Abstract
Background
There is increasing requests of Vitamin D test in many clinical settings in recent years. However, immunoassay performance is still a controversial topic. Several diagnostic manufacturers have launched automated 25-hydroxyvitamin D (25-OH D) immunoassays in the past decade. We compared the performance of Abbott Architect 25-OH D Vitamin immunoassay with liquid chromatography-tandem mass spectrometry systems (LCMS/MS) to evaluate immunoassay performance, especially in deficient groups.
Methods
Eighty human serum samples were analyzed with Architect 25-OH D vitamin kit (Abbott Diagnostics, Lake Forest, IL, USA) and LC-MS/MS systems (Zivak Technology, Istanbul, Turkey). The results of the immunoassay method were compared with the LC-MS/MS using Passing-Bablok regression analysis, Bland-Altman plots and correlation coefficient analysis. We also evaluated results in four levels of D vitamin as a severe deficiency, deficiency, insufficiency, and sufficiency.
Results
Architect showed 9.59% bias from LC-MS/MS with smaller mean. Passing-Bablok regression analysis demonstrated the value of 0.95 slope and had a constant bias with an intercept value of -4.25. Concordance correlation coefficient showed moderate agreement with the value of 0.918 (95% CI 0.878-0.945). Two methods revealed good interrater agreement (kappa = 0.738). While the smallest bias determined in deficiency (9.95%) group, the biggest was in insufficiency (15.15%).
Conclusions
Architect 25-OH D vitamin immunoassay can be used in routine measurements but had potential misclassification of vitamin D status in insufficient and deficient groups. Although there are recent standardization attempts in 25-OH D measurements, clinical laboratories must be aware of this method.
Keywords: vitamin D, method comparison, deficiency, insufficiency, 25-Hydroxyvitamin D
Abstract
Uvod
Poslednjih godina povećan je zahtev za određivanjem vitamina D u mnogim kliničkim slučajevima. Međutm, imuno određivanje je i dalje kontraverzno. Nekoliko dijagnostičkih proizvođača razvilo je poslednjih godina automatsko imunoodređivanje 25-hidroksivitamina D (25-OH D). Mi smo poredili imunohemijsko određivanje 25-OH vitamina D pomoću Abbott Architect analizatora sa tandem sistemom tečna hromatografija-masena spektrometrija (LS-MS/MS) kako bi procenili imunohemijsko određivanje naročito u deficijentnim grupama.
Metode
Analizirano je 80 humanih uzoraka seruma primenom Architect 25-OH D vitamin reagensom (Abbott Diagnostics, Lake Forest, IL, USA) i LC-MS/MS (Zivak Technology, Istanbul, Turkey). Dobijeni rezultati primenom imunoodređivanja bili su u korelaciji sa rezultatima dobijenim sistemom tečna hromatografija-masena spektrometrija (LSMS/MS) korišćenjem Passing-Bablok regresionom analizom, Bland-Altman metodom i analizom korelacionog koeficijenta. Takođe smo procenjivali rezultate četiri nivoa vitamina D kao tešku deficijenciju, deficijenciju, insuficijenciju i suficit.
Rezultati
Arhitect je prikazao 9,59% odstupanje od LCMS/MS sa neznatnim značajem. Passing-Bablok regresiona analiza ukazala je na vrednost nagiba od 0,95 i konstantom Korelacioni koeficijent se umereno slagao sa vrednostima 0,918 (95% CI 0,878-0,945). Dve metode su imale dobro slaganje (kappa = 0,738). Najmanje odstupanje je određeno u grupi sa deficijencijom (0,95%), a najveće u grupi sa insuficijencijom (15,15%).
Zaključak
Imunodređivanje sa Arhitect 25-OH D vitaminom može da se koristi kao rutinsko merenje mada ima potencijalni nedostatak pri klasifikaciji statusa vitamina D u grupama sa insuficijencijom i deficijencijom. Mada postoje skorašnji pokušaji standardizacije merenja 25-OH D kliničke laboratorije moraju ovo da imaju na umu.
Keywords: 25-hiodroksivitamin D, insuficijencija, deficijencija, poređenje metoda, vitamin
Introduction
Vitamin D deficiency is a worldwide health problem caused mainly by insufficient exposure to sunlight and dietary consumption [1]. It is affecting more than one billion people, especially prevalent among elderly with the several clinical findings such as muscle weakness, orthostatic hypotension, eczema etc. [2]. Some studies suggested that Vitamin D deficiency were related to rheumatologic and autoimmune diseases, cancer and the other clinical conditions [3] [4].
