Abstract
Objective:
Anxiety and depression in epilepsy are prevalent, associated with poor outcomes, under-recognized, undertreated, and thus a key area of need for treatment research. The objective of this study was to assess factors associated with research participation among epilepsy clinic patients who screened positive for anxiety or depression. This was accomplished by characterizing clinical and psychiatric factors among patients seen in an epilepsy clinic and evaluating which factors were associated with consent for potential research participation, via a combined clinical and research screening model.
Methods:
In a pragmatic trial of anxiety and depression treatment in epilepsy, individuals with a positive screen for anxiety and/or depression at a routine epilepsy clinic visit were invited to opt-in (via brief electronic consent) to further eligibility assessment for a randomized treatment study. Information on psychiatric symptoms and treatment characteristics were collected for dual clinical care and research screening purposes. Cross-sectional association of demographic, clinical, and psychiatric factors with opting-in to research was analyzed by multiple logistic regression.
Results:
Among N=199 unique adults with a first positive screen for anxiety and/or depression among 786 total screening events, 154 (77.4%) opted-in to further potential research assessment. Higher depression scores and current treatment with an antidepressant were independently associated with opting-in to research (depression OR=1.13 per 1-point increase in Neurological Disorders Depression Inventory-Epilepsy score, p=0.028, 95% CI 1.01–1.26; antidepressant OR=2.37, p=0.041, CI 1.04–5.41). Nearly half of the 199 individuals (43.7%) with anxiety and/or depression symptoms were already being treated with an antidepressant, and 46.7% were receiving neither antidepressant therapy nor mental health specialty care. One quarter (24.1%) reported a past psychiatric hospitalization, yet only half of these individuals were receiving mental health specialty care.
Significance:
Our results demonstrate a high willingness to participate in research using a brief electronic consent approach at a routine clinic visit. Adults with persistent anxiety or depression symptoms despite antidepressant therapy and those with higher depression scores were more willing to consider a randomized treatment study. This has implications for future study design, as individuals already on treatment or those with more severe symptoms are often excluded from traditional research designs. We also found a high burden of psychiatric disease and high prevalence of persistent symptoms despite ongoing antidepressant treatment.
Keywords: research participation, epilepsy, anxiety, depression, treatment, pragmatic trial
1. Introduction
In epilepsy, anxiety and depression are highly prevalent and associated with poor quality of life, mortality due to suicide, increased health care utilization, increased adverse effects of antiseizure drugs, and increased perceived cognitive dysfunction[1–6]. Despite the clinical importance of mental health in epilepsy and willingness among many epileptologists to prescribe antidepressants, minimal efficacy data exist in epilepsy populations[7–10]. Also, real-world data are lacking for the standard treatment approaches including medication treatment or mental health referrals[9]. Given the gap in evidence for mental health treatment in epilepsy, the Institute of Medicine recommended a major research priority be studies identifying effective interventions for mental health comorbidities in epilepsy[11]. Individuals with epilepsy and mental health comorbidities including anxiety and depression are similar to other populations with chronic illness who fall outside most standard randomized controlled trial inclusion criteria, resulting in a weak treatment evidence base[12, 13]. Pragmatic studies conducted in routine practice settings are especially needed to fill the evidence gap for those with multiple complex illnesses including anxiety and/or depression in epilepsy[13].
To plan and conduct research on anxiety and depression in epilepsy, it is important to understand the influence of interest in research on the composition of a clinical trial sample. Furthermore, since research interest-related bias may contribute to differences between clinical trial samples and the real-world clinic populations targeted by an intervention, investigation in routine clinic samples is needed to examine factors associated with research participation. In non-epilepsy populations, research on willingness to participate in mental health treatment trials suggests those with more severe psychiatric disease are more willing to participate[14, 15]. Among people with epilepsy, it is unknown how psychiatric symptoms or treatment characteristics may influence willingness to participate in mental-health focused treatment trials. To design future studies to improve mental health in epilepsy, it is important to characterize individuals with anxiety or depression in the epilepsy clinic and determine factors associated with interest in research.
The aims of this pragmatic study were to assess clinical and psychiatric characteristics of individuals who screened positive for anxiety or depression in a real-world epilepsy clinic sample, and to examine factors independently associated with potential desire to participate in an anxiety and depression treatment study.
