Some food protein-derived peptides have antihypertensive activity, which is conventionally characterized by the inhibition of angiotensin-converting-I enzyme (ACE-I). ACE-I is a key component of renin–angiotensin system (RAS) that cleaves angiotensin (ANG) I into the potent vasoconstrictor ANG-II (see Fig. 1). Transcriptomics studies on cell culture and animal models have recently shown that the peptides derived from food protein sources like egg white (namely the peptide IRW, Ile-Arg-Trp, derived from ovotransferrin) [1–3] and pea (namely the parent peptide AKSLSDRFSY, and offspring peptides LSDRFS and SDRFSY) [4] can regulate blood pressure also by stimulating ACE-II secretion. ACE-II, a membrane-bound aminopeptidase and close homologue of ACE-I, is massively present on the blood vessels of the lungs and other tissues [5]. It counter-regulates RAS via degrading ANG-II into the vasodilator heptapeptide ANG-(1–7) and ANG-I into ANG-(1–9), which can be further hydrolyzed into ANG-(1–7). We believe that the stimulatory effect of food protein-derived antihypertensive peptides on ACE-II expression might have implications on coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome corona virus-2 (SARS-CoV-2).
FIGURE 1.
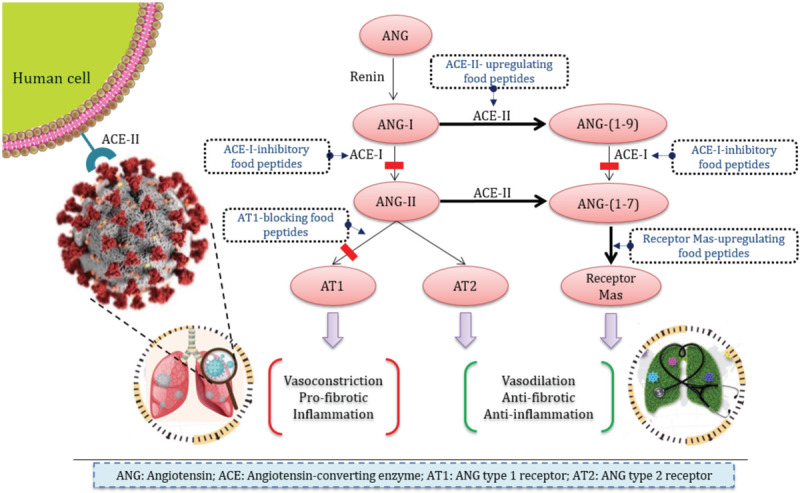
Modulatory effects of the food-derived antihypertensive peptides on renin--angiotensin system and consequences on SARS-CoV-2 infection.
ACE-II is exploited by SARS-CoV-2 as a key entry receptor to hijack the cells [6]. Animal studies have shown that RAS inhibitory drugs, such as ACE inhibitors and ANG type-I receptor blockers (ARBs), which are the first choice in treatment of hypertension [5], may upregulate ACE-II expression, thereby increasing the virus receptor. Though at the moment, there is no direct evidence based on COVID-19 patient studies to confirm that the administration of RAS inhibitor drugs is associated with the risk of COVID-19 [5,7], the concern is still high. The role of RAS inhibitory drugs and food peptides on promotion of COVID-19 remains to be explored definitively by randomized trials [7]. This is particularly important because from the biochemical perspective the upregulation of ACE-II expression can be advantageous.
SARS-CoV-2 cellular entry, which occurs through binding of its spike protein to the ACE-II is concomitant with ACE-II shedding (cleavage of the membrane anchor) from the host-cell surface. Following cell entry, SARS-CoV-2 downregulates ACE-II expression. It has been postulated that the decreased ACE-II activity may be a cause for the lung injury in COVID-19 [8]. As a consequence of reduction of ACE-II activity, the balance between the levels of ANG-II and ANG-(1–7) is shifted in favour of the former. ANG-II causes inflammatory responses in the lungs and fibrosis, whereas, ANG-(1–7) is a vasodilator having opposed functions to those of ANG-II. In this framework, it is particularly relevant that the administration of recombinant ACE-II is able to reverse the lung injury process [8]. The balance shift between ANG-II and ANG-(1–7) concentrations in favour of ANG-II caused by the viral infection can be restored by stimulating the ACE-II expression by the food-derived peptides. This issue is a future research agenda.