Chemiluminescence immunoassays (CLIA), radioimmunoassay (RIA), high performance liquid chromatography (HPLC) and liquid chromatographytandem mass spectrometry (LC-MS/MS) are the most common techniques used for measuring of vitamin D [5] [6].
National Health and Nutritional Examination Survey (NHANES) recommended LC-MS/MS as the best method for quantifying vitamin D metabolites due to improved sensitivity, accuracy, and reproducibility [7]. Although LC-MS/MS is not simple to use, inherit some limitations; those are high costly requirement specifications, complex assay procedures, requiring of an experienced analyst. LC-MS/MS is still the advanced method in determining D vitamin status, hence the new methods have more technique restrictions [8] [9].
Serum 25-hydroxyvitamin D (25-OH D) levels have been determined by using Chemiluminescent Immunoassay (CLIA) in the recent years. It is simple, cost and time-effective method when compared to LC-MS/MS systems [6]. The main limitation of immuno chemical methods is the cross reactivity of the antibodies used in the assay. This method has an inability to discriminate the D2 and D3 forms of the 25-OH D metabolite [9].
Vitamin D Standardization Program (VDSP) [10] is an international effort collaborated with many constitutions etc. Central Disease Center (CDC), National Institute of Standards and Technology (NIST), NHANES, Belgian Laborator y for Analytical Chemistry, Faculty of Pharmaceutical Sciences, Ghent University [11]. One of the objectives of VSDP is standardize 25-OH D assays with NIST traceable measurement procedures [12]. Standard reference materials (SRM) 2972 and 972 has been recommended for improving traceability and harmonization of 25-OH vitamin D measurement [10] [12].
Recently, Abbott diagnostic claimed, their 25-OH Vitamin D kit could determine 25(OH) Vitamin D metabolites, with excellent accuracy and sensitivity. This kit also calibrated against NIST SRM 2972. Some studies in literature, investigate the verification of Architect 25-OH Vitamin D assay (Catalog no: 3L52-25, 510k Abbott) [13] [14] [15]. In the present study, we aim to compare NIST traceable Architect 25-OH Vitamin D assay with LC-MS/MS systems. To the best of our knowledge in the literature there is not any study evaluated these methods in different levels of Vitamin D. For further examination, immuno assay performance evaluated in severe deficiency/deficiency/insufficiency/sufficiency groups.
Material and Methods
Serum specimens
We randomly chosen 80 serum specimens from the patients' samples pool during four days period. The sample consisted of 40 male and 40 female, with a mean age of 50 ± 16.4 years, ranging from 18 to 83 years old without any chronic diseases. Serum specimen aliquot into three parts. First part was measured with Architect 25-OH D immunoassay. Within 24 h period, second and third parts were analyzed with LC-MS/MS methods in another laboratory. All samples stored at -80 °C until the transfer period. While transferring period, sun exposure and heating avoided. There was not any calcium/phosphor metabolism defect. One patients' sample was excluded who received vitamin D supplement. The Non-Interventional Medical Ethical Committee of Pamukkale University of Faculty of Medicine approved the study protocol.
Architect 25-OH D immunoassay
Immunochemical assays analyzed on Abbott Architect i-2000 (Abbott Park, IL, USA). The original Architect (Abbott Diagnostics, Lake Forest, IL, USA) 25-OH Vitamin D assay determine 25(OH) D2 and D3 in human serum and plasma. It is a delayed one-step immunoassay including a sample pre-treatment for the quantitative determination of vitamin D in com petitive chemiluminescent microparticle immuno assay (CMIA) technology with flexible assay protocols. This chemiflex method was used microparticles coated with anti-vitamin D IgG antibody, and biotinylated Vitamin D antibiotin IgG acridinium-labelled conjugated complex. Architect 25-OH Vitamin D 5p02 calibrators [16] were standardized against NIST SRM 2972 and traceable to the LC-MS/MS Vitamin D reference measurement procedure (University of Ghent). In Architect kit insert, limit of blank (LOB), limit of detection (LOD), limit of quantitation (LOQ) and reportable range of the assay were given as 4, 5.5, 6, 8.5-389.75 nmol/L respectively [16].
