Abstract
Background
Previous literatures have implied that the liver fat deposition plays a crucial role in the development and progression of insulin resistance. In the present study, we aimed to investigate the association of liver fat content (LFC) with glucose metabolism status in the population of newly diagnosed type 2 diabetes mellitus (nT2DM), prediabetes mellitus (PDM) and normal controls (NC), and assessing if the LFC could as an indicator for the prediction of T2DM.
Methods
A total of 242 subjects (including 141 nT2DM patients, 48 PDM subjects and 53 NC) were enrolled. The levels of LFC were quantified by using the proton magnetic resonance spectroscopy ([1H]-MRS) technique. Clinical and laboratory parameters of study subjects were collected by medical records and biochemical detection. One-way ANOVA or nonparametric test (Kruskal–Wallis) was applied for intergroup comparisons; intergroup comparison was performed in using of Bonferroni multiple-significance-test correction.
Results
There were significantly increased LFC levels in nT2DM (14.72% ± 6.37%) than in PDM (9.62% ± 4.41%) and that of NC groups (5.11% ± 3.66%) (all p < 0.001). The prevalence of nonalcoholic fatty liver disease (NAFLD) was also found to be increased in nT2DM (91.48%) than in PDM (85.41%) and that of NC (32.07%) groups. Correlation analysis revealed that the increase of LFC positively associated with fast plasma glucose (FPG), 2 h plasma glucose (PG), Delta G30 and homeostatic model assessment of insulin resistance (HOMA-IR), negatively associated with Delta Ins30, Delta C30, Ins30/G30 AUC, CP30/G30 AUC, Ins AUC/G AUC, CP AUC/G AUC, homeostatic model assessment for β-cell function index (HOMA-β) and matsuda insulin sensitivity index (Matsuda ISI). Multilinear regression analysis showed that LFC, body mass index (BMI) and diastolic blood pressure (DBP) contributed for the prediction of HOMA-IR, and total cholesterol (TC), age, waist circumference (WC) and low-density lipoprotein cholesterol (LDL-C) were the significant contributors for HOMA-β.
Conclusions
Our study revealed an increased LFC level and prevalence of NAFLD in nT2DM than in PDM and that of NC groups, the increase of LFC was closely associated with insulin resistance and impaired glucose metabolism status, may be regarded as potential indicator contributing to the development and progression of T2DM.
Keywords: Liver fat content, Insulin resistance, Magnetic resonance imaging, T2DM
Background
Type 2 diabetes mellitus (T2DM) is a complex, multifactorial, chronic metabolic disease characterized as insulin resistance and impaired pancreatic β-cell function [1, 2]. Up to date, the etiology of T2DM is still not clear. Non-alcoholic fatty liver disease (NAFLD) is defined as the presence of a significant amount of fat deposition in the liver after excluding the secondary causes of fat accumulation in the liver (alcohol consumption, medications or other causes of liver diseases, such as viral hepatitis, autoimmune hepatitis, etc.) [3]. It has been disclosed that NAFLD associated with different types of diseases, such as obesity, diabetes, hypertension and metabolic syndrome [4–7]. The incidence of NAFLD in the general population is approximately 20–30%, but reaches nearly 75% in patients with T2DM [8]. In the past few years, emerging evidence has revealed that the association of NAFLD with an increased risk for T2DM and metabolic syndrome [9, 10].
Liver fat content (LFC) has been regarded as an important clinical indicator for evaluation and diagnosis of NAFLD [11]. Liver biopsy with direct histological visualization remains the current golden standard to evaluate the LFC and diagnose NAFLD. However, due to the invasive nature of the procedure, the clinical application of liver biopsy is limited [12, 13].
The proton magnetic resonance spectroscopy ([1H]-MRS) has been recently demonstrated as an accurate, non-invasive option for quantification of LFC [14, 15]. In addition, studies have shown that [1H]-MRS had a high consistency with liver biopsy in quantification of LFC, and could be regarded as a reliable and accurate method in assessing LFC [16, 17].
In the present study, we used [1H]-MRS to quantify the LFC in nT2DM and PDM and normal controls (NC), investigating the prevalence of NAFLD and exploring the association of LFC with glucose metabolism status and several clinical or laboratory parameters among those groups. In addition, we also evaluate if the LFC could as a reliable and effective indicator for the prediction of T2DM.
Materials and methods
Study subjects and methods
This is a single-center, observational study. A total of 242 subjects (141 nT2DM patients, 48 PDM subjects and 53 NC) were recruited from the Department of Endocrinology and medical examination center at the Second People’s Hospital of Hefei, when they first visited the DM clinic. For the clinical diagnosis of PDM and T2DM, the American Diabetes Association diagnostic criteria 2018 was applied [18]. PDM was defined as those without DM but fasting plasma glucose (FPG) value ≥ 5.6 mmol/l and FPG < 6.9 mmol/l or the 2 h plasma glucose (PG) value ≥ 7.8 mmol/l and 2hPG < 11.1 mmol/l after a 75-g oral glucose tolerance test (OGTT) using a glucose load containing the equivalent of 75-g anhydrous glucose dissolved in water. Patients with alcohol consumption, medications or other causes of liver diseases (viral hepatitis, autoimmune hepatitis, Wilson’s disease, hemochromatosis, drug-induced hepatitis) were excluded. NC subjects, without any history of liver or metabolic diseases, were enrolled from the medical examination center. Anthropometric measurement, clinical manifestations and routine laboratory results were obtained from hospital medical records.
The height and weight of each participant clothed in a light gown was measured. Body mass index (BMI) was computed as weight (kg) divided by height (m) squared. Waist circumference (WC) was assessed with a soft tape at the midpoint between the lowest rib margin and iliac crest, and the hip circumference was scaled at the widest level over the greater trochanters. The waist-to-hip ratio (WHR) was calculated as the WC divided by the hip circumference. After a preliminary 5-min rest in the sitting position, blood pressure was measured three times on right arm using an automated sphygmomanometer (OMRON Model HEM-752 FUZZY, Omron Co., Dalian, China), and the average systolic and diastolic blood pressure was calculated.
