Abstract
Objectives
To determine the effect of contemporary exercise-based cardiac rehabilitation on generic and disease-specific health related quality of life for people with coronary artery disease.
Design
Systematic review and meta-analysis.
Study eligibility criteria
Randomised controlled trials testing exercise-based cardiac rehabilitation versus no exercise control that recruited after 31 December 1999. On 30 July 2019, we searched the Cochrane Central Register of Controlled Trials, MEDLINE (Ovid), Embase (Ovid) and CINAHL (EBSCO) databases.
Study appraisal and synthesis
Studies were screened for inclusion by two independent reviewers. Risk of bias was assessed using the Cochrane risk of bias tool. Data were reported as pooled means (95% CI for between-group difference.
Results
We identified 24 studies (n=4890). We performed meta-analyses for 15 short-term and 9 medium-term outcomes (36-Item Short Form Survey Instrument (SF-36), EuroQol-5D (EQ-5D) and MacNew, a cardiac-specific outcome). Six short-term and five medium-term SF-36 domains statistically favoured exercise-based cardiac rehabilitation. Only for two short-term SF-36 outcomes, ‘physical function’ (mean difference 12.0, 95% CI 4.4 to 19.6) and ‘role physical’ (mean difference 16.9, 95% CI 2.4 to 31.3), did the benefit appear to be clinically important. Meta-analyses of the short-term SF-36 physical and mental component scores, EQ-5D and MacNew and the medium-term SF-36 physical component score, did not show statistically significant benefits. Only two studies had a low risk of bias (n=463 participants).
Conclusions and implications of key findings
There is some evidence of a short-term benefit of contemporary exercise-based cardiac rehabilitation on quality of life for people with coronary artery disease. However, the contemporary data presented in this review are insufficient to support its routine use.
Keywords: coronary heart disease, rehabilitation medicine, coronary intervention, ischaemic heart disease, myocardial infarction
Strengths and limitations of this study.
To our knowledge, this is the most comprehensive systematic review and meta-analysis of contemporary exercise-based cardiac rehabilitation for people with all manifestations of coronary artery disease.
We conducted meta-analyses for 15 short-term and 9 medium-term outcomes.
We assessed risk of bias for all included studies using the Cochrane risk of bias tool.
Data had a high level of statistical heterogeneity and the majority of studies were identified as having ‘some concerns’ or ‘high risk’ in relation to the risk of bias assessment.
Data were insufficient to analyse at distinct time-points, thus were pooled as short-term (1–6 months) or medium term (8–12 months).
Introduction
Coronary artery disease (CAD) is the leading cause of death worldwide.1 Over the past 30 years, advances in interventional and secondary preventative cardiology have dramatically improved survival for people with CAD.2 3 In high-income countries, living with CAD, as a long-term condition, is now common. Of the 200 000 people who have a myocardial infarction annually in the UK, 7 out of 10 survive. In 2018, there were over 900 000 survivors of myocardial infarction and 2.3 million people living with CAD in the UK.4 This longevity after myocardial infarction represents a substantial and increasing burden on healthcare resource. There is a need for medical and lifestyle interventions that improve quality of life (QoL), maintain physical and psychosocial independence, and reduce long-term health and social care utilisation.
Cardiac rehabilitation (CR) has long been considered integral to the management of CAD.5 Exercise training in conjunction with cardiovascular risk factor management, psychosocial support and behaviour change (‘comprehensive’ CR) are the core components of a complex health and lifestyle intervention, which is unreservedly advocated in international guidelines and policy.6 7 Multiple meta-analyses incorporating trials spanning 1975–2018 reported favourable effects on functional capacity, hospital readmissions and mortality.8–12
Nevertheless, our 2018 systematic review (22 randomised controlled trials (RCTs), N=4834), which only included RCTs of ‘contemporary’ exercise-based CR that recruited after the end of 1999, found that the CR programmes tested had no effect on all-cause mortality (risk difference 0.0, 95% CI −0.02 to 0.01), and only a small effect on hospital readmissions of borderline statistical significance.13 A 2018 network meta-analysis, while showing a reduction in mortality when including studies from 1975 to present day, found a non-significant reduction in mortality for studies published after 2001.12 Existing data do not support the continued delivery of exercise-based CR in its current form where the intention is to reduce mortality or prevent hospital readmissions in CAD. For the continued use of these programmes to be justified for people with CAD, a paradigm shift in their stated aims is required.
In an ageing, multimorbid population, QoL, defined by WHO as ‘an individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns’,14 is a key priority for patients and healthcare providers. Patient-reported outcomes such as QoL are unique in providing the patient’s perspective on the efficacy of medical or lifestyle interventions.15 Furthermore, any change is tangible and subjective, thus patients can themselves perceive and report any benefit associated with CR. Therefore, CR should perhaps be judged on its ability to add ‘life to years’ rather than ‘years to life’. Nearly all previous systematic reviews have considered QoL data for exercise-based CR to be insufficient or unsuitable for meta-analysis due to considerable heterogeneity in outcome measures and reporting. A 2016 Cochrane review9 concluded that present data demonstrated improvement in at least one QoL domain in 65% of studies, and improvement in half or more of the reported domains in 25% of studies.
A 2018 meta-analysis (41 RCTs, N=11 747), pooling a range of measures and CR interventions from studies between 1975 and 2017, found that exercise training was associated with a small positive effect on QoL, but, overall, ‘psychosocial management’ was more effective.16 A 2018 Cochrane review of exercise-based CR for angina pectoris was unable to draw conclusions on the impact of this intervention on QoL.17 Subsequently, a 2019 meta-analysis reported exercise to be effective in reducing anxiety and depression following myocardial infarction.18 However, in a review of prospective cohort studies,19 people with depression were four times less likely to participate in CR and seven times more likely to drop out. A 2019 systematic review (14 RCTs, N=1739) of CR for people following acute coronary syndrome, published when this paper was being prepared for submission, included eight studies in a meta-analysis and concluded that there were clinically important positive effects on two 36-Item Short Form Survey Instrument (SF-36) domains at 6 months (role physical and general health) and one domain at 12 months (physical function).20
The objective of this systematic review and meta-analysis was to determine the effect of exercise-based CR on health-related QoL in all people with CAD, in the era of modern medical management.
Methods
The methodology for our systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines and the study was registered on PROSPERO (CRD42018110197).
Search strategy and methodology
First, we reviewed all studies included in the most recent Cochrane systematic review of exercise-based CR in coronary heart disease.9 Second, we assessed the 93 studies listed as ‘excluded’ in the Cochrane review to identify any additional studies that met our inclusion criteria. Third, on 30 July 2019, we searched the Cochrane Central Register of Controlled Trials, MEDLINE (Ovid), Embase (Ovid) and CINAHL (EBSCO) databases using the strategy used in the Cochrane Systematic Review (online supplementary appendix 1).
bmjopen-2019-036089supp001.pdf (56.4KB, pdf)
Results from our three predefined sources were individually examined to determine inclusion or exclusion. We retrieved abstracts, full-text manuscripts and supplementary material where necessary, and hand searched reference lists of the subsequently included articles (and other recent systematic reviews) to identify additional studies of interest. Two reviewers (GMcG and RP) independently undertook screening of the resultant citations, abstracts and manuscripts, with disputes mediated by a third reviewer (MU). Where data were missing or inappropriately presented, we requested additional information from lead and corresponding authors by email, on multiple occasions.
Study inclusion criteria
Our overall aim was to identify RCTs testing an exercise-based CR intervention against non-exercise usual care, with QoL as an outcome measure, which recruited after 31 December 1999. The rationale for excluding studies recruiting prior to the year 2000 is detailed elsewhere.13 Briefly, we defined contemporary CR as postdating the widespread adoption of primary percutaneous coronary intervention and the ‘modern’ pharmacology outlined in the Joint British Society recommendations for the Prevention of Coronary Heart Disease in Clinical Practice.21 Including studies that recruited after the end of 1999 allowed sufficient time for these innovations to become commonplace.
Design
We identified RCTs testing an exercise-based CR intervention against non-exercise usual care, which reported outcomes at any time-point following completion of the intervention. Previous reviews assessing mortality and hospitalisation have only included studies with at least 6 months follow-up.9 For QoL indicators, we considered outcome measures at any time-point following completion of CR to be of interest. We excluded abstracts, conference proceedings, theses and non-English language publications.
Participants
We included all studies where participants either had CAD confirmed with coronary angiography, had a diagnosis of angina pectoris, had undergone coronary revascularisation via either percutaneous coronary intervention or coronary artery bypass grafting, or had sustained a myocardial infarction.
Interventions
We defined interventions as exercise-based CR undertaken with or without supervision as a hospital inpatient, as a hospital outpatient, in a community venue or at home. Furthermore, the exercise programme could have been delivered in isolation or in combination with other educational, behavioural or psychosocial components constituting a ‘comprehensive’ multicomponent CR programme. We defined usual care as any intervention delivered to people with CAD that did not include a structured exercise component, that is, disease-specific education, smoking cessation, dietary advice or psychosocial support, delivered without supervised exercise training. We excluded studies in which both groups had completed a CR exercise training intervention prior to randomisation to an exercise intervention or a non-exercise control.