2. Methods
2.1. Study Overview and Design: dual clinical-research screening model and brief description of parent study enrollment
This was a prospective, cross-sectional study of adults in a tertiary care epilepsy clinic during screening/enrollment for a pragmatic trial of anxiety and depression management from April 30, 2018 to March 12, 2019[16]. A combined clinical care and research screening method was employed, with anxiety and depression symptom screening in all patients along with further psychiatric symptom and treatment questions among those with positive anxiety or depression scores. Specifically, all patients presenting for clinic visits with one of three adult-focused epileptologists at a single level IV epilepsy center completed tablet-based anxiety and depression instruments after clinic check-in. If anxiety or depression scores indicated a positive screen (defined below in measurement section), a brief research consent was activated on the tablet. The electronic research consent provided brief information about the randomized treatment study and gave individuals the opportunity to opt-out of further research eligibility assessment. All patients with positive anxiety or depression scores then completed additional tablet-based psychiatric symptom and treatment questions for clinical use; their responses were also used to assess trial eligibility if they opted-in to research on the brief electronic consent. Individuals who opted-in and met preliminary eligibility for the treatment study were interviewed and, if eligible, offered the opportunity for full consent and enrollment in the treatment study that same day, as part of the routine epilepsy clinic visit. Results of anxiety and depression scales and psychiatric symptom and treatment questions were provided to the treating epileptologist for clinical care in real-time and were entered into the medical record by study staff; among those who opted-in to research, responses were also examined for trial eligibility as described above.
This combined clinical care and research screening/enrollment process was approved by the institutional review board. Research use of clinical information collected was also approved by the institutional review board, with full waiver of informed consent for those who opted out of the pragmatic treatment study. More information on the design of the parent pragmatic treatment study can be found at clinicaltrials.gov (NCT03464383). The parent study was a 12-week, randomized feasibility trial of epileptologist-prescribed medication for anxiety and/or depression with biweekly chronic care management, versus psychiatry referral under real-world circumstances[16].
2.2. Study Sample
This sample includes those who were screened for anxiety and depression using the tablet-based screening model and met the following criteria: age ≥18 years, able to complete the screening questionnaires independently based on observation by trained study staff, first-time positive screen for anxiety or depression during the study period, and completed psychiatric symptom and treatment questions. A total of 199 unique individuals were included in this sample, out of 900 clinic visits with screening attempted. Of the 786 total visits with anxiety and depression screening completed, there were 283 adult visits with positive anxiety and/or depression scores which triggered the research electronic consent and psychiatric symptom and treatment questions. Of these 283 visits, 84 were excluded because they were repeat clinic visits among individuals with prior positive screens. Figure 1 is a flow diagram outlining more details of the screening process and eligibility criteria which resulted in the final study sample of N=199 adults.
Figure 1:
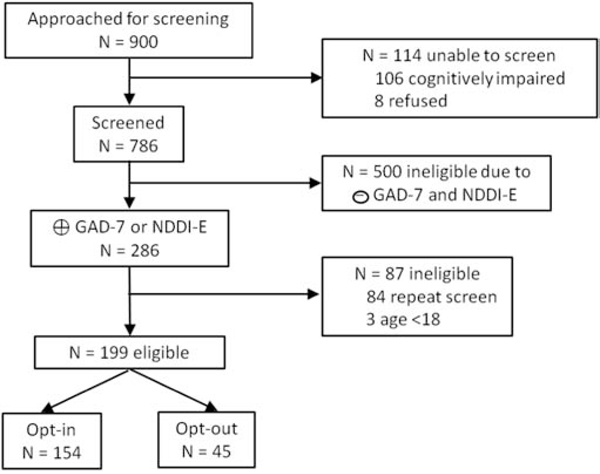
Eligibility flow diagram
This shows reasons for exclusion from the study sample.
2.3. Measurements
Anxiety and depression screening was completed via brief, freely available, and well-validated instruments in epilepsy: the Generalized Anxiety Disorder-7 (GAD-7) and Neurological Disorders Depression Inventory-Epilepsy (NDDI-E)[17–19]. Using original validation literature for these instruments, scores of 10 or higher for the GAD-7 and >15 on the NDDI-E were considered a positive screen[17, 19, 20].
The following psychiatric symptoms/treatment characteristics were collected via self-report: history of a past psychiatric hospitalization, current mental health specialty treatment (psychiatrist or therapist), current antidepressant treatment (selective serotonin reuptake inhibitor (SSRI), serotonin norepinephrine reuptake inhibitor (SNRI), mirtazapine, bupropion, or buspirone), screen for potential past manic episode (SCID-I for DSM-IV-TR screening questions or the MINI-IV past manic episode module), and presence of auditory or visual hallucinations[21, 22].
The following clinical and demographic variables were also collected: age, sex, race, ethnicity, insurance type (proxy for socioeconomic status), epilepsy diagnosis, psychogenic nonepileptic seizure diagnosis if present, type of epilepsy among those with epilepsy, and seizure freedom (seizure free for past 6 months). These were collected by chart abstraction, except for age and sex which were collected by self-report. Individuals were considered to have a diagnosis of epilepsy if epileptiform discharges or seizure(s) were recorded on prior EEG, or if epilepsy was the epileptologist’s documented leading diagnosis. A diagnosis of psychogenic nonepileptic seizures (PNES) was coded if EEG during spell recording suggested this diagnosis (clinically established or documented based on International League Against Epilepsy criteria)[23]. PNES was considered uncertain if it was suspected by the epileptologist but not confirmed by EEG spell recording. Epilepsy type was determined based on EEG findings when available, or clinician impression and semiology description (if EEG unavailable or without epileptiform abnormalities).
