Abstract
Background
Although previous studies have evaluated the prognostic role of the systemic immune-inflammation index (SII) in patients with breast cancer, the results were inconsistent. Therefore, in this context, we aimed to identify the prognostic and clinicopathological value of the SII in patients with breast cancer by performing a meta-analysis.
Methods
A literature search was using PubMed, Web of Science, EMBASE, and Cochrane Library databases for relevant articles, from their inception to May 12, 2020. The prognostic value of the SII in breast cancer was assessed by pooling the hazard ratios (HRs) with 95% confidence intervals (CIs). The clinical outcomes included the overall survival (OS), disease-free survival (DFS), recurrence-free survival (RFS), and distant metastasis-free survival (DMFS). The methodological quality of all the included studies was evaluated using the Newcastle–Ottawa quality assessment scale. The odds ratios (ORs) with 95% CIs were combined to evaluate the correlation between the SII and clinicopathological characteristics of patients with breast cancer. Publication bias was evaluated using the Begg funnel plot and the Egger linear regression test. All statistical analyses were performed using Stata software, version 12.0 (Stata Corporation, College Station, TX, USA). A p value of < 0.05 was considered statistically significant.
Results
Eight studies involving 2642 patients were included in the current meta-analysis. The combined data showed that patients with a high SII had worse OS (HR = 1.79, 95% CI 1.33–2.42, p < 0.001), poorer DFS/RFS (HR = 1.79, 95% CI 1.31–2.46, p < 0.001), and inferior DMFS (HR = 1.64, 95% CI 1.32–2.03, p < 0.001) than patients with a low SII. In addition, a high SII was correlated with the presence of lymph node metastasis (OR = 1.38, 95% CI 1.12–1.69, p = 0.002), higher T stage (OR = 1.49, 95% CI 1.17–1.89, p < 0.001), advanced TNM stage (OR = 1.37, 95% CI 1.07–1.77, p = 0.014), and higher histological grade (OR = 3.71, 95% CI 1.00–13.73, p = 0.049). However, there was no significant association between the SII and the pathological type (OR = 0.82, 95% CI 0.55–1.23, p = 0.345) or lymphatic invasion (OR = 1.30, 95% CI 0.82–2.08, p = 0.266).
Conclusions
The results of our meta-analysis suggest that an elevated SII predicts poor survival outcomes and is associated with clinicopathological features that indicate tumor progression of breast cancer.
Keywords: Breast cancer, Meta-analysis, Systemic immune-inflammation index, Prognosis, Tumor microenvironment
Background
Breast cancer is the most common malignancy and the leading cause of cancer-related deaths in women worldwide [1]. In 2018, approximately 2,088,849 new cases of breast cancer and 626,679 deaths occurred worldwide [1]. Over the past several decades, the mortality due to breast cancer has decreased in Europe and in the United States because of early diagnosis and systemic treatments [2]. For patients with breast cancer with local and metastatic disease, the treatment approaches include surgery, radiotherapy, and systematic treatment with chemotherapy, endocrine therapy, and targeted therapy, or a combination of these [3]. However, the clinical outcomes of patients with breast cancer remain unsatisfactory owing to a lack of effective prognostic factors. Therefore, novel and reliable prognostic parameters need to be identified for designing personalized treatment regimens and for improving the survival of patients with breast cancer.
Tumor environment and inflammation play important roles in tumor development [4]. The components of the tumor microenvironment include the response cells, such as neutrophils, monocytes, lymphocytes, platelets, and cytokines. Several inflammatory cell parameters, including the neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, C-reactive protein/albumin ratio, and systemic immune-inflammation index (SII), are derived using these meditators. The SII is an index that is calculated on the basis of the platelet, neutrophil, and lymphocyte counts. The SII has been used to evaluate the pretreatment balance between inflammatory factors and immune status of patients with cancer [5–8]. The SII is associated with the prognosis of patients with breast cancer, although the results are controversial [9–16]. Therefore, we performed the current meta-analysis to identify the prognostic impact of the SII in patients with breast cancer by aggregating all available data.
Materials and methods
Search strategy
The current meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement [17]. A literature search was using PubMed, Web of Science, EMBASE, and Cochrane Library databases for relevant articles, from their inception to May 12, 2020. The following search terms were used: (systemic immune-inflammatory index or SII or systemic-immune-inflammation index or systemic immune-inflammation index) and (breast carcinoma or breast tumor or Breast Cancer or Breast Tumors or Cancer of Breast or Cancer of the Breast or Human Mammary Carcinoma or Mammary Carcinoma, Human or Mammary Neoplasm, Human or Mammary Neoplasms, Human or Neoplasms, Breast or Tumors, Breast or Breast Neoplasm or Breast Tumor or Cancer, Breast or Carcinoma, Human Mammary or Carcinomas, Human Mammary or Human Mammary Carcinomas or Human Mammary Neoplasm or Human Mammary Neoplasms or Mammary Carcinomas, Human or Neoplasm, Breast or Neoplasm, Human Mammary or Neoplasms, Human Mammary or Tumor, Breast). The references of the searched articles were also manually checked for additional relevant records. The language of publication was restricted to English. There were no restrictions on the study design (prospective or retrospective), location, or ethnicity. The current meta-analysis collected data from previously published studies; therefore, approval was not required from the ethical committee or medical institutional board.