Outcome measures
Data were extracted from studies reporting between-group difference in QoL, collected with a generic or cardiovascular disease-specific, validated measure, for example, the SF-36 at any time-point post-CR. Measures were considered to be validated if there was evidence in the peer-reviewed literature that the instrument had been psychometrically tested for reliability, validity and/or sensitivity.
Data extraction and statistical analysis
Any QoL data available at baseline and follow-up were extracted from studies that met the inclusion criteria. For each of the exercise-based CR and non-exercise usual care arms, the data extracted at each visit were the mean QoL score and the SD of the QoL score. If the means and SDs were not explicitly reported, they were extracted from line graphs (where possible) or derived from CIs. The SDs were computed assuming the CIs were obtained taking the point estimates to be normally distributed.
To pool the results from all studies for each QoL measure at a particular time-point, we fitted a random-effects meta-analysis model in the R statistical program.22 The pooled results were summarised in forest plots. Where means and SDs in each arm were available for all included studies, the ‘meta’ package, with a command that requires specifying the mean and SD for each arm, was used to perform the meta-analysis.23 For the short-term SF-36 Mental Health Component and Physical Health Component scores, two studies24 25 reported the mean difference and the SE, thus it was not possible to extract the means and SDs. Therefore, the ‘metafor’ package, with a command that requires specifying mean difference and SE for each study, was used to perform the meta-analysis.26 This was the same approach to extracting EuroQol-5D (EQ-5D) data in another study.27 In these cases, if a study reported means and SDs, these were used to calculate the mean difference and the SE.
There were two options for defining the outcomes to be used to compare usual care and exercise-based CR at any particular time-point: (1) taking the outcome as the QoL score at each time-point or (2) taking the outcome as the change in QoL score from baseline. Some studies did not report baseline values or changes, and so the former definition was chosen. This enabled the inclusion of more studies in the meta-analyses.
Assessment of risk of bias
We performed a risk of bias assessment for all studies included in our meta-analyses using V.2 of the Cochrane risk of bias tool for randomised trials.28 Accordingly, risk of bias was assessed for general trial procedures and specifically for the QoL outcome of interest. Each trial was assessed against five domains of bias: (1) bias arising from the randomisation process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in measurement of the outcome and (5) bias in selection of the reported result. As per the Cochrane Handbook,28 an overall risk of bias score of ‘low’, ‘some concerns’ or ‘high’ was determined for each trial. ‘Low’ risk of bias was implied when all domains were scored ‘low’. ‘Some concerns’ was implied when at least one domain was scored as ‘some concerns’. ‘High’ risk of bias was implied when at least one domain was scored as ‘high’, or multiple domains were scored ‘some concerns’. All studies were assessed independently by two reviewers (GMcG and RP) with discrepancies resolved by a third (MU).
Patient and public involvement
There was no patient and public involvement in this systematic review.
Results
Studies retrieved
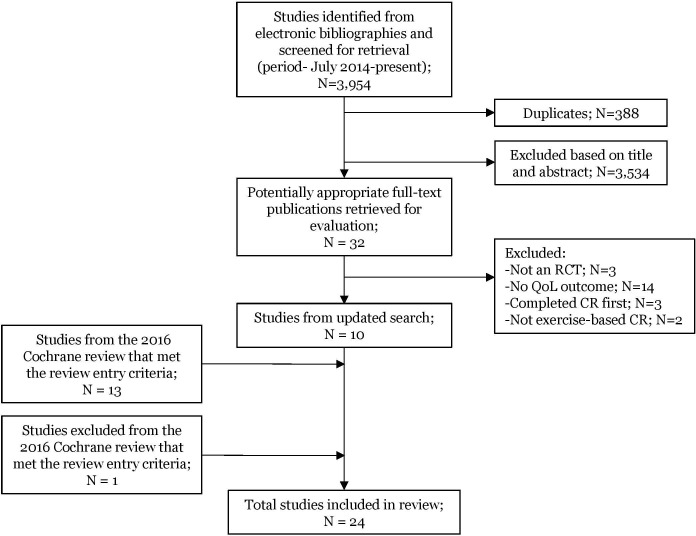
Thirteen studies in the Cochrane review24 27 29–39 met our criteria and one study was identified from the Cochrane excluded studies list.40 Of 32 studies retrieved for full evaluation from our updated literature search, 3 were excluded as they were not RCTs, 14 because they did not use QoL as an outcome measure, 3 because participants completed structured CR prior to being randomised to a CR intervention or control group and 2 because they did not qualify as ‘exercise-based CR’: this left 10 studies.25 41–49 A total of 24 studies (N=4,890) were suitable for inclusion (figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram. CR, cardiac rehabilitation; QoL, quality of life; RCT, randomised controlled trial.
All studies reported QoL using at least one validated measure, and seven studies used two measures. Six different generic measures were used: SF-36 (14 studies), 12-item Short Form Survey (SF-12, 2 studies), EQ-5D (2 studies) and 20-item Short Form Survey (SF-20), 15 D Questionnaire, Time Trade Off Questionnaire 1 study each. Six cardiac-specific measures were used: MacNew QoL Questionnaire (four studies), HeartQoL Questionnaire (two studies) and Duke Activity Status Index (DASI), the Seattle Angina Questionnaire, Quality of Life Index-cardiac version III and Myocardial Infarction Dimensional Assessment Scale all one study each.
Data were reported at varying time-points and presented in numerous statistical formats, thus reducing the number of point estimates we could reliably include in each analysis. We contacted authors from eight studies24 27 30 33 37 40 42 44 to request provision of data in a format consistent with our meta-analysis protocol. A response was received from two authors stating that the data were not available.37 42
We performed meta-analyses at two time-points: short-term (immediately postintervention, or as soon as possible thereafter, up to 6 months (1–6 months)) and medium-term (8–12 months postrandomisation). This allowed us to assess both the immediate postintervention effect of exercise-based CR, and the long-term effect. Where data were reported twice within the short-term timescale (ie, 3 and 6 months),35 data recorded closest to the end of the intervention period were included in the meta-analysis. We pooled data from studies using SF-36 and SF-12, henceforth SF-36. Data were sufficient to undertake meta-analysis for three measures: the SF-36 (eight domains and physical component score for the short-term and medium-term time-points, plus the physical component score for the short-term time-point only), the EQ-5D (short-term only) and the MacNew (short-term only).
Excluded studies and erroneous data
Despite the SF-20 Questionnaire being broadly a derivative of the SF-36 and SF-12, we did not include one study29 in the SF-36 analyses as the questions and scoring algorithms are not sufficiently comparable. One study46 described exercise performed as an inpatient prior to randomisation. We included this study as the prerandomisation exercise involved gentle mobilisation only, as opposed to a structured CR exercise intervention, thus fitting with our inclusion criteria. For the same reasons, we excluded a study50 in which both groups did complete a structured exercise-based CR intervention prior to randomisation. Following full-text retrieval, we excluded two studies51 52 which, although aimed to increase participation in physical activity, employed general lifestyle interventions as opposed to exercise-based CR as defined in our protocol.
For one study,41 only selected SF-36 variables were reported; physical function domain, and mental component score and physical component score. We were able to include the physical function domain data but were unable to include the mental and physical component score subscales data as the mean values reported were out of range for the measure. Data from another study53 reporting the SF-36 could not be accurately extracted from a line-graph, and for another,31 could not be meta-analysed, as the way in which the data were reported meant multiple assumptions would have been required. For the EQ-5D, we performed meta-analysis at the short-term time-point using data from two studies.27 34 We could not include EQ-5D data from one study,47 as all the information required for a meta-analysis was not reported. For another study,49 mean values were out of the measurement range for the MacNew Questionnaire, thus, while otherwise the study met our inclusion criteria, the data could not be included. Online supplementary appendix 2 shows how data were extracted and included in the meta-analyses where means or SDs were not explicitly reported.
bmjopen-2019-036089supp002.pdf (27.5KB, pdf)
Sample size, gender, age and study origin
We included 4890 randomised participants in our analyses. Of the 24 studies, 21 included both male and female participants, 1 study included males only33 and 1 study did not specify43 the gender of participants recruited (table 1). The mean age of participants in each study was 62 years, range 53–77 years. One study reported an incorrect mean age.49 Three studies were conducted in the UK,36 40 42 9 elsewhere in Europe24 25 29 30 34 38 44 45 47 and 12 outside Europe.27 31–33 35 37 39 41 43 46 48 49
Table 1.
