediate the development of cancer (53,54); therefore, as gga-miR-375 and YAP1 can affect the expression levels of these two proteins, this may be the mechanism through which gga-miR-375 targets YAP1 to promote tumor formation.
The present results identified that YAP1 may be a mediator of gga-miR-375, and that gga-miR-375 could affect cell proliferation and the cell cycle by targeting YAP1. Thus, the results provided evidence that the cell cycle and cell proliferation may be affected by gga-miR-375 targeting YAP1 in DF-1 cells. In addition, it was demonstrated that after ALV-J infection or knockdown of gga-miR-375, p-YAP1 expression was downregulated, thus indicating that YAP1 is not degraded in the cytoplasm and this leads to an increase of YAP1. It was further found that the overexpression of gga-miR-375 had no effect on the protein expression levels of MST1, salvador family WW domain containing protein 1, MOB1 and LATS1. Therefore, the results suggested that gga-miR-375 directly affected YAP1, which in turn affected the cell cycle and cell proliferation, and ultimately tumor formation.
In conclusion, it was identified that gga-miR-375 inhibited proliferation and the cell cycle by maintaining DF-1 cells in G1 phase. Moreover, YAP1 could promote cell proliferation and the cell cycle by increasing cellular transition from G1 to S/G2 phase. The results also indicated that gga-miR-375 inhibited these cellular processes by targeting YAP1. Furthermore, downregulation of gga-miR-375 and upregulation of YAP1 by ALV-J infection affected the cell cycle by altering the protein expression levels of cyclin D1 and cyclin E, which may be the possible mechanism through which gga-miR-375 targets YAP1 to promote tumor formation. Therefore, the results of the present study provided further evidence of the role between gga-miR-375 and YAP1 in proliferation and the cell cycle in DF-1 cells, thus suggesting that these cellular processes are affected by gga-miR-375 targeting of YAP1, which may be an important regulator of tumorigenesis.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (grant nos. 31902252, 31972659, 31802185, 31672564 and 31472217), the China Postdoctoral Science Foundation (grant no. 2019M652922), the International Science and Technology Cooperation Project of Guangdong Province (grant no. 2016A050502042), the Construction Project of Modern Agricultural Science and Technology Innovation Alliance in Guangdong Province (grant no. 2018LM1112) and the Natural Science Foundation of Guangdong Province (grant nos. 2019A1515012006, 2018B030315009 and S2013030013313).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZL, YY, YW, SC and QX conceived and designed the original study. XZ conducted the statistical analysis and drafted the manuscript. ZL, YY, YW, SC and QX contributed to the acquisition and interpretation of data. SL and FC contributed to the data interpretation and analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Indiková I, Much P, Stipkovits L, Siebert-Gulle K, Szostak MP, Rosengarten R, Citti C. Role of the GapA and CrmA cytadhesins of mycoplasma gallisepticum in promoting virulence and host colonization. Infect Immun. 2013;81:1618–1624. doi: 10.1128/IAI.00112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenkins C, Geary SJ, Gladd M, Djordjevic SP. The mycoplasma gallisepticum OsmC-like protein MG1142 resides on the cell surface and binds heparin. Microbiology. 2007;153:1455–1463. doi: 10.1099/mic.0.2006/004937-0. [DOI] [PubMed] [Google Scholar]
- 3.Kato M, Slack FJ. microRNAs: Small molecules with big roles-C. elegans to human cancer. Biol Cell. 2008;100:71–81. doi: 10.1042/BC20070078. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Wang Z, Hou Y, Zhang K, Peng X. gga-miR-99a targets SMARCA5 to regulate mycoplasma gallisepticum (HS strain) infection by depressing cell proliferation in chicken. Gene. 2017;627:239–247. doi: 10.1016/j.gene.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 5.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 6.Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y, Yao H, Liu X, Ke Y, Si J, Zhou T. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784–793. doi: 10.1038/cr.2010.79. [DOI] [PubMed] [Google Scholar]