2.4. Statistical analysis and data management
Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted by the Wake Forest Clinical and Translational Science Institute [24]. REDCap is a secure, web-based application designed to support data capture for research studies by providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources[24].
Analyses were conducted using SAS 9.4 and RStudio version 1.1.453. We examined the distribution of data prior to further analyses. For preliminary comparison of the research opt-in to opt-out groups, two-sample t-test was used for continuous variables and chi square test for categorical variables. We also used the chi square test to compare individuals who screened positive for anxiety only, depression only, or anxiety and depression, as well as other bivariable analyses. Logistic regression modeling was then conducted, with research opt-in status as the dependent variable. This was carried out in a two-step process. Initially a simple logistic regression was conducted, with individual demographic, epilepsy-related, and psychiatric treatment variables as the covariable. Then stepwise multiple logistic regression modeling was completed among those with epilepsy (N=170). Independent variables were added initially if p<0.1 in the simple logistic regression process, and variables with p<0.1 were kept in the multivariable model. The epilepsy sample size was considered appropriate for the number of variables considered in the multiple logistic regression modeling process.
3. Results
3.1. Demographics and clinical seizure characteristics of the sample
Table 1 shows demographic and epilepsy/seizure characteristics of the sample (N=199). None of the demographic factors or epilepsy/seizure characteristics were associated with research opt-in status based on the bivariable analysis (Table 1). Seizure frequency for psychogenic seizures was also examined among the 20 individuals with PNES or suspected PNES; none were free of seizures/spells for the prior 6 months.
Table 1:
Demographic and Epilepsy Characteristics of Study Population by Research Opt-in Status
| All Subjects (N=199) | Opt-in (N=154) | Opt-out (N=45) | P-value* | |
|---|---|---|---|---|
| Age (yrs) (mean SD) | 42.9 ± 15.5 | 42.1 ± 15.9 | 45.8 ± 13.5 | 0.16 |
| Female | 114 (57.3%) | 89 (57.8%) | 25 (55.6%) | 0.79 |
| Race | 0.91 | |||
| White or Caucasian | 169 (84.9%) | 130 (84.4%) | 39 (86.7%) | |
| Black or African American | 24 (12.1%) | 19 (12.3%) | 5 (11.1%) | |
| Other | 6 (3.0%) | 5 (3.2%) | 1 (2.2%) | |
| Hispanic/Latino | 6 (3.0%) | 4 (2.6%) | 2 (4.4%) | 0.52 |
| Primary insurance type | 0.19 | |||
| Private/commercial | 80 (40.2%) | 63 (40.9%) | 17 (37.8%) | |
| Medicaid | 38 (19.1%) | 34 (22.1%) | 4 (8.9%) | |
| Medicare | 60 (30.2%) | 41 (26.6%) | 19 (42.2%) | |
| None | 17 (8.5%) | 13 (8.4%) | 4 (8.9%) | |
| Other | 4 (2.0%) | 3 (1.9%) | 1 (2.2%) | |
| Leading diagnosis of epilepsy | 0.36 | |||
| Yes | 170 (85.4%) | 129 (83.8%) | 41 (91.1%) | |
| No | 20 (10.1%) | 18 (11.7%) | 2 (4.4%) | |
| Uncertain | 9 (4.5%) | 7 (4.5%) | 2 (4.4%) | |
| Epilepsy type among N=170 with epilepsy | N=170 | N=129 | N=41 | 0.08 |
| Focal | 127 (74.7%) | 91 (70.5%) | 36 (87.8%) | |
| Generalized | 30 (17.6%) | 26 (20.2%) | 4 (9.8%) | |
| Unknown | 13 (7.6%) | 12 (9.3%) | 1 (2.4%) | |
| Diagnosis of psychogenic nonepileptic seizures | 0.13 | |||
| Yes: clinically established or documented | 7 (3.5%) | 7 (4.5%) | 0 (0.0%) | |
| Uncertain: includes possible or probable | 13 (6.5%) | 12 (7.8%) | 1 (2.2%) | |
| No | 179 (89.9%) | 135 (87.7%) | 44 (97.8%) | |
| Seizure free 6 months among N=170 with epilepsy | N=170 | N=129 | N=41 | 0.27 |
| Yes | 66 (38.8%) | 48 (37.2%) | 18 (43.9%) | |
| No | 97 (57.1%) | 74 (57.4%) | 23 (56.1%) | |
| Uncertain | 7 (4.1%) | 7 (5.4%) | 0 (0.0%) |
two sample t-test or chi square test
chi square p value shown at top of category label for categorical variables having more than 2 categories
3.2. Psychiatric treatment characteristics in the study sample
Table 2 demonstrates psychiatric symptom and treatment characteristics of the sample overall and in the opt-in and opt-out groups. Nearly one-quarter of the study sample reported a past psychiatric hospitalization, and less than one-third overall were under current treatment by a psychiatrist or therapist (Table 2). The distribution of treatment type differed among those with prior psychiatric hospitalization versus those without (p=0.007, χ 2=12.1). Individuals with a past psychiatric hospitalization were more likely receiving medication plus mental health specialty care (37.5% vs 15.9%), yet half of these individuals were not under mental health specialty care (24 of 48), and one-third (16 of 48) were not receiving