Abstract
Introduction
Crohn’s disease diagnosis and monitoring remains a great clinical challenge and often requires multiple testing modalities. Assessing Crohn’s disease activity in the entire gastrointestinal (GI) tract using a panenteric capsule endoscopy (CE) system could be used as an alternative to colonoscopy and cross-sectional imaging. This study assessed the accuracy and safety of panenteric CE in Crohn’s disease as compared with ileocolonoscopy (IC) and/or magnetic resonance enterography (MRE).
Methods
A prospective, multicentre study was performed in subjects with established Crohn’s disease. Individuals with proven small bowel patency underwent a standardised bowel preparation, followed by CE ingestion and IC either the same or following day. MRE, IC, and CE interpretations were performed by blinded central readers using validated scoring systems. The primary endpoint was the overall sensitivity of CE vs MRE and/or IC in Crohn’s disease subjects.
Results
Study enrolment included 158 subjects from 21 sites in the USA, Austria, and Israel. Of those, 99 were included in the analysis. Imaging modality scores indicated none to mild inflammation in the proximal small bowel and colon, but discrepant levels of inflammation in the terminal ileum. Overall sensitivity for active enteric inflammation (CE vs MRE and/or IC) was 94% vs 100% (p=0.125) and specificity was 74% vs 22% (p=0.001). Sensitivity of CE was superior to MRE for enteric inflammation in the proximal small bowel (97% vs 71%, p=0.021), and similar to MRE and/or IC in the terminal ileum and colon (p=0.500–0.625). There were seven serious adverse advents of which three were related to the CE device.
Conclusion
Panenteric CE is a reliable tool for assessing Crohn’s disease mucosal activity and extent compared with more invasive methods. This study demonstrates high performance of the panenteric CE as compared to MRE and/or IC without the need for multiple tests in non-stricturing Crohn’s disease.
Trial registration number
ClinicalTrials.gov NCT03241368
Keywords: Crohn's disease, small bowel disease, inflammatory bowel disease, colonoscopy, endoscopy
Summary box.
What is already known about this subject?
Mucosal healing is the preferred treatment target in the Crohn’s disease management algorithms. Unfortunately, Crohn’s disease assessments require multiple tests due to multifocal, patchy inflammation throughout the gastrointestinal tract with a predilection for the small bowel.
What are the new findings?
The PillCam Crohn’s system (panenteric video capsule endoscopy) offers the opportunity to evaluate the entire intestinal mucosal surface with a high degree of sensitivity using a single minimally invasive test.
How might it impact clinical practice in the foreseeable future?
The monitoring of Crohn’s disease could be directed by the use of this new capsule system, potentially reducing invasive testing and the number of procedures performed, in addition to reducing healthcare costs and providing a new alternative for disease monitoring.
Introduction
Crohn’s disease is an idiopathic chronic autoimmune disorder that typically involves the gastrointestinal (GI) tract. The worldwide disease incidence continues to increase.1 The natural history of Crohn’s disease is a progression from inflammatory lesion(s) to often stricturing and/or penetrating complications in the majority of patients.2 Early diagnosis and aggressive treatment to a target of mucosal healing has been associated with improved clinical outcomes.3 4
The diagnosis and monitoring of Crohn’s disease extent and severity is challenging. Non-specific symptoms may include fatigue, bloating, diarrhoea, abdominal pain, and clinical symptoms often do not correlate with biological activity.5 In addition, Crohn’s disease has a special predilection for the small bowel that is beyond the reach of standard endoscopic interrogations and can involve the small bowel mesentery and adjacent structures.6 For these reasons, clinicians often rely on magnetic resonance enterography (MRE), CT enterography (CTE), and/or ultrasound.7 8 These imaging tools are complementary to colonoscopy, which is invasive but remains the gold standard for colonic evaluations and tissue acquisition. This approach to Crohn’s disease activity assessment often requires multiple tests completed over several days.
Despite its high sensitivity for the detection of active small bowel inflammation, many clinicians have been reticent to employ capsule endoscopy (CE) as a diagnostic and monitoring tool in patients with Crohn’s disease owing to the small risk of capsule retention.9 A growing body of literature, however, has highlighted the safety of CE when used in conjunction with a patency capsule or cross-sectional enterography and has established criteria for improving observer performance.10–13 In addition, a paediatric Crohn’s disease study suggested that using CE to guide management results in higher rates of mucosal healing and deep remission.4
Recently, the advent of the pan-intestinal CE system, the PillCam Crohn’s system, has provided a mechanism to explore the entire GI tract mucosa with a single test. Unlike previous CE systems, data acquisition is not limited to the small bowel (PillCam SB 3 system) or the colon (PillCam COLON 2 system). Its application to Crohn’s disease management algorithms may allow for non-invasive simplified disease assessment strategies of the oesophagus, stomach, small bowel, and the colon. In addition, the PillCam Crohn’s capsule may provide critical information about proximal small bowel (PSB) disease that is not identified by other testing modalities.12 There is a paucity of data, however, on the performance of this CE system when compared with MRE and ileocolonoscopy (IC).4 14 The purpose of the BLINK study was to prospectively assess the performance and safety of the PillCam Crohn’s capsule in patients undergoing IC and MRE.
Materials and methods
Study design and population
BLINK was a multicentre, prospective study that evaluated the accuracy of CE versus IC and/or MRE for detecting active intestinal Crohn’s disease in subjects with established Crohn’s disease. All subjects had a history (within 2 years) of active mucosal disease (based on radiological, endoscopic, or histological evidence) or were felt to have a high clinical suspicion for current active disease. The study was designed to include up to 145 subjects at 21 investigational sites in the USA, Israel, and Austria. Eligible subjects ≥18 years of age who met all inclusion/exclusion criteria (online supplementary table 1) were enrolled between January 2018 and June 2019. All subjects underwent MRE, CE, and IC sequentially. Patients unable to complete all the required testing were excluded (figure 1).