All study subjects have undergone tests for: total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), lactate dehydrogenase (LDH), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), creatinine, uric acid (UA), apolipoprotein-A1 (ApoA1) and apolipoprotein-B (ApoB). The blood biochemical indices were determined by a model 7600 automated bio-analyzer (Hitachi, Tokyo, Japan) or immunoturbidimetry (Roche/Cobas Integra Tinaquant, Roche Diagnostics).
After 10–12 h in the fasting state, the standard 75-g OGTT test was performed in all study subjects (including nT2DM, PDM and NC), then, FPG, 30 min PG, 60 min PG, 120 min (2 h) PG, fasting insulin, 30 min insulin, 60 min insulin, 120 min insulin, fasting C-peptide, 30 min insulin C-peptide, 60 min C-peptide and 120 min insulin C-peptide were measured by the hexokinase method (Audit Diagnostics, Ireland) or the direct chemical luminescence method (Siemens, USA).
The formulas for calculating insulin resistance, β-cell function and insulin sensitivity were as follows: homeostatic model assessment of insulin resistance (HOMA-IR) = fasting serum insulin (mmol/ml) × FPG (mmol/l)/22.5 [19], homeostatic model assessment for β-cell function (HOMR-β) = (fasting insulin (μU/ml) × 20/FPG (mmol/l) − 3.5) [20], and Matsuda insulin sensitivity index (Matsuda ISI) = 10000/(Glu0 × Ins0 × Glumean × Insmean)1/2 [21].
Standard protocol approvals and patient consents
This study was approved by the Ethical Committee of the Second People’s Hospital of Hefei (Hefei, Anhui, China) (approval number 1523). All the study subjects provided informed consent to participate in this study.
[1H]-MRS quantify LFC
All participants underwent the liver 1H-MRS to quantify the LFC (GE Signa HDxT 3.0T scanning system, GE Medical Systems, Inc., Waukesha, WI, USA). Sagittal, coronal and axial slices covering the whole liver were preliminarily acquired for positioning of the spectroscopy acquisition voxel. Three independent 20 × 20 × 20 mm voxels were placed within the right lobe of the liver. During the voxel placement, the vessels, bile ducts and focal lesions should be avoided. The proton spectrum was acquired using the body coil after shimming over the volume of interest by means of a point-resolved spectroscopy (PRESS) sequence with the following parameters: repetition time = 3, 333 ms, echo time = 144 ms. The operator calculated the LFC by determining the signal intensity of the in-phase (IP) and out of phase (OP) images at identical locations within regions of interest (ROI). Each ROI was measured three times and the average ROI was calculated as the final value. The captured IP and OP images of the liver were transferred to the GE SAGE software (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) for further data processing. LFC was calculated as fat fraction 100 × (area under the curve [AUC] fat peak/[AUC fat peak + water peak]) [22–24]. All study subjects were carefully instructed to hold their breath during the end of inspiration to ensure the consistency among subjects. The cut-off value for diagnosis of NAFLD was set as above 5.56% [25].
The repeated measures LFC in using [1H]-MRS were independently performed in 100 study subjects within the two different ROI regions by a skilled operator. Variability analysis, by calculating intraclass correlation coefficients (ICC) and depicting Bland–Altman plots, was implemented to evaluate the consistency and reliability of [1H]-MRS quantified LFC by the same operator [26]. The results indicated that the repeated quantified LFC by the same operator showed relative high degrees of consistencies (ICC = 0.997) (Additional file 1: Figure S1).
Statistical analysis
Continuous data were presented as mean ± standard deviation (SD) or the median (interquartile range, IQR) if they were not in normal distribution. One-way ANOVA or nonparametric test (Kruskal–Wallis test) was applied for intergroup comparisons; intragroup comparisons were performed in using Bonferroni multiple-significance-test correction. Chi square test or Fisher’s exact test was used to analyze categorical variables. Statistical correlation analysis was determined by Pearson’s correlation or Spearman’s rank correlation. To identify the contribution of LFC and traditional risk factors on the influence of HOMA-IR or HOMA-β, multivariate linear regression (MLR) analyses were used to detect independent associations of HOMA-IR or HOMA-β with LFC and traditional risk factors of age, BMI, WC, WHR, systolic blood pressure (SBP), diastolic blood pressure (DBP), TC, TG, HDL-C, LDL-C, VLDL-C. Receiver operating characteristic (ROC) analysis was constructed and the area under the curve (AUC) was calculated. Statistical analysis was performed with the use of SPSS software, version 23.0 (SPSS Inc., Chicago, IL, USA). All results with a two tailed p < 0.05 were considered to be statistically significant.
Results
Characteristics of the study population
Demographic and clinical characteristics of study subjects were displayed in Table 1. There were significant differences in BMI, WC, hip circumference, WHR, SBP, DBP, UA, IBIL, AST, ALT, GGT, LDH, TC, TG, LDL-C, HDL-C, VLDL-C, ApoA1, fasting insulin, FPG, 2hPG, HOMA-IR, HOMA-β and Matsuda ISI among nT2DM, PDM and NC groups (all p < 0.05). However, we did not find significant differences in age and gender distributions among those groups (all p > 0.05).
Table 1.