Characteristics of included studies and interventions
| Study | Country | Recruitment period | N randomised | Mean age (years) | Male participants (%) | Participant diagnosis | Exercise intervention | Comparator (control) | Medication |
| Asbury et al 40 | UK | Not specified | 42 | 65 | 83 | Angina pectoris and/or previous history of single or multiple MI, CABG, PCI. | Outpatient group-based circuit, home exercise programme and 8-week symptom monitoring diary. | Standard treatment, 8-week symptom monitoring diary. | Full description and breakdown. |
| Belardinelli et al 29 | Italy | Not specified | 118 | 57 | 84 | CAD, post-PCI or coronary stenting. | Outpatient group cycling. | Recommendations to perform basic daily mild physical activities but avoid physical training. | Full description and breakdown, lipid-lowering drugs were not allowed. |
| Bettencourt et al 30 | Portugal | 2001–2002 | 126 | 57 | 84 | Acute coronary syndrome (unstable angina or MI). Revascularisation procedure not specified. | Outpatient group treadmill or cycling. | Standard cardiological follow-up. | No description. |
| Briffa et al 31 | Australia | Not specified | 113 | 61 | 74 | Uncomplicated acute myocardial infarction or unstable angina who underwent PCI or CABG or treated by thrombolytic therapy. | Outpatient group aerobic circuit training interspaced with resistance training. | Pharmacotherapy and lifestyle counselling. | Full description and breakdown. |
| Chen et al 41 | China | Not specified | 44 | 69 | 78 | CAD who underwent PCI or CABG. | Outpatient group cardiopulmonary exercise training, strength and balance training. | Medical management and clinic visits as needed. | Reference to beta-blocker but no other medication. |
| Devi et al 42 | UK | 2008–2010 | 94 | 66 | 75 | Stable angina. Stents or CABG. | Web-based exercise intervention including individualised goal setting, exercise diary and feedback. | Usual treatment from their GP. | Reference to medication but no breakdown. |
| Firouzabadi et al 43 | Iran | Not specified | 70 | 59 | Not specified (similar in gender) | Cardiovascular patients after CABG. | Hospital-based group aerobic exercise. | No intervention. | No description. |
| Hassan and Nahas48 | Egypt | Not specified | 60 | 53 | 68 | After PCI. | Hospital-based aerobic exercise training. | Instruction about risk factors after PCI. | Reference to medication but no breakdown. |
| Hautala et al 44 | Finland | 2011–2014 | 204 | 61 | 72 | Patients with CAD who suffered from acute coronary syndrome with PCI or CABG. | Outpatient gym-based group and home-based aerobic exercise training, strength training and exercise diary. | No individualised tailored exercise prescriptions. | Full description and breakdown. |
| Højskov et al 45 | Denmark | Not specified | 60 | 65 | 78 | Elective CABG. | Hospital-based, two groups including exercise:
|
Two groups of no exercise:
|
Full description and breakdown. |
| Højskov et al 25 | Denmark | Not specified | 326 | 65 | 86 | Elective CABG. | Hospital-based, physical exercise plus usual care and exercise diary. | Usual care procedures, which included medical follow-up and standard. | Full description and breakdown. |
| Houle et al 32 | Canada | 2007–2008 | 65 | 59 | 79 | Unstable angina, non-ST-elevation or ST-elevation myocardial infarction with PCI or CABG. | Home-based pedometer-based programme | Usual advice regarding physical activity, diet and medication. | Reference to medication for control group but no breakdown. |
| Maddison et al 27 | New Zealand | 2011–2012 | 171 | 60 | 81 | Angina, MI, revascularisation including angioplasty, stent or CABG. | Home-based moderate to vigorous personalised exercise programme, automated text messages to increase exercise behaviour, technical support. | Free to participate in any other CR service or support that they wished to. | No description. |
| Mutwalli et al 33 | Saudi Arabia | 2008–2010 | 49 | 57 | 100 | Following CABG. | In-patient and home-based walking programme. | Usual hospital care and advice. | Reference to medication but no breakdown. |
| Oerkild et al 24 | Denmark | 2007–2008 | 40 | 77 | 58 | MI, PCI, CABG. | Home-based walking programme. | Risk factor intervention and medical adjustment. | Full description and breakdown. |
| Peixoto et al 46 | Brazil | 2010–2013 | 100 | 56 | 70 | MI and PCI with or without chemical reperfusion therapy. | Inpatient early mobilisation exercise programme and outpatient walking programme. | Education regarding physical activity, diet and medication. | Full description and breakdown. |
| Reid et al 39 | Canada | 2004–2007 | 223 | 56 | 84 | Acute coronary syndrome, post-PCI. | Home-based, web-based ‘CardioFit’ programme including physical activity plan, expert advice and motivational feedback. | Physical activity guidance and education booklet. | Full description and breakdown in supplemental table. |
| Salavati et al 49 | Iran | 2013 | 110 | Specified but incorrect | Specified but incorrect | Post-CABG. | Home-based walking programme, including home visits and telephone calls. | Usual education. | Reference to medication for intervention group but no breakdown. |
| Sandström and Ståhle34 | Sweden | Not specified | 101 | 71 | 80 | Acute coronary event. Number of patients with previous PCI or CABG. | Outpatient group aerobic training programme. | Information meetings about disease, importance and recommendations of physical activity and pharmacological therapy. | No description. |
| Santaularia et al 47 | Spain | 2010–2012 | 86 | 60 | 85 | MI, pre-infarct angina, angina pectoris, specific or unspecified ischaemic heart disease. Revascularisation procedure not specified. | Outpatient exercise training programme. | Standard hospital care, oral and written information on risk factors, advice and guidance on returning to physical activity. | Reference to medication but no breakdown. |
| Wang et al 35 | China | 2005–2007 | 160 | 58 | 83 | MI and PCI. | Home-based rehabilitation programme using a self-help manual. | Encouragement and general advice on self-management. | Full description and breakdown. |
| West et al 36 | UK | 1997–2000 | 1813 | 64 | 74 | MI. Number of patients with previous PCI or CABG. | Outpatient comprehensive exercise-based cardiac rehabilitation as delivered in the UK. | Usual care including access to booklets and routine outpatient follow-up. | Full description and breakdown. |
| Yu et al 37 | Hong Kong | Not specified | 269 | 64 | 76 | MI, PCI performed for angina pectoris. | Four phases:
|
Cardiac clinic, conventional therapy, risk factor education. | Full description and breakdown. |
| Zwisler et al 38 | Denmark | 2000–2003 | 770 (446 with diagnosis of IHD) | 66 | 64 | MI, angina pectoris, PCI, CABG. | Hospital-based intensive cardiac rehabilitation programme. | Usual care and pharmaceutical treatment. | Full description and breakdown. |
CABG, coronary artery bypass graft; CAD, coronary artery disease; IHD, ischaemic heart disease; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Participant diagnosis of coronary artery disease
Participant diagnoses, that is, manifestations of CAD, was described in all studies (table 1). Fourteen trials included participants with a range of diagnoses including CAD confirmed with coronary angiography, angina pectoris, myocardial infarction, percutaneous coronary intervention and/or coronary artery bypass grafting.27 29–32 34 37–41 44 47 Four studies included participants following myocardial infarction only,24 35 36 46 and one study, angina pectoris only.42 Five studies recruited participants ‘following coronary artery bypass grafting’25 33 43 45 49 and one study ‘after percutaneous coronary intervention’.48
Treatment received
Twelve studies included participants who had been revascularised by percutaneous coronary intervention or coronary artery bypass grafting following a recent or past cardiac event24 27 31 32 35–38 40–42 44 (table 1). Five studies recruited participants following percutaneous coronary intervention only29 35 39 46 48 and five studies recruited participants following coronary artery bypass grafting only.25 33 43 45 49 It was unclear in two studies whether participants had been revascularised before randomisation and, if so, by which specific procedure.30 47
Medication
Thirteen studies provided a full description of medication (table 1). Six studies referred to medication but provided no specific detail.32 33 42 47–49 One study reported beta-blocker usage without reference to other medications.41 The remaining four studies did not provide any information about medication.27 30 34 43
Recruitment period
Thirteen studies recruited participants after 31 December 1999 (table 1). In one study, participants were recruited between 1997 and 2000.36 On the basis that participant diagnosis, treatment received and co-existing medical therapies indicated ‘contemporary’ medical care, it was agreed by all reviewers to include this study. This is consistent with the approach used in our previous review of survival.13 For the remaining 10 studies, a recruitment period could not be clearly determined from the manuscripts. However, given the description of participant diagnosis, medical treatment, pharmacological therapies and CR interventions, it was agreed by all reviewers that they met our criteria for inclusion.
Content of the interventions
Intervention content varied considerably between studies (tables 1 and 2). Nineteen studies compared exercise training in combination with additional therapies (education and psychosocial components), two studies compared exercise training as a stand-alone intervention,29 41 while one study combined exercise and relaxation.43 The exercise components of the interventions varied with respect to the setting, training modality, duration, session length, frequency and intensity. The majority of studies incorporated walking and/or cycling as the main exercise modality, delivered for a period ranging from 4 to 12 months, in either an inpatient, home-based or out-patient setting.
Table 2.