Figure 1.
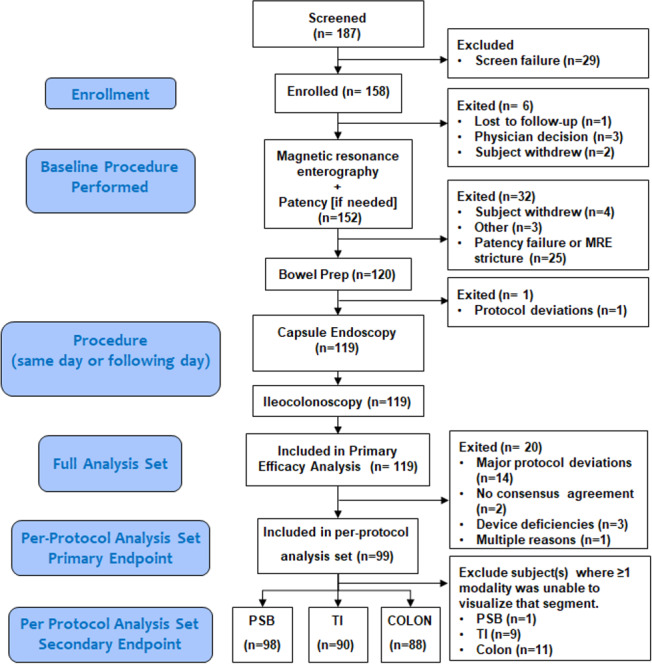
Flow chart of the subjects included and excluded in the study. MRE, magnetic resonance enterography; PSB, proximal small bowel; TI, terminal ileum.
bmjgast-2019-000365supp001.pdf (192.9KB, pdf)
Study objectives and endpoints
The objective of the study was to assess the accuracy of CE vs MRE and/or IC for detecting active inflammatory Crohn’s disease within the GI tract.
Primary endpoint
The primary endpoint was the overall sensitivity of CE versus MRE and/or IC for active intestinal Crohn’s disease in the small bowel and colon.
Secondary endpoints
Secondary endpoints were overall specificity, negative predictive value (NPV), positive predictive value (PPV) as well as segmental sensitivity and performance in the PSB to the terminal ileum (TI) (CE vs MRE), TI (CE vs IC and MRE), and colon (CE vs IC). The PSB was defined as the small bowel minus the TI segment. The TI was defined as the last 10 cm of small bowel on MRE and IC, or the last 10 min of the CE video before entering the caecum. In addition, patient satisfaction was also assessed.
Additional analysis
Additional analysis included correlation of disease severity with laboratory markers including C reactive protein (CRP) and faecal calprotectin (FC). Adverse events (AEs), bowel cleansing levels, CE completion rates, study deviations, and device deficiencies were also collected.
Study workflow
After providing informed consent, subjects underwent screening which included laboratory tests and a negative pregnancy test if applicable (online supplementary table 1). Demographic data, medical/surgical history, previous GI procedures, Montreal classification and medication information were collected. MRE was performed first in the series of tests and in accordance with a standardised imaging protocol (online supplementary table 2), which was used to identify strictures (defined as an unequivocal proximal upstream dilation ≥2.5 cm). If there was a clinical suspicion of a stricture despite negative MRE findings, the patient then underwent a patency capsule procedure (PillCam patency capsule; Medtronic, Yoqneam, Israel). If patency was confirmed, subjects received a bowel preparation (online supplementary table 3) prior to CE evaluation. IC was performed after the CE on the same or next day. If IC was not performed on the same day as CE, patient remained on clear liquids until the IC was performed.
Central readers
A skilled group of central readers was used to interpret the MRE, CE, and colonoscopy examinations. Central IC and MRE readers were experienced gastroenterologists and gastroenterology (GI) radiologists. CE, IC, and MRE images were anonymised and uploaded to an imaging core laboratory (ICON, North Wales, Pennsylvania) and randomly distributed to central readers. All readers were blinded to the site, subject data, and were required to read each video or image independently without external input. Images from each modality were evaluated using standardised, validated scoring systems, as described below. CE central readers were experienced gastroenterologists, who were trained by the sponsor to read and evaluate PillCam software videos. Clinical site radiologists and endoscopists were also trained prior to the trial to ensure consistency of data acquisition.
Imaging modalities
All enrolled subjects underwent MRE using either a 1.5T or 3T MR scanners using the parameters described in online supplementary table 2, and images were reviewed by central readers, according to the criteria for active Crohn’s disease in the small bowel as previously described.15 The PillCam Crohn’s system (Medtronic) can visualise the entire small bowel and colon and includes a regimen alert system to remind subjects to take the prescribed bowel preparation materials, as outlined in online supplementary table 3. All IC procedures were completed per the standard care at each facility and in accordance with the study protocol for video recording. During colonoscopy, TI intubation could not be achieved in 17 patients.
