Abstract
Thyroid hormones are essential for normal growth and development in children. Nutritional factors are closely related to thyroid dysfunction due to deviation from normal physiology of the gland. Iodine, a main constituent of thyroid hormones (T3 and T4), deficiency is one of the commonest causes of hypothyroidism in children and adults, worldwide. Other micronutrients, such as Cruciferous vegetables, Pearl Millet, Soy products and Cassava, were also attributed to cause thyroid dysfunction. Environmental factors, namely, contamination of water with goitrogens could also contribute to the aetiology of goitre in some endemic areas. Dietary advice and avoidance of excessive use of goitrogens in diet are part of guidance on nutritional safety that needs to be established, especially in the areas of endemicity.
Keywords: Cruciferous vegetables, Goitrogens, Iodine, Micronutrients, Millet, Soy, Thyroid
INTRODUCTION
The thyroid is an endocrine gland with a pivotal role in regulating different metabolic processes in foetal, childhood and adult life. Several diseases affect the thyroid function and deem the body either deficient or in excess of the thyroid hormones; causing hypo or hyperthyroidism. While both of the diseases could be caused by different mechanisms, nutritional factors play an important role in affecting the function of the thyroid gland [1,2]. Among these factors are several micronutrients including iodine, which is a major constituent of T3 and T4 hormones.
While autoimmune thyroiditis is the main aetiology in paediatric hypothyroidism in children, mainly adolescents, iodine deficiency is still an important preventable cause of the disease in this age group [3]. In adults, iodine deficiency is considered the commonest cause of hypothyroidism worldwide [4]. This does impose a risk of poor neurodevelopmental outcome in newborns because of the effect of maternal hypothyroidism on foetal brain maturation [5].
Iodine deficiency disorders decreased significantly in the United States by the 1920s as a result of salt iodisation programs that aimed to eliminate iodine deficiency [1]. Although replacing iodine has decreased the incidence of hypothyroidism, some cases still persisted owing to the fact that other micronutrients that affects thyroid hormones function could also be involved [6]. Goitrogens, including cruciferous vegetables and soy product, have been shown to inhibit thyroid hormones synthesis in several ways, mostly by inhibiting iodine utilisation. Those micronutrients have to be taken in consideration specially in those with thyroid diseases or who are iodine deficient.
In this paper, we reviewed the physiology of thyroid hormones and the role of micronutrients, including dietary goitrogens, such as pearl millet, in affecting the thyroid function. We initially highlighted the crucial role of thyroid hormone in the neurodevelopment of foetuses and newborns. Then, we shed some light on the synthesis and metabolism of thyroid hormone before elaborating in the pathophysiological role of goitrogens in thyroid diseases. Some of the substances discussed in this paper, such as peanut oil and water hyacinth, are also previously described as potential goitrogens with postulated mechanisms of pathogenicity [7,8]. We thought this should be a focus of future research that could elucidate better the thyroid dietary relationship and inform the nutritional management of children with thyroid diseases.
PHYSIOLOGY
Thyroid hormone and brain development
Thyroid hormone is important for different body functions; however, it has a crucial role in the development of foetal brain (Table 1). The process of foetal hormonal maturation is controlled by the thyroid receptors. Those receptors, including TRα and TRβ subtypes, are expressed on cell nucleus and regulate production of thyroid hormones through gene expression. The foetus depends upon maternal thyroid hormone up until 12 weeks of gestation, when the thyroid receptors start to appear in the cerebral cortex and the cerebellum. By then, the foetal thyroid is able to concentrate iodine in order to synthesise thyroxine [9].
Table 1.
Role of thyroid hormones in different functions of the body.
|
The fact that thyroxine was found to be detectable in infants with complete thyroid agenesis, indicates that transplacental passage of maternal thyroid hormones remain an important source for the foetus throughout the pregnancy. Maternal hormones are probably the reason behind maintaining a normal IQ in those infants whom been treated early after newborn screening [10].