Intervention components of included studies
| Study | Exercise type (modality) |
Intervention duration | Session frequency (per week) | Session time | Session intensity | Supervised/Non-supervised (exercise provider) | Additional components | Fidelity to exercise intervention described |
| Asbury et al 40 | Circuit of cardiovascular and rest stations | 8 weeks | Not specified | 80 min | 60%–75% of HRR (normal LV) or 40%–60% HRR (LV<40%). | Not specified. | Health promotion seminars. | Not specified. |
| Belardinelli et al 29 | Cycling | 6 months | 3 | 53 min | 60% of peak oxygen uptake. | Supervised (cardiologist). | Not specified. | Not specified. |
| Bettencourt et al 30 | Cycling or treadmill | 12 weeks | 3 | 20–30 min | Based on maximum HR reached on exercise test prior. | Supervised (under qualified supervision). | Standard cardiological follow-up. One session a month for 12 months. | Not specified. |
| Briffa et al 31 | Circuit (aerobic/resistance) | 6 weeks | 3 | 60–90 min | Not specified. | Supervised (treating doctor). | Education on lifestyle and risk factor management. | 23 (40%) completed 75% or more of all sessions offered. |
| Chen et al 41 | Cycling | 12 weeks | 3 | 50 min | 60%–80% of HRR. | Supervised (physician and a physical therapist). | Resistance and balance training. | Not specified. |
| Devi et al 42 | Physical exercise, most commonly walking | 6 weeks | Daily | Advice to be physically active for 30 min five times a week | Not specified. | Unsupervised. | Information about secondary prevention and education on lifestyle and risk factor management. | Compliance assessed by exercise diaries, questionnaires and electronic feedback on performance. |
| Firouzabadi et al 43 | Cycling and treadmills | 8–10 weeks | 3 | 60–90 min | Not specified. | Supervised (medical and CCU nurses. | Relaxation. | Not specified. |
| Hassan and Nahas48 | Cycling | 6 months | 3 | 50 min | Mild-to-moderate intensity based on Borg’s RPE scale. | Not specified. | Education on lifestyle and risk factor management. | Not specified. |
| Hautala et al 44 | Walking, running, cycling, cross-country skiing | 1 year (6 months gym and home-based, 6 months home-based) |
4–5 | 30–45 min strength (gym-based), 30–40 min aerobic and 30–40 min strength (home-based) | 12–15 Borg’s RPE. | Supervised (in the gym by a physical therapist). Unsupervised at home. | Accelerometry, strength exercise, dietary counselling or a check-up by a medical doctor. | Use of exercise diaries to prescribe target duration/intensity and record trained mode, duration and mean RPE. |
| Højskov et al 45 | Walking, cycling | 4 weeks | Walking: daily Cycling: twice daily |
Walking: not specified Cycling: 2×10 min. |
13–15 on Borg’s RPE. | Supervised (physiotherapist). | Deep breathing exercises. Muscle and endurance exercises. Psychoeducation and mindfulness. |
Acceptability, adherence and attrition to the intervention and study was measured and reported. Safety and tolerability also reported. |
| Højskov et al 25 | Walking, cycling | 4 weeks | Walking: twice daily then 3× daily. Cycling: daily then twice daily. |
Walking: 2×5 min to 3×10 min. Cycling: 2×10 min. |
Walking: low-to-moderate. Cycling:13–15 on Borg’s RPE. |
Supervised (physiotherapist). | Respiratory physiotherapy, neck and shoulder exercises. Psychoeducation and mindfulness. |
Intervention adherence was defined as completing at least 75% of exercise sessions. |
| Houle et al 32 | Walking | 12 months | Daily | Not specified | Not specified. | Unsupervised. | Socio-cognitive intervention. Access to exercise specialist, nutritionist, psychologist and physician. | Monitoring through a pedometer and exercise diary. |
| Maddison et al 27 | Physical exercise, in particular walking | 24 weeks | At least 5 | Minimum 30 min | Moderate-to-vigorous aerobic-based exercise (11–13 on Borg’s RPE in early stages then 13–15 in latter stages). | Not specified. | Behavioural change strategies and technical support. | Self-reported physical activity. |
| Mutwalli et al 33 | Walking | 6 months | 7 | 30 min | Not specified. | Supervised on ward then unsupervised at home. | Talks and workshops on lifestyle and risk factor management. | Not specified. |
| Oerkild et al 24 | Individualised | 12 months | 6 | 30 min | 13–15 on Borg’s RPE. | Unsupervised. | Consultations with cardiologist. Dietary counselling and smoking cessation (if required). | Self-reported physical activity. |
| Peixoto et al 46 | Walking | 1 month | 4 | 30–50 min | 4 and 5 on RPE scale rating. | Unsupervised. | Education on lifestyle and risk factor management. | Not specified. |
| Reid et al 39 | Personally tailored physical activity plan. | 20 weeks | Not specified | Not specified | Not specified. | Unsupervised. | Psychological support. | Monitoring through pedometer. Self-reported physical activity. Reference to online tutorials completed and email correspondence. |
| Salavati et al 49 | Not specified | 5 weeks | 4 | Not specified | Not specified. | Unsupervised. | Education on lifestyle and risk factor management. | Not specified. |
| Sandström and Ståhle34 | Not specified | 3 months | 3 | 50 min | Not specified. | Supervised (specialist physiotherapist). | Relaxation sessions. Education on lifestyle and risk factor management. | Not specified. |
| Santaularia et al 47 | Cycling | 10 weeks | 3 | 60 min | 75%–90% of max HR (11–15 on Borg’s RPE. | Supervised (physiotherapist). | Resistance training, education on lifestyle and risk factor management. | Intensity measured using a pulse oximeter. |
| Wang et al 35 | Not specified | 6 weeks | Not specified | Not specified | Not specified. | Unsupervised. | Health education. | Not specified. |
| West et al 36 | Varied by centre (exercise equipment in physiotherapy gyms) | 6–8 weeks | 1–2 | Average 20 hours over intervention duration | Not specified. | Supervised (nurse, occupational therapists or physiotherapists plus one other Allied Health Professional). | Education on lifestyle and risk factor management. | Not specified. |
| Yu et al 37 | Treadmill, cycling, rowing, stepper, arm ergometry | 8 weeks (phase II) | 2 | 2 hours | 65%–85% of maximal aerobic capacity. | Supervised (physiotherapist and occupational therapist). | Resistance training, education on lifestyle and risk factor management. | Not specified. |
| Zwisler et al 38 | Not specified | 6 weeks | 2 | Not specified | Not specified. | Supervised (multidisciplinary team). | Psychosocial support, education on lifestyle and risk factor management. | Not specified. |
CCU, coronary care unit; HR, heart rate; HRR, heart rate reserve; LV, left ventricular; RPE, rating or perceived exertion.
Overall effects of interventions
SF-36 short-term
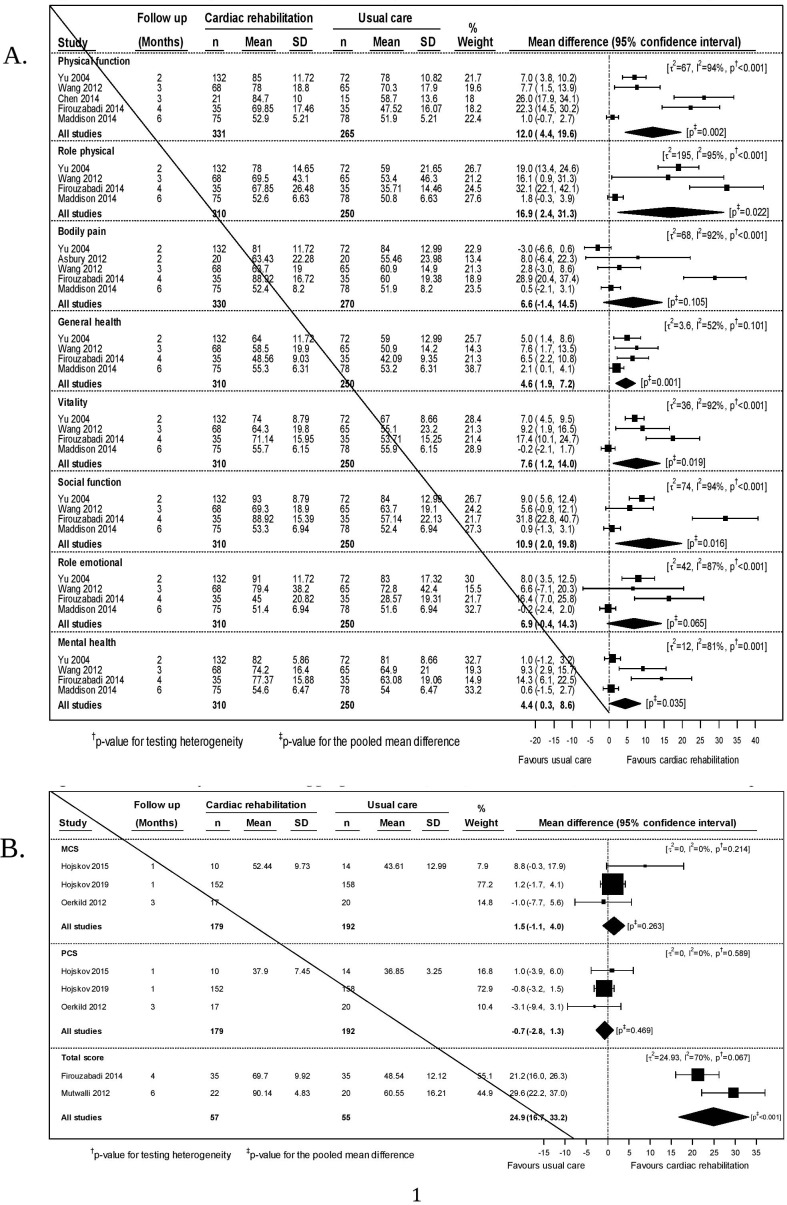
We included data from four trials (N= 560) for six SF-36 domains27 35 37 43 and data from five trials for the physical function27 35 37 41 43 and bodily pain27 35 37 40 43 domains (N= 596 and 600) in our meta-analyses (figure 2A). The data were heterogeneous (I2 >80% for seven of eight domains). Point estimates favoured exercise-based CR in all domains. In six domains, physical function (12.0 (95% CI 4.4 to 19.6)), role physical (16.9 (2.4 to 31.3)), general health (4.6 (1.9 to 7.2)), vitality (7.6 (1.2 to 14.0)), social function (10.9 (2.0 to 19.8)) and mental health (4.4 (0.3 to 8.6)), these differences were statistically significant.