antidepressant therapy or mental health specialty care. Nearly half of the 199 individuals overall with positive screen for anxiety and/or depression were already receiving antidepressant therapy, and those on antidepressant therapy were more likely to opt-in to research. Most were taking a single antidepressant (75 of 87 total taking an antidepressant, 86.2%). The most common antidepressants were sertraline (24%), escitalopram (19.5%), and citalopram (14.9%; N=21, 17, and 13, respectively). Overall, 46.7% of the study sample was untreated (not receiving antidepressant medication or mental health specialist treatment, N=93), while 22.6%, 9.6%, and 21.1% received medication alone, mental health specialty care alone, or both treatment types, respectively (N=45, 19, and 42). Compared with untreated individuals, those treated with an antidepressant and/or by a mental health specialist were more likely to opt-in to research (OR=2.9, p=0.003, 95% CI 1.4–5.8).
Table 2:
Mental Health Characteristics of Study Population by Research Opt-in Status
| All Subjects (N=199) | Opt-in (N=154) | Opt-out (N=45) | P-value* | |
|---|---|---|---|---|
| Prior psychiatric hospitalization | 48 (24.1%) | 39 (25.3%) | 9 (20.0%) | 0.46 |
| Current treatment by psychiatrist or therapist? | 61 (30.7%) | 51 (33.1%) | 10 (22.2%) | 0.16 |
| Currently on antidepressant medication?** | 87 (43.7%) | 75 (48.7%) | 12 (26.7%) | 0.009 |
| GAD-7 anxiety score (mean SD) | 12.6 ± 4.5 | 12.7 ± 4.5 | 12.2 ± 4.5 | 0.44 |
| Positive anxiety screen (GAD-7 ≥10) | 154 (77.4%) | 120 (77.9%) | 34 (75.6%) | 0.74 |
| NDDI-E depression score (mean SD) | 16.0 ± 3.6 | 16.4 ± 3.5 | 14.7 ± 3.8 | 0.007 |
| Positive depression screen (NDDI-E ≥16) | 128 (64.3%) | 105 (68.2%) | 23 (51.1%) | 0.04 |
| Passive suicidality screen positive (NDDI-E)*** | 34 (17.1%) | 29 (18.8%) | 5 (11.1%) | 0.23 |
| Irritability screen positive (SCID-IV or MINI-IV past manic episode screen) [N=198] | 102 (51.5%) | 86 (56.2%) | 16 (35.6%) | 0.01 |
| Elevated mood screen positive (SCID-IV or MINI-IV past manic episode screen) [N=198] |
58 (29.3%) | 50 (32.7%) | 8 (17.8%) | 0.05 |
| Past manic episode (MINI-IV past manic episode module) |
0.52 | |||
| Yes | 17 (8.5%) | 15 (9.7%) | 2 (4.4%) | |
| No | 159 (79.9%) | 121 (78.6%) | 38 (84.4%) | |
| Uncertain | 23 (11.6%) | 18 (11.7%) | 5 (11.1%) | |
| Visual hallucinations (in past month) | 22 (11.1%) | 19 (12.3%) | 3 (6.7%) | 0.29 |
| Auditory hallucinations(in past month) [N=198] | 0.87 | |||
| Auditory-tones | 22 (11.1%) | 17 (11.1%) | 5 (11.1%) | |
| Auditory-voices | 12 (6.1%) | 10 (6.5%) | 2 (4.4%) | |
| No auditory hallucinations | 164 (82.8%) | 126 (82.4%) | 38 (84.4%) |
two-sample t-test or chi square test; chi square p value shown at top of category label for categorical variables having more than 2 categories
SSRI, SNRI, mirtazapine, bupropion, or buspirone
positive passive suicidality screen: response of 3 or 4 (“sometimes” or “always or often” on item 4 of the NDDI-E “I’d be better off dead”)[34]
3.3. Psychiatric symptom characteristics of the study sample
Although more than half of the 199 individuals in the study sample screened positive for past episode of irritability/angry mood and nearly one-third acknowledged a period of elevated mood (Table 2), only 9.7% of those fully assessed for past manic episode met MINI-IV criteria (17 of 176). Individuals with positive irritability screen were more likely to opt-in to research than those without (OR=2.32, p=0.02, 95% CI 1.2–4.6), and more individuals with past manic episode or positive screen for elevated mood opted-in to research, though these findings were not statistically significant. Positive depression screen and higher NDDI-E scores were associated with greater likelihood of opting-in, while other symptoms examined were not associated with research opt-in status (see Table 2). The largest proportion of the study sample screened positive for both anxiety and depression (41.7%), followed by anxiety only (35.7%), and then depression only (22.6%; Table 3). The distribution of demographics, psychiatric treatment status, and research opt-in status by symptom screening category is shown in Table 3. Individuals who screened positive for both anxiety and depression were more likely to be under treatment with a psychiatrist or therapist than those who screened positive for anxiety alone (p<0.001, χ 2=13.8), and they were also more likely to opt-in to research than those with anxiety alone (OR=2.7, p=0.02, 95% CI 1.2–5.9). While 33.7% of those with both anxiety and depression (28 of 83) were receiving neither antidepressant therapy nor specialty mental health care, individuals with anxiety or depression alone were more likely untreated (57.8% and 53.3% respectively).