Demographic and clinical characteristic of study subjects
| Parameters | nT2DM (n = 141) |
PDM (n = 48) |
NC (n = 53) |
p value |
|---|---|---|---|---|
| Age (year) | 50.8 ± 10.1 | 49.7 ± 13.0 | 47.0 ± 8.0 | 0.078 |
| Gender (female/male) | 69/72 | 23/25 | 25/28 | 0.974 |
| BMI (kg/cm2) | 26.5 (25.4, 27.7) | 25.7 (24.1, 26.8) | 23.6 (22.1, 24.6) | 0.000 |
| WC (cm) | 88.72 ± 9.94 | 91.87 ± 9.00 | 83.08 ± 7.81 | 0.000 |
| HIP (cm) | 92.06 ± 8.14 | 98.63 ± 8.39 | 95.19 ± 6.96 | 0.000 |
| WHR | 0.95 (0.92, 1.04) | 0.90 (0.88, 0.94) | 0.86 (0.83, 0.88) | 0.000 |
| SBP (mmHg) | 135 ± 16 | 136 ± 16 | 125 ± 14 | 0.001 |
| DBP (mmHg) | 83 ± 11 | 83 ± 11 | 77 ± 10 | 0.001 |
| Urea Nitrogen (mmol/l) | 5.10 ± 1.48 | 5.09 ± 1.56 | 4.82 ± 1.18 | 0.464 |
| Cr (umol/l) | 57.97 ± 14.37 | 59.98 ± 14.20 | 62.85 ± 13.80 | 0.101 |
| UA (umol/l) | 342.04 ± 63.36 | 319.25 ± 75.46 | 294.15 ± 54.47 | 0.000 |
| TBIL(umol/l) | 15.9 (13.2, 21.2) | 15.7 (11.5, 20.5) | 13.6 (11.3, 16.6) | 0.074 |
| DBIL(umol/l) | 4.2 (3.3, 5.5) | 3.7 (2.7, 5.1) | 4.2 (3.0, 5.1) | 0.540 |
| IBIL(umol/l) | 11.9 (9.6, 16.3) | 12.0 (8.4, 15.1) | 9.6 (7.9, 12.5) | 0.045 |
| ALP (U/l) | 76 ± 21 | 76 ± 21 | 71 ± 22 | 0.289 |
| AST (U/l) | 20 (16, 28) | 24 (18, 29) | 19 (16, 24) | 0.019 |
| ALT (U/l) | 35 (32, 38) | 31 (26, 36) | 18 (13, 27) | 0.000 |
| GGT (U/l) | 36 (26, 54) | 34 (19, 68) | 23 (16, 34) | 0.007 |
| LDH (U/l) | 166 ± 33 | 189 ± 38 | 184 ± 32 | 0.000 |
| TG (mmol/l) | 2.4 ± 0.6 | 1.9 ± 0.7 | 1.4 ± 0.6 | 0.000 |
| TC (mmol/l) | 5.4 ± 0.3 | 4.8 ± 0.2 | 4.1 ± 0.4 | 0.000 |
| HDL-C (mmol/l) | 1.4 ± 0.3 | 1.5 ± 0.3 | 1.6 ± 0.4 | 0.000 |
| LDL-C (mmol/l) | 3.3 ± 0.4 | 2.9 ± 0.7 | 2.2 ± 0.2 | 0.000 |
| VLDL-C (mmol/l) | 0.4 (0.3, 0.6) | 0.3 (0.2, 0.5) | 0.2 (0.1, 0.4) | 0.003 |
| ApoB (g/l) | 0.9 (0.7, 1.1) | 0.9 (0.7, 1.0) | 0.9 (0.8, 1.0) | 0.403 |
| ApoA1 (g/l) | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.000 |
| FPG (mmol/l) | 7.44 ± 1.07 | 5.54 ± 0.65 | 4.84 ± 0.62 | 0.000 |
| 2hPG (mmol/l) | 16.90 (14.00, 17.88) | 8.93 (8.05, 9.75) | 6.05 (5.34, 6.71) | 0.000 |
| Fasting Insulin (mU/l) | 6.31 (5.24, 8.47) | 7.77 (6.05, 8.70) | 6.63 (5.54, 8.26) | 0.021 |
| HOMA-IR | 2.20 (1.73, 2.78) | 1.82 (1.45, 2.22) | 1.44 (1.19, 1.86) | 0.000 |
| HOMA-β | 32.89 (24.32, 45.60) | 81.09 (59.16, 109.50) | 117.11 (74.55, 158.77) | 0.000 |
| Matsuda ISI | 93.38 ± 38.31 | 78.76 ± 24.14 | 114.64 ± 44.28 | 0.000 |
ApoA1 Apolipoprotein A1, ApoB Apolipoprotein B, ALP alkaline phosphatase, AST aspartate aminotransferase, ALT alanine transaminase, BMI body mass index, Cr creatine, DBIL direct bilirubin, DBP diastolic blood pressure, FPG fasting plasma glucose, GGT γ-glutamyltransferase, HOMA-IR homeostatic model assessment of insulin resistance, HOMA-β homeostatic model assessment for β-cell function, HDL-C high-density lipoprotein cholesterol, IBIL indirect bilirubin, LDH lactate dehydrogenase, LDL-C low-density lipoprotein cholesterol, nT2DM newly diagnosed type 2 diabetes mellitus, NC normal control, PDM prediabetes mellitus, SBP systolic blood pressure, TC total cholesterol, TG triglycerides, TBIL total bilirubin, UA uric acid, VLDL-C very low-density lipoprotein cholesterol, WC waist circumference, WHR waist-to-hip ratio
Comparisons and distribution of LFC among study groups
There was a significant difference of LFC level among three groups (all p < 0.05) (Fig. 1). In compared to NC group (5.11% ± 3.66%), a significantly increased LFC level was observed in nT2DM (14.72% ± 6.37%) and PDM groups (9.62% ± 4.41%) (both p < 0.05). Furthermore, a significantly higher LFC level was found in nT2DM group than in PDM group (14.72% ± 6.37% vs 9.62% ± 4.41%) (p < 0.05).
Fig. 1.