Zivak LC-MS/MS Multitasker LC-MS/MS system
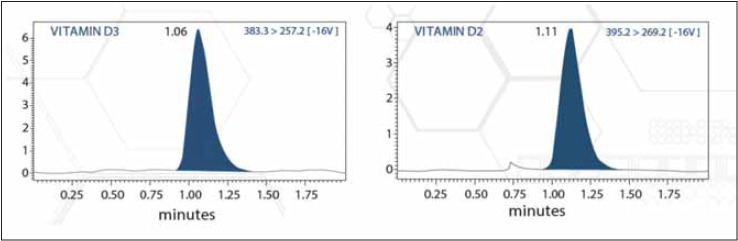
LC-MS/MS analysis were performed in Zivak Multitasker Fully Automated Sample Preparation and Injection System coupled to a MS detector. This spectrometer equipped with a Macherey-Nagel Nucleoder C18 Gravity column (125×2 mmi.d., 5 mm particle size). This system can report patient data within 6 minutes including the solid phase extraction (SPE) and sample preparation and mass spectrometry analysis time. Gören et al. [17] has been verified this method, and determined uncertainties in 2015. After verification and standardization of this method started to use in routine laboratory analysis. Certified standard reference material SRM 972a was used to mo nitor the LC-MS/MS traceability. Sample Chromato grams and validation results for Zivak LC-MS/MS Multi tasker LC-MS/MS system were given at Figure 1 and Table 1.
Figure 1. Sample Chromatograms of Zivak 25-OH Vitamin D2/D3 LC-MS/MS Analysis Kit.
Table 1. Diagnostic performance characteristics of Zivak 25-OH Vitamin D2/D3 LC-MS/MS Analysis Kit.
| 25-OH Vitamin D3 | 25-OH Vitamin D2 | |
|---|---|---|
| LOD (nmol/L) | 3.7 | 2.65 |
| LOQ (nmol/L) | 12.25 | 8.75 |
| Accuracy (%) | 94.7 | 96.4 |
| Intra-assay precision %CV) | 2.16 | 4.6 |
| Inter-assay precision (%CV) | 2.74 | 4.74 |
| Linearity (R2) | 0–200 (0.993) | 0–200 (0.992) |
Measurement comparison
Method comparison was designed according to CLSI EP09-A3 guideline. Inter-assay imprecision of the Architect 25-OH D assay was estimated according to CLSI EP15-A3 guidelines by measuring three levels commercial quality control samples during 5 days with five replicates per day (n = 25). Intra-assay imprecision estimation was conducted on measuring five replicates on the same day. We determined trueness via Biorad External Quality System running three sample from different levels. We determined severe deficiency/deficiency/insufficiency/sufficiency as 0-25, 25-50, 50-75, >75, respectively (nmol/L) [4] [17] [18] [19].
Statistical Analysis
Concordance correlation analysis, Passing-Bablok regression analysis, Box Whisker graphics and the Bland-Altman method were performed to determine agreement between two methods. Lin's concordance correlation coefficient for continuous data and Kappa interrater agreement for categorical data wasused to determine consistency of two methods (k < 0.4 poor, 0.4-0.70 fair to good, and >0.70 excellent agreement). The level of statistical significance was set at p ≤ 0.05. Statistical analysis was performed using SPSS v.24.0 for Windows (SPSS Inc., Armonk, NY, USA), R (version 3.4.3, Vienna, Austria) in R Studio (Version 1.1.463 -© 2009-2018 RStudio, Inc.). Packages used for the analysis were mcr and epiR [20] [21].
Results
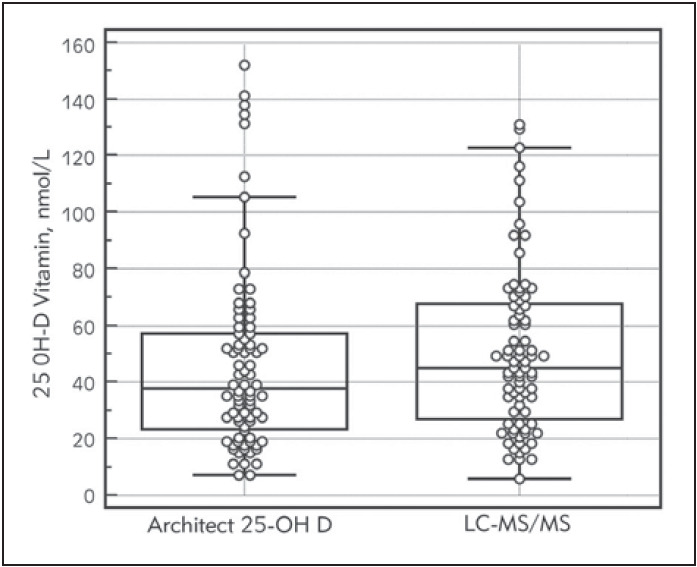
We studied 80 samples by two methods within 48-hour period. The median value of 25-OH D (2.5-97.5 percentiles; nmol/L) of eighty samples 37.75 nmol/L (9.68 -139.7 nmol/L), and 45 nmol/L (12.86-126.16 nmol/L) based on the values obtained from the immunoassay, and LC-MS/MS systems. Box and whisker plots show the distribution of results for the two methods in Figure 2. At three concentration of 25-OH D internal quality control inter-assay CV% was 4.9%, 3.0%, and 2.4% and intra-assay CV% was 1.5%, 1.1%, and 1.0%. Deviation percent results from the three external quality reports were -4.2%, 8.6% and -0.9%. Architect 25-0H D Vitamin assay deviated negatively from gold standard (11.5%). Architect 25-OH D vitamin assay demonstrated a good correlation with the results obtained with the LC-MS/MS method (r2 =0.871, p < 0.001).