Table 3:
Demographic and Mental Health Treatment Characteristics by Symptom Screen Type
| All Subjects (N=199) | Anxiety positive only (N=71) | Depression positive only (N=45) | Anxiety & depression positive (N=83) | P-value* | |
|---|---|---|---|---|---|
| Age (yrs) (mean SD) | 42.9 ± 15.5 | 41.4 ± 15.4 | 44.8 ± 13.7 | 43.2 ± 16.4 | 0.50 |
| Female | 114 (57.3%) | 45 (63.4%) | 24 (53.3%) | 45 (54.2%) | 0.43 |
| Race | 0.40 | ||||
| White or Caucasian | 169 (84.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Black or African American | 24 (12.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Other | 6 (3.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Hispanic/Latino | 6 (3.0%) | 5 (7.0%) | 0 (0.0%) | 1 (1.2%) | 0.04 |
| Opt-in to research | 154 (77.4%) | 49 (69.0%) | 34 (75.6%) | 71 (85.5%) | 0.05 |
| Currently on antidepressant medication?** | 87 (43.7%) | 25 (35.2%) | 18 (40.0%) | 44 (53.0%) | 0.07 |
| Current treatment by psychiatrist or therapist? | 61 (30.7%) | 11 (15.5%) | 13 (28.9%) | 37 (44.6%) | <0.001 |
| Prior psychiatric hospitalization | 48 (24.1%) | 14 (19.7%) | 12 (26.7%) | 22 (26.5%) | 0.56 |
chi square test; chi square p value shown at top of category label for categorical variables having more than 2 categories
SSRI, SNRI, mirtazapine, bupropion, or buspirone
3.4. Factors associated with willingness to participate in research: multivariable analyses
The results of a multiple regression analysis among those with epilepsy are presented in Table 4 (N=170 individuals). Higher depression score on the NDDI-E and current antidepressant use were independently associated with opting-in to research. Those with generalized epilepsy had higher odds of opting-in to research than those with focal epilepsy in the final multivariable model (p=0.046), though the full epilepsy type variable (focal, generalized, unknown epilepsy types) did not meet statistical significance, as shown in Table 4. The other factors associated with opt-in status in bivariable analysis were not independently associated with research participation in the multivariable modeling.
Table 4:
Multivariable analysis: Research screening participation by patient characteristics among those with epilepsy
| Unadjusted Results | Final Multivariable Model N=170 | |||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio* | 95% Confidence Interval | P-value | Odds Ratio* | 95% Confidence Interval | P-value |
| Epilepsy type | 0.10 | 0.08 | ||||
| Generalized versus focal | 2.57 | 0.84–7.89 | 0.10 | 3.30 | 1.02–10.66 | 0.05 |
| Unknown versus focal | 4.75 | 0.60–37.85 | 0.14 | 3.62 | 0.44–29.94 | 0.23 |
| Depression score** | 1.13 | 1.03–1.25 | 0.008 | 1.13 | 1.01–1.26 | 0.03 |
| Current antidepressant therapy*** | 2.61 | 1.26–5.43 | 0.01 | 2.37 | 1.04–5.41 | 0.04 |
Odds of opting-in to research screening
Odds per one-point increase in score on the NDDI-E
SSRI, SNRI, mirtazapine, bupropion, or buspirone
4. Discussion
This study demonstrated more than 75% of patients with a positive anxiety or depression screen at a tertiary care epilepsy clinic visit were willing to consider participating in a pragmatic trial of epileptologist-prescribed antidepressant versus psychiatry referral. Independent predictors of opting-in to research were markers of potentially more severe psychiatric illness, specifically higher scores on the depression screening instrument (NDDI-E) and current therapy with an antidepressant. Other markers of more severe psychiatric symptoms were also associated with opting-in to research in bivariable analyses, including past irritability/anger episodes and presence of positive screen for both anxiety and depression symptoms (versus anxiety alone). Indeed, the present study findings are consistent with general population research linking willingness to participate in mental health treatment trials with more severe symptoms, though this has not previously been demonstrated in epilepsy[14, 15]. Individuals with more severe symptoms or lack of symptom relief despite treatment may be motivated to participate in research due to perceived potential personal benefit, consistent with studies in epilepsy associating participation in seizure treatment research with interest in personal benefit[25, 26]. Another potential reason for the association of antidepressant treatment with research interest could be a tendency for those who have refused antidepressant therapy to refuse/opt-out of other interventions including research. In our sample, it is unknown how many individuals were not taking antidepressants due to refusal.