Assessment of Crohn’s disease activity
Active Crohn’s disease was assessed using clinical, laboratory, stool, radiological, and endoscopic measures of disease activity. Laboratory tests included complete blood count with differential, serum albumin, CRP, and FC. Disease activity was assessed by three separate scoring systems for IC, MRE, and CE according to the modality and location. Any evidence of active disease regardless of the level of severity (ie, mild, moderate, and severe) or location was considered active disease. The Simple Endoscopic Score for Crohn’s Disease (SES-CD) score was used to evaluate the TI and colon by CE or IC according to the following cut-off values: 0–2=inactive, 3–6=mild, 7–15=moderate, and >15=severe.16 The Lewis Score (LS) assessed active Crohn’s disease in the PSB by CE with the following cut-off values: <135=inactive, 135≤ LS ≤790=mild, and >790=moderate or severe.17 An adapted Magnetic Resonance Index of Activity (MaRIA) scoring system was used for assessing the small bowel and TI by MRE, taking into account wall thickness, relative contrast enhancement, presence of intramural oedema and ulcerations, with activity graded according to the following cut-off values: inactive <7, mild 7–9, moderate 9–11, and severe >11.18 19
Reference standard
Anticipating potential disagreement among the numerical indices used to indicate disease activity, a standardised method for resolving discrepancies was created prior to the initiation of the study, as has been done previously.20 In cases of agreement, the reference diagnosis was determined by the presence or absence of inflammation as assigned by the modality-specific scoring systems at prospective interpretation by expert central readers—MaRIA (MRE), SES-CD (colonoscopy), and the LS (CE). In cases with discrepant numerical indices for any bowel segment, MRE, CE, and IC were reviewed and resolved by a consensus panel which consisted of three gastroenterologists (Cristiano Spada, Fondazione Poliambulanza; Bruno Rosa, Hospital da Senhora da Oliveira; and Daniel Mishkin, Beth Israel Deaconess Hospital) and a radiologist (Jordi Rimola, Hospital Clínic of Barcelona). To establish the reference diagnosis in cases of discrepancy between modality indices, the images from MRE, videos (CE and colonoscopy), laboratory, and clinical data were reviewed by all panel members and discussed. The panel then determined the presence or absence of Crohn’s inflammation based on all images, videos, and evidence, with gastroenterologist and radiologist panellists indicating which scoring systems reflected the reference diagnosis and which did not. This comparison was performed for every bowel segment in cases, which were reviewed.
Statistical analysis
Subjects enrolled by 30 April 2019 were selected through the convenience sampling method. The study was non-powered, and all continuous data were summarised using descriptive statistics, specifically the number of observations (N), mean, SD, median, minimum, maximum, and 95% CIs were calculated as appropriate. Kappa test and Spearman’s coefficient were used to assess correlation. Safety and baseline demographic analysis was based on all subjects who completed assessments by MRE, CE, and IC modalities. The analysis was based on the per-protocol analysis, which included data of all subjects who completed assessments by MRE, CE, and IC modalities without major protocol deviations at baseline or who did not withdraw, have capsule retention or have system technical failures. Missing data were not included in the primary analysis.
Safety assessment
Type, incidence, severity, duration, and procedure/device relationship of AEs were collected. AEs for all enrolled subjects were collected from the start of baseline imaging procedures (MRE) and through week following completion of the last imaging procedure (IC). Subjects with AEs at 1 week following the IC procedure were followed for 30 days or until the event resolved.
Results
Patient characteristics
A total of 187 patients were screened and 158 enrolled from 19 sites in the USA, 1 in Austria, and 1 in Israel from January 2018 through June 2019. Baseline characteristics were as follows: 40% males, 60% females with a mean (±SD) age of 40 (±12) years, and a mean (±SD) body mass index of 29.0 (±7.53) kg/m2. A complete list of baseline demographics, clinical features, Montreal classification, and concurrent Crohn’s disease-related medications are listed in online supplementary table 4. Clinical activity scores of inflammation were collected by each test and classified according to the scoring indices.
Overall mean measures of inflammation were assessed and revealed mostly none to mild inflammation in the PSB and colon (by LS and MaRIA score), and discrepant levels of inflammation in the TI (table 1).
Table 1.
Clinical activity scores by segment according to modality and the scoring index (LS, MaRIA, or SES-CD)
| Test | Scoring index* | Proximal small bowel, n=98 | Terminal ileum, n=90 | Colon, n=88 |
| CE | LS, mean (SD) |
599.2 (1291.3) | – | – |
| SES-CD, mean (SD) |
– | 3.1 (2.9) | 1.2 (1.8) | |
| MRE | MaRIA score, mean (SD) |
7.8 (3.8) | 12.0 (6.9) | – |
| IC | SES-CD, mean (SD) |
– | 2.6 (2.9) | 1.4 (2.2) |
*LS <135=absent, 135≤ LS ≤790=mild, and >790=moderate or severe; SES-CD index 0–2=absent, 3–6=mild, 7–15=moderate, and >15=severe; MaRIA score <7=inactive, 7–9=mild,>9–11=moderate, and >11=severe.
CE, capsule endoscopy; IC, ileocolonoscopy; LS, Lewis Score; MaRIA, Magnetic Resonance Index of Activity; MRE, magnetic resonance enterography; SES-CD, Simple Endoscopic Score for Crohn’s Disease.
Patient population for analysis
Of the 158 patients enrolled, 119 underwent all three tests with MRE, CE, and colonoscopy. Of those, 119 were included in the safety and demographic analysis. A total of 66% (78/119) of subjects went to the consensus panel when there were discrepancies in the numerical modality-specific activity scores. Discrepancies between the modality scores and the final consensus panel determination were further analysed by segment (online supplementary table 5). The per-protocol overall analysis was performed in 99 patients who had at least one segment with acceptable comparative imaging modalities.
Disease activity
According to the reference standard, the overall active disease was found in 73% (72/99) of subjects in the overall GI tract. In the segmental analysis, 32% (31/98) of subjects had active disease present in the PSB, 58% (52/90) in the TI, and 26% (23/88) in the colon. When we evaluated disease activity based on the total number of segments visualised by CE, 39% (39/99) of PSB segments, 61% (56/92) of TI segments, and 30% (27/90) of colon segments had active disease. CE demonstrated a similar trend to the reference standard with 40% (39/98) of subjects having active disease in the PSB, 62% (56/90) in the TI, and 31% (27/88) in the colon.
