Abstract
Advanced imaging studies in neurodegenerative disease have yielded new insights into subtypes of disease, progression of disease in various brain regions, and changes in structural and functional connectivity between brain regions related to symptom progression. However, few studies have revealed imaging markers at baseline that correlate with rate or degree of decline in function. Here we tested the hypothesis that imaging features at baseline correlate with outcome of naming in primary progressive aphasia. We obtained longitudinal multimodal imaging in 15 individuals with primary progressive aphasia at the same time points as assessment of naming. We found that functional connectivity between particular brain regions (measured with resting state functional connectivity magnetic resonance imaging) is strongly associated with accuracy of naming 21 months later, independently of baseline severity of naming impairment. These data indicate that functional connectivity may carry information about later performance in naming, and is potentially useful for refining prognosis.
1. Introduction
Primary progressive aphasia (PPA) is a clinical syndrome, caused by various neurodegenerative diseases, that has a highly variable course. There are distinct clinical variants of PPA that have different profiles of impairments across language tasks. Nearly all cases in each of the variants have naming impairment, although a naming impairment is not required for the diagnosis of PPA. Within variants, there are slow decliners and rapid decliners (Sebastian et al., 2018). However, it is difficult to identify at baseline whether the course will be rapid or slow. Focal brain atrophy is a marker of PPA and its variants, indicating that imaging can reveal the neurocircuits affected by the underlying disease (see Rohrer and Rosen (2013) for review), suggesting that baseline imaging potentially carries information about prognosis.
Recently, other imaging techniques, such as Diffusion Tensor Images (DTI) and resting state (or task free) functional connectivity MRI (rsfMRI) have been explored in order to clarify different facets of the disease. Previous studies of rsfMRI in PPA have revealed that language networks and other networks are disrupted in this syndrome (Sonty et al., 2007), and the degree of disruption correlates with specific language impairments (Gola et al., 2015). However, none of these functional or structural imaging studies have provided prognostic information about future decline in language in PPA. Here, we tested the hypothesis that imaging markers at baseline correlate with subsequent decline in naming in patients with PPA.
2. Methods
2.1. Participants and Language assessment
Fifteen patients with PPA were recruited from one author’s (AH) cognitive disorders clinic, and were diagnosed by AH using a battery of tests used in the National Alzheimer’s Coordinating Center (NACC) to diagnose and classify frontotemporal lobar degeneration and PPA, along with MRI. Some patients also had FDG PET, which when available was consistent with the diagnosis of PPA. No part of the study procedures or analyses was preregistered prior to the research being conducted.
The patients were administered a battery of tests, including the Boston Naming Test (BNT), at their first visit, and nine and 21 months later. We chose the BNT as the primary outcome measure for language, as naming objects is the one language task that is nearly always impaired in all three variants of PPA, albeit for different reasons. People with nonfluent agrammatic PPA often have impaired object naming due to apraxia of speech and/or anomia (although naming actions is sometimes even more impaired); people with semantic variant PPA have impaired object naming due to semantic deficits, and people with logopenic variant PPA have impaired object naming due to anomia or phonological paraphasias. The BNT is therefore a common outcome measure in both treatment studies of PPA and observational studies of PPA. All patients were asked to respond verbally, or if unable to speak at all, were permitted to respond in writing or using a keyboard. We accepted responses that were recognizable as the correct word, even if there were articulatory distortions. The demographic and BNT scores are summarized in Table 1, as well as additional language tests, performed at the first visit.
Table 1.
Demographics and behavioral scores at baseline.
| Case | PPA Variant | Age at initial evaluation | Gender | Years of education | Symptoms duration (years) | Baseline BNT | BNT 9 months later | BNT 21 months later | FBI/63 1 | MoCA/302 |
| 1 | LV | 51 | F | 18 | 0.4 | 50 | 48 | 47 | 3 | 19 |
| 2 | LV | 66 | F | 18 | 5.5 | 46 | 35 | 10 | 0 | 20 |
| 3 | LV | 68 | M | 18 | 2.5 | 34 | 29 | 24 | 3 | 18 |
| 4 | LV | 67 | F | 14 | 7 | 13 | 9 | 2 | 8 | 12 |
| 5 | LV | 69 | F | 18 | 3.5 | 15 | 9 | 3 | 11 | 12 |
| 6 | LV | 73 | F | 18 | 4 | 31 | 29 | 4 | 11 | 21 |
| 7 | NFV | 48 | M | 12 | 7 | 24 | 13 | 0 | 17 | 17 |
| 8 | NFV | 76 | F | 16 | 1.2 | 44 | 27 | 0 | 4 | 19 |
| 9 | NFV | 55 | M | 16 | 2 | 46 | 41 | 35 | 20 | 24 |
| 10 | NFV | 75 | M | 18 | 2 | 51 | 50 | 49 | 11 | 19 |
| 11 | NFV | 68 | M | 15 | 2.75 | 57 | 55 | 49 | 28 | 27 |
| 12 | NFV | 74 | M | 18 | 1 | 44 | 43 | 39 | 16 | 26 |
| 13 | SV | 68 | F | 16 | 13 | 10 | 6 | 0 | 18 | 1 |
| 14 | SV | 69 | M | 16 | 1.8 | 5 | 4 | 4 | 2 | 20 |
| 15 | SV | 61 