Overall, the study findings highlight the feasibility and importance of conducting pragmatic, routine care-embedded research on treatment of anxiety and depression in epilepsy. The high opt-in rates observed may suggest that similar trial recruitment strategies incorporating brief electronic screening consents can achieve robust recruitment. The results also demonstrate the need to study anxiety and depression treatment in epilepsy, and especially new treatment strategies, since most of the sample had active psychiatric symptoms despite ongoing antidepressant or mental health specialty treatment. The present results suggest that they are quite willing to participate in research, though their characteristics would exclude them from many traditional research studies, especially those designed to examine initial medication treatment for anxiety or depression in epilepsy[12, 13].
In addition to demonstrating high willingness for research participation among those with potentially more severe psychiatric disease, this prospective, real-world clinic study demonstrated a high burden of psychiatric disease in the sample. This burden of psychiatric disease includes factors that excluded many potential participants from our pragmatic treatment study, and which would likely exclude even more individuals from traditional treatment efficacy study designs for mental health in epilepsy[16]. This is exemplified by the nearly one-quarter of subjects who had a prior psychiatric hospitalization, and the finding that almost 45% of the study sample had a positive screen for anxiety and/or depression despite already taking an antidepressant medication. The presence of symptoms despite current antidepressant therapy could potentially be due to under-dosing of antidepressants, suboptimal selection of antidepressant agents (perhaps by non-psychiatry providers), severe initial symptoms with persistent positive symptom screen despite some improvement with therapy, or high prevalence of treatment resistant symptoms in the study sample. Further research is needed to assess longitudinal outcomes of antidepressant prescribing in epilepsy clinics during real-world practice and to further examine source of antidepressant prescriptions and dosing in epilepsy populations. Randomized trials assessing efficacy and/or effectiveness of antidepressants commonly used in current practice may be required in this specific population. This is important given the overall paucity of data for antidepressant efficacy specifically among people with epilepsy. The only published double blind randomized trial examined non-SSRI older antidepressants (amitriptyline versus nomifensine, a drug withdrawn from the market more than 30 years ago for safety concerns)[27]. Except for one recently published unblinded randomized trial of sertraline versus cognitive behavioral therapy for depression, other studies have been small open-label trials with unselected patients[10, 27, 28].
Along with the potential severity of anxiety and depression in this sample and potential under-treatment with antidepressants, our results also demonstrate substantial under-treatment of anxiety and depression. Nearly half of the sample was not receiving antidepressant medication or specialty mental health care despite symptoms, and only half of those with a past psychiatric hospitalization were receiving specialty mental health care. The overall untreated rate in this sample is similar to prior studies, though more specific treatment rates among individuals with a past psychiatric hospitalization in an epilepsy clinic have not been previously reported[29–31]. Based on the cross-sectional nature of this analysis, it is unclear how many individuals in the study population had new anxiety and/or depression symptoms at the time of the visit and may have received initial treatment immediately following symptom identification. It is also unclear whether individuals may have been untreated due to refusal of treatment, or whether past treatment may have been interrupted for other reasons. There is scant literature on treatment preferences with respect to mental health care among patients with epilepsy, and further research in this area is needed.
4.1. Strengths and limitations
The strengths of this study are the prospective assessment of anxiety and depression symptoms, other psychiatric symptom and treatment factors, and the novel embedded research consent and eligibility assessment incorporated in routine clinical practice. Limitations include the use of brief validated screening instruments to assess for anxiety and/or depression rather than a standardized interview, sample recruited from a single tertiary care epilepsy center, and research opt-in decision focused on participation in a single specific study/design. These last two factors may limit the generalizability of our findings to other centers, non-tertiary care settings, and to other potential research studies. Due to the tertiary care setting, our results may reflect more severe illness than would be seen in a community clinic. Validated psychiatric interviews are not practical for administration in most epilepsy clinics by neurologists; the screening instruments used in this study are well-validated in epilepsy, brief, and freely available, making them advantageous for routine clinical practice and potential use in pragmatic trials[9, 20, 32]. Although the GAD-7 has been validated in epilepsy, it was not designed specifically for epilepsy, and until recently no validated epilepsy-specific anxiety instrument existed. In future research, use of the newly published Epilepsy Anxiety Survey Instrument may be advantageous[33].