Primary and secondary analyses
The primary endpoint was the overall sensitivity of CE vs MRE and/or IC for active intestinal Crohn’s disease in the small bowel and colon. Based on the per-protocol analysis (table 2), CE demonstrated a very high overall intestinal sensitivity for evaluating the entire GI tract with a sensitivity of 94% (95% CI: 86% to 98%) compared with 100% (95% CI: 95% to 100%) for MRE and/or IC, p=0.125. Specificity was significantly higher for CE compared with MRE and/or IC (74% (95% CI: 55% to 87%) vs 22% (95% CI: 10% to 41%), p=0.001). CE had a higher PPV than MRE and/or IC but lower NPV.
Table 2.
Accuracy measures of CE compared with the modality in the entire GI tract and per segmental analysis
| Segment | Test | Sensitivity % (95% CI) |
Specificity % (95% CI) | PPV% (95% CI) |
NPV% (95% CI) |
| Overall | CE (n=99) | 94 (86 to 98) | 74 (55 to 87) | 91 (82 to 96) | 83 (64 to 94) |
| MRE and/or IC (n=99) | 100 (95 to 100) | 22 (10 to 41) | 77 (68 to 85) | 100 (54 to 100) | |
| P value | 0.125 | 0.001 | – | – | |
| Proximal small bowel* | CE (n=98) | 97 (82 to 100) | 87 (76 to 93) | 77 (62 to 88) | 98 (90 to 100) |
| MRE (n=98) | 71 (53 to 84) | 66 (54 to 76) | 49 (35 to 63) | 83 (71 to 91) | |
| P value | 0.021 | 0.020 | – | – | |
| Terminal ileum | CE (n=90) | 94 (84 to 99) | 82 (66 to 91) | 88 (76 to 94) | 91 (76 to 98) |
| MRE and/or IC (n=90) | 98 (89 to 100) | 37 (23 to 53) | 68 (57 to 78) | 93 (68 to 100) | |
| P value | 0.625 | <0.001 | – | – | |
| CE (n=83) | 94 (82 to 98) | 81 (66 to 91) | 86 (74 to 93) | 91 (76 to 98) | |
| IC (n=83) | 89 (77 to 96) | 92 (78 to 98) | 93 (81 to 98) | 87 (73 to 95) | |
| P value | 0.688 | 0.289 | – | – | |
| CE (n=92) | 94 (84 to 99) | 82 (67 to 91) | 88 (76 to 94) | 91 (77 to 98) | |
| MRE (n=92) | 79 (66 to 88) | 44 (29 to 59) | 66 (53 to 76) | 61 (42 to 77) | |
| P value | 0.057 | 0.001 | – | – | |
| Colon | CE (n=88) | 83 (62 to 94) | 88 (77 to 94) | 70 (51 to 84) | 93 (84 to 98) |
| IC (n=88) | 91 (72 to 99) | 89 (79 to 95) | 75 (56 to 88) | 97 (88 to 100) | |
| P value | 0.500 | 1.00 | – | – |
CE, capsule endoscopy; GI, gastrointestinal; IC, ileocolonoscopy; MRE, magnetic resonance enterography; NPV, negative predictive value; PPV, positive predictive value.
Segmental analysis was performed for the PSB, TI, and colon (table 2). In regard to the PSB, CE had a significantly higher sensitivity and specificity compared with MRE (97% vs 71%, p=0.021) and (87% vs 66%, p=0.020), respectively. In the TI, no significant difference in sensitivity was found between CE and the combined modalities (MRE and/or colonoscopy) or between CE compared with either MRE or IC alone. Specificity of CE was significantly higher than MRE combined with IC (82% vs 37%, p<0.001) and MRE alone in the TI. Similarly, when analysing the colon, CE and IC had no significant difference in sensitivity or specificity.
Correlation of disease severity
Correlation of disease severity (absent, mild, moderate, or severe) using the scoring indices LS, SES-CD, and the MaRIA obtained by CE, IC, and MRE were assessed. Patients who did not have that segment visualised by that modality were excluded from the analysis. In the PSB, CE LS classified 17% (17/98) of subjects as having moderate/severe disease compared with 20% (20/98) of subjects classified as severe according to MaRIA score; however, no statistical significance was identified (table 3). In the TI, no subjects were classified as having severe disease by either IC or CE; however, 42% (38/90) of subjects were classified as severe disease by the MRE MaRIA score. This trend was further confirmed by imaging modalities comparing MRE to both CE and IC in the TI (figure 2).
Table 3.
Correlation of disease severity in the proximal small bowel, terminal ileum, and colon using CE versus MRE plus IC based on Lewis, SES-CD, and MaRIA scores
| CE score | Kappa (95% CI) | P value | ||||||
| Proximal small bowel Lewis Score | ||||||||
| Absent | Mild | Moderate/severe | Total | |||||
| MRE | MaRIA score | Absent | 35 (36%) | 12 (12%) | 6 (6%) | 53 (54%) | 0.105 (−0.046 to 0.257) | 0.153 |
| Mild | 12 (12%) | 7 (7%) | 6 (6%) | 25 (26%) | ||||
| Moderate/severe | 12 (12%) | 3 (3%) | 5 (5%) | 20 (20%) | ||||
| Total | 59 (60%) | 22 (22%) | 17 (17%) | 98 (100%) | ||||
| Terminal ileum SES-CD | ||||||||
| Absent | 13 (14%) | 12 (13%) | 1 (1%) | 26 (29%) | 0.009 (−0.097 to 0.080) | 0.849 | ||
| Mild | 9 (10%) | 5 (6%) | 1 (1%) | 15 (17%) | ||||
| Moderate | 6 (7%) | 5 (6%) | 0 | 11 (12%) | ||||
| Severe | 6 (7%) | 22 (24%) | 10 (11%) | 38 (42%) | ||||
| Total | 34 (38%) | 44 (49%) | 12 (13%) | 90 (100%) | ||||
| IC | SES-CD | Terminal ileum SES-CD | ||||||
| Absent | 28 (34%) | 10 (12%) | 0 | 38 (46%) | <0.001 | 0.579 (0.423 to 0.736) | ||
| Mild | 3 (4%) | 30 (37%) | 4 (5%) | 37 (45%) | ||||
| Moderate | 1 (1%) | 2 (2%) | 4 (5%) | 7 (9%) | ||||
| Total | 32 (39%) | 42 (51%) | 8 (10%) | 82 (100%) | ||||
| Colon SES-CD | ||||||||
| Absent | 52 (59%) | 8 (9%) | 0 | 60 (68.2%) | <0.001 | 0.440 (0.260 to 0.619) | ||
| Mild | 9 (10%) | 14 (16%) | 1 (1%) | 24 (27%) | ||||
| Moderate | 0 | 4 (5%) | 0 | 4 (5%) | ||||
| Total | 61 (69%) | 26 (30%) | 1 (1%) | 88 (100%) | ||||
*Lewis score <135=absent, 135≤ Lewis Score ≤790=mild, and >790=moderate or severe; SES-CD index 0–2=absent, 3–6=mild, 7–15=moderate, and >15=severe; MaRIA score <7=inactive, 7–9=mild, >9–11=moderate, and >11=severe.