Figure 2. Box and whisker plots of LC-MS/MS and Architect 25-OH D assay for 25-OH D measurements.. Each box shows 25 to 75 percentile range. Horizontal line in each boxrepresents group median.
The Passing-Bablok regression analyses for immunoassay against LC-MS/MS system shown in Figure 3. Architect 25-OH D assay deviated from the linearity with the value of 0.95 slope and had a constant bias with an intercept value of -4.25 Table 2.
Figure 3. Passing-Bablok regression analysis of Architect 25-OH D immunoassay compared to LC-MS/MS. The regression equation is presented as y = a + bx, y = -4.2512+0.9575 x.Significant deviation from linearity (P<0.05).
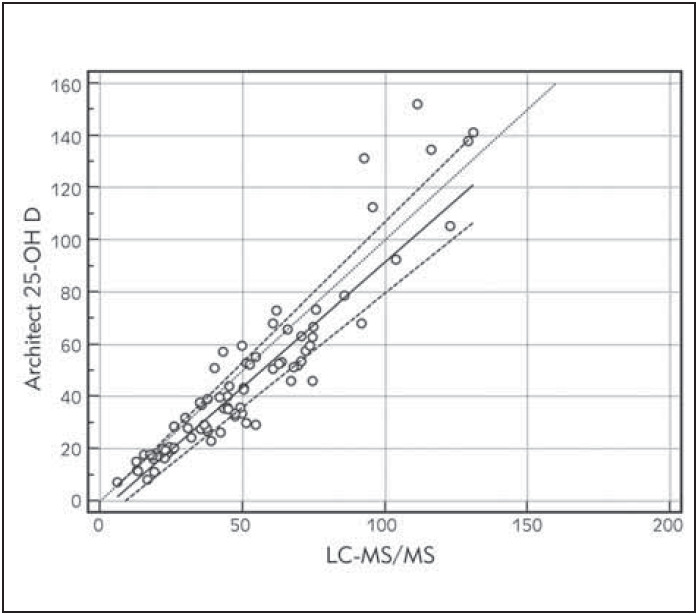
Table 2.
| Method | Passing-Bablok Regression Analyses | Concordance Correlation Analyses | Bland-Altman Analyses | Interrater agreement | |||
| Slope | Intercept | CCC(CI) | P | Cb | Bias (%) | Kappa (Cl) | |
| LC-MS/MS | 0.95+(0.87–1.07) | -4.2512 (-7.54–0.55) | 0.918 (0.878–0.945) | 0.934 | 0.983 | 9.59 | 0.738* (0.604–0.848) |
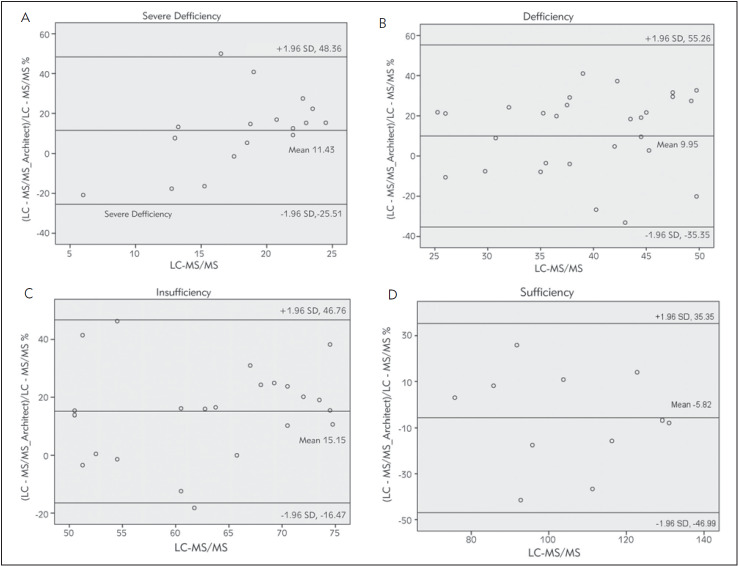
Bland-Altman plots for all results and four groups were given in Figures 4‒5 A-B-C-D. The first one was for all sample results. The other four graphics for four different Vitamin D level groups. Architect 25-OH showed 9.59% bias when compared to LC-MS/MS system. Agreement between two methods under the four levels of 25-OH D vitamin were given in Table 3. Classified results under four headings revealed biases as 11.43%, 9.95%, 15.5%, -5.82% for severe deficiency, deficiency, insufficiency and suf-ficiency groups respectively. However, one of them was < %5 that VSDP recommended. Concordance correlation analyses showed moderate agreement with LC-MS/MS with 0.918 concordance coefficient (95% CI, 0.87 to 0.94). Precision between two methods was 0.93 with the 0.98 corrected bias factor. Kappa coefficients of interrater agreement was found 0.738 (95% CI 0.604-0.848) indicated excellent agreement. Method comparison data were shown in Table 2.