Figure 2.
(A) Meta-analysis for quality of life (36-Item Short Form Survey Instrument (SF-36) domains) at the short-term time-point. (B) Meta-analyses of SF-36 aggregate scores (MCS, mental component score; PCS, physical component score and total) at the short-term time-point.
We included data from three trials (N=371)24 25 45 that reported the physical and mental component scores of the SF-36. No statistical significant differences were found (figure 2B). We meta-analysed data from two studies (N=112)33 43 reporting an overall SF-36 score. A statistically significant benefit (24.9 (95% CI 16.7 to 33.2)) was found.
SF-36 medium-term
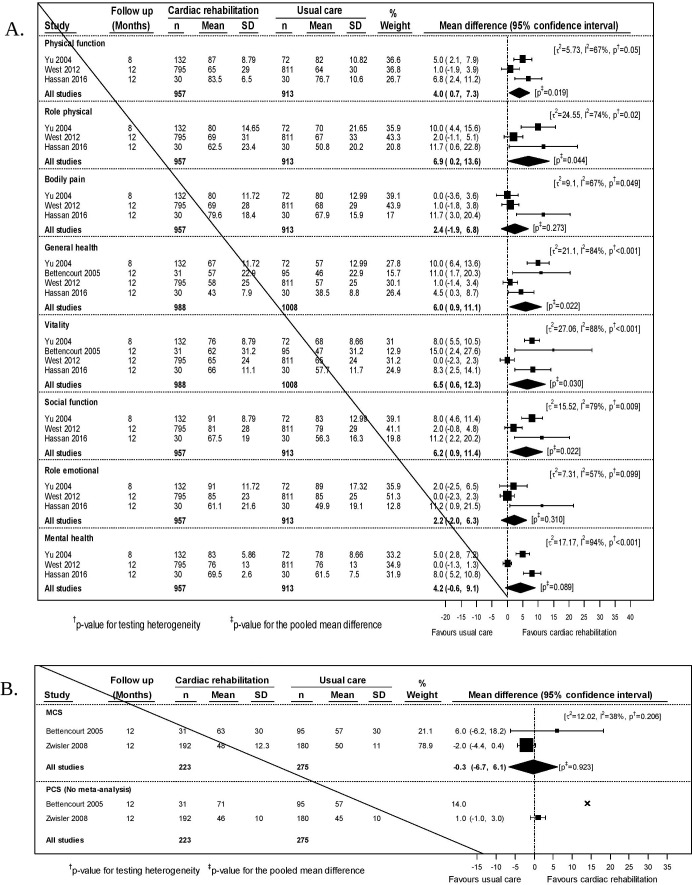
In our meta-analyses, we included data from three trials (N= 1870)36 37 48 for six SF-36 domains, and data from four trials (N=1996)30 36 37 48 for the general health and vitality domains. The data were less heterogeneous than the short-term data (I2 >80% for three of eight domains). Point estimates favoured exercise-based CR in all domains. In five domains, physical function (4.0 (95% CI 0.7 to 7.3)), role physical (6.9 (0.2 to 13.6)), general health (6.0 (0.9 to 11.1)), vitality (6.5 (0.6 to 12.3)) and social function (6.2 (0.9 to 11.4)), these differences were statistically significant (figure 3A).
Figure 3.
(A) Meta-analysis for quality of life (36-Item Short Form Survey Instrument (SF-36) domains) at the medium-term time-point. (B) Meta-analyses of SF-36 aggregate scores (MCS and PCS) at the medium-term time-point.
We included data from two trials (N=478)30 38 that reported the mental component score of the SF-36, one of which also reported the physical component score (N = 372).38 No statistically significant differences were found (figure 3B).
EuroQol-5D
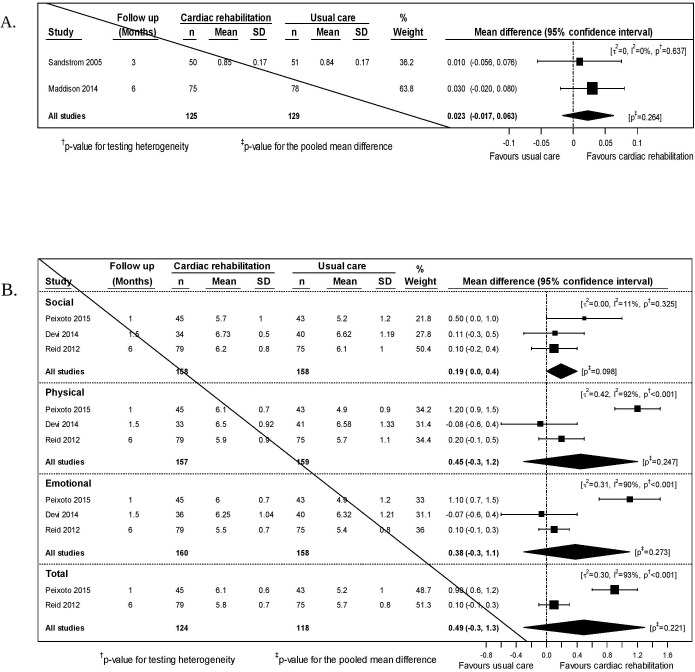
We included data from two studies that reported short-term outcomes for the EQ-5D (N=254).27 34 The point estimate favoured exercise-based CR but was not statistically significant (figure 4A). No studies reported medium-term outcomes for the EQ-5D.
Figure 4.
(A) Meta-analysis for EuroQol-5D (EQ-5D) at the short-term time-point. (B) Meta-analysis for quality of life (MacNew) at the short-term time-point.
MacNew Questionnaire
We included data from three studies that reported short-term outcomes for the MacNew subscales (N=316, 316 and 318).39 42 46 Two of these also reported an overall score (N=242).39 46 All point estimates favoured exercise-based CR but there were no statistically significant differences (figure 4B).
Other measures
Eight other QoL measures were each reported by one study. Statistically significant benefits were found in seven out of eight domains of the MOS (Medical Outcomes Study 20-Item Short Form Survey) 20 at 12 months, the EQ-5D mobility subscale at 12 months, the DASI at 4 and 12 months, the Seattle Angina Questionnaire emotional score at 6 weeks, the overall Quality of Life Index-cardiac version III and the same five out of seven of MIDAS subscales at 3 and 12 months (online supplementary appendix 3).
bmjopen-2019-036089supp003.pdf (103.7KB, pdf)
Risk of bias assessment
We assessed two of the studies included in our meta-analyses as having a low risk of bias (N=463),25 27 nine as having ‘some concerns’ (N=2493)24 34–36 38 42 45 46 48 and the remaining seven as high risk (N=671)30 33 37 39–41 43 (online supplementary appendix 4, figure 5). Methodological issues leading to a classification of high risk of bias related primarily to two domains: (1) deviations from the intended interventions and (2) missing outcome data. For the former, lack of intention-to-treat analysis and inadequate blinding were common issues. For the latter, high loss to follow-up was a common issue.
Figure 5.