Synthesis and metabolism of thyroid hormones
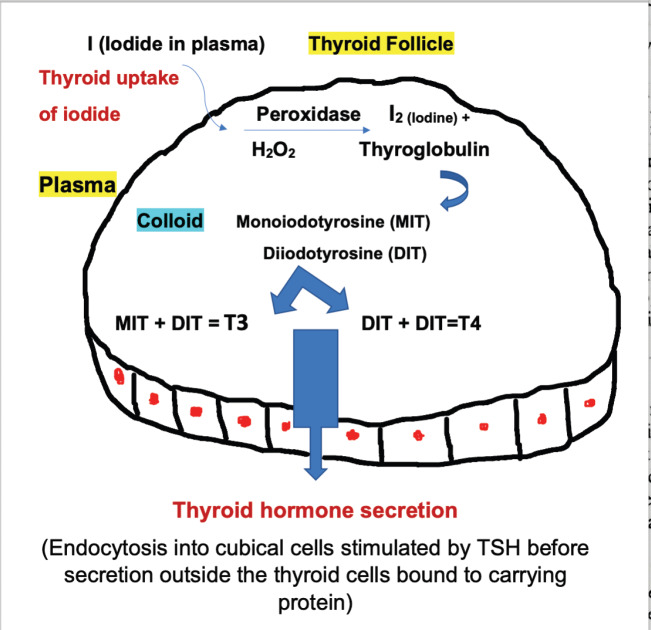
The thyroid gland synthesises two hormones: Thyroxine (T4) and triiodothyronine (T3) (Figure 1). After the absorption of Iodine, it circulates in blood stream in the form of Iodide (I) and enters the thyroid follicular cells through the sodium-iodine symporter which is regulated by the thyroid stimulating hormone (TSH). TSH also stimulate the production of thyroglobulin, which is a glycoprotein produced inside the thyroid follicular cells and stored within the follicular lumen (the colloid space). Inside the cell, the iodide is oxidised via the thyroid peroxidase enzyme (TPO), then goes through organification process that incorporate iodide into thyroglobulin. Thus, forming monoiodotyrosine and diiodotyrosine residues that couple to form T4 and T3 (Figure 1). Finally, TSH stimulates thyroglobulin proteolysis and subsequently releases both hormones into the bloodstream [11]. Each step in the synthesis of thyroid hormone is catalysed by enzymes which, when deficient, may lead to goitre and/or hypothyroidism. Naturally occurring autosomal recessive mutations in many of the genes which encode these enzymes have been shown to cause dyshormonogenic congenital hypothyroidism [12].
Figure 1.
Synthesis and secretion of thyroid hormones.
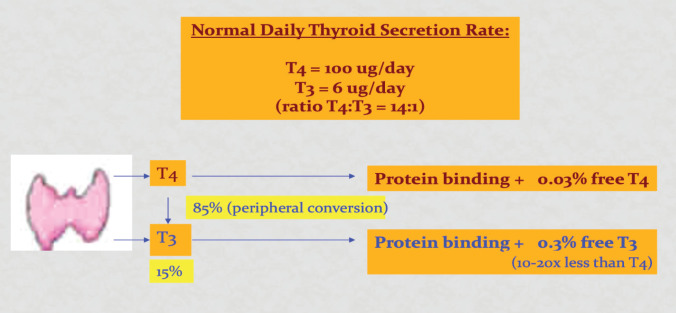
The vast majority of thyroid hormone in the circulation is bound to serum proteins which include thyroglobulin binding protein, transthyretin and albumin. In euthyroid individuals, only 0.03% of T4 and 0.3% of T3 is unbound or ‘free’ and immediately available to enter cells and mediate thyroid hormone signalling (Figure 2). In the circulation, most of T4 are converted to T3, the active form of thyroid hormone, via deiodination process of the outer ring (Figure 2). While inner-ring deiodination of T4 or T3 inactivate both hormones, forming reverse triiodothyronine (rT3) and diiodothyronine (T2) metabolites.
Figure 2.
Thyroid hormones in the circulation.
ROLE OF MICRONUTRIENTS
Several nutritional factors have been shown to affect the thyroid in addition to iodine. Goitrogens refer to a group of micronutrients that can cause enlargement of the thyroid gland. It includes two main categories which are cruciferous vegetables and soy products. Although big clinical studies in this area is lacking, several animal studies and human case reports highlighted the effect of these micronutrients and their possible contribution to better thyroid disease management [1,2,13].
Dietary effect on thyroid dysfunction and suggested pathogenesis
Iodine
Iodised salt, seafood (such as fish and seaweed), and some grains and breads are common dietary sources of iodine [2]. In routine clinical practice, patients will often inquire about dietary changes they can make to treat or reverse their thyroid dysfunction. In general, there is reasonable evidence that adequate but not excessive iodine intake is beneficial for thyroid health as well as selenium supplementation for patients with Graves’ disease [5,6,8,14]. Aside from these, the scientific data showing that dietary changes can significantly benefit hypo- or hyperthyroidism is sparse [1,2].