Figure 4. Bland-Altman plots of Architect 25-OH D and LC-MS/MS measurements for four different groups. Mean (thick solid line) – percentage bias (means of paired differences). Dashed lines demonstrate the 95% limits of agreement (bias ± 1.96 standard deviation).
Figure 5. Bland-Altman plots of Architect 25-OH D and LCMS/MS measurements for all results. Mean (thick solid line)- percentage bias (means of paired differences). Dashed linesdemonstrate the 95% limits of agreement (bias ± 1.96 standard deviation).
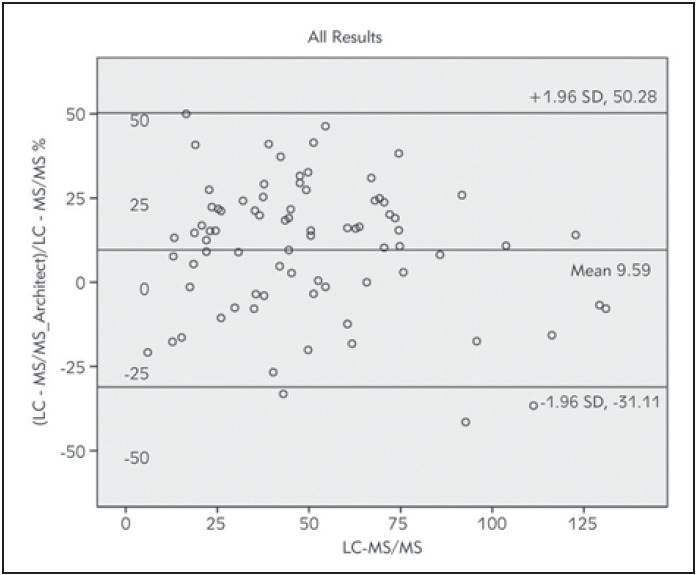
Table 3. Agreement between two methods under the four levels of 25-OH D vitamin.
Kappa coefficient: 0.738; Standard error: 0.062 p=0.000
| Architect 25-OH D Vitamin Assay Number of samples (percentage %) | ||||||
| Severe deficiency | Deficiency | Insufficiency | Sufficiency | Sum | ||
| LC-MS/MS system Number of samples (percentage %) | Severe deficiency | 17 (21.3%) | - | - | - | 17 (21.3%) |
| Deficiency | 4 (5.1%) | 21 (26.6%) | 3 (3.8%) | - | 28 (35.5%) | |
| Insufficiency | - | 6 (7.6%) | 17 (21.5%) | - | 23 (29.1%) | |
| Sufficiency | - | - | 2 (2.5%) | 9 (11.4%) | 11 (13.9%) | |
| Sum | 21 (26.4%) | 27 (34.2%) | 22 (27.8%) | 9 (11.4%) | 79 (100%) | |
Discussion
Architect 25-OH D vitamin assay revealed excellent precision with a total coefficient of variance (CV %) <5%. When we compared to LC-MS/MS as the reference method, NIST traceable immunoassay showed a higher r-value (r2 =0.871) and a proportional bias (9.59%). Nonetheless, Architect 25-OH D vitamin assay revealed moderate agreement based on the value CCC (0.918). Two method showed excel-Many immunoassays are suitable to determine 25-OH D3 form. Cross reaction between total 25-OH D and 25-OH D2 is the major problem [6]. In Abbott kit insert manufacturer claimed 25-OH D vitamin concentrations, at the level of 51.5 nmol/L assay has 80.5% and with the level of 140 nmol/L assay has 82.4% cross reactivity with 25-OH D2 [15]. Abbott 25-OH D has 100% cross reactivity with 25-OH D3. However, failure to detect the 25-OH D2 form is not an important issue in our country, hence the supplements mostly used do not contain Vit D2. Anyway, we could not detect measurable amounts of 25-OH D2 in any patients' sera. This issue might be a problem in France or USA, because of treating D vitamin deficiency with D2 supplements [14]. Probability of 3-epi-25-OH D3 epimers-presence in the pediatric population [22], we did not include children and patients given D vitamin treatment in this study. On the other hand, both immunoassay and LC-MS/MS cannot separate 3-epi-25(OH) D3 epimer from the D3 form, so negative bias for immunoassay systems cannot be explained on the 3-epimer contribution [14].