Inclusion and exclusion criteria
The inclusion criteria for eligible studies were as follows: (1) all patients were diagnosed with breast cancer; (2) studies reported the association between the SII and prognosis of patients with breast cancer; (3) a cutoff value was given for defining a high and a low SII; (4) the hazard ratios (HRs) with 95% confidence intervals (CIs) for survival outcomes were reported or sufficient data were given for calculating the HRs with 95% CIs. The following studies were excluded: (1) letters, reviews, and case reports; (2) duplicate studies; (3) studies with insufficient data; and (4) animal studies.
Data extraction and quality assessment
Two independent investigators (Y.Z. and Y.S.) extracted the data from eligible studies by using a standardized form. Any disagreements were resolved via discussion with a third investigator (Q.Z.). The extracted information included the name of the first author, year of publication, country of study origin, study duration, molecular stratification of breast cancer, sample size, median age, clinical stage, ethnicity, treatment methods, SII cutoff value, method for cutoff determination, follow-up, survival outcomes, and HRs with 95% CIs. The clinical outcomes included the overall survival (OS), disease-free survival (DFS), recurrence-free survival (RFS), and distant metastasis-free survival (DMFS). The methodological quality of all the included studies was evaluated by using the Newcastle–Ottawa quality assessment scale (NOS) [18]. The NOS assesses the quality of the included studies by using a score of 0 to 9 points. Studies with a NOS score of ≥ 6 points were regarded as high-quality studies.
Statistical analysis
The prognostic value of the SII in patients with breast cancer was assessed by pooling the HRs and 95% CIs. The odds ratios (ORs) with 95% CIs were combined to evaluate the correlation between the SII and clinicopathological characteristics of patients with breast cancer. The heterogeneity among studies was evaluated using the Cochran Q test [19] and the Higgins I2 statistics [20]. Significant heterogeneity was defined as p < 0.10 and/or I2 > 50%, and then, a random-effects model was applied for pooling the data. Otherwise, a fixed-effects model was applied. Subgroup analysis—stratified by the molecular stratification, cutoff value of the SII, method for cutoff determination, and treatment—was performed to explore the sources of heterogeneity. Publication bias was evaluated using the Begg funnel plot [21] and the Egger linear regression test [22]. All statistical analyses were performed using Stata software, version 12.0 (Stata Corporation, College Station, TX, USA). A p-value of < 0.05 was considered statistically significant.
Results
Search results and study characteristics
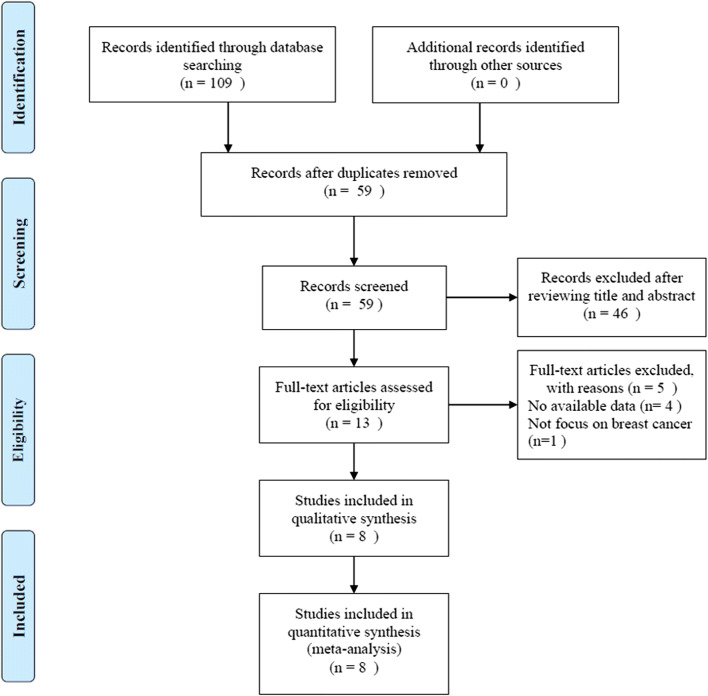
A total of 109 studies were identified after the initial search of the databases, and then, 50 duplicate records were removed. After screening the title and/or the abstract, 46 studies were eliminated on the basis of the inclusion criteria. Then, 13 full-text articles were evaluated for eligibility [9–16, 23–27]. A total of 5 studies were removed owing to the following reasons: 4 studies [23–25, 27] did not provide sufficient data for the current meta-analysis, and 1 study [26] included patients with different cancers, rather than breast cancer only. Finally, 8 studies [9–16] involving 2642 patients were included in the current meta-analysis. A flowchart of the literature search is shown in Fig. 1. The general characteristics of the enrolled studies are summarized in Table 1. The included studies were published from 2019 to 2020 and were mainly conducted in 2 countries, including 1 in Italy [9] and 7 in China [10–16]. The total sample size was 2642 patients, ranging from 147 to 1026 patients. Seven studies reported the prognostic value of the SII considering OS [9, 11–16], 7 studies provided data on the association between the SII and DFS/RFS [10–16], and 3 studies reported the correlation between the SII and DMFS [11, 12, 16]. The cutoff values of the SII ranged from 422 to 836 in the included studies. Considering the quality assessment of the eligible studies, all the studies had a NOS score of ≥ 6 and the median value was 7, indicating that all the included studies were high-quality studies.
Fig. 1.