Cruciferous vegetables
Cruciferous vegetables are vegetables of Brassicaceae family, including kale, turnips, cauliflower and others, which are rich in indole glucosinolate. In animal studies, this compound was found to degrade into a goitrin metabolite called thiocyanate that inhibits iodine uptake by the thyroid cell. It works by competitive inhibition of the sodium/iodide symporter, and hence inhibiting the synthesis of thyroid hormones [15]. However, data are lacking in examining the serum concentrations of goitrin following the ingestion of cruciferous vegetables.
There is no enough data available that define the amount of cruciferous vegetable consumption that is needed to cause thyroid dysfunction. One small study on five healthy participants showed a decrease by 25% of radioiodine uptake from baseline after 6-hour of 15.2 of consumption of kale juice twice per day for 7 days, while serum thyroid function tests were unchanged. Another case report of an elderly woman showed that longer period of consuming large amount of raw bok choy (1–1.5 kg) was associated with myxedema. Further clinical studies to explore the minimum duration and amount of consumption is warranted. While cruciferous vegetables might be heavily promoted for their anticarcinogenic effects, those benefits could come at the risk of inducing a state of hypothyroidism, especially if accompanied by iodine deficiency. Heating could be a beneficial way since it denatures the goitrogenic compound into a less harmful metabolite.
Soy products
Goitrogens has been also found in soy products. Several studies on isoflavone, that is the most abundant phytoestrogens in soy, have found that it inhibits the thyroid hormones in people with iodine deficiency while unlikely to affect those with euthyroid. The mechanism behind could be due to inhibition of the activity of TPO enzyme as seen in animal studies [16]. A case report from 1960s described a gaiter in an infant who was on soybean formula for 4 months, which noticed to improve 1 week after substitution of the soy formula with whole milk [17].
Pearl millet
Another soy product that can cause gaiter is Millet grain; namely, of Pearl Millet (Pennisetum glaucum or a synonym P. americanum), a tall cereal grass that has large leaves and dense round spikes, which is widely grown for its seeds and for forage [18]. Even in people with adequate iodine intake, millet still can suppress the thyroid gland function. Many rural areas of Africa and Asia that have a semi-arid climate depend on millet as their main source of food energy. Endemic goitre noticed in these areas raised this possibility to investigate antithyroid activities of millets. Pearl millet is a type of millet that is rich in C-glycosylflavones, which was shown to have similar activities to methimazole in animal studies. In vitro studies in rats, C-glycosylflavones inhibited 85% of Thyroid Peroxidase enzyme [13,19].
Other micronutrients
Another micronutrient that affects the thyroid function is selenium. A metanalysis looked into the effect of selenium on patient with Graves’ disease. Patients who received selenium supplementation showed a temporary improvement in thyroid function at 6 months follow-up. This improvement though didn’t last by 9 months in comparison to those who were on placebo. Although adjuvant selenium supplementation holds some benefits for patient with gravis disease, it cannot be part of the standard treatment until further supportive evidence [14].
Cassava, a starchy tuberous root of a tropical tree, consumed as a routine diet in some African countries, is also another previously reported goitrogen [20]. The aetiology could be related to, though still controversial, a high concentration of linamarin, a cyanogenic glucoside, in Cassava that can be metabolised to thiocyanate [20,21]. Peanut oil and water hyacinth, are also previously described as potential goitrogens in Kosti city in Sudan when Eltom et al. [7,8] explored the possible causes of endemic goitre in central Sudan. A postulated mechanism of pathogenesis included the strong absorptive capacity of water hyacinth (Eiccornia Crossipes), a floating aquatic weed that form a perennial mat that covers most of the White Nile river, the main supply of drinking water in Kosti [22]. Onion and garlic, previously attributed as vegetables containing potential dietary goitrogens, were also suspected to be behind the endemicity beside peanut oil as all are well consumed in the city [7,23–25]. Likewise, Vougt et al. reported on endemic goiter in Virginia, United States of America, in relation to water pollution with Escherichia Coli bacteria that has antithyroid activity [26]. Moreover, Gaitan et al. findings suggested intervention of certain disulphides contained in drinking water [27,28].
Effect of micronutrients on absorption of thyroid medications
It is important for general practitioners and paediatricians to know that common nutritional supplements can interact with the absorption of thyroid medications. For instance, calcium supplements have the potential to interfere with proper absorption of thyroid medications. Therefore, spacing the intake of both of the medications at least 4 hours apart is recommended [29]. Coffee and fibre supplements also should precede thyroid hormone intake by at least an hour so as not to interfere with the absorption of the thyroid medication [30]. Chromium picolinate, which is used as an alternative medicine to reduce blood sugar in children with diabetes and in prediabetes status as well as for cholesterol lowering and for weight loss, impairs the absorption of thyroid medications. Hence, the two medications should be consumed three to 4 hours apart. Flavonoids in fruits, vegetables and tea that could potentially affect the cardiovascular in a positive way, do suppress thyroid function in high-doses. The Natural Standards Database provides an extensive list of supplements that have a potential impact on thyroid function that consumers can refer to for guidance [31]. Dieticians should confirm that patients are adhering to these guidelines for optimal absorption of thyroid hormone supplements.