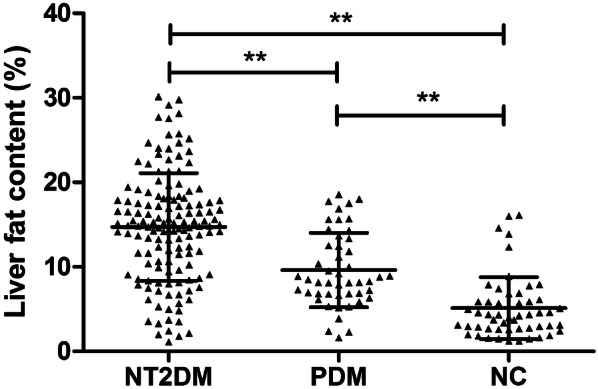
The comparison of LFC in nT2DM, PDM and NC groups. LFC liver fat content, nT2DM newly diagnosed type 2 diabetes mellitus, PDM pre-diabetes mellitus, NC normal controls
The frequency distribution of LFC among nT2DM, PDM and NC groups was shown in Fig. 2. We could observe that the frequency of LFC among NC, PDM and nT2DM groups mainly distributed in LFC of 0–5% (68.75%), LFC of 5–10% (41.54%) and LFC of 10–20% (53.81%), respectively. In addition, the detection of NAFLD among nT2DM, PDM and NC groups were 91.48%, 85.41% and 32.07%, respectively.
Fig. 2.
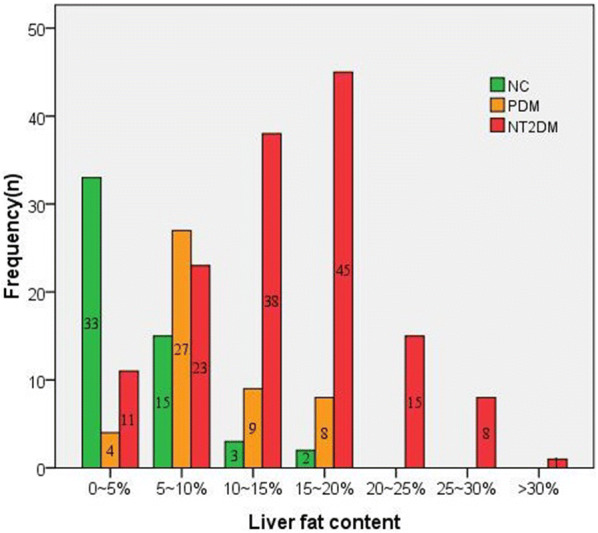
The frequency distribution of LFC in nT2DM, PDM and NC groups. LFC liver fat content, nT2DM newly diagnosed type 2 diabetes mellitus, PDM pre-diabetes mellitus, NC normal controls
Correlation analysis of LFC with clinical and laboratory parameters among study groups
Univariate correlation analysis revealed that LFC was positively correlated with BMI, WHR, SBP, DBP, FPG, HOMA-IR and negatively correlated with TBIL, DBIL and IBIL in nT2DM group (all p < 0.05). In PDM group, there was a significantly positive association of LFC with BMI, FPG, UA and HOMA-IR (all p < 0.05). Moreover, in NC group, LFC showed a positively association with FPG, urea nitrogen, ALT, ALP and HOMA-IR, and a negatively association with ApoA1 (all p < 0.05). However, no significant correlations of LFC with other clinical and quantitative laboratory parameters among those three groups were observed (all p > 0.05) (Table 2).
Table 2.
Correlation coefficients between LFC with demographic and laboratory parameters
| Parameters | nT2DM (n = 141) |
PDM (n = 48) |
Normal Control (n = 53) |
|||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age | − 0.024 | 0.781 | 0.072 | 0.626 | 0.151 | 0.280 |
| BMI | 0.268 | 0.001 | 0.416 | 0.003 | 0.223 | 0.108 |
| WHR | 0.314 | 0.000 | 0.183 | 0.214 | 0.107 | 0.446 |
| SBP | 0.190 | 0.024 | 0.256 | 0.079 | 0.223 | 0.109 |
| DBP | 0.178 | 0.035 | 0.074 | 0.616 | 0.114 | 0.414 |
| FPG | 0.222 | 0.008 | 0.385 | 0.007 | 0.398 | 0.003 |
| 2hPG | 0.096 | 0.258 | 0.110 | 0.458 | 0.094 | 0.505 |
| Urea Nitrogen | − 0.042 | 0.617 | 0.227 | 0.121 | 0.439 | 0.001 |
| Cr | − 0.071 | 0.403 | 0.215 | 0.142 | 0.036 | 0.800 |
| UA | 0.121 | 0.151 | 0.390 | 0.006 | 0.178 | 0.203 |
| TBIL | − 0.244 | 0.004 | 0.022 | 0.880 | − 0.186 | 0.183 |
| DBIL | − 0.213 | 0.011 | 0.099 | 0.502 | − 0.267 | 0.053 |
| IBIL | − 0.225 | 0.007 | 0.005 | 0.974 | − 0.040 | 0.774 |
| ALT | 0.119 | 0.161 | − 0.030 | 0.840 | 0.304 | 0.027 |
| AST | 0.060 | 0.483 | 0.038 | 0.800 | 0.140 | 0.316 |
| GGT | 0.014 | 0.867 | 0.122 | 0.408 | 0.135 | 0.336 |
| ALP | 0.038 | 0.653 | − 0.009 | 0.951 | 0.291 | 0.035 |
| LDH | 0.062 | 0.469 | − 0.033 | 0.823 | 0.015 | 0.915 |
| TG | − 0.059 | 0.486 | − 0.109 | 0.461 | 0.195 | 0.162 |
| TC | − 0.042 | 0.621 | − 0.056 | 0.705 | 0.186 | 0.182 |
| HDL-C | − 0.038 | 0.657 | − 0.157 | 0.286 | − 0.262 | 0.058 |
| LDL-C | − 0.008 | 0.922 | − 0.041 | 0.780 | 0.192 | 0.169 |
| VLDL-C | 0.119 | 0.161 | − 0.077 | 0.602 | 0.219 | 0.116 |
| ApoB | − 0.112 | 0.185 | − 0.163 | 0.267 | 0.023 | 0.868 |
| ApoA1 | − 0.008 | 0.922 | − 0.193 | 0.189 | − 0.289 | 0.036 |
| HOMA-IR | 0.262 | 0.002 | 0.400 | 0.005 | 0.274 | 0.047 |
| HOMA-β | − 0.014 | 0.873 | − 0.073 | 0.621 | − 0.221 | 0.112 |
| Matsuda ISI | − 0.149 | 0.078 | − 0.056 | 0.706 | − 0.234 | 0.092 |
ApoA1 Apolipoprotein A1, ApoB Apolipoprotein B, ALP alkaline phosphatase, AST aspartate aminotransferase, ALT alanine transaminase, BMI body mass index, Cr creatine, DBIL direct bilirubin, DBP diastolic blood pressure, FPG fasting plasma glucose, GGT γ-glutamyltransferase, HOMA-IR homeostatic model assessment of insulin resistance, HOMA-β homeostatic model assessment for β-cell function, HDL-C high-density lipoprotein cholesterol, IBIL indirect bilirubin, LFC liver fat content, LDH lactate dehydrogenase, LDL-C low-density lipoprotein cholesterol, nT2DM newly diagnosed type 2 diabetes mellitus, NC normal control, PDM prediabetes mellitus, SBP systolic blood pressure, TC total cholesterol, TG triglycerides, TBIL total bilirubin, UA uric acid, VLDL-C very low-density lipoprotein cholesterol, WC waist circumference, WHR waist-to-hip ratio