- 7.Wang P, Xu LL, Li L, Ren S, Tang J, Zhang M, Xu M. The microRNA-375 as a potentially promising biomarker to predict the prognosis of patients with head and neck or esophageal squamous cell carcinoma: A meta-analysis. Eur Arch Otorhinolaryngol. 2019;276:957–968. doi: 10.1007/s00405-019-05325-8. [DOI] [PubMed] [Google Scholar]
- 8.Winther M, Alsner J, Tramm T, Baeksgaard L, Holtved E, Nordsmark M. Evaluation of miR-21 and miR-375 as prognostic biomarkers in esophageal cancer. Acta Oncol. 2015;54:1582–1591. doi: 10.3109/0284186X.2015.1064161. [DOI] [PubMed] [Google Scholar]
- 9.Fan YP, Liao JZ, Lu YQ, Tian DA, Ye F, Zhao PX, Xiang GY, Tang WX, He XX. MiR-375 and doxorubicin co-delivered by liposomes for combination therapy of hepatocellular carcinoma. Mol Ther-Nucleic Acids. 2017;7:181–189. doi: 10.1016/j.omtn.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Xie G, Tong J, Peng Y, Huang H, Li J, Wang N, Liang H. Overexpression of microRNA-122 re-sensitizes 5-FU-resistant colon cancer cells to 5-FU through the inhibition of PKM2 in vitro and in vivo. Cell Biochem Biophys. 2014;70:1343–1350. doi: 10.1007/s12013-014-0062-x. [DOI] [PubMed] [Google Scholar]
- 11.Shaker OG, Mohammed SR, Mohammed AM, Mahmoud Z. Impact of microRNA-375 and its target gene SMAD-7 polymorphism on susceptibility of colorectal cancer. J Clin Lab Anal. 2018;32 doi: 10.1002/jcla.22215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Z, Liu J, Cui Z, Zhang L. Tumors associated with avian leukosis virus subgroup J in layer hens during 2007 to 2009 in China. J Vet Med Sci. 2010;72:1027–1033. doi: 10.1292/jvms.09-0564. [DOI] [PubMed] [Google Scholar]
- 13.Payne LN, Howes K, Gillespie AM, Smith LM. Host range of Rous sarcoma virus pseudotype RSV(HPRS-103) in 12 avian species: Support for a new avian retrovirus envelope subgroup, designated J. J Gen Virol. 1992;73:2995–2997. doi: 10.1099/0022-1317-73-11-2995. [DOI] [PubMed] [Google Scholar]
- 14.Meng F, Li Q, Zhang Y, Cui Z, Chang S, Zhao P. Isolation and characterization of subgroup J Avian Leukosis virus associated with hemangioma in commercial Hy-Line chickens. Poult Sci. 2018;97:2667–2674. doi: 10.3382/ps/pey121. [DOI] [PubMed] [Google Scholar]
- 15.Liang X, Gu Y, Chen X, Li T, Gao Y, Wang X, Fang C, Fang S, Yang Y. Identification and characterization of a novel natural recombinant avian leucosis virus from Chinese indigenous chicken flock. Virus Genes. 2019;55:726–733. doi: 10.1007/s11262-019-01695-7. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Lin W, Chang S, Zhao P, Zhang X, Liu Y, Chen W, Li B, Shu D, Zhang H, et al. Isolation, identification and evolution analysis of a novel subgroup of avian leukosis virus isolated from a local Chinese yellow broiler in south China. Arch Virol. 2016;161:2717–2725. doi: 10.1007/s00705-016-2965-x. [DOI] [PubMed] [Google Scholar]
- 17.Meng F, Li Q, Zhang Y, Zhang Z, Tian S, Cui Z, Chang S, Zhao P. Characterization of subgroup J avian Leukosis virus isolated from Chinese indigenous chickens. Virol J. 2018;15(33) doi: 10.1186/s12985-018-0947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Shang H, Shu D, Zhang H, Ji J, Sun B, Li H, Xie Q. gga-miR-375 plays a key role in tumorigenesis post subgroup J Avian Leukosis virus infection. PLos One. 2014;9 doi: 10.1371/journal.pone.0090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, He J, Dong J, Meyer TF, Xu T. The HIPPO pathway in gynecological malignancies. Am J Cancer Res. 2020;10:610–629. [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Yang Y, Wang F, Wei Q, Qin H. Hippo-YAP signaling pathway: A new paradigm for cancer therapy. Int J Cancer. 2015;137:2275–2286. doi: 10.1002/ijc.29073. [DOI] [PubMed] [Google Scholar]