Schematic flow diagram for selection of included studies
Table 1.
Basic characteristics of all included studies
| Author | Year | Country | Molecular stratification | Included period | Sample size | Age, years Median (range) |
Clinical stage | Ethnicity | Treatment | Survival outcomes | Cut-off value (10^9) | Cut-off determination | Follow-up, months Median (range) |
NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De Giorgi | 2019 | Italy | Mixed | 2004–2009 | 516 | 59 | IV | Caucasian | No‐surgery | OS | 836 | ROC analysis | 24 | 8 |
| Li | 2019 | China | Luminal BC | 2008–2013 | 161 | 58 | I–III | Asian | No-surgery | DFS | 518 | ROC analysis | 28.4 (1–79) | 7 |
| Liu | 2019 | China | TNBC | 2000–2012 | 160 | NA | I–III | Asian | Mixed | OS, DFS, DMFS | 557 | ROC analysis | 61.7 (5.9–159.0) | 6 |
| Sun | 2019 | China | HER2+ | 2002–2012 | 155 | NA | I–III | Asian | Mixed | OS, DFS, DMFS | 578 | Median value | 57.6 (10.4–158.2) | 6 |
| Wang | 2019 | China | TNBC | 2008–2016 | 215 | NA | I–III | Asian | Mixed | OS, DFS | 624 | Median value | 49.2 (4–105) | 7 |
| Chen | 2020 | China | Mixed | 1999–2014 | 262 | 48 (27–73) | II–III | Asian | No-surgery | OS, DFS | 602 | ROC analysis | NA | 8 |
| Hua | 2020 | China | Mixed | 2010–2012 | 1026 | 47 (22–87) | I–III | Asian | Mixed | OS, RFS, DMFS | 601.7 | ROC analysis | 68.5 (0.9–87.5) | 7 |
| Jiang | 2020 | China | HER2+ | 2011–2015 | 147 | NA | I–III | Asian | Mixed | OS, DFS | 442 | ROC analysis | 42 (15–78) | 7 |
BC breast cancer, TNBC triple-negative breast cancer, HER2 human epidermal growth factor receptor-2, OS overall survival, DFS disease-free survival, RFS recurrence-free survival, DMFS distant metastasis-free survival, ROC receiver operating characteristics, NA not available, NOS Newcastle–Ottawa Scale
Association between the SII and OS of patients with breast cancer
The data regarding the association between the SII and OS were available in 7 studies with 2481 patients [9, 11–16]. As shown in Fig. 2, the pooled HRs and 95% CIs revealed that patients with a high SII had worse OS (HR = 1.79, 95% CI 1.33–2.42, p < 0.001) than patients with a low SII. A random-effects model was used because of significant heterogeneity (I2 = 77.3%, p < 0.001; Fig. 2; Table 2). Subgroup analysis of OS was conducted on the basis of the molecular stratification, cutoff value of the SII, method for cutoff determination, and treatment. The subgroup analysis showed that an elevated SII was associated with poor OS of patients with triple-negative breast cancer (TNBC; HR = 2.82, 95% CI 2.22–3.59, p < 0.001), patients with breast cancer that was positive for human epidermal growth factor receptor-2 (HR = 1.71, 95% CI 1.23–2.39, p = 0.002), and patients with mixed molecular stratification (HR = 1.33, 95% CI 1.09–1.63, p = 0.005; Table 2). Considering the cut-off value of the SII, an SII cut-off value of ≤ 600 (HR = 2.03, 95% CI 1.57–2.62, p < 0.001) and an SII cut-off value of > 600 (HR = 1.63, 95% CI 1.04–2.56, p = 0.033) showed prognostic value for poor OS. Regarding the cut-off determination methods, both receiver operating characteristic curve analysis (HR = 1.65, 95% CI 1.20–2.26, p = 0.002) and the median value (HR = 2.14, 95% CI 1.11–4.14, p = 0.024) to determine the cut-off value were correlated with poor OS. Considering the treatment, a high SII showed a prognostic value for worse OS of patients receiving mixed treatments (HR = 2.15, 95% CI 1.61–2.86, p < 0.001; Table 2).
Fig. 2.
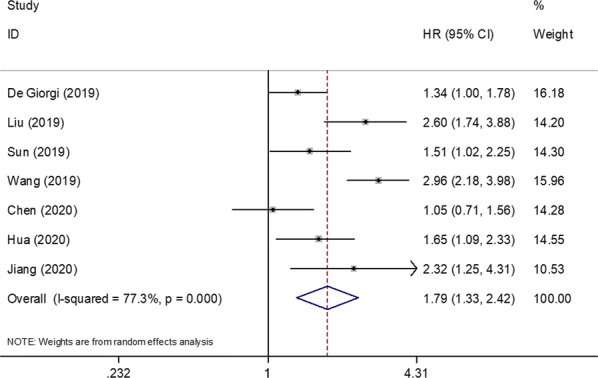
Forest plot of the correlation between SII and overall survival in patients with breast cancer
Table 2.