In the literature, immunoassay methods showed good agreement when compared reference methods, however revealed negative biases likely our study [11] [14] [22]. Annema et al. [15] reported, Architect assay showed a good correlation (r=0.901) in both vitamin D-insufficient and vitamin D-supplemented subjects when compared to the LC-MS/MS method, but had negative biases (17.4% and 8.9%, respectively). Madenci [11] and Ozcan [23] revealed different negative biases for Access immunoassay system (-19.2% and -2.9% respectively), but had good concordance (0.901, 0.952) with LC-MS/MS systems. Topçuoğlu and her colleagues (24) found r and CCC value 0.957 and 0.916 with the bias of 9.5% for Access Total 25(OH) Vitamin D immunoassay on the Beckman Coulter Unicel DXI 800 analyzer.
Different from previous studies, we classified our results under four categories as; severe deficiency (< 25 nmol/L), deficiency (26-50 nmol/L), insufficiency (51-75 nmol/L and sufficiency (>75 nmol/L) [18] [19]. While the smallest bias between Architect 25-OH D and LC-MS/MS system was in deficiency group (9.95%), the biggest was determined in insufficiency group (15.15%) Figure 5C). Architect 25-OH D vitamin assay determine some sufficient samples in insufficient area. This might lead unnecessary treatment for patients. However, World Health Organization (WHO) defined vitamin D insufficiency as serum 25-OH D is below 50 nmol/L [24]. Some studies show a large variance in the plateau level of PTH, ranging from a serum 25-OH D of 45 nmol/L to 75 nmol/L [19]. Lack of PTH test as a limitation of our study, sufficiency cut-off was taken as 75 nmol/L [25]. Some experts recommended deficiency cut-off should be taken as < 50 nmol/L, based upon evidence related to bone health. Because of the necessity in older adults to minimize the risk of falls and fracture, the others suggested < 75 nmol/L as the deficiency cutoff [26]. We divide <75 nmol/L results into three groups, to take the attention capability of immunoassay detection in these groups. Based on the LC-MS/MS data, 8.9% in deficiency, 7.6% of insufficiency, and 2.5% of sufficiency group data were misclassified as having vitamin D deficiency by the Architect 25-OH D vitamin immunoassay Table 3.
Annema et al. [15] revealed negative bias (-17.2%) for new restandardized Abbott Architect 25-OH Vitamin D assay, on the Vitamin D deficiency population. Nevertheless, Annema gave proportional small bias in the Vitamin D supplementation group. Evaluation of Abbott 25-OH D assay against LC-MS/MS system, Cavalier et al. [27] has evaluated; immunoassay showed good agreement with the bias of −3.2% (±1.96 SD 8.6 to −15.1). Both two studies dwelled on the misdiagnosis of Architect immunoassay systems in the deficiency groups similar to our study.
In our study, we pointed out NIST traceable Architect 25-OH D assay failed to diagnose deficiency and insufficiency. This issue might be a problem in several clinical conditions such as osteoporosis, osteomalacia etc. Recently, Cavalier et al. [28] reported the analytical and clinical validation of new developed Abbot Architect 25-OH D assay named as »5P02«. They revealed the new standardized 5P02 clearly improved diagnosis especially in osteoporotic patients and in patients from the intensive care unit. LC-MS/MS is still currently the best method for the precise quantification of 25-OH D3 and 25-OH D2 [9].