Comparisons of several glucose metabolism indicators among LFC quartile groups
Given the distribution of LFC among study subjects, we calculated the quartile of LFC, a quartile divides LFC into three points (a lower quartile, median, and upper quartile) to inform four groups of the LFC (Q1: LFC < 5.89%, Q2: 5.89% ≤ LFC < 11.62%, Q3: 11.62% ≤ LFC < 16.26% and Q4: LFC ≥ 16.26%).
The results indicated significant differences of FPG, 2hPG, Delta G30, Delta Ins30, Delta C30, Ins30/G30 AUC, CP30/G30 AUC, Ins AUC/G AUC, CP AUC/G AUC, HOMA-IR, HOMA-β and Matsuda ISI among four groups (all p < 0.05) (Table 3). With the increase of LFC, the FPG, 2hPG and HOMA-IR showed a comparable increase. Nevertheless, Matsuda ISI, Delta Ins30, Delta C30, Ins30/G30 AUC, CP30/G30 AUC, Ins AUC/G AUC, CP AUC/G AUC and HOMA-β showed a tendency of decrease. Delta G30 exerted an increased trend from Q1 to Q3, then showed a descent trend in Q4.
Table 3.
Comparison of insulin resistance and β-cell function among groups divided by LFC quartile
| Parameters | LFC < 5.89% (n = 61) |
5.89% ≤ LFC < 11.62% (n = 61) |
11.62% ≤ LFC < 16.26% (n = 60) |
16.26% ≤ LFC (n = 60) |
p value |
|---|---|---|---|---|---|
| FPG (mmol/l) | 5.26 ± 1.00 | 6.28 ± 1.35a | 6.99 ± 1.36bd | 7.45 ± 1.09ce | 0.000 |
| 2hPG (mmol/l) | 6.70 (5.73, 9.59) | 10.42 (8.01, 17.16)a | 15.23 (10.49, 17.68)b | 16.89 (12.90, 17.86)ce | 0.000 |
| Delta G30 | 4.23 ± 1.41 | 5.52 ± 1.59a | 5.60 ± 1.58b | 5.51 ± 1.58c | 0.000 |
| Delta Ins30 | 39.96 (18.02, 56.95) | 24.03 (12.28, 47.62) | 13.70 (5.87, 31.97)b | 10.60 (4.67, 23.61)ce | 0.000 |
| Delta C30 | 2.69 (1.80, 4.27) | 2.09 (1.10, 3.79) | 1.40 (0.63, 2.80)b | 1.05 (0.71, 1.78)ce | 0.000 |
| Ins30/G30 AUC | 9.26 (4.49, 14.24) | 4.49 (2.17, 9.55)a | 2.36 (1.01, 6.39)b | 2.11 (0.84, 4.08)ce | 0.000 |
| CP30/G30 AUC | 0.65 (0.38, 1.06) | 0.38 (0.19, 0.81)a | 0.28 (0.12, 0.52)b | 0.21 (0.11, 0.33)ce | 0.000 |
| Ins AUC/G AUC | 5.52 (3.52, 7.79) | 3.20 (1.33, 5.24)a | 1.86 (1.20, 4.50)b | 1.51 (1.04, 3.56)ce | 0.000 |
| CP AUC/G AUC | 0.57 (0.38, 0.85) | 0.44 (0.22, 0.70) | 0.31 (0.19, 0.55)b | 0.29 (0.17, 0.43)c | 0.000 |
| HOMA-IR | 1.62 ± 0.58 | 1.98 ± 0.76 | 2.31 ± 0.78b | 2.64 ± 1.14ce | 0.000 |
| HOMA-β | 93.38 (56.90, 141.29) | 55.61 (32.92, 94.99)a | 40.48 (25.21, 78.90)b | 34.74 (26.58, 56.84)c | 0.000 |
| Matsuda ISI | 110.23 ± 42.91 | 95.46 ± 38.55 | 88.92 ± 34.31b | 85.69 ± 36.26c | 0.005 |
AUC area under the curve, PG plasma glucose, FPG fasting plasma glucose, HOMA-IR homeostatic model assessment of insulin resistance, HOMR-β homeostatic model assessment for β-cell function, LFC liver fat content, Matsuda ISI Matsuda insulin sensitivity index
aSignificant difference in LFC < 5.89% versus 5.89% ≤ LFC < 11.62
bSignificant difference in LFC < 5.89% versus 11.62% ≤ LFC < 16.26%
cSignificant difference in LFC < 5.89% versus 16.26% ≤ LFC
dSignificant difference in 5.89% ≤ LFC < 11.62 versus 11.62% ≤ LFC < 16.26%
eSignificant difference in 5.89% ≤ LFC < 11.62 versus 16.26% ≤ LFC
fSignificant difference in 11.62% ≤ LFC < 16.26% versus 16.26% ≤ LFC
Correlation analysis of LFC with several glucose metabolism indicators
Univariate correlation analysis indicated that LFC was positively correlated with FPG, 2hPG, Delta G30 and HOMA-IR, negatively correlated with Delta Ins30, Delta C30, Ins30/G30 AUC, CP30/G30 AUC, Ins AUC/G AUC, CP AUC/G AUC, HOMA-β and Matsuda ISI (all p < 0.05). (Additional file 2: Table S1).