Stratified analysis of pooled HR of breast cancer patients with SII on OS, DFS, RFS, and DMFS
| Subgroup analysis | No. of studies | No. of patients | HR (95% CI) | p | Effects model | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2 (%) | p | ||||||
| OS | |||||||
| Total | 7 | 2481 | 1.79 (1.33–2.42) | < 0.001 | Random | 77.3 | <0.001 |
| Molecular stratification | |||||||
| Mixed | 3 | 1804 | 1.33 (1.09–1.63) | 0.005 | Fixed | 21.9 | 0.278 |
| TNBC | 2 | 375 | 2.82 (2.22–3.59) | < 0.001 | Fixed | 0 | 0.612 |
| HER2+ | 2 | 302 | 1.71 (1.23–2.39) | 0.002 | Fixed | 23.9 | 0.252 |
| Cut-off value of SII | |||||||
| ≤ 600 | 3 | 462 | 2.03 (1.57–2.62) | < 0.001 | Fixed | 47.3 | 0.150 |
| > 600 | 4 | 2019 | 1.63 (1.04–2.56) | 0.033 | Random | 85.9 | < 0.001 |
| Method for cut-off determination | |||||||
| ROC analysis | 5 | 2111 | 1.65 (1.20–2.26) | 0.002 | Random | 68.7 | 0.012 |
| Median value | 2 | 370 | 2.14 (1.11–4.14) | 0.024 | Random | 85.8 | 0.008 |
| Treatment | |||||||
| Mixed | 5 | 1703 | 2.15 (1.61–2.86) | <0.001 | Random | 60.5 | 0.038 |
| No-surgery | 2 | 778 | 1.23 (0.98–1.56) | 0.080 | Fixed | 0 | 0.330 |
| DFS/RFS | |||||||
| Total | 7 | 2126 | 1.79 (1.31–2.46) | <0.001 | Random | 66.0 | 0.007 |
| Molecular stratification | |||||||
| Mixed | 2 | 1288 | 1.34 (0.89–2.01) | 0.160 | Random | 56.6 | 0.129 |
| Luminal BC | 1 | 161 | 6.04 (1.82–19.98) | 0.003 | – | – | – |
| TNBC | 2 | 375 | 2.03 (1.06–3.88) | 0.033 | Random | 81.0 | 0.022 |
| HER2+ | 2 | 302 | 1.94 (0.83–4.56) | 0.128 | Random | 52.0 | 0.149 |
| Cut-off value of SII | |||||||
| ≤ 600 | 4 | 623 | 1.92 (1.20–3.08) | 0.006 | Random | 57.2 | 0.072 |
| > 600 | 3 | 1503 | 1.70 (1.01–2.88) | 0.047 | Random | 81.1 | 0.005 |
| Method for cut-off determination | |||||||
| ROC analysis | 5 | 1756 | 1.68 (1.14–2.47) | 0.008 | Random | 60.7 | 0.038 |
| Median value | 2 | 370 | 2.02 (1.05–3.89) | 0.035 | Random | 81.5 | 0.020 |
| Treatment | |||||||
| Mixed | 5 | 1703 | 1.82 (1.36–2.42) | < 0.001 | Random | 52.5 | 0.078 |
| No-surgery | 2 | 423 | 2.31 (0.43–12.43) | 0.328 | Random | 86.1 | 0.007 |
| DMFS | |||||||
| Total | 3 | 1341 | 1.64 (1.32–2.03) | < 0.001 | Fixed | 0 | 0.590 |
Association between the SII and DFS/RFS of patients with breast cancer
A total of 7 studies consisting of 2126 patients [10–16] investigated the association between the SII and DFS/RFS. Owing to significant heterogeneity (I2 = 66.0%, p = 0.007), a random-effects model was applied (Fig. 3; Table 2). The combined data showed that an elevated SII was correlated with poor DFS/RFS of patients with breast cancer (HR = 1.79, 95% CI 1.31–2.46, p < 0.001; Fig. 3). Subgroup analysis stratified by molecular stratification showed that a high SII was associated with poor DFS/RFS of patients with luminal breast cancer (HR = 6.04, 95% CI 1.82–19.98, p = 0.003) and patients with TNBC (HR = 2.03, 95% CI 1.06–3.88, p = 0.033; Table 2). In addition, an SII cut-off value of ≤ 600 (HR = 1.92, 95% CI 1.20–3.08, p = 0.006) and > 600 (HR = 1.70, 95% CI 1.01–2.88, p = 0.047) predicted poor DFS/RFS. A high SII was associated with poor DFS/RFS of patients receiving mixed treatments (HR = 1.8, 95% CI 1.36–2.42, p < 0.001; Table 2).
Fig. 3.
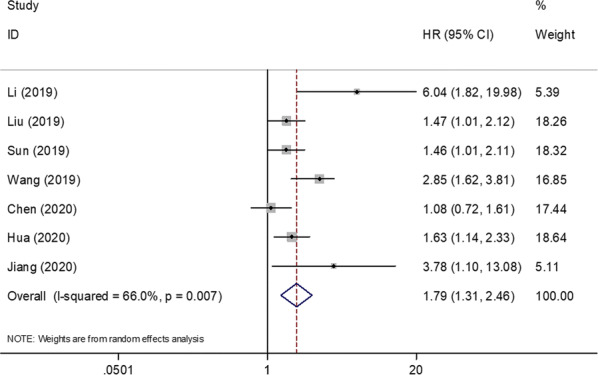
Forest plot of the correlation between SII and disease-free survival/recurrence-free survival in patients with breast cancer
Association between the SII and DMFS of patients with breast cancer
Three studies involving 1341 patients [11, 12, 16] provided data regarding the prognostic impact of the SII for DMFS. The pooled HR and 95% CI were 1.64 and 1.32–2.03, respectively (p < 0.001), with no significant heterogeneity (I2 = 0%, p = 0.590; Fig. 4; Table 2). Owing to the limited sample size, subgroup analysis was not performed for DMFS.