Food peptides with ACE-I inhibitory effects can substantially reduce the ANG-II generation (Fig. 1), and this is another way, they can indirectly remediate the pulmonary function in COVID-19 patients. Nonetheless, also this action has its own drawback. ACE-I inhibition will as well reduce the generation of ANG-(1–7) from ANG-(1–9). The actual effect, particularly in the circumstances of infection is unknown. In addition to the upstream approach, which includes reducing ANG-II generation by inhibiting ACE-I, food-derived peptides can act at the downstream by blocking the ANG-II action. The tetrapeptide RPYL, released from lactoferrin, has an inhibitory effect on ANG-II-induced vasoconstriction. The peptide blocks the binding of ANG-II to ANG type 1 receptor (AT1) on cell surface, in a dose-dependent manner up to 62% at a PRYL concentration of 300 μmol/l [9]. Moreover, food peptides are capable of antagonizing ANG-II through stimulation of G-protein-coupled receptor, Mas. ANG-(1–7) binds to Mas for induction of vasodilatory effects. Three peptides from rapeseed protein, including LY, GHS, and RALP increased the mRNA levels of the receptor Mas in rats, leading to higher ANG-(1–7) levels in the rat myocardium after 5-week oral administration of the peptides. Noticeably, although ANG-II mRNA expression was inhibited significantly by the three peptides, only LY, which exclusively consists of hydrophobic amino acid residues, significantly downregulated ANG-II level [10].
No study has been conducted on the impact of food-derived peptides and hydrolysed food proteins on the SARS-CoV and SARS-CoV-2-induced acute lung injury. The ability of food-derived peptides to increase ACE-II levels, which causes two opposite effects (favouring the virus internalization or reversing the lung injury) depends on the exact nature of the peptide. Inhibition of ACE-I and ANG-II is also dependent on peptide sequence. Although the stimulation of ACE-II expression and the inhibition of ACE-I have two possible opposite effects, the inhibition of ANG-II and upregulation of the receptor Mas are surely beneficial, leading to an improvement of the respiratory function. A controlled proteolysis process followed by purification of bioactive peptides and tailor-made design of peptide-enriched foods can be a sound strategy. An ideal platform would yield peptides lacking ACE-II stimulatory effect, while inhibiting the ANG-II functionality and over-expressing Mas. Food-derived peptides are probably not the ultimate solution to this pandemic; however, they represent a sustainable long-term strategy for COVID-19 remediation.
ACKNOWLEDGEMENTS
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Majumder K, Liang G, Chen Y, Guan L, Davidge ST, Wu J. Egg ovotransferrin-derived ACE inhibitory peptide IRW increases ACE2 but decreases proinflammatory genes expression in mesenteric artery of spontaneously hypertensive rats. Mol Nutr Food Res 2015; 59:1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao W, Bhullar KS, Chakrabarti S, Davidge ST, Wu J. Egg white-derived tripeptide IRW (Ile-Arg-Trp) is an activator of angiotensin converting enzyme 2. J Agric Food Chem 2018; 66:11330–11336. [DOI] [PubMed] [Google Scholar]
- 3.Liao W, Fan H, Davidge ST, Wu J. Egg white-derived antihypertensive peptide IRW (Ile-Arg-Trp) reduces blood pressure in spontaneously hypertensive rats via the ACE2/Ang (1-7)/Mas receptor axis. Mol Nutr Food Res 2019; 63:e1900063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao W, Fan H, Liu P, Wu J. Identification of angiotensin converting enzyme 2 (ACE2) up-regulating peptides from pea protein hydrolysate. J Funct Foods 2019; 60:103395. [Google Scholar]
- 5.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of covid-19. N Engl J Med 2020; NEJMoa2006923 [Epub ahead ofx print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarcho JA, Ingelfinger JR, Hamel MB, D’Agostino RB, Harrington DP. Inhibitors of the renin–angiotensin–aldosterone system and Covid-19. N Engl J Med 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020; 382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-Musoles R, Castelló-Ruiz M, Arce C, Manzanares P, Ivorra MD, Salom JB. Antihypertensive mechanism of lactoferrin-derived peptides: angiotensin receptor blocking effect. J Agric Food Chem 2014; 62:173–181. [DOI] [PubMed] [Google Scholar]
- 10.He R, Yang Y-J, Wang Z, Xing C, Yuan J, Wang L-F, et al. Rapeseed protein-derived peptides, LY, RALP, and GHS, modulates key enzymes and intermediate products of renin–angiotensin system pathway in spontaneously hypertensive rat. NPJ Sci Food 2019; 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]