CE, capsule endoscopy; IC, ileocolonoscopy; MaRIA, Magnetic Resonance Index of Activity; MRE, magnetic resonance enterography; SES-CD, Simple Endoscopic Score for Crohn’s Disease.
Figure 2.
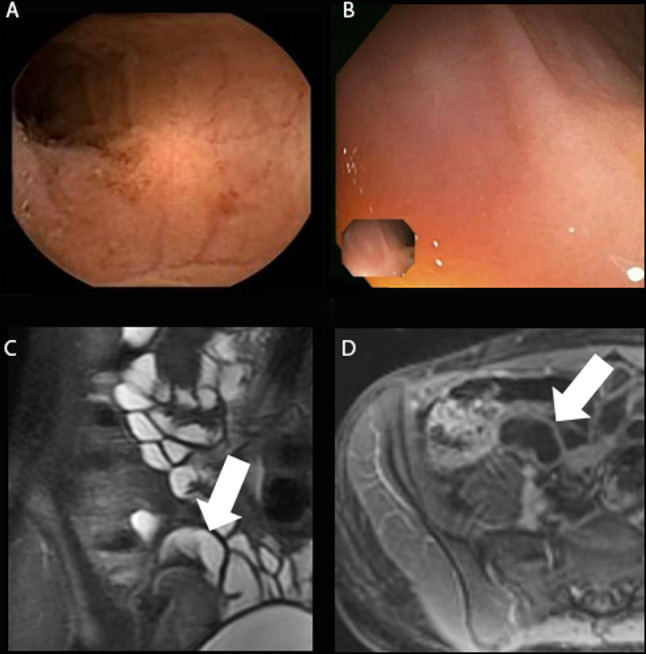
(A–D) Subject with active disease in the terminal ileum by MRE (MaRIA score 15.74) and negative findings according to both CE and IC (SES-CD of 0 in the terminal ileum and colon). (A) and (B) demonstrate normal CE and IC images. (C) and (D) demonstrate MRE images of the terminal ileum. After review of all images and laboratory data, the consensus panel determined that the terminal ileum did not have active inflammatory Crohn’s disease. CE, capsule endoscopy; IC, ileocolonoscopy; MaRIA, Magnetic Resonance Index of Activity; MRE, magnetic resonance enterography; SES-CD, Simple Endoscopic Score for Crohn’sDisease.
There was moderate correlation in the TI (k=0.579, 95% CI: 0.423 to 0.736, p<0.001) and colon (k=0.440, 95% CI: 0.260 to 0.619, p<0.001) between CE and IC when SES-CD scoring index was used by both modalities. No other significant disease severity correlation was found between any of the tests. Finally, when assessing disease correlation between CE and laboratory parameters (FC and CRP), only a moderate positive agreement (ρ=0.505, p<0.001) was found between FC levels and CE SES-CD in the colon and a low positive agreement (ρ=0.320, p=0.003) in the TI. No correlation was found between CRP levels and disease severity levels in any other segment of the bowel.
Patency and capsule completion rate
MRE was completed in 151 subjects and 37/151 (25%) had evidence of strictures. A patency capsule study was further completed in 34 subjects. Patency was confirmed in 19/34 (56%) subjects. Of the 119 patients who swallowed the CE, 1 patient requested and had CE removed from the stomach with an oesophagogastroduodenoscopy due to worsening anxiety with testing (documented as an AE). In 16 patients, the CE was removed at the time of colonoscopy (CE had not passed spontaneously at the time of planned IC). One patient retained the capsule endoscopy (more than 14 days). In this patient, CE was retrieved at the time of second colonoscopy with dilation of a narrowing at the ileocolonic anastomosis. The median duration of capsule intestinal transit time was 6 hours 49 min.
Patient compliance
Subjects received a baseline and three additional bowel preparation alerts per the CE system alert regimen (online supplementary table 3). Out of 119 subjects who underwent the CE procedure, 99.2% (118/119) of patients received the alerts. Of 118 subjects who received the alerts, 98% (117/118) took the appropriate actions for the first alert (ie, additional bowel preparation solution or booster according to protocol within the allotted time), 77% (91/117) were compliant with the second alert, and 60% (71/117) were compliant with the third alert. Two patients excluded in this analysis were subjects who had device deficiencies and did not receive partial or all alerts.
Colon cleansing level
CE cleansing levels were considered adequate by the CE central readers in 79% of cases in the PSB, 90% in the TI, and 64% in the colon, using a 4-point grading system (inadequate=poor/fair and adequate=good/excellent).21 The IC central readers rated the cleansing levels similarly adequate, 88% in the TI and 77% in the colon. There were no cases where an assessment could not be completed due to inadequate cleansing levels.