Fig. 4.
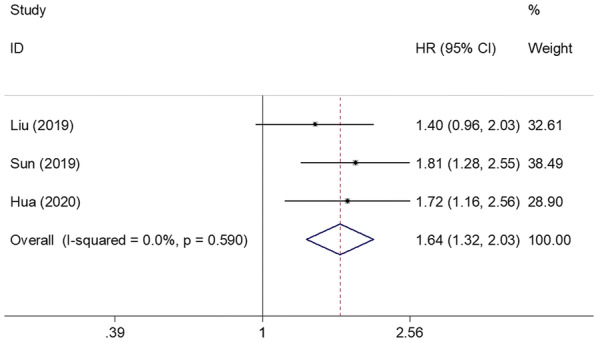
Forest plot of the correlation between SII and distant metastasis-free survival in patients with breast cancer
Association between the SII and clinicopathological features of patients with breast cancer
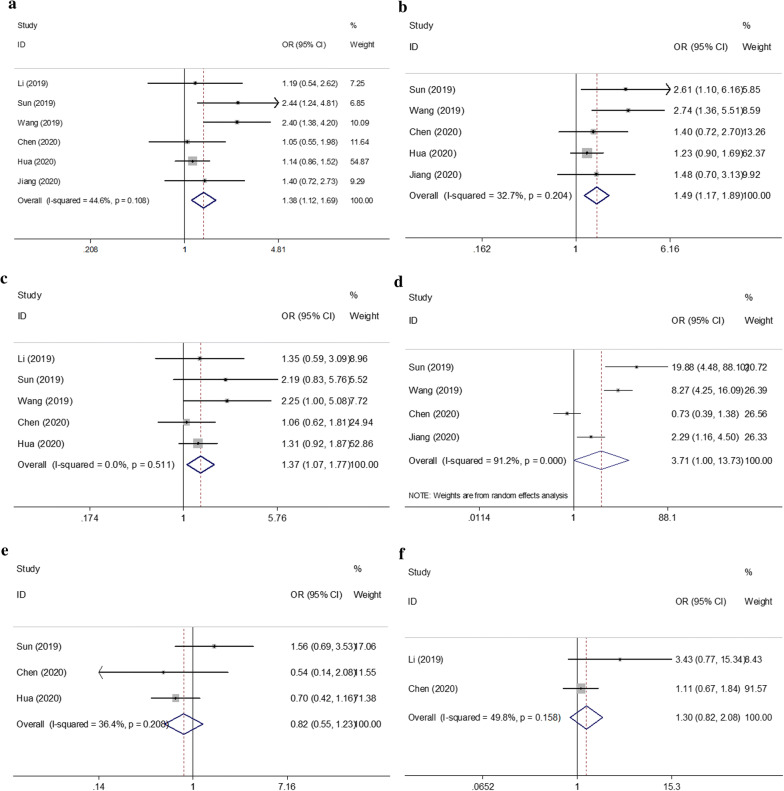
The relationship between the SII and clinicopathological characteristics was analyzed by using data from 6 studies with 1966 patients [10, 12–16]. Six clinicopathological factors were investigated, including lymph node metastasis (presence vs. absence), T stage (T2–T4 vs. T1), TNM stage (II–III vs. 0–I), histological grade (G3 vs. G1–G2), pathological type (intralobular carcinoma vs. intraductal carcinoma), and lymphatic invasion (presence vs. absence). The combined ORs and 95% CIs indicated that a high SII was correlated with the presence of lymph node metastasis (OR = 1.38, 95% CI 1.12–1.69, p = 0.002), higher T stage (OR = 1.49, 95% CI 1.17–1.89, p < 0.001), advanced TNM stage (OR = 1.37, 95% CI 1.07–1.77, p = 0.014), and higher histological grade (OR = 3.71, 95% CI 1.00–13.73, p = 0.049; Fig. 5; Table 3). However, there was no significant association between the SII and pathological type (OR = 0.82, 95% CI 0.55–1.23, p = 0.345) or lymphatic invasion (OR = 1.30, 95% CI 0.82–2.08, p = 0.266; Fig. 5; Table 3).
Fig. 5.
Forest plots for association between SII and various clinicopathological features in breast cancer. a presence of lymph node (LN) metastasis (yes vs no); b T stage (T2–T4 vs T1); c TNM stage (II–III vs 0–I); d histological grade (G3 vs G1–G2); e pathological type (intralobular carcinoma vs intraductal carcinoma) and f lymphatic invasion (yes vs no)
Table 3.