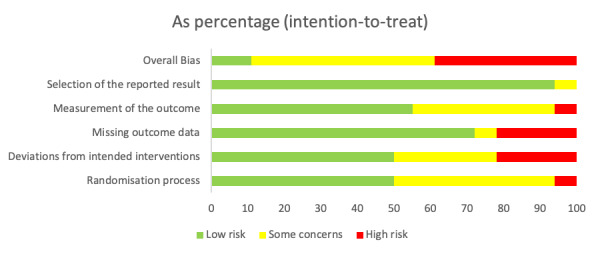
Risk of bias assessment. Does exercise-based cardiac rehabilitation improve quality of life in coronary artery disease? A contemporary systematic review and meta-analysis.
bmjopen-2019-036089supp004.pdf (49.3KB, pdf)
Discussion
We performed meta-analyses of 15 short-term and 9 medium-term outcomes. With such a large number of comparisons, some statistically significant findings could be expected due to random chance. Two-thirds of the analyses (16/24) were for the eight SF-36 domains. Multiple individual SF-36 domain scores showed statistically significant positive results from exercise-based CR, both in the short-term and medium-term. However, the domains in which a statistically significant effect was observed were different for the short-term and medium-term outcomes. These findings should be interpreted with considerable caution; the quality of the included trials was generally poor and there was substantial statistical heterogeneity. Nevertheless, the overall picture for all domains at both time points favours exercise-based CR, suggesting that there may be an overall benefit on SF-36 domain scores.
The meta-analyses of the SF-36 physical and mental component scores at 12 months, in contrast, did not show any benefit from exercise-based CR. We have for completeness included a meta-analysis of an overall SF-36 score showing a clear benefit from exercise-based CR. The SF-36 overall score is not an accepted metric.54 55 While these studies met our inclusion criteria, we attach very little weight to this finding because of the non-standard approach to the analysis of the SF-36.
The meta-analysis for the MacNew Questionnaire, a cardiovascular disease-specific outcome, did not find any statistically significant short-term benefit. Nevertheless, for all three domains, the direction of change favoured exercise-based CR. We found no data on the medium-term or long-term benefits of exercise-based CR on cardiovascular disease-specific QoL. Similarly, the meta-analysis of EQ-5D data found a non-significant difference in favour of exercise-based CR.
A broadly similar pattern was seen in the trials not suitable for meta-analysis, with some statistically significant findings on certain outcomes but with no consistent support for benefit. Taking all of these data into account, our interpretation is that there is some evidence of a beneficial effect of exercise-based CR on QoL in the short-term and insufficient data to comment on the medium-term or long-term benefits. In combination, therefore, undertaking an exercise programme, risk factor modification and behavioural education as part of a comprehensive CR programme may have some impact on individual domains of health-related QoL.
Our observations are limited by the quality of the included studies and the heterogeneity of both the trial participants and the interventions tested. We cannot exclude the possibility that there are subgroups for whom exercise-based CR is effective. To contextualise this observation, data from our previous review of mortality13 in exercise-based CR should be considered (mortality was not assessed in the current review). The review was criticised for not considering the potentially greater benefit for those who adhere to treatment.56 Since the overall effect on mortality in our previous review was zero, any reduced mortality in the subgroup that adhered, would inevitably mean an equal increase in mortality in participants who did not adhere. In contrast, given the positive effect of exercise-based CR on QoL in the current analyses, it is plausible that poor adherence to the intervention is attenuating the benefits. If there is a zero effect in those who do not adhere, there may be a worthwhile effect in those who do adhere. None of our included studies presented an analysis that would allow the effect size in adherent participants to be estimated appropriately.57 The approach of comparing outcomes in the adherent group with overall outcomes in the control group used by some authors25 is potentially misleading. An appropriate approach, such as a complier average causal effect analysis, would adjust for non-compliance, thus providing more reliable results.
Our previous review13 has been criticised for including the RAMIT (Rehabilitation After Myocardial Infarction Trial) trial.36 Other recent reviews20 using the same recruitment period criteria as us, excluded the RAMIT trial whose recruitment straddled the end of 1999. In a post hoc sensitivity analysis we excluded RAMIT data. It did not materially affect our conclusions (online supplementary appendix 5). For completeness, we also provide our previous mortality analysis with RAMIT excluded (online supplementary appendix 6). Again, this does not materially change our previous conclusions.
bmjopen-2019-036089supp005.pdf (227.4KB, pdf)
bmjopen-2019-036089supp006.pdf (95.1KB, pdf)
Post hoc, to inform a discussion on the clinical relevance of our findings, we searched unsuccessfully for established values of clinically important between-group differences, following CR, for the outcomes included in our meta-analyses. For the SF-36, the minimal clinically important within-person change has been reported as an increase in any domain score of three to five points.58 All of our statistically significant differences in the SF-36 domains met this threshold. However, caution is strongly advised with this arbitrary value as the clinically important within-person change varies considerably, dependent on diagnosis and duration and severity of disease, among other confounders.59 60 Therefore, we looked for values of a clinically important within-person change in SF-36 domains following CR. On the basis that an improvement equating to half of the within-person change can be considered a worthwhile outcome for an appreciable number of people,61 we set this as a criterion for a clinically important between-group difference. Usefully, for our current purpose, a heart disease expert consensus62 suggested SF-36 domain-specific changes that should be considered minimal, moderate and large for an individual. Minimal changes ranged from 15 to 25 points, and moderate changes from 25 to 50 points (online supplementary appendix 7). Using this approach, the only clinically important differences in the SF-36 domains were the short-term effects on ‘physical function’ and ‘role physical’. These are above, or close to half of, the consensus values for a moderate change. No other point estimates met the criteria for a clinically important change.
bmjopen-2019-036089supp007.pdf (29.7KB, pdf)
For the MacNew Questionnaire, a within-person change of 0.5 points for any specific domain or the overall score has been proposed.63–65 Using the same approach, we would set a between-group difference of 0.25 points as a clinically important benefit. Although none of the analyses was statistically significant, the point estimates for physical and emotional subscales and the overall score are consistent with a clinically important short-term benefit on the MacNew Questionnaire.
Strengths and limitations
We identified 24 studies, 18 of which we could include in meta-analyses. Due to wider inclusion criteria in terms of time-points for outcome reporting, this is >14 studies identified (8 meta-analysed) in another recent review.20 Also, our search date was more recent, and we included studies testing exercise-based CR interventions in all manifestations of CAD rather than just those with acute coronary syndrome±revascularisation, angina or angiographically documented CAD. We did, however, exclude one study53 from our meta-analyses that was included by the previous authors. We were unable to extract data from the line graph, and values presented in the text were not between groups differences so could not be used. We also differ from the other recent review in our interpretation of a clinically important benefit for the SF-36. This is because we used consensus values for minimal and moderate change62 to define clinical importance, rather than the smallest measurable change in an SF-36 domain. We would interpret their findings as showing that there were no clinically important benefits on any SF-36 domains and that only role physical achieved a minimal benefit.
Given the heterogeneity and paucity of data included in our review, strengths and weaknesses should be considered. We performed rigorous and transparent systematic review, with meta-analysis where possible. Where there was any doubt as to data compatibility, we opted to exclude studies from the analysis, helping to ensure the integrity of the results. We included only data from studies recruiting after 31 December 1999 to ensure that findings were applicable in the era of contemporary medical care. Defining the era of contemporary medical care can be problematic due to difficulties in identifying exactly when data were collected for each trial, and the nature of medical practice at the time. Therefore, it is not possible to be certain that included and excluded trials exactly match our criteria for what constitutes contemporary medical care. However, meticulous examination of each trial provides a high level of confidence that the most appropriate studies have been included.
Our findings are limited by a number of inconsistencies in the CR literature and data. While baseline data were always collected prior to randomisation, follow-up data were reported at varying times postrandomisation. Our short-term data included studies reporting their first follow-up at anything between 1 and 6 months. Equally, our medium-term data covered studies reporting between 8 and 12 months. Data were too scarce and heterogeneous to assess time-points more accurately. It is also worth noting that only 5/24 analyses in our review included studies using CAD-specific QoL measures. It is possible that generic QoL instruments are insufficiently sensitive to detect change in people with CAD. Disease-specific tools are more likely to accurately reflect QoL in this population.
Exercise interventions and other core components of comprehensive CR varied widely in their composition and delivery, and these may have fallen short of what would be considered ‘optimal’ or ‘gold standard’ care. Equally, usual care was inconsistent which may dilute any benefit associated with exercise-based CR. Furthermore, the overall quality of studies included in our meta-analyses was poor, with the majority scoring ‘some concerns’ or ‘high’ on the risk of bias assessment. Numerous sources of potential bias were identified including poor reporting of key methodological information such as randomisation, blinding and statistical analyses.
Data reporting in some studies is a potential source of bias. First, results from one study46 showed a vastly superior improvement in MacNew QoL scores compared with others39 42 in the short-term analysis. Second, one study33 reported only the total score for the SF-36 at 6 months; this is not a validated or recommended measure.54 55 Third, for one study,38 we only included n=372 (58%) participants who had IHD at 12 months follow-up, however, the SF-36 values included a proportion of participants with heart failure (12%) or at high risk of ischaemic heart disease (30%). However, this only affected the medium-term data for SF-36 MCS and PCS, as these were the only data reported in the trial. Finally, we could not perform a meta-analysis for the SF-36 PCS at 12 months in one study30 as an exact p value was not provided.