Patient satisfaction
After completing the last procedure (IC), subjects were requested (either in person or via telephone) to answer a patient satisfaction questionnaire on which procedure they preferred (CE, IC, or IC±MRE combined) as a disease monitoring procedure. Of 118 subjects who completed the questionnaire, 54% of patients preferred CE, 36% preferred IC, and 9% preferred the combined modalities of IC+MRE. The most common reasons for those who preferred CE were no need for sedation (33%), no need for a driver (33%), single procedure to see all intestines (32%), no need for intravenous access (32%), and were able to move around during the procedure (32%). The most common reasons for preferring IC were most familiar with it (25%), ability to biopsy (16%), and it is a standard procedure (16%).
Summary of AEs
There were a total of seven serious AEs (SAEs) in 16/119 (13%) subjects who underwent all three tests (online supplementary table 6). Of the SAEs, two were severe; one subject who had abdominal pain developed a partial bowel obstruction which was later deemed to be related to the capsule with retention at an ulcerated stricture. The second subject had worsening pain and was hospitalised for sigmoid resection due to perforation; however, this was determined to not be capsule related as the subject had passed CE and completed IC without complications. Both subjects had recovered/resolved from the events. No AEs were related to the patency capsule or the MRE procedure.
Discussion
Treating, diagnosing, and monitoring patients with Crohn’s disease is a daunting clinical challenge. IC often must be combined with MRE or CTE to assess the small bowel and colon.7 Despite this strategy, concerns exist about missing subtle upper gut and PSB disease. When active Crohn’s disease is unrecognised, alternate diagnostic tests are often wrongfully ordered to evaluate for other conditions. In addition, the current Crohn’s disease assessment approach requires multiple tests typically performed on separate days.22 With this in mind, CE may allow clinicians and patients a simplified method to better evaluate what is often under evaluated and underappreciated: small bowel disease activity.
This study revealed the exceptional performance characteristics of panenteric CE when compared with MRE and/or IC. In regard to the primary aim of the study, the sensitivity of the CE was equal (no statistical difference) or better than MRE and/or IC in the overall intestinal assessments and in each of the analysed segments. This is the first prospective multicentre study to reveal these findings. Additionally, the specificity of CE was superior to MRE in the small bowel, and similar to IC in the TI and colon. These results would suggest the need to create a prominent role for panenteric CE in Crohn’s disease diagnostic and monitoring algorithms as well as provide additional evidence on the role of using panenteric CE in patients with Crohn’s disease, which is currently scarce.4 23–25
Additional potential cost and risk benefits to patients with Crohn’s disease are suggested by this trial. Patients with inflammatory bowel disease have a significant number of workdays lost per year just due to disease activity, testing preparation, test administration, and recovery.26–30 CE could potentially attenuate this burden by reducing the number and days of testing. These results also demonstrate panenteric CE to be safe in patients with non-stricturing Crohn’s disease as well as having a high patient preference. Only one case of CE retention occurred and was retrieved after a second colonoscopy with stricture dilation. In addition, the inherent risks of sedation and endoscopy are avoided with CE.
Several limitations to this study should be noted. First, the results apply to patients with Crohn’s disease with mild non-stricturing disease. Bias could exist for the reported performance estimates for all modalities given the mild disease (eg, patients demonstrating strictures with inflammation were excluded by MRE prior to CE assessment). Moderate to severe disease was only identified by MRE MaRIA in the TI. Second, the low specificity (high discrepancy rate) for MRE in the TI is reported. This could be the result of altered perfusion (previous ileocecal surgery, n=22), high MaRIA scores in the absence of convincing wall thickening (eg, potentially due to near-field artefacts), differences in the identification of ulcerations or intramural oedema, and the relatively small number of patients without TI inflammation. The concept of intramural disease has now been well described with MRE and CTE,6 31 and isolated findings of intramural inflammation by MRE with normal ileoscopic findings can exist.32 However, in this study, the reference standard in cases of discrepant numerical indices required radiologist review of MRE images, and acknowledgement that the images and preponderance of evidence indicated inflammation was absent. The use of a consensus panel as the reference standard when discrepancies occurred is to take advantage of the diagnostic advantages of each modality (eg, mucosal visualisation for IC, display of the intestinal wall, and perienteric tissues for MRE), but should also be acknowledged as a potential limitation given questions about validity and reproducibility of this methodology.
Lastly, the potential non-specific features of Crohn’s disease on CE and other testing modalities should be noted as a potential limitation.
This study demonstrates the powerful potential of panenteric CE to provide accurate Crohn’s disease assessments. Furthermore, CE in this population was noted to be a safe alternative to multiple modality testing. Panenteric CE should be considered for utilisation in non-stricturing Crohn’s disease evaluations.
Acknowledgments
We acknowledge Jessica Carlson (Medtronic) for clinical support; Delila Peri (Medtronic) for medical writing support; Ming Teng (Medtronic) and Sylvain Anselme (Medtronic) for statistical analysis; Ari Bergwerk (Medtronic) for medical advisement. The authors also thank the BLINK study investigators and staff at all participating sites.