CONCLUSION
A balanced diet is an important component of maintaining a healthy thyroid gland function. While iodine being on top of the list, it is not the sole micronutrient affecting the thyroid gland. Since the current evidence does not provide a maximum limit of intake of these micronutrients inhibiting the thyroid function, dietary regulation should be based upon clinical correlation and experienced dietetic advice. Further studies on exploring the toxic limits of these micronutrients and better understanding of the surrounding environment and potential goitrogens may help establishing guidance on nutritional safety.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
FUNDING
None.
ETHICAL APPROVAL
No ethical approval was required.
REFERENCES
- 1.Harris C. Thyroid disease and diet—nutrition plays a part in maintaining thyroid health. Todays Diet. 2012;14:40. Available from: https://www.todaysdietitian.com/newarchives/070112p40.shtm . [Google Scholar]
- 2.Leung AM. The thyroid diet: is there such a thing? Medscape. 2018 Available from: https://www.medscape.com/viewarticle/901118 .
- 3.Sağlam H, Büyükuysal L, Köksal N, Ercan I, Tarim O. Increased incidence of congenital hypothyroidism due to iodine deficiency. Pediatr Int. 2007;49(1):76–9. doi: 10.1111/j.1442-200X.2007.02297.x. https://doi.org/10.1111/j.1442-200X.2007.02297.x. [DOI] [PubMed] [Google Scholar]
- 4.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. American Association of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200–35. doi: 10.1089/thy.2012.0205. https://doi.org/10.1089/thy.2012.0205. [DOI] [PubMed] [Google Scholar]
- 5.Andersson M, de Benoist B, Delange F, Zupan J WHO Secretariat. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10(12A):1606–11. doi: 10.1017/S1368980007361004. https://doi.org/10.1017/S1368980007361004. [DOI] [PubMed] [Google Scholar]
- 6.Menon PS. Prevention of iodine deficiency disorders in children in India—the way forward. Indian J Pediatr. 2019;86(2):113–5. doi: 10.1007/s12098-018-02849-5. https://doi.org/10.1007/s12098-018-02849-5. [DOI] [PubMed] [Google Scholar]
- 7.Eltom M, Elmahdi EM, Salih MA, Mukhtar E, Omer MI. A new focus of endemic goitre in the Sudan. Trop Geogr Med. 1985;37(1):15–21. [PubMed] [Google Scholar]
- 8.Elnagar B, Eltom M, Karlsson FA, Ermans AM, Gebre-Medhin M, Bourdoux PP. The effects of different doses of oral iodized oil on goiter size, urinary iodine, and thyroid-related hormones. J Clin Endocrinol Metab. 1995;80(3):891–7. doi: 10.1210/jcem.80.3.7883848. https://doi.org/10.1210/jcem.80.3.7883848. [DOI] [PubMed] [Google Scholar]
- 9.Bernal J. Thyroid hormones in brain development and function. Endotext. 2000 Available from: https://www.ncbi.nlm.nih.gov/books/NBK285549/
- 10.Sun F, Zhang JX, Yang CY, Gao GQ, Zhu WB, Han B, et al. The genetic characteristics of congenital hypothyroidism in China by comprehensive screening of 21 candidate genes. Eur J Endocrinol. 2018;178(6):623–33. doi: 10.1530/EJE-17-1017. https://doi.org/10.1530/EJE-17-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kappy M, Geffner M, Allen D. 1st. New York: McGraw-Hill Education; 2010. Pediatric practice; pp. 107–10. Chapter 4: Thyroid. [Google Scholar]
- 12.Sperling M. 4th. 400 Elsevier, Philadelphia, United States of America: 2014. Pediatric endocrinology; pp. 186–92. Disorders of the thyroid in the newborn 401 and infant. Chapter 7. [Google Scholar]
- 13.Rome, Italy: Food and Agriculture Organization of the United Nations; 1995. Sorghum and millets in human nutrition. Chapter 6: Nutritional inhibitors and toxic factors. [Google Scholar]
- 14.Zheng H, Wei J, Wang L, Wang Q, Zhao J, Chen S, et al. Effects of selenium supplementation on Graves’ disease: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2018;2018:3763565. doi: 10.1155/2018/3763565. https://doi.org/10.1155/2018/3763565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felker P, Bunch R, Leung AM. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr Rev. 2016;74(4):248–58. doi: 10.1093/nutrit/nuv110. https://doi.org/10.1093/nutrit/nuv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang HC, Doerge DR. Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol Appl Pharmacol. 2000;168(3):244–52. doi: 10.1006/taap.2000.9019. https://doi.org/10.1006/taap.2000.9019. [DOI] [PubMed] [Google Scholar]
- 17.Hydovitz JD. Occurrence of goiter in an infant on a soy diet. N Engl J Med. 1960;262(7):351–3. doi: 10.1056/NEJM196002182620707. https://doi.org/10.1056/NEJM196002182620707. [DOI] [PubMed] [Google Scholar]
- 18.Pearl millet. Merriam-Webster. Available from: https://www.merriam-webster.com/dictionary/pearl%20millet .