Conclusions
For people with CAD participating in exercise-based CR, our meta-analyses show statistically significant improvements in multiple individual SF-36 domain scores, but only 2/24 comparisons (both short-term outcomes) can be deemed clinically important. Exercise-based CR shows promise as an approach to improve QoL for people with CAD. However, the contemporary data presented in this review are insufficient to support its routine use. Given the critical importance of QoL to people living with long-term conditions, future research should optimise CR programmes to target improvement in QoL domains.
Supplementary Material
Acknowledgments
The authors would like to thank Petra Meeson, Specialist Medical Librarian, UHCW NHS Trust for her expert assistance with database searching.
Footnotes
Twitter: @HIITorMISSUK
Contributors: Conceptualisation: MU and GMcG; methodology: GMcG, PK, RP and MU; formal analysis: PK; investigation: GMcG, RP and MU; data curation: GMcG and RP; supervision: MU; writing—original draft preparation: GMcG; writing—review and editing: GMcG, RP, PK and MU.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MU was Chair of the NICE accreditation advisory committee until March 2017 for which he received a fee. He is chief investigator or co-investigator on multiple previous and current research grants from the UK National Institute for Health Research, Arthritis Research UK and is a co-investigator on grants funded by the Australian NHMRC. He is co-applicant on two studies of cardiopulmonary rehabilitation. He is an NIHR Senior Investigator. He has received travel expenses for speaking at conferences from the professional organisations hosting the conferences. He is a director and shareholder of Clinvivo Ltd that provides electronic data collection for health services research. He is part of an academic partnership with Serco Ltd related to return to work initiatives. He is a co-investigator on a study receiving support in kind from Stryker Ltd. He has accepted honoraria for teaching/lecturing from CARTA & Sterling Events. He is an editor of the NIHR journal series, and a member of the NIHR Journal Editors Group, for which he receives a fee. GM is a practising exercise physiologist. He is chief investigator and co-investigator on multiple studies of cardiopulmonary rehabilitation funded by NIHR and BHF. RP is a practising exercise physiologist.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. Data are available in supplementary material.
References
- 1. World Health Organisation Global health estimates 2016: disease burden by cause, age, sex, by country and by region, 2000-2016. Geneva: World Health Organization, 2018. [Google Scholar]
- 2. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20. 10.1016/S0140-6736(03)12113-7 [DOI] [PubMed] [Google Scholar]
- 3. Gaziano TA, Bitton A, Anand S, et al. . Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol 2010;35:72–115. 10.1016/j.cpcardiol.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. British Heart Foundation Cardiovascular Disease Statistics - BHF UK Factsheet. London, England, 2018. [Google Scholar]
- 5. NICE Secondary prevention after a myocardial infarction. NICE quality standard [QS99]., 2015. Available: https://www.nice.org.uk/guidance/qs99
- 6. NICE Cardiac rehabilitation after myocardial infarction, 2018. Available: https://pathways.nice.org.uk/pathways/myocardial-infarction-rehabilitation-and-preventing-further-cardiovascular-disease/cardiac-rehabilitation-after-myocardial-infarction
- 7. Piepoli MF, Hoes AW, Agewall S, et al. . 2016 European guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur Heart J 2016;37:2315–81. 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valkeinen H, Aaltonen S, Kujala UM. Effects of exercise training on oxygen uptake in coronary heart disease: a systematic review and meta-analysis. Scand J Med Sci Sports 2010;20:545–55. 10.1111/j.1600-0838.2010.01133.x [DOI] [PubMed] [Google Scholar]
- 9. Anderson L, Thompson DR, Oldridge N, et al. . Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016:CD001800. 10.1002/14651858.CD001800.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heran BS, Chen JM, Ebrahim S, et al. . Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2011:CD001800. 10.1002/14651858.CD001800.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kabboul NN, Tomlinson G, Francis TA, et al. . Comparative effectiveness of the core components of cardiac rehabilitation on mortality and morbidity: a systematic review and network meta-analysis. J Clin Med 2018;7:514. 10.3390/jcm7120514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xia T-L, Huang F-Y, Peng Y, et al. . Efficacy of different types of Exercise-Based cardiac rehabilitation on coronary heart disease: a network meta-analysis. J Gen Intern Med 2018;33:2201–9. 10.1007/s11606-018-4636-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Powell R, McGregor G, Ennis S, et al. . Is exercise-based cardiac rehabilitation effective? A systematic review and meta-analysis to re-examine the evidence. BMJ Open 2018;8:e019656. 10.1136/bmjopen-2017-019656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The World Health Organization quality of life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med 1995;41:1403–9. 10.1016/0277-9536(95)00112-K [DOI] [PubMed] [Google Scholar]
- 15. Mercieca-Bebber R, King MT, Calvert MJ, et al. . The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas 2018;9:353–67. 10.2147/PROM.S156279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Francis T, Kabboul N, Rac V, et al. . The effect of cardiac rehabilitation on health-related quality of life in patients with coronary artery disease: a meta-analysis. Can J Cardiol 2019;35:352–64. 10.1016/j.cjca.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 17. Long L, Anderson L, Dewhirst AM, et al. . Exercise-based cardiac rehabilitation for adults with stable angina. Cochrane Database Syst Rev 2018;2:CD012786. 10.1002/14651858.CD012786.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng X, Zheng Y, Ma J, et al. . Effect of exercise-based cardiac rehabilitation on anxiety and depression in patients with myocardial infarction: a systematic review and meta-analysis. Heart Lung 2019;48:1–7. 10.1016/j.hrtlng.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 19. Resurrección DM, Moreno-Peral P, Gómez-Herranz M, et al. . Factors associated with non-participation in and dropout from cardiac rehabilitation programmes: a systematic review of prospective cohort studies. Eur J Cardiovasc Nurs 2019;18:38–47. 10.1177/1474515118783157 [DOI] [PubMed] [Google Scholar]
- 20. Candelaria D, Randall S, Ladak L, et al. . Health-related quality of life and exercise-based cardiac rehabilitation in contemporary acute coronary syndrome patients: a systematic review and meta-analysis. Qual Life Res 2020;29:579–92. 10.1007/s11136-019-02338-y [DOI] [PubMed] [Google Scholar]
- 21. Wood D, De Backer G, Faergeman O, et al. . Prevention of coronary heart disease in clinical practice: recommendations of the second joint Task force of European and other societies on coronary prevention. Atherosclerosis 1998;140:199–270. 10.1016/s0021-9150(98)90209-x [DOI] [PubMed] [Google Scholar]
- 22. Schwarzer G. Meta: an R package for meta-analysis, 2007: 40–5. [Google Scholar]
- 23. Team RC R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2018. https://www.R-project.org/ [Google Scholar]
- 24. Oerkild B, Frederiksen M, Hansen JF, et al. . Home-based cardiac rehabilitation is an attractive alternative to no cardiac rehabilitation for elderly patients with coronary heart disease: results from a randomised clinical trial. BMJ Open 2012;2:e001820. 10.1136/bmjopen-2012-001820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Højskov IE, Moons P, Egerod I, et al. . Early physical and psycho-educational rehabilitation in patients with coronary artery bypass grafting: a randomized controlled trial. J Rehabil Med 2019;51:136–43. 10.2340/16501977-2499 [DOI] [PubMed] [Google Scholar]
- 26. Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Softw 2010;36:1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 27. Maddison R, Pfaeffli L, Whittaker R, et al. . A mobile phone intervention increases physical activity in people with cardiovascular disease: results from the heart randomized controlled trial. Eur J Prev Cardiol 2015;22:701–9. 10.1177/2047487314535076 [DOI] [PubMed] [Google Scholar]
- 28. Cumpston M, Li T, Page MJ, et al. . Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for systematic reviews of interventions. Cochrane Database Syst Rev 2019;10:ED000142. 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Belardinelli R, Paolini I, Cianci G, et al. . Exercise training intervention after coronary angioplasty: the ETICA trial. J Am Coll Cardiol 2001;37:1891–900. 10.1016/S0735-1097(01)01236-0 [DOI] [PubMed] [Google Scholar]
- 30. Bettencourt N, Dias C, Mateus P, et al. . Impact of cardiac rehabilitation on quality of life and depression after acute coronary syndrome. Rev Port Cardiol 2005;24:687–96. [PubMed] [Google Scholar]