- 21.Johnson R, Halder G. The two faces of Hippo: Targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao YJ, Hata Y, Ikeda M, Withanage K. Mammalian Hippo pathway: From development to cancer and beyond. J Biochem. 2011;149:361–379. doi: 10.1093/jb/mvr021. [DOI] [PubMed] [Google Scholar]
- 23.Machado-Neto JA, Lazarini M, Favaro P, Franchi-GC Jr, Nowill AE, Saad ST, Traina F. ANKHD1, a novel component of the Hippo signaling pathway, promotes YAP1 activation and cell cycle progression in prostate cancer cells. Exp Cell Res. 2014;324:137–145. doi: 10.1016/j.yexcr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–273. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Ramakrishnan SK, Triner D, Centofanti B, Maitra D, Győrffy B, Sebolt-Leopold JS, Dame MK, Varani J, Brenner DE, et al. Tumor-selective proteotoxicity of verteporfin inhibits colon cancer progression independently of YAP1. Sci Signal. 2015;8(ra98) doi: 10.1126/scisignal.aac5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Du YC, Zhou XJ, Liu H, Tang SC. The dual functions of YAP-1 to promote and inhibit cell growth in human malignancy. Cancer Metastasis Rev. 2014;33:173–181. doi: 10.1007/s10555-013-9463-3. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno T, Murakami H, Fujii M, Ishiguro F, Tanaka I, Kondo Y, Akatsuka S, Toyokuni S, Yokoi K, Osada H, Sekido Y. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012;31:5117–5122. doi: 10.1038/onc.2012.5. [DOI] [PubMed] [Google Scholar]
- 28.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Yan Y, Lin W, Li A, Zhang H, Lei X, Dai Z, Li X, Li H, Chen W, et al. Circular RNA Vav3 sponges gga-miR-375 to promote epithelial-mesenchymal transition. RNA Biol. 2019;16:118–132. doi: 10.1080/15476286.2018.1564462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frampton D, Schwenzer H, Marino G, Butcher LM, Pollara G, Kriston-Vizi J, Venturini C, Austin R, de-Castro KF, Ketteler R, et al. Molecular signatures of regression of the canine transmissible venereal tumor. Cancer Cell. 2018;33(620-633.e6) doi: 10.1016/j.ccell.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan TM, Mehra R, Tiemeny P, Wolf JS, Wu S, Sangale Z, Brawer M, Stone S, Wu CL, Feldman AS. A multigene signature based on cell cycle proliferation improves prediction of mortality within 5 yr of radical nephrectomy for renal cell carcinoma. Eur Urol. 2018;73:763–769. doi: 10.1016/j.eururo.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Sun F, Zhang Y, Xu L, Li S, Chen X, Zhang L, Wu Y, Li J. Proteasome inhibitor MG132 enhances cisplatin-induced apoptosis in osteosarcoma cells and inhibits tumor growth. Oncol Res. 2018;26:655–664. doi: 10.3727/096504017X15119525209765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Y, Gu J, Li Y, Peng C, Shi M, Wang X, Wei G, Ge O, Wang D, Zhang B, et al. MiR-17-5p enhances pancreatic cancer proliferation by altering cell cycle profiles via disruption of RBL2/E2F4-repressing complexes. Cancer Lett. 2018;412:59–68. doi: 10.1016/j.canlet.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 34.Liao Z, Dai Z, Cai C, Zhang X, Li A, Zhang H, Yan Y, Lin W, Wu Y, Li H, et al. Knockout of Atg5 inhibits proliferation and promotes apoptosis of DF-1 cells. In Vitro Cell Dev Biol Anim. 2019;55:341–348. doi: 10.1007/s11626-019-00342-7. [DOI] [PubMed] [Google Scholar]