Footnotes
Collaborators: BLINK Collaborators: The participating investigators for the BLINK study group were: Dr Linda Cummings, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA; Dr Mukund Venu, Loyola University, Chicago, Illinois, USA; Dr Laurel Fisher, University of Pennsylvania Hospital, Philadelphia, Pennsylvania, USA; Dr Tuba Esfandyari, University of Kansas Hospital, Kansas City, Kansas, USA; Dr Kara De Felice, Louisiana State University Hospital, New Orleans, Louisiana, USA; Dr Michael Chiorean, Virginia Mason Hospital, Seattle, Washington, USA; Dr Dario Sorrentino, Carrillion Clinic, Roanoke, Virginia, USA; Dr David Pound, Indianapolis Gastroenterology, Indianapolis, Indiana, USA; Dr Felix Tiongco, Gastroenterology Associates of Tidewater, Norfolk, Virginia, USA; Dr Stanley Cohen, Children’s Center for Digestive Healthcare, Atlanta, Georgia, USA; Dr Michael Rice, University of Michigan Hospital, Ann Arbor, Michigan, USA; Dr Pramod Malik, Virginia Research Institute Hospital, Suffolk, Virginia, USA; Dr Jack DiPalma, University of South Alabama Hospital, Mobile, Alabama, USA; Dr Razvan Arsenescu, Morristown Memorial Hospital/Atlantic Health, Morristown, New Jersey, USA; Dr Brian Garvin, Asheville Gastroenterology Associates – a division of Digestive Health Partners, Asheville, North Carolina, USA; Dr Carl Raczkowski, Digestive Disease Specialists, Oklahoma City, Oklahoma, USA; Dr Werner Dolak, Medical University of Vienna, Vienna, Austria; Dr Abraham Eliakim, Sheba Medical Center, Tel-Aviv, Israel; Central Readers and/or consensus panel: Dr Cristina Carretero Ribon, Clinical Universidad de Navarra, Spain; Dr Leung Wai-Keung, Queen Mary Hospital, University of Hong Kong, Hong Kong; Dr Bruno Rosa, Hospital da Senhora da Oliveira – Guimarães, Portugal; Dr Cristiano Spada, Fondazione Poliambulanza, Roma, Italy; Dr Mattitiahu Waterman, Rambam Health Care Campus, Haifa, Israel; Dr Daniel Mishkin, Crown Colony Medical Center, Quincy, Massachusetts, USA; Dr Blair Lewis, Carnegie Hill Endoscopy, New York City, New York; Dr Jordi Rimola, University of Barcelona, Spain; Dr Rendon Nelson, Duke Radiology, Durham, North Carolina.
Contributors: The following authors made substantial contributions to the conception or design of the work (DHB, JGF, SO), interpretation of data (DHB, JGF, SO, MRF, MF), drafting the article (DHB, JGF, SO, MRF, MF); critical revisions of the article (DHB, JGF, SO, MRF, MF); and approved the version to be published (DHB, JGF, SO, MRF, MF).
Funding: The study was sponsored by Given Imaging, a Medtronic company (Minneapolis, Minnesota), which contributed to the study design, data collection and data analysis, and assisted with manuscript writing. All authors had full access to all study data and final responsibility for the decision to submit for publication.
Competing interests: DHB declares consulting for Medtronic. SO declares research funding from Medtronic.
Patient consent for publication: Not required.
Ethics approval: This study was conducted in accordance with the Declaration of Helsinki and all local regulatory requirements. Institutional review board approval was first received on 26 October 2017 and subsequently thereafter from all participating clinical sites. All subjects provided written informed consent prior to participation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. No individual participant data will be shared.
Contributor Information
BLINK study group:
Linda Cummings, Mukund Venu, Laurel Fisher, Tuba Esfandyari, Kara De Felice, Michael Chiorean, Dario Sorrentino, David Pound, Felix Tiongco, Stanley Cohen, Michael Rice, Pramod Malik, Jack DiPalma, Razvan Arsenescu, Brian Garvin, Carl Raczkowski, Abraham Eliakim, Cristina Carretero Ribon, Leung Wai-Keung, Bruno Rosa, Cristiano Spada, Mattitiahu Waterman, Daniel Mishkin, Blair Lewis, Jordi Rimola, Rendon Nelson, and Werner Dolak
References
- 1. Shivashankar R, Tremaine WJ, Harmsen WS, et al. Incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol 2017;15:857–63. 10.1016/j.cgh.2016.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cosnes J, Cattan S, Blain A, et al. Long-Term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis 2002;8:244–50. 10.1097/00054725-200207000-00002 [DOI] [PubMed] [Google Scholar]
- 3. Colombel J-F, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn's disease (calm): a multicentre, randomised, controlled phase 3 trial. Lancet 2018;390:2779–89. 10.1016/S0140-6736(17)32641-7 [DOI] [PubMed] [Google Scholar]
- 4. Oliva S, Aloi M, Viola F, et al. A treat to target strategy using panenteric capsule endoscopy in pediatric patients with Crohn's disease. Clin Gastroenterol Hepatol 2019;17:2060–7. 10.1016/j.cgh.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 5. Cellier C, Sahmoud T, Froguel E, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut 1994;35:231–5. 10.1136/gut.35.2.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Samuel S, Bruining DH, Loftus EV, et al. Endoscopic skipping of the distal terminal ileum in Crohn's disease can lead to negative results from ileocolonoscopy. Clin Gastroenterol Hepatol 2012;10:1253–9. 10.1016/j.cgh.2012.03.026 [DOI] [PubMed] [Google Scholar]
- 7. Bruining DH, Zimmermann EM, Loftus EV, et al. Consensus Recommendations for Evaluation, Interpretation, and Utilization of Computed Tomography and Magnetic Resonance Enterography in Patients With Small Bowel Crohn's Disease. Gastroenterology 2018;154:1172–94. 10.1053/j.gastro.2017.11.274 [DOI] [PubMed] [Google Scholar]