However, reported in literature the LC-MS/MS systems have some major problems as matrix related issues, analytical, instrumental, epimeric and isobaric interferences. Insufficiency in separating epimers and isobars resulted in overlapping with Vitamin D metabolites in chromatograms [29]. Accuracy of LC-MS/MS systems were performed by DEQAS with the acceptable biases varied from -8.9% to 1.9% in our study. In the DEQAS reports we could see a large discrepancy of CV% for LC-MS/MS systems varied from 2% to 10.6%. Biases for Architect 25-OH D vitamin assay from the EQAS external quality program were -12.2%, -8.6% and -0.9% during three month period.
Based on the present study findings, Architect 25-OH D vitamin assay can be used in routine 25-OH D measurements, still properly making the diagnosing of patients' status. Standardization efforts in improving immunoassay techniques do not seem to contribute too much to clinical diagnosis. Especially, in deficient/insufficient samples, laboratory experts should be aware of the misinterpretation of results. Our study offered an insight into D vitamin deficiency analyzing and further examinations.
Footnotes
Conflict of Interest: The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Sahota O. Understanding vitamin D deficiency. Age Ageing. 2014;43(5):589. doi: 10.1093/ageing/afu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speeckaert M M, Speeckaert R, van Geel N, Delanghe J R. Makowski Gregory S., editor. Vitamin D Binding Protein: A Multifunctional Protein of Clinical Importance. Advances in Clinical Chemistry. 2014;63:1–57. doi: 10.1016/b978-0-12-800094-6.00001-7. [DOI] [PubMed]
- 3.Clarke N M, Page J E. Vitamin D deficiency: A paediatric orthopaedic perspective. Curr Opin Pediatr. 2012;24(1):46. doi: 10.1097/MOP.0b013e32834ec8eb. [DOI] [PubMed] [Google Scholar]
- 4.Damiati S. A pilot study to assess Kidney functions and toxic dimethyl-arginines as risk biomarkers in women with low vitamin D levels. J Med Biochem. 2019;38(2):145. doi: 10.2478/jomb-2018-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiani A, Abedini A, Adcock I M, Sadat-Mirenayat M, Taghavi K, Mortaz E, Kazempour-Dizaji M. Association between vitamin D deficiencies in sarcoidosis with disease activity, course of disease and stages of lung involvements. J Med Biochem. 2018;37(2):103. doi: 10.1515/jomb-2017-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrell C, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-Art Vitamin D Assays: A Comparison of Automated Immunoassays with Liquid Chromatography-Tandem Mass Spectrometry Methods. Clin Chem. 2012;58(3):531. doi: 10.1373/clinchem.2011.172155. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich A, Volmer L. Analysis of Vitamin D Metabolic Markers by Mass Spectrometry: Current Techniques, Limitations of the 'Gold Standard' Method, and Anticipated Future Directions. Mass Spectrom Rev. 2015;34:2–23. doi: 10.1002/mas.21408. [DOI] [PubMed] [Google Scholar]
- 8.Atef S H. Vitamin D assays in clinical laboratory: Past, present and future challenges. J Steroid Biochem Mol Biol. 2018;175:136–137. doi: 10.1016/j.jsbmb.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Grebe S K G, Singh R J. LC-MS/MS in the Clinical Laboratory: Where to from Here? Clin Biochem Rev. 2011;32(1):5. [PMC free article] [PubMed] [Google Scholar]
- 10.Stepman H C M, Vanderroost A, van Uytfanghe K, Thienpont L M. Candidate Reference Measurement Procedures for Serum 25-Hydroxyvitamin D3 and 25-Hydroxyvitamin D2 by Using Isotope-Dilution Liquid Chromatography-Tandem Mass Spectrometry. Clin Chem. 2011;57(3):441. doi: 10.1373/clinchem.2010.152553. [DOI] [PubMed] [Google Scholar]
- 11.Çakır M Ö, Orçun A, Yıldız Z, Sirmali R. Evaluation of new Beckman Coulter 25(OH) Vitamin D assay and potential improvement of clinical interpretation. Biochem Med (Zagreb) 2017:332–273. doi: 10.11613/BM.2017.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakobsen J, Bysted A, Andersen R, Bennett T, Brot C, Bügel S, Cashman K D, Denk E, Harrington M, Teucher B, Walczyk T, Ovesen L. Vitamin D status assessed by a validated HPLC method: Within and between variation in subjects supplemented with vitamin D3. Scand J Clin Lab Invest. 2009;69(2):190. doi: 10.1080/00365510802471570. [DOI] [PubMed] [Google Scholar]