Relationship between SII and clinicopathological variables in breast cancer
| Clinicopathological features | No. of studies | No. of patients | OR (95% CI) | p | Effects model | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2 (%) | p | ||||||
| LN metastasis (yes vs no) | 6 | 1966 | 1.38 (1.12–1.69) | 0.002 | Fixed | 44.6 | 0.108 |
| T stage (T2–T4 vs T1) | 5 | 1805 | 1.49 (1.17–1.89) | 0.001 | Fixed | 32.7 | 0.204 |
| TNM stage (II–III vs 0–I) | 5 | 1819 | 1.37 (1.07–1.77) | 0.014 | Fixed | 0 | 0.511 |
| Histological grade (G3 vs G1–G2) | 4 | 779 | 3.71 (1.00–13.73) | 0.049 | Random | 91.2 | < 0.001 |
| Pathological type (ILC vs IDC) | 3 | 1443 | 0.82 (0.55–1.23) | 0.345 | Fixed | 36.4 | 0.208 |
| Lymphatic invasion (yes vs no) | 2 | 423 | 1.30 (0.82–2.08) | 0.266 | Fixed | 49.8 | 0.158 |
OR odds ratio, G grade, ILC intralobular carcinoma, IDC intraductal carcinoma
Publication bias
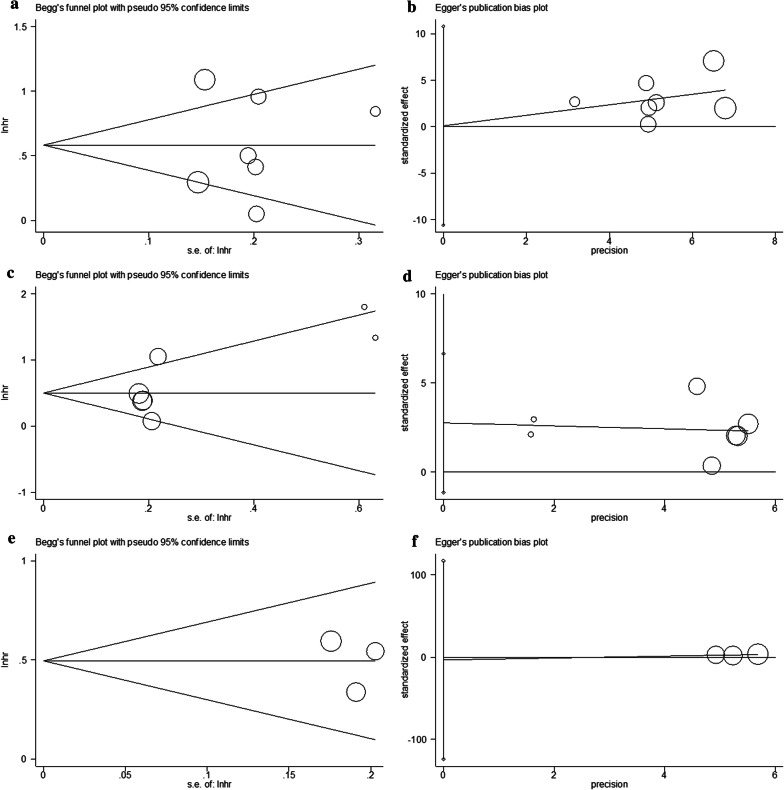
The Begg funnel plot and the Egger test were conducted to evaluate potential publication bias for OS, DFS/RFS, and DMFS analysis. For OS, the test results suggested that the potential publication bias was negative (p = 0.881 on the Begg test, and p = 0.981 on the Egger test; Fig. 6). Similarly, there was no significant publication bias for DFS/RFS (p = 0.548 on the Begg test, and p = 0.128 on the Egger test) or DMFS (p = 0.602 on the Begg test, and p = 0.785 on the Egger test; Fig. 6).
Fig. 6.
Begg’s funnel plot and Egger’s test for the assessment of publication bias in the meta-analysis. a Begg’s test for OS; b Egger’s test for OS; c Begg’s test for DFS/RFS; d Egger’s test for DFS/RFS; e Begg’s test for DMFS; and f Egger’s test for DMFS
Discussion
In the current meta-analysis, we evaluated the prognostic influence of the SII in patients with breast cancer. Our results showed that the SII was associated with worse OS, DFS/RFS, and DMFS. Moreover, the prognostic effect of the SII remained consistent for patients with TNBC as well as when the cutoff value of the SII was ≤ 600. We also found that a high SII was associated with the clinical characteristics that indicated tumor progression and high malignancy, including the presence of lymph node metastasis, a higher T stage, advanced TNM stage, and higher histological grade. As the SII is a blood-derived parameter and is easily available, it is an optimal tool for aiding in the prognostication of patients with breast cancer. To the best of our knowledge, the present study is the first meta-analysis to evaluate the prognostic and clinicopathological value of the SII in patients with breast cancer.
The SII is calculated by using the following formula: neutrophil count × platelet count/lymphocyte count; the SII was developed as a prognostic factor for determining the survival outcomes of patients with various cancers in clinical practice [28–34]. As the SII is an index of the combination of neutrophil, platelet, and lymphocyte counts, a high SII could be attributed to the changes in the counts of these cells. Neutrophils can exert tumor-promoting activity by secreting a variety of inflammatory mediators, including vascular endothelial growth factor, interleukin (IL)-6, IL-10, and IL-22 [35]. Platelets can protect cancer cells from lysis by natural killer cells [36] and promote cancer cell arrest in the endothelium, supporting the formation of secondary lesions [37]. In contrast, lymphocytes are involved in cancer immune-surveillance to inhibit cancer progression [38]. Therefore, low lymphocyte counts may result in inadequate immunological reactions in patients with cancer [39].