- 31. Briffa TG, Eckermann SD, Griffiths AD, et al. . Cost‐effectiveness of rehabilitation after an acute coronary event: a randomised controlled trial. Med J Aust 2005;183:450–5. 10.5694/j.1326-5377.2005.tb07121.x [DOI] [PubMed] [Google Scholar]
- 32. Houle J, Doyon O, Vadeboncoeur N, et al. . Effectiveness of a pedometer-based program using a socio-cognitive intervention on physical activity and quality of life in a setting of cardiac rehabilitation. Can J Cardiol 2012;28:27–32. 10.1016/j.cjca.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 33. Mutwalli HA, Fallows SJ, Arnous AA, et al. . Randomized controlled evaluation shows the effectiveness of a home-based cardiac rehabilitation program. Saudi Med J 2012;33:152–9. [PubMed] [Google Scholar]
- 34. Sandström L, Ståhle A. Rehabilitation of elderly with coronary heart disease – improvement in quality of life at a low cost. Adv Physiother 2005;7:60–6. 10.1080/14038190510010287 [DOI] [Google Scholar]
- 35. Wang W, Chair SY, Thompson DR, et al. . Effects of home-based rehabilitation on health-related quality of life and psychological status in Chinese patients recovering from acute myocardial infarction. Heart Lung 2012;41:15–25. 10.1016/j.hrtlng.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 36. West RR, Jones DA, Henderson AH. Rehabilitation after myocardial infarction trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart 2012;98:637–44. 10.1136/heartjnl-2011-300302 [DOI] [PubMed] [Google Scholar]
- 37. Yu C-M, Lau C-P, Chau J, et al. . A short course of cardiac rehabilitation program is highly cost effective in improving long-term quality of life in patients with recent myocardial infarction or percutaneous coronary intervention. Arch Phys Med Rehabil 2004;85:1915–22. 10.1016/j.apmr.2004.05.010 [DOI] [PubMed] [Google Scholar]
- 38. Zwisler A-DO, Soja AMB, Rasmussen S, et al. . Hospital-based comprehensive cardiac rehabilitation versus usual care among patients with congestive heart failure, ischemic heart disease, or high risk of ischemic heart disease: 12-month results of a randomized clinical trial. Am Heart J 2008;155:1106–13. 10.1016/j.ahj.2007.12.033 [DOI] [PubMed] [Google Scholar]
- 39. Reid RD, Morrin LI, Beaton LJ, et al. . Randomized trial of an internet-based computer-tailored expert system for physical activity in patients with heart disease. Eur J Prev Cardiol 2012;19:1357–64. 10.1177/1741826711422988 [DOI] [PubMed] [Google Scholar]
- 40. Asbury EA, Webb CM, Probert H, et al. . Cardiac rehabilitation to improve physical functioning in refractory angina: a pilot study. Cardiology 2012;122:170–7. 10.1159/000339224 [DOI] [PubMed] [Google Scholar]
- 41. Chen C-H, Chen Y-J, Tu H-P, et al. . Benefits of exercise training and the correlation between aerobic capacity and functional outcomes and quality of life in elderly patients with coronary artery disease. Kaohsiung J Med Sci 2014;30:521–30. 10.1016/j.kjms.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 42. Devi R, Powell J, Singh S. A web-based program improves physical activity outcomes in a primary care angina population: randomized controlled trial. J Med Internet Res 2014;16:e186. 10.2196/jmir.3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Firouzabadi MG, Sherafat A, Vafaeenasab M. Effect of physical activity on the life quality of coronary artery bypass graft patients. J Med Life 2014;7:260–3. [PMC free article] [PubMed] [Google Scholar]
- 44. Hautala AJ, Kiviniemi AM, Mäkikallio T, et al. . Economic evaluation of exercise-based cardiac rehabilitation in patients with a recent acute coronary syndrome. Scand J Med Sci Sports 2017;27:1395–403. 10.1111/sms.12738 [DOI] [PubMed] [Google Scholar]
- 45. Højskov IE, Moons P, Hansen NV, et al. . Early physical training and psycho-educational intervention for patients undergoing coronary artery bypass grafting. The SheppHeart randomized 2 × 2 factorial clinical pilot trial. Eur J Cardiovasc Nurs 2016;15:425–37. 10.1177/1474515115594524 [DOI] [PubMed] [Google Scholar]
- 46. Peixoto TCA, Begot I, Bolzan DW, et al. . Early exercise-based rehabilitation improves health-related quality of life and functional capacity after acute myocardial infarction: a randomized controlled trial. Can J Cardiol 2015;31:308–13. 10.1016/j.cjca.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 47. Santaularia N, Caminal J, Arnau A, et al. . The efficacy of a supervised exercise training programme on readmission rates in patients with myocardial ischemia: results from a randomised controlled trial. Eur J Cardiovasc Nurs 2017;16:201–12. 10.1177/1474515116648801 [DOI] [PubMed] [Google Scholar]
- 48. Hassan A, Nahas N. Efficacy of cardiac rehabilitation after percutaneous coronary intervention. Int J Pharmtech Res 2016;9:134–41. [Google Scholar]
- 49. Salavati M, Fallahinia G, Vardanjani AE, et al. . Comparison between effects of home based cardiac rehabilitation programs versus usual care on the patients' health related quality of life after coronary artery bypass graft. Glob J Health Sci 2015;8:196–202. 10.5539/gjhs.v8n4p196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vieira Ágata, Melo C, Machado J, et al. . Virtual reality exercise on a home-based phase III cardiac rehabilitation program, effect on executive function, quality of life and depression, anxiety and stress: a randomized controlled trial. Disabil Rehabil Assist Technol 2018;13:112–23. 10.1080/17483107.2017.1297858 [DOI] [PubMed] [Google Scholar]
- 51. Duan YP, Liang W, Guo L, et al. . Evaluation of a web-based intervention for multiple health behavior changes in patients with coronary heart disease in home-based rehabilitation: pilot randomized controlled trial. J Med Internet Res 2018;20:e12052. 10.2196/12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wong EM, Chair SY, Leung DY, et al. . Home-based interactive e-health educational intervention for middle-aged adults to improve total exercise, adherence rate, exercise efficacy, and outcome: a randomised controlled trial. Hong Kong Med J 2018;24:34-38. [PubMed] [Google Scholar]
- 53. Seki E, Watanabe Y, Sunayama S, et al. . Effects of phase III cardiac rehabilitation programs on health-related quality of life in elderly patients with coronary artery disease: juntendo cardiac rehabilitation program (J-CARP). Circ J 2003;67:73–7. 10.1253/circj.67.73 [DOI] [PubMed] [Google Scholar]
- 54. SF-36.org FAQ: is there one summary score that is a combined score for the various subscales in the SF-36® so that a single score could be used for each patient? Available: http://www.webcitation.org/6cfeefPkf [Accessed 01 Oct 2019].
- 55. Lins L, Carvalho FM. Sf-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med 2016;4:205031211667172. 10.1177/2050312116671725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abell B, Zecchin R, Gallagher R. Making sense of the unfavourable systematic review of exercise-based cardiac rehabilitation in the modern era: how should we proceed? Heart Lung Circ 2019;28:204–6. 10.1016/j.hlc.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 57. Shrier I, Steele RJ, Verhagen E, et al. . Beyond intention to treat: what is the right question? Clin Trials 2014;11:28–37. 10.1177/1740774513504151 [DOI] [PubMed] [Google Scholar]
- 58. Stewart AL, Greenfield S, Hays RD, et al. . Functional status and well-being of patients with chronic conditions. results from the medical outcomes study. JAMA 1989;262:907–13. [PubMed] [Google Scholar]
- 59. Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med 2001;33:350–7. 10.3109/07853890109002089 [DOI] [PubMed] [Google Scholar]
- 60. Wyrwich KW, Bullinger M, Aaronson N, et al. . Estimating clinically significant differences in quality of life outcomes. Qual Life Res 2005;14:285–95. 10.1007/s11136-004-0705-2 [DOI] [PubMed] [Google Scholar]
- 61. Johnston BC, Patrick DL, Thorlund K, et al. . Patient-reported outcomes in meta-analyses-part 2: methods for improving interpretability for decision-makers. Health Qual Life Outcomes 2013;11:211. 10.1186/1477-7525-11-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wyrwich KW, Spertus JA, Kroenke K, et al. . Clinically important differences in health status for patients with heart disease: an expert consensus panel report. Am Heart J 2004;147:615–22. 10.1016/j.ahj.2003.10.039 [DOI] [PubMed] [Google Scholar]
- 63. Dixon T, Lim LLY, Oldridge NB. The MacNew heart disease health-related quality of life instrument: reference data for users. Qual Life Res 2002;11:173–83. 10.1023/A:1015005109731 [DOI] [PubMed] [Google Scholar]
- 64. Benzer W, Höfer S, Oldridge NB. Health-related quality of life in patients with coronary artery disease after different treatments for angina in routine clinical practice. Herz 2003;28:421–8. 10.1007/s00059-003-2388-9 [DOI] [PubMed] [Google Scholar]
- 65. Oldridge N, Perkins A, Marchionni N, et al. . Number needed to treat in cardiac rehabilitation. J Cardiopulm Rehabil 2002;22:22–30. 10.1097/00008483-200201000-00003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-036089supp001.pdf (56.4KB, pdf)
bmjopen-2019-036089supp002.pdf (27.5KB, pdf)
bmjopen-2019-036089supp003.pdf (103.7KB, pdf)
bmjopen-2019-036089supp004.pdf (49.3KB, pdf)
bmjopen-2019-036089supp005.pdf (227.4KB, pdf)
bmjopen-2019-036089supp006.pdf (95.1KB, pdf)
bmjopen-2019-036089supp007.pdf (29.7KB, pdf)