- 13.Sempos C T, Vesper H W, Phinney K W, Thienpont L M, Coates P M. Vitamin D status as an international issue: National surveys and the problem of standardization. Scand J Clin Lab Invest. 2012;72(suppl 243):32. doi: 10.3109/00365513.2012.681935. [DOI] [PubMed] [Google Scholar]
- 14.Hutchinson K, Healy M, Crowley V, Louw M, Rochev Y. Verification of Abbott 25-OH-vitamin D assay on the architect system. Pract Lab Med. 2017;7:27–35. doi: 10.1016/j.plabm.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annema W, Nowak A, von Eckardstein A, Saleh L. Evaluation of the new restandardized Abbott Architect 25-OH Vitamin D assay in vitamin D-insufficient and vitamin D-supplemented individuals. J Clin Lab Anal. 2018;32(4):e22328. doi: 10.1002/jcla.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A. V. D. package insert 510k 25-OH D, Decision Sum - mary Assay Only Template. 1825;510:1–17. [Google Scholar]
- 17.Gören A C, Şahin G, Gümilcineli İ, Binici B. Rapid and reliable 25-OH vitamin D2 and 25-OH vitamin D3 measurements by multitasker LC-MS/MS Journal of Chemical Metrology. 2018;12(1):17. doi: 10.25135/jcm.13.18.02.070. [DOI] [Google Scholar]
- 18.Thacher T D, Clarke B L. Vitamin D Insufficiency. Mayo Clin Proc. 2011;86(1):50. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher C J, Sai A J. Vitamin D Insufficiency, Deficiency, and Bone Health. J Clin Endocrinol Metab. 2010;95(6):2630. doi: 10.1210/jc.2010-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manuilova E, Schuetzenmeister A, Model F. 2014 [Google Scholar]
- 21.Marshall J, Nunes T, Heuer C, Marshall J. RStudio: Integrated Development for R. Boston, MA: RStudio, Inc-RStudio Team; 2015. [Google Scholar]
- 22.Dreier N, Lykkedegn S, Back P. S-25-hydroxyvitamin D and C3-epimers in pregnancy and infancy: An Odense Child Cohort study. Clin Biochem. 2017;(2):1. doi: 10.1016/j.clinbiochem.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Ozcan N, Ucar F, Arzuhal A E, Bulut E, Ozturk A, Taslipinar Y M, Temel I, Erden G. Evaluation of the analytical performance of Unicel DXI 800 for the Total 25 (OH) Vitamin D measurements. Clin Biochem. 2016;49(6):486. doi: 10.1016/j.clinbiochem.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Topçuoğlu C, Sezer S, Yılmaz F M, Kösem A, Ercan M, Turhan T. Evaluation of the analytical performance of the Beckman Coulter Unicel DXI 800 Access Total 25(OH) Vitamin D immunoassay Laboratoriums Medizin. 2018;42(5):205. doi: 10.1515/labmed-2018-0068. [DOI] [Google Scholar]
- 25.Prevention and management of osteoporosis: Report of a WHO scientific group. Geneva, Switzerland: World Health Organization-WHO Scientific Group on the Prevention and Management of Osteoporosis; 2003. [Google Scholar]
- 26.Sezgin G, Ozturk G, Turkal R, Caykara B. Vitamin D levels of outpatients admitted to a University Hospital in the Marmara region of Turkey over 3 years. J Med Biochem. 2019;38(2):181. doi: 10.2478/jomb-2018-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bess D. Vitamin D deficiency in adults: Definition, clinical manifestations, and treatment. UpToDate Inc; [Google Scholar]
- 28.Cavalier E, Carlisi A, Bekaert A, Rousselle O, Chapelle J, Souberbielle J. Analytical evaluation of the new Abbott Architect 25-OH vitamin D assay. Clin Biochem. 2012;45(6):505. doi: 10.1016/j.clinbiochem.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Cavalier E, Lukas P, Bekaert A, Carlisi A, Le G C, Delanaye P, Souberbielle J. Analytical and clinical validation of the new Abbot Architect 25(OH)D assay: Fit for purpose? Clin Chem Lab Med. 2017;55(3):378. doi: 10.1515/cclm-2016-0566. [DOI] [PubMed] [Google Scholar]
- 30.Shah I J, James R, Barker J, Petroczi A, Naughton D P. Misleading measures in Vitamin D analysis: A novel LC-MS/MS assay to account for epimers and isobars. Nutr J. 2011;10(1):46. doi: 10.1186/1475-2891-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]