The prognostic effect of the SII has been studied in many human tumors by using a meta-analysis approach [40–43]. A comprehensive meta-analysis containing 15 articles showed that an SII greater than the cutoff predicted poor OS in various cancers [40]. Moreover, another meta-analysis including 9 studies with 2441 patients revealed that an elevated pretreatment SII indicated significantly poorer OS, DFS/progression-free survival, and cancer-specific survival of patients with non-small cell lung cancer [42]. A recent meta-analysis published in 2020 demonstrated that an elevated SII was a poor prognostic factor for patients with hepatocellular carcinoma [43]. The results of the current meta-analysis extend the prognostic role of the SII for breast cancer. Therefore, we recommend that the SII be used to predict the prognosis of patients with breast cancer.
The current meta-analysis has several limitations. First, significant heterogeneity was detected even though we selected a random-effects model for calculation. Second, most eligible studies were from China; therefore, the results may be more relevant to Chinese patients. The prognostic value of the SII for patients of other nationalities still needs to be verified. Third, the cutoff value of the SII was not uniform among the studies, which may have introduced a selection bias in the meta-analysis.
Conclusions
In summary, the results of our meta-analysis suggest that an elevated SII predicts poor survival outcomes and is associated with clinicopathological features that indicate tumor progression of breast cancer. However, owing to the several limitations, more prospective studies including patients with diverse ethnicities are needed to confirm our results.
Acknowledgements
Not applicable.
Abbreviations
- SII
Systemic immune-inflammation index
- HR
Hazard ratio
- CI
Confidence interval
- OS
Overall survival
- DFS
Disease-free survival
- RFS
Recurrence-free survival
- DMFS
Distant metastasis-free survival
- NLR
Neutrophil–lymphocyte ratio
- PLR
Platelet-lymphocyte ratio
- CAR
C-reactive protein/albumin ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement
- NOS
Newcastle–Ottawa quality assessment scale
- OR
Odds ratio
- TNBC
Triple-negative breast cancer
- HER2
Human epidermal growth factor receptor-2
- ILC
Intralobular carcinoma
- IDC
Intraductal carcinoma
- IL-6
Interleukin-6
- PFS
Progression-free survival
- CSS
Cancer-specific survival
Authors’ contributions
YZ and YS wrote the paper by analyzing the above data; YZ and QZ collected and draw out data. YS and QZ conducted quality assessment. YZ and YS designed this study. All authors accepted the eventual manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N, Gnant M. Breast Cancer. Lancet. 2017;389(10074):1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 3.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A, Giordano SH, et al. Breast Cancer, Version 4.2017 Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(3):310–320. doi: 10.6004/jnccn.2018.0012. [DOI] [PubMed] [Google Scholar]
- 4.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 5.Zhang K, Hua YQ, Wang D, Chen LY, Wu CJ, Chen Z, Liu LM, Chen H. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J Transl Med. 2019;17:30. doi: 10.1186/s12967-019-1782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang WT, Wang RL, Ma WC, Wu Y, Maskey N, Guo YD, Liu J, Mao SY, Zhang JF, Yao XD, et al. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy. Annals of Translational Medicine. 2019;20(23):6212–6222. doi: 10.21037/atm.2019.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murthy P, Zenati MS, Al Abbas AI, Rieser CJ, Bahary N, Lotze MT, Zeh HJ, 3rd, Zureikat AH, Boone BA. Prognostic Value of the Systemic Immune-Inflammation Index (SII) after neoadjuvant therapy for patients with resected pancreatic cancer. Ann Surg Oncol. 2020;27(3):898–906. doi: 10.1245/s10434-019-08094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Jin S, Xu S, Cao S. High Systemic Immune-Inflammation Index (SII) represents an unfavorable prognostic factor for small cell lung cancer treated with etoposide and platinum-based chemotherapy. Lung. 2020;198(2):405–414. doi: 10.1007/s00408-020-00333-6. [DOI] [PubMed] [Google Scholar]
- 9.De Giorgi U, Mego M, Scarpi E, Giordano A, Giuliano M, Valero V, Alvarez RH, Ueno NT, Cristofanilli M, Reuben JM. Association between circulating tumor cells and peripheral blood monocytes in metastatic breast cancer. Ther Adv Med Oncol. 2019;11:1758835919866065. doi: 10.1177/1758835919866065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li QX, Shi DJ, Zhang LX, Wang DM, Zhao J, Wang T, Deng XN, Fan XY. Association of body mass and systemic immune-inflammation indices with endocrine therapy resistance in luminal breast cancers. J Int Med Res. 2019;47(5):1936–1947. doi: 10.1177/0300060519831570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu JX, Shi ZZ, Bai YS, Liu L, Cheng KL. Prognostic significance of systemic immune-inflammation index in triple-negative breast cancer. Cancer Manag Res. 2019;11:4471–4480. doi: 10.2147/CMAR.S197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Li WQ, Li AJ, Su HC, Yue JB, Yu JM. Increased systemic immune-inflammation index independently predicts poor survival for hormone receptor-negative, HER2-positive breast cancer patients. Cancer Manag Res. 2019;11:3153–3162. doi: 10.2147/CMAR.S190335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P, Yue W, Li WY, Luo YQ, Li ZK, Shao Y, He ZZ. Systemic immune-inflammation index and ultrasonographic classification of breast imaging-reporting and data system predict outcomes of triple-negative breast cancer. Cancer Manag Res. 2019;11:813–819. doi: 10.2147/CMAR.S185890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J Cell Mol Med. 2020;24(5):2993–3021. doi: 10.1111/jcmm.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang L, Fang JJ, Ding JH. High systemic immune-inflammation index predicts poor survival in patients with human epidermal growth factor receptor-2 positive breast cancer receiving adjuvant trastuzumab. Cancer Manag Res. 2020;12:475–484. doi: 10.2147/CMAR.S231444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua X, Long ZQ, Zhang YL, Wen W, Guo L, Xia W, Zhang WW, Lin HX. Prognostic value of preoperative systemic immune-inflammation index in breast cancer: a propensity score-matching study. Front Oncol. 2020;10:580. doi: 10.3389/fonc.2020.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 19.Cochran W. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 20.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Giorgi U, Mego M, Scarpi E, Giordano A, Giuliano M, Valero V, Alvarez RH, Ueno NT, Cristofanilli M, Reuben JM: Correlation of circulating tumor cells (CTCs) with peripheral blood leukocytes to predict outcome in metastatic breast cancer (MBC). J Clin Oncol. 2016; 34.