MLR to identify the contributors for HOMA-IR and HOMA-β
First, HOMA-IR was set as dependent variable, independent variables of LFC and traditional risk factors (age, gender, BMI, WC, SBP, DBP, TC, TG, HDL-C, LDL-C and VLDL-C) were included in MLR model, the results indicated that BMI, LFC and DBP were the significant contributors that closely associated with HOMA-IR (Additional file 3: Table S2).
Second, we also analyzed the contribution of LFC and traditional risk factors (age, gender, BMI, WC, SBP, DBP, TC, TG, HDL-C, LDL-C, VLDL-C) on HOMA-β, the MLR model suggested that TC, age, WC, LDL-C were the significant contributors for HOMA-β (Additional file 3: Table S2).
Discussion
Although previous studies showed that LFC may be closely associated with several clinical and laboratory parameters like BMI or HOMA-IR, however, limited study has investigated the LFC and its relationship with clinical and laboratory parameters in nT2DM and PDM. In the present study, we have used [1H]-MRS to measure the LFC among nT2DM, PDM and NC groups, and the results revealed that there was an increased LFC level and detection rate of NAFLD in patients with nT2DM than in PDM and those of NC. LFC was shown to be positively associated with FPG and HOMA-IR in all three groups. In addition, we found that there were significant differences of several glucose metabolism indicators among four LFC quartile groups; from Q1 to Q4, the levels of FPG, 2hPG and HOMA-IR showed a comparable increase, however, Matsuda ISI, Delta Ins30, Delta C30, Ins30/G30 AUC, CP30/G30 AUC, Ins AUC/G AUC, CP AUC/G AUC and HOMA-β showed a decedent trend. Correlation analysis also supported a positive correlation of LFC with FPG, 2hPG and HOMA-IR, and a negatively correlation of LFC with Matsuda ISI, Delta Ins30, Delta C30, Ins30/G30 AUC, CP30/G30 AUC, Ins AUC/G AUC, CP AUC/G AUC and HOMA-β. It has been demonstrated that increasing accumulation of intrahepatic triglyceride (IHTG) was associated with a step-wise increase in plasma fasting insulin levels and continuous reduction in hepatic insulin extraction, however, the level of FPG showed no association with the increase of IHTG [27]. Given that HOMA-IR was mainly driven by plasma insulin levels, HOMA-IR levels increased with worsening IHTG accumulation. Furthermore, the possibility therefore arises that the relationship between hepatic steatosis and insulin resistance is a vicious cycle, in which systemic insulin resistance leads to hepatic steatosis, and hepatic steatosis then leads to an exacerbation of hepatic insulin resistance.
There is a widely held perception that liver steatosis is associated with increased production of insulin from the beta cell in order to compensate for whole-body insulin resistance, insulin resistance is not thought to influence beta cell function per se, it just leads to more insulin being produced. Study has suggested that, in apparently healthy older adults, liver steatosis is associated with reduced hepatic insulin extraction and beta cell dysfunction after adjusting confounding factors of age, sex and alcohol consumption [28]. In our study, there are several explanations that may cause the decreased trend of HOMA-β. First, the time of newly diagnosis T2DM and PDM patient’s recruitment fall behind the disease onset, thus, may cause the different status on impaired β-cell function. Second, the toxicity of lipid could impair the pancreatic function and decrease the insulin compensatory secretion, and lead to a decrease of HOMA-β. In addition, the study sample size of among study groups is differed, the relatively small sample size of PDM and NC groups may also cause the decrease of HOMA-β. Furthermore, although we did not quantify pancreatic fat content in the present study, the accumulation of ectopic fat in the pancreas is increasingly recognized as a cause of beta-cell dysfunction.
MLR analysis indicated that, LFC and traditional risk factors of BMI and DBP represented the significant contributors for the presence of HOMA-IR. BMI has been demonstrated as the marker for evaluation of overweight or obesity, and was also considered to be the strongest influencing factor for the peripheral insulin resistance [29]. HOMA-IR mainly reflects insulin sensitivity in fasting state, that is, the degree to which insulin inhibits liver sugar output, and also the severity of liver insulin resistance. Although BMI reflects an individual’s overall obesity and associated with blood pressure, it does not accurately reflect the extent to which fat is deposited in organs. Therefore, compared with other traditional factors, LFC can accurately and truly assess the extent of fat heterotopic deposition and more directly reflect insulin resistance in liver.
As for the MLR analysis of HOMA-β, the results revealed that TC, age, WC and LDL-C were the greater contributor associated with HOMA-β. The potential influence of TC and LDL-C on HOMA-β may be attributed to the inhibited pancreatic function caused by the toxicity of lipid [30]. In addition, the pancreatic function gradually declined with the increase of age, and then affects the HOMA-β. Increase of WC has been demonstrated to be associated with increased HOMA-IR and decreased insulin sensitivity, thus could lead to insulin compensatory secretion and impair pancreatic function.