- 8. Taylor SA, Mallett S, Bhatnagar G, et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn's disease (metric): a multicentre trial. Lancet Gastroenterol Hepatol 2018;3:548–58. 10.1016/S2468-1253(18)30161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Voderholzer WA, Beinhoelzl J, Rogalla P, et al. Small bowel involvement in Crohn's disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut 2005;54:369–73. 10.1136/gut.2004.040055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boivin ML, Lochs H, Voderholzer WA. Does passage of a patency capsule indicate small-bowel patency? A prospective clinical trial? Endoscopy 2005;37:808–15. 10.1055/s-2005-870220 [DOI] [PubMed] [Google Scholar]
- 11. Al-Bawardy B, Locke G, Huprich JE, et al. Retained capsule endoscopy in a large tertiary care academic practice and radiologic predictors of retention. Inflamm Bowel Dis 2015;21:2158–64. 10.1097/MIB.0000000000000482 [DOI] [PubMed] [Google Scholar]
- 12. Hansel SL, McCurdy JD, Barlow JM, et al. Clinical benefit of capsule endoscopy in Crohn's disease: impact on patient management and prevalence of proximal small bowel involvement. Inflamm Bowel Dis 2018;24:1582–8. 10.1093/ibd/izy050 [DOI] [PubMed] [Google Scholar]
- 13. Pasha SF, Pennazio M, Rondonotti E, et al. Capsule retention in Crohn's disease: a meta-analysis. Inflamm Bowel Dis 2019. [DOI] [PubMed] [Google Scholar]
- 14. Melmed GY, Dubinsky MC, Rubin DT, et al. Utility of video capsule endoscopy for longitudinal monitoring of Crohn's disease activity in the small bowel: a prospective study. Gastrointest Endosc 2018;88:947–55. 10.1016/j.gie.2018.07.035 [DOI] [PubMed] [Google Scholar]
- 15. Bruining DH, Zimmermann EM, Loftus EV, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance Enterography in patients with small bowel Crohn's disease. Radiology 2018;286:776–99. 10.1148/radiol.2018171737 [DOI] [PubMed] [Google Scholar]
- 16. Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. 10.1016/S0016-5107(04)01878-4 [DOI] [PubMed] [Google Scholar]
- 17. Gralnek IM, Defranchis R, Seidman E, et al. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther 2008;27:146–54. 10.1111/j.1365-2036.2007.03556.x [DOI] [PubMed] [Google Scholar]
- 18. Rimola J, Ordás I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn's disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis 2011;17:1759–68. 10.1002/ibd.21551 [DOI] [PubMed] [Google Scholar]
- 19. Coimbra AJF, Rimola J, O'Byrne S, et al. Magnetic resonance enterography is feasible and reliable in multicenter clinical trials in patients with Crohn's disease, and may help select subjects with active inflammation. Aliment Pharmacol Ther 2016;43:61–72. 10.1111/apt.13453 [DOI] [PubMed] [Google Scholar]
- 20. Solem CA, Loftus EV, Fletcher JG, et al. Small-Bowel imaging in Crohn's disease: a prospective, blinded, 4-way comparison trial. Gastrointest Endosc 2008;68:255–66. 10.1016/j.gie.2008.02.017 [DOI] [PubMed] [Google Scholar]
- 21. Leighton JA, Rex DK. A grading scale to evaluate colon cleansing for the PillCam colon capsule: a reliability study. Endoscopy 2011;43:123–7. 10.1055/s-0030-1255916 [DOI] [PubMed] [Google Scholar]
- 22. Lichtenstein GR, Loftus EV, Isaacs KL, et al. Acg clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol 2018;113:481–517. 10.1038/ajg.2018.27 [DOI] [PubMed] [Google Scholar]
- 23. Eliakim R, Spada C, Lapidus A, et al. Evaluation of a new pan-enteric video capsule endoscopy system in patients with suspected or established inflammatory bowel disease - feasibility study. Endosc Int Open 2018;6:E1235–46. 10.1055/a-0677-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adler SN, González Lama Y, Matallana Royo V, et al. Comparison of small-bowel colon capsule endoscopy system to conventional colonoscopy for the evaluation of ulcerative colitis activity. Endosc Int Open 2019;7:E1253–61. 10.1055/a-0982-2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leighton JA, Helper DJ, Gralnek IM, et al. Comparing diagnostic yield of a novel pan-enteric video capsule endoscope with ileocolonoscopy in patients with active Crohn's disease: a feasibility study. Gastrointest Endosc 2017;85:196–205. 10.1016/j.gie.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 26. Kawalec P. Indirect costs of inflammatory bowel diseases: Crohn's disease and ulcerative colitis. A systematic review. Arch Med Sci 2016;12:295–302. 10.5114/aoms.2016.59254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Everhov Åsa H, Khalili H, Askling J, et al. Sick leave and disability pension in prevalent patients with Crohn's disease. J Crohns Colitis 2018;12:1418–28. 10.1093/ecco-jcc/jjy123 [DOI] [PubMed] [Google Scholar]
- 28. Mehta F. Report: economic implications of inflammatory bowel disease and its management. Am J Manag Care 2016;22:s51–60. [PubMed] [Google Scholar]
- 29. Wernli KJ, Brenner AT, Rutter CM, et al. Risks associated with anesthesia services during colonoscopy. Gastroenterology 2016;150:888–94. 10.1053/j.gastro.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Navaneethan U, Parasa S, Venkatesh PGK, et al. Prevalence and risk factors for colonic perforation during colonoscopy in hospitalized inflammatory bowel disease patients. J Crohns Colitis 2011;5:189–95. 10.1016/j.crohns.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 31. Mansuri I, Fletcher JG, Bruining DH, et al. Endoscopic skipping of the terminal ileum in pediatric Crohn disease. AJR Am J Roentgenol 2017;208:W216–24. 10.2214/AJR.16.16575 [DOI] [PubMed] [Google Scholar]
- 32. Nehra AK, Sheedy SP, Wells ML, et al. Imaging findings of ileal inflammation at computed tomography and magnetic resonance Enterography: what do they mean when Ileoscopy and biopsy are negative? J Crohns Colitis 2020. 10.1093/ecco-jcc/jjz122. [Epub ahead of print: 21 Jan 2020]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2019-000365supp001.pdf (192.9KB, pdf)