- 24.Merloni F, Pistelli M, Cantini L, Della Mora A, Bastianelli L, De Lisa M, Burattini M, Maccaroni E, Ballatore Z, Savini A, et al. Role of inflammation parameters in locally advanced breast cancer: the debate is still open. Ann Oncol. 2017;28:37. [Google Scholar]
- 25.van der Willik KD, Koppelmans V, Hauptmann M, Compter A, Ikram MA, Schagen SB. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: a cohort study. Breast Cancer Res. 2018;20(1):135. doi: 10.1186/s13058-018-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fest J, Ruiter R, Mulder M, Groot Koerkamp B, Ikram MA, Stricker BH, van Eijck CHJ. The systemic immune-inflammation index is associated with an increased risk of incident cancer—a population-based cohort study. Int J Cancer. 2020;146(3):692–698. doi: 10.1002/ijc.32303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miklikova S, Minarik G, Sedlackova T, Plava J, Cihova M, Jurisova S, Kalavska K, Karaba M, Benca J, Smolkova B, et al. Inflammation-Based Scores Increase the Prognostic Value of Circulating Tumor Cells in Primary Breast Cancer. Cancers (Basel) 2020;12:5. doi: 10.3390/cancers12051134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao YB, Guo W, Cai SH, Zhang F, Shao F, Zhang GC, Liu TJ, Tan FW, Li N, Xue Q, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected esophageal squamous cell carcinoma. J Cancer. 2019;10(14):3188–3196. doi: 10.7150/jca.30281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang HP, Liu Q, Zhu LX, Zhang Y, Lu XJ, Wu YW, Liu L. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9:3284. doi: 10.1038/s41598-019-39150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X, Hu HZ, Zhang WY, Shao ZW. Prognostic value of prognostic nutritional index and systemic immune-inflammation index in patients with osteosarcoma. J Cell Physiol. 2019;234(10):18408–18414. doi: 10.1002/jcp.28476. [DOI] [PubMed] [Google Scholar]
- 31.Man YN, Chen YF. Systemic immune-inflammation index, serum albumin, and fibrinogen impact prognosis in castration-resistant prostate cancer patients treated with first-line docetaxel. Int Urol Nephrol. 2019;51(12):2189–2199. doi: 10.1007/s11255-019-02265-4. [DOI] [PubMed] [Google Scholar]
- 32.Mirili C, Paydas S, Kapukaya TK, Yilmaz A. Systemic immune-inflammation index predicting survival outcome in patients with classical Hodgkin lymphoma. Biomark Med. 2019;13(18):1565–1575. doi: 10.2217/bmm-2019-0303. [DOI] [PubMed] [Google Scholar]
- 33.Nie D, Gong H, Mao XG, Li ZY. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: a retrospective study. Gynecol Oncol. 2019;152(2):259–264. doi: 10.1016/j.ygyno.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 34.Wang DY, Guo D, Shi F, Zhu Y, Li AJ, Kong L, Teng FF, Yu JM. The predictive effect of the systemic immune-inflammation index for patients with small-cell lung cancer. Future Oncol. 2019;15(29):3367–3379. doi: 10.2217/fon-2019-0288. [DOI] [PubMed] [Google Scholar]
- 35.Tan KW, Chong SZ, Wong FH, Evrard M, Tan SM, Keeble J, Kemeny DM, Ng LG, Abastado JP, Angeli V. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood. 2013;122(22):3666–3677. doi: 10.1182/blood-2012-11-466532. [DOI] [PubMed] [Google Scholar]
- 36.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–1300. [PubMed] [Google Scholar]
- 37.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Chen L, Zhang F, Sheng XG, Zhang SQ. Decreased pretreatment lymphocyte/monocyte ratio is associated with poor prognosis in stage Ib1-IIa cervical cancer patients who undergo radical surgery. Onco Targets Ther. 2015;8:1355–1362. doi: 10.2147/OTT.S82174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8(43):75381–75388. doi: 10.18632/oncotarget.18856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–3302. doi: 10.7150/jca.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Li Y, Chen P, Xu W, Wu Y, Che G. Prognostic value of the pretreatment systemic immune-inflammation index (SII) in patients with non-small cell lung cancer: a meta-analysis. Ann Transl Med. 2019;7(18):433. doi: 10.21037/atm.2019.08.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang B, Huang Y, Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2020;99(1):e18571. doi: 10.1097/MD.0000000000018571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.