Our results revealed an association between LFC and glucose metabolism status, where the excessive accumulation of liver fat strongly correlated with insulin resistance, impaired insulin secretive function and abnormality of glucose metabolism. It remains always controversial whether fat deposition in the liver is a cause or consequence of insulin resistance. Some investigators have illustrated that liver fat accumulation closely associated with BMI, LDL, TG, insulin resistance and FPG, suggesting that ectopic fat accumulation in the liver affects the normal metabolism of lipids and may contribute to the development and progression of diabetes [28, 31, 32]. However, several observations indicated that the intrahepatic alterations in glucose and fat metabolism could also cause liver steatosis, and the liver fat accumulation does not seem to be sufficient or necessary to induce hepatic insulin resistance [33–35].
There are some shortcomings in the present study that need to be acknowledged. First, this study is an observational study with a case–control design that could not prove the causal relationship due to the lack of clear time logic. Second, the selection of study sample is based on single hospital, and may have selection bias. Third, [1H]-MRS is time consuming to perform and can depict the fat content of only a portion of the organs; the placement of voxels requires operator expertise, especially in small organs of irregular shape, thus the accuracy of MRS can be compromised. Furthermore, due to a relatively small sample size, especially in PDM and NC, it may impair the reliability of our results. Hence, further community-based studies with a large sample size are still required to confirm our results.
Conclusions
In summary, our study has indicated that the increase of LFC plays an important role in insulin resistance, abnormal glucose metabolism status and eventually diabetes, and may be regarded as potential indicators for abnormal glucose tolerance and T2DM. Early intervention, ideally as soon as abnormalities in LFC are detected, is of great importance for the prevention of T2DM.
Supplementary information
Additional file 1: Figure S1. Bland–Altman analysis for intra-session repeatability for [1H]-MRS defined LFC in two ROI regions of 100 subjects.
Additional file 2: Table S1. Correlation of LFC with parameters regarding insulin resistance and β-cell function.
Additional file 3: Table S2. Multilinear regression analysis of HOMA-IR and HOMA-β with different predictors.
Acknowledgements
The authors thank the study participants as well as the staff involved in the collection of blood samples.
Abbreviations
- AUC
Area under the curve
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- AST
Aspartate aminotransferase
- ApoA1
Apolipoprotein A1
- ApoB
Apolipoprotein B
- BMI
Body mass index
- DBP
Diastolic blood pressure
- DBIL
Direct bilirubin
- FPG
Fasting plasma glucose
- GGT
γ-Glutamyltransferase
- HDL-C
High-density lipoprotein cholesterol
- [1H]-MRS
Proton magnetic resonance spectroscopy
- HOMA-IR
Homeostatic model assessment of insulin resistance
- HOMR-β
Homeostatic model assessment for β-cell function
- IP
In-phase
- ICC
Intraclass correlation coefficients
- IBIL
Indirect bilirubin
- IQR
Interquartile range
- LDL-C
Low-density lipoprotein cholesterol
- LDH
Lactate dehydrogenase
- LFC
Liver fat content
- Matsuda ISI
Matsuda insulin sensitivity index
- MLR
Multivariate linear regression
- NAFLD
Non-alcoholic fatty liver disease
- NC
Normal controls
- nT2DM
Newly diagnosed T2DM
- OGTT
Oral glucose tolerance test
- OP
Out of phase
- PDM
Prediabetes mellitus
- PG
Plasma glucose
- PRESS
Point-resolved spectroscopy
- ROC
Receiver operating characteristic
- ROI
Regions of interest
- SD
Standard deviation
- SBP
Systolic blood pressure
- TBIL
Total bilirubin
- TC
Total cholesterol
- TG
Triglyceride
- T2DM
Type 2 diabetes mellitus
- UA
Uric acid
- VLDL-C
Very low-density lipoprotein cholesterol
- WC
Waist circumference
- WHR
Waist-to-hip ratio
Authors’ contributions
Study design and concept: YW, WD, QZ. Performance of experiment: GZ, JY, YC, RZ, LZ, JX. Data collection and analysis: YW, LK, JZ, HL. Writing of manuscript: YW, GZ. Revising the manuscript: WD and QZ. All authors read and approved the final manuscript.
Funding
This study was supported by Anhui Science and Technology Project (Grant Number 1604a0802099).
Availability of data and materials
The data and material that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This study was approved by the Ethical Committee of the Second People’s Hospital of Hefei (Hefei, Anhui, China) (approval number 1523). All the study subjects provided informed consent to participate in this study. All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form).
Consent for publication
All the study subjects provided informed consent to participate in this study.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13098-020-00558-8.