- 35.Giotis ES, Ross CS, Robey RC, Nohturfft A, Goodbourn S, Skinner MA. Constitutively elevated levels of SOCS1 suppress innate responses in DF-1 immortalised chicken fibroblast cells. Sci Rep. 2017;7(17485) doi: 10.1038/s41598-017-17730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abu-Bonsrah KD, Zhang D, Newgreen DF. CRISPR/Cas9 targets chicken embryonic somatic cells in vitro and in vivo and generates phenotypic abnormalities. Sci Rep. 2016;6(34524) doi: 10.1038/srep34524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Warren JSA, Xiao YX, Lamar JM. YAP/TAZ activation as a target for treating metastatic cancer. Cancers. 2018;10 doi: 10.3390/cancers10040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan SW, Lim CJ, Chen L, Chong YF, Huang C, Song H, Hong W. The Hippo pathway in biological control and cancer development. J Cell Physiol. 2011;226:928–939. doi: 10.1002/jcp.22435. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Guan X, Chen Z, Cao D, Kang Z, Shen Q, Lei Q, Li F, Li H, Leghari MF, et al. The high conserved cellular receptors of avian leukosis virus subgroup J in Chinese local chickens contributes to its wide host range. Poult Sci. 2018;97:4187–4192. doi: 10.3382/ps/pey331. [DOI] [PubMed] [Google Scholar]
- 41.Ross CL, Kaushik S, Valdes-Rodriguez R, Anvekar R. MicroRNAs in cutaneous melanoma: Role as diagnostic and prognostic biomarkers. J Cell Physiol. 2018;233:5133–5141. doi: 10.1002/jcp.26395. [DOI] [PubMed] [Google Scholar]
- 42.Panagiotakopoulou M, Lendenmann T, Pramotton FM, Giampietro C, Stefopoulos G, Poulikakos D, Ferrari A. Cell cycle-dependent force transmission in cancer cells. Mol Biol Cell. 2018;29:2528–2539. doi: 10.1091/mbc.E17-12-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen BW, Chen W, Liang H, Liu H, Liang C, Zhi X, Hu LQ, Yu XZ, Wei T, Ma T, et al. Inhibition of mTORC2 induces cell-cycle arrest and enhances the cytotoxicity of doxorubicin by suppressing MDR1 expression in HCC cells. Mol Cancer Ther. 2015;14:1805–1815. doi: 10.1158/1535-7163.MCT-15-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li JL, Fu FQ, Wan XC, Huang SS, Wu DL, Li Y. Up-regulated miR-29c inhibits cell proliferation and glycolysis by inhibiting SLC2A3 expression in prostate cancer. Gene. 2018;665:26–34. doi: 10.1016/j.gene.2018.04.086. [DOI] [PubMed] [Google Scholar]
- 45.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 46.Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb Perspect Med. 2016;6(a026104) doi: 10.1101/cshperspect.a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Ji J, Xie Q, Shang H, Zhang H, Xin X, Chen F, Sun B, Xue C, Ma J, Bi Y. Aberrant expression of liver microRNA in chickens infected with subgroup J avian leukosis virus. Virus Res. 2012;169:268–271. doi: 10.1016/j.virusres.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Gise A, Lin ZQ, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, Pu WT. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci USA. 2012;109:2394–2399. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Z, Zhang M, Xu K, Liu L, Hou WK, Cai YZ, Xu P, Yao JF. Knockdown of YAP1 inhibits the proliferation of osteosarcoma cells in vitro and in vivo. Oncol Rep. 2014;32:1265–1272. doi: 10.3892/or.2014.3305. [DOI] [PubMed] [Google Scholar]
- 51.Oak C, Khalifa AO, Isali I, Bhaskaran N, Walker E, Shukla S. Diosmetin suppresses human prostate cancer cell proliferation through the induction of apoptosis and cell cycle arrest. Int J Oncol. 2018;53:835–843. doi: 10.3892/ijo.2018.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu HF, Chen YS, Yang JS, Chen JC, Lu KW, Chiu TH, Liu KC, Yeh CC, Chen GW, Lin HJ, Chung JG. Gypenosides induced G0/G1 arrest via inhibition of cyclin E and induction of apoptosis via activation of caspases-3 and -9 in human lung cancer A-549 cells. In Vivo. 2008;22:215–221. doi: 10.1177/1534735410377198. [DOI] [PubMed] [Google Scholar]
- 53.Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med (Berl) 2016;94:1313–1326. doi: 10.1007/s00109-016-1475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luhtala S, Staff S, Tanner M, Isola J. Cyclin E amplification, over-expression, and relapse-free survival in HER-2-positive primary breast cancer. Tumour Biol. 2016;37:9813–9823. doi: 10.1007/s13277-016-4870-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.