- 19.Gaitan E, Lindsay RH, Reichert RD, Ingbar SH, Cooksey RC, Legan J, et al. Antithyroid and goitrogenic effects of millet: role of C-glycosylflavones. J Clin Endocrinol Metab. 1989;68(4):707–14. doi: 10.1210/jcem-68-4-707. https://doi.org/10.1210/jcem-68-4-707. [DOI] [PubMed] [Google Scholar]
- 20.Cliff J, Lundquist P, Rosling H, Sörbo B, Wide L. Thyroid function in a cassava-eating population affected by epidemic spastic paraparesis. Acta Endocrinol (Copenh) 1986;113(4):523–8. doi: 10.1530/acta.0.1130523. https://doi.org/10.1530/acta.0.1130523. [DOI] [PubMed] [Google Scholar]
- 21.Kittivachra R. Effects of cassava on thyroid gland in rats. Thaiphesatchasan. 2006;30:57–62. [Google Scholar]
- 22.Chigbo FE, Smith RW, Shore FL. Uptake of arsenic, cadmium, lead and mercury from polluted water by the water hyacinth Eichornia-crassipes. Environ Pollut Ser A Echol Biol. 1982;27:31–6. https://doi.org/10.1016/0143-1471(82)90060-5. [Google Scholar]
- 23.Moudgal NR, Raghupathy E, Sarma PS. Studies on goitrogenic agents in food. III. Goitrogenic action of some glycosides isolated from edible nuts. J Nutr. 1958;66(2):291–303. doi: 10.1093/jn/66.2.291. https://doi.org/10.1093/jn/66.2.291. [DOI] [PubMed] [Google Scholar]
- 24.Saghir AR, Cowan JW, Salji JP. Goitrogenic activity of onion volatiles. Nature. 1966;211(5044):87. doi: 10.1038/211087a0. https://doi.org/10.1038/211087a0. [DOI] [PubMed] [Google Scholar]
- 25.Hassan AS, Dawood KS, Abo-Turkey YY, El Khatim AS. An epidemiological account of the 1976 outbreak of enteric fever in Kosti town. Sudan Med J. Forthcoming. [Google Scholar]
- 26.Vought RL, London WT, Stebbing GE. Endemic goiter in Northern Virginia. J Clin Endocrinol Metab. 1967;27(10):1381–9. doi: 10.1210/jcem-27-10-1381. https://doi.org/10.1210/jcem-27-10-1381. [DOI] [PubMed] [Google Scholar]
- 27.Gaitán E. Water-borne goitrogens and their role in the etiology of endemic goiter. World Rev Nutr Diet. 1973;17:53–90. https://doi.org/10.1159/000393077. [PubMed] [Google Scholar]
- 28.Gaitan E, Cooksey RC, Matthews D, Presson R. In vitro measurement of antithyroid compounds and environmental goitrogens. J Clin Endocrinol Metab. 1983;56(4):767–73. doi: 10.1210/jcem-56-4-767. https://doi.org/10.1210/jcem-56-4-767. [DOI] [PubMed] [Google Scholar]
- 29.Mazokopakis EE, Giannakopoulos TG, Starakis IK. Interaction between levothyroxine and calcium carbonate. Can Fam Physician. 2008;54(1):39. [PMC free article] [PubMed] [Google Scholar]
- 30.Benvenga S, Bartolone L, Pappalardo MA, Russo A, Lapa D, Giorgianni G, et al. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid. 2008;18(3):293–301. doi: 10.1089/thy.2007.0222. https://doi.org/10.1089/thy.2007.0222. [DOI] [PubMed] [Google Scholar]
- 31.The Natural Medicines Research Collaboration. Available from: https://naturalmedicines.therapeuticresearch.com .