5. Conclusions
Overall, this epilepsy clinic study of anxiety and depression screening with simultaneous research eligibility assessment demonstrates that individuals with higher depression scores and pre-existing antidepressant treatment are more likely to participate in research on mental health treatment in epilepsy. Further research to assess why individuals already on antidepressants do not have symptom relief is important, and future pragmatic studies of treatment optimization among individuals already receiving some treatment for anxiety and depression are needed.
Highlights.
In an epilepsy clinic, 77% agreed to research screening for a mental health study
Antidepressant therapy was ongoing in 43% at time of + anxiety or depression screen
Higher depression score was independently associated with research participation
Antidepressant therapy was independently associated with research participation
Nearly 50% with + anxiety or depression screen were untreated at time of screen
Acknowledgements/Funding:
We would like to acknowledge Brittany Briceno, Deanna Oates, Mysha Sissine, and Jianyi Li for data collection and other technical assistance.
Funding: This work was supported in part by the National Institutes of Health, grant numbers: R25 NS088248; UL1 TR001420; U24 NS107197; 2KL2TR001421-05; PCS-11403-14531; RO1 HS025723-01.
Study sponsor had no role in study design, collection, analysis and interpretation of data, writing the report, or in the decision to submit this article for publication.
Footnotes
Declaration of Interest: Kelly Conner has served as a paid consultant for SK Life Sciences. Pamela Duncan has received support from the following entities: Moleac, Duke-NUS Singapore Medical School, Care Directions, Woods-Duncan, University of Kansas for SIS-impact license, University of Pittsburgh-PRIMA DSMB, Maine Medical Center Research Institute’s COBRE, CoHstar, and she serves as Chair of the NINDS Stroke Common Data Elements-Outcomes.
The remaining authors have nothing to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kwan P, Yu E, Leung H, Leon T, Mychaskiw MA. Association of subjective anxiety, depression, and sleep disturbance with quality-of-life ratings in adults with epilepsy. Epilepsia 2009;50: 1059–66. [DOI] [PubMed] [Google Scholar]
- [2].Choi H, Hamberger MJ, Munger Clary H, Loeb R, Onchiri FM, Baker G, Hauser WA, Wong JB. Seizure frequency and patient-centered outcome assessment in epilepsy. Epilepsia 2014;55: 1205–12. [DOI] [PubMed] [Google Scholar]
- [3].Fazel S, Wolf A, Langstrom N, Newton CR, Lichtenstein P. Premature mortality in epilepsy and the role of psychiatric comorbidity: a total population study. Lancet 2013;382: 1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Caller TA, Chen JJ, Harrington JJ, Bujarski KA, Jobst BC. Predictors for readmissions after video-EEG monitoring. Neurology 2014;83: 450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kanner AM, Barry JJ, Gilliam F, Hermann B, Meador KJ. Depressive and anxiety disorders in epilepsy: do they differ in their potential to worsen common antiepileptic drug-related adverse events? Epilepsia 2012;53: 1104–8. [DOI] [PubMed] [Google Scholar]
- [6].Au A, Leung P, Kwok A, Li P, Lui C, Chan J. Subjective memory and mood of Hong Kong Chinese adults with epilepsy. Epilepsy Behav 2006;9: 68–72. [DOI] [PubMed] [Google Scholar]
- [7].Kanner AM. The treatment of depressive disorders in epilepsy: what all neurologists should know. Epilepsia 2013;54 Suppl 1: 3–12. [DOI] [PubMed] [Google Scholar]
- [8].Mula M. Treatment of anxiety disorders in epilepsy: an evidence-based approach. Epilepsia 2013;54 Suppl 1: 13–8. [DOI] [PubMed] [Google Scholar]
- [9].Bermeo-Ovalle A. Psychiatric Comorbidities in Epilepsy: We Learned to Recognize Them; It Is Time to Start Treating Them. Epilepsy Currents 2016;16: 270–272. [Google Scholar]
- [10].Gilliam FG, Black KJ, Carter J, Freedland KE, Sheline YI, Tsai WY, Lustman PJ. A Trial of Sertraline or Cognitive Behavior Therapy for Depression in Epilepsy. Ann Neurol 2019;86: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Institute of Medicine. Epilepsy Across the Spectrum: Promoting Health and Understanding. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- [12].Steinhoff BJ, Staack AM, Hillenbrand BC. Randomized controlled antiepileptic drug trials miss almost all patients with ongoing seizures. Epilepsy Behav 2017;66: 45–48. [DOI] [PubMed] [Google Scholar]