References
- 1.Lin Y, Sun Z. Current views on type 2 diabetes. J Endocrinol. 2010;204(1):1–11. doi: 10.1677/JOE-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. doi: 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- 3.Puri P, Sanyal AJ. Nonalcoholic fatty liver disease: definitions, risk factors, and workup. Clin Liver Dis (Hoboken). 2012;1(4):99–103. doi: 10.1002/cld.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almobarak AO, Barakat S, Suliman EA, et al. Prevalence of and predictive factors for nonalcoholic fatty liver disease in Sudanese individuals with type 2 diabetes: is metabolic syndrome the culprit? Arab J Gastroenterol. 2015;16(2):54–58. doi: 10.1016/j.ajg.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi Y, Ito H, Komatsu Y, et al. Non-alcoholic fatty liver disease is an independent predictor for macroangiopathy in Japanese type 2 diabetic patients: a cross-sectional study. Intern Med. 2012;51(13):1667–1675. doi: 10.2169/internalmedicine.51.7307. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto Y, Hamaguchi M, Fukuda T, et al. Fatty liver as a risk factor for progression from metabolically healthy to metabolically abnormal in non-overweight individuals. Endocrine. 2017;57(1):89–97. doi: 10.1007/s12020-017-1313-6. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 8.Leite NC, Salles GF, Araujo AL, et al. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29(1):113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Byrne CD, Bonora E, et al. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care. 2018;41(2):372–382. doi: 10.2337/dc17-1902. [DOI] [PubMed] [Google Scholar]
- 10.Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(5):936–944. doi: 10.1111/jgh.13264. [DOI] [PubMed] [Google Scholar]
- 11.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Sertoglu E, Ercin CN, Celebi G, et al. The relationship of serum uric acid with non-alcoholic fatty liver disease. Clin Biochem. 2014;47(6):383–388. doi: 10.1016/j.clinbiochem.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Chuah K, Chan W. Quantification of liver fat in NAFLD: available modalities and clinical significance. Curr Hepatol Rep. 2019;18:492–502. doi: 10.1007/s11901-019-00493-x. [DOI] [Google Scholar]
- 14.Perman WH, Balci NC, Akduman I. Review of magnetic resonance spectroscopy in the liver and the pancreas. Top Mag Reson Imaging TMRI. 2009;20(2):89–97. doi: 10.1097/RMR.0b013e3181c422f1. [DOI] [PubMed] [Google Scholar]
- 15.McPherson S, Jonsson JR, Cowin GJ, et al. Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J Hepatol. 2009;51(2):389–397. doi: 10.1016/j.jhep.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Ligabue G, Besutti G, Scaglioni R, et al. MR quantitative biomarkers of non-alcoholic fatty liver disease: technical evolutions and future trends. Quant Imaging Med Surg. 2013;3(4):192–195. doi: 10.3978/j.issn.2223-4292.2013.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv S, Jiang S, Liu S, et al. Noninvasive quantitative detection methods of liver fat content in nonalcoholic fatty liver disease. J Clin Transl Hepatol. 2018;6(2):217–221. doi: 10.14218/JCTH.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes A 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. doi: 10.2337/dc18-s002. [DOI] [PubMed] [Google Scholar]
- 19.Lansang MC, Williams GH, Carroll JS. Correlation between the glucose clamp technique and the homeostasis model assessment in hypertension. Am J Hypertens. 2001;14(1):51–53. doi: 10.1016/S0895-7061(00)01229-2. [DOI] [PubMed] [Google Scholar]
- 20.Takai T, Sakura H. Insulinogenic index, HOMA-beta, disposition index. Nihon Rinsho Japn J Clin Med. 2012;70(Suppl 3):459–464. [PubMed] [Google Scholar]
- 21.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 22.Bian H, Yan H, Zeng M, et al. Increased liver fat content and unfavorable glucose profiles in subjects without diabetes. Diabetes Technol Ther. 2011;13(2):149–155. doi: 10.1089/dia.2010.0101. [DOI] [PubMed] [Google Scholar]
- 23.Xia MF, Bian H, Yan HM, et al. Assessment of liver fat content using quantitative ultrasonography to evaluate risks for metabolic diseases. Obesity. 2015;23(9):1929–1937. doi: 10.1002/oby.21182. [DOI] [PubMed] [Google Scholar]
- 24.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 25.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 26.Cleophas TJ, Droogendijk J, van Ouwerkerk BM. Validating diagnostic tests, correct and incorrect methods, new developments. Curr Clin Pharmacol. 2008;3(2):70–76. doi: 10.2174/157488408784293697. [DOI] [PubMed] [Google Scholar]
- 27.Bril F, Barb D, Portillo-Sanchez P, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132–1144. doi: 10.1002/hep.28985. [DOI] [PubMed] [Google Scholar]
- 28.Finucane FM, Sharp SJ, Hatunic M, et al. Liver fat accumulation is associated with reduced hepatic insulin extraction and beta cell dysfunction in healthy older individuals. Diabetol Metab Syndr. 2014;6(1):43. doi: 10.1186/1758-5996-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margariti E, Deutsch M, Manolakopoulos S, et al. Non-alcoholic fatty liver disease may develop in individuals with normal body mass index. Ann Gastroenterol. 2012;25(1):45–51. [PMC free article] [PubMed] [Google Scholar]
- 30.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142(4):711–25 e6. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Rossi AP, Fantin F, Zamboni GA, et al. Predictors of ectopic fat accumulation in liver and pancreas in obese men and women. Obesity. 2011;19(9):1747–1754. doi: 10.1038/oby.2011.114. [DOI] [PubMed] [Google Scholar]
- 32.Patel NS, Peterson MR, Brenner DA, et al. Association between novel MRI-estimated pancreatic fat and liver histology-determined steatosis and fibrosis in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37(6):630–639. doi: 10.1111/apt.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.den Boer M, Voshol PJ, Kuipers F, et al. Hepatic steatosis: a mediator of the metabolic syndrome. Lessons from animal models. Arterioscler Thromb Vasc Biol. 2004;24(4):644–649. doi: 10.1161/01.atv.0000116217.57583.6e. [DOI] [PubMed] [Google Scholar]
- 34.Schonfeld G, Yue P, Lin X, et al. Fatty liver and insulin resistance: not always linked. Trans Am Clin Climatol Assoc. 2008;119:217–223. [PMC free article] [PubMed] [Google Scholar]
- 35.Farese RV, Jr, Zechner R, Newgard CB, et al. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab. 2012;15(5):570–573. doi: 10.1016/j.cmet.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Bland–Altman analysis for intra-session repeatability for [1H]-MRS defined LFC in two ROI regions of 100 subjects.
Additional file 2: Table S1. Correlation of LFC with parameters regarding insulin resistance and β-cell function.
Additional file 3: Table S2. Multilinear regression analysis of HOMA-IR and HOMA-β with different predictors.
Data Availability Statement
The data and material that support the findings of this study are available from the corresponding author upon reasonable request.