- [13].Institute of Medicine. The Learning Healthcare System: Workshop Summary. Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- [14].Moran-Sanchez I, Maurandi-Lopez A, Perez-Carceles MD. Assessment of Motivations and Willingness to Participate in Research of Outpatients With Anxiety, Mood, and Psychotic Disorders. J Empir Res Hum Res Ethics 2018;13: 546–560. [DOI] [PubMed] [Google Scholar]
- [15].Funderburk JS, Spinola S, Maisto SA. Mental health predictors of veterans willingness to consider research participation. Mil Med 2015;180: 697–701. [DOI] [PubMed] [Google Scholar]
- [16].Munger Clary H CR, Snively B, Wong M, O’Donovan C, Conner K, Kimball J, Duncan P. Epileptologist-treated anxiety and depression: Methods and feasibility of a learning health system trial. In: American Epilepsy Society Annual Meeting Baltimore, MD; 2019. [Google Scholar]
- [17].Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166: 1092–7. [DOI] [PubMed] [Google Scholar]
- [18].Micoulaud-Franchi JA, Lagarde S, Barkate G, Dufournet B, Besancon C, Trebuchon-Da Fonseca A, Gavaret M, Bartolomei F, Bonini F, McGonigal A. Rapid detection of generalized anxiety disorder and major depression in epilepsy: Validation of the GAD-7 as a complementary tool to the NDDI-E in a French sample. Epilepsy Behav 2016;57: 211–216. [DOI] [PubMed] [Google Scholar]
- [19].Gilliam FG, Barry JJ, Hermann BP, Meador KJ, Vahle V, Kanner AM. Rapid detection of major depression in epilepsy: a multicentre study. Lancet Neurol 2006;5: 399–405. [DOI] [PubMed] [Google Scholar]
- [20].Munger Clary HM, Salpekar JA. Should adult neurologists play a role in the management of the most common psychiatric comorbidities? Practical considerations. Epilepsy Behav 2019;98: 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].First MBS, Robert L; Gibbon Miriam; Williams Janet B W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- [22].Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59 Suppl 20: 22–33;quiz 34–57. [PubMed] [Google Scholar]
- [23].LaFrance WC Jr., Baker GA, Duncan R, Goldstein LH, Reuber M. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia 2013;54: 2005–18. [DOI] [PubMed] [Google Scholar]
- [24].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42: 37–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Canvin K, Jacoby A. Duty, desire or indifference? A qualitative study of patient decisions about recruitment to an epilepsy treatment trial. Trials 2006;7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reijula E, Halkoaho A, Pietila AM, Selander T, Kalviainen R, Keranen T. Therapeutic misconception correlates with willingness to participate in clinical drug trials among patients with epilepsy; need for better counseling. Epilepsy Behav 2015;48: 29–34. [DOI] [PubMed] [Google Scholar]
- [27].Robertson MM, Trimble MR. The treatment of depression in patients with epilepsy. A double-blind trial. J Affect Disord 1985;9: 12–36. [DOI] [PubMed] [Google Scholar]
- [28].Mula M. Epilepsy and Psychiatric Comorbidities: Drug Selection. Curr Treat Options Neurol 2017;19: 44. [DOI] [PubMed] [Google Scholar]
- [29].Thompson AW, Kobau R, Park R, Grant D. Epilepsy care and mental health care for people with epilepsy: California Health Interview Survey, 2005. Prev Chronic Dis 2012;9: E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Reid AY, Metcalfe A, Patten SB, Wiebe S, Macrodimitris S, Jette N. Epilepsy is associated with unmet health care needs compared to the general population despite higher health resource utilization--a Canadian population-based study. Epilepsia 2012;53: 291–300. [DOI] [PubMed] [Google Scholar]
- [31].Fiest KM, Patten SB, Altura KC, Bulloch AG, Maxwell CJ, Wiebe S, Macrodimitris S, Jette N. Patterns and frequency of the treatment of depression in persons with epilepsy. Epilepsy Behav 2014;39: 59–64. [DOI] [PubMed] [Google Scholar]
- [32].Kanner AM. Anxiety disorders in epilepsy: the forgotten psychiatric comorbidity. Epilepsy Curr 2011;11: 90–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Scott AJ, Sharpe L, Thayer Z, Miller LA, Hunt C, MacCann C, Parratt K, Nikpour A, Wong T, Gandy M. Design and validation of two measures to detect anxiety disorders in epilepsy: The Epilepsy Anxiety Survey Instrument and its brief counterpart. Epilepsia 2019;60: 2068–2077. [DOI] [PubMed] [Google Scholar]
- [34].Mula M, McGonigal A, Micoulaud-Franchi JA, May TW, Labudda K, Brandt C. Validation of rapid suicidality screening in epilepsy using the NDDIE. Epilepsia 2016;57: 949–55. [DOI] [PubMed] [Google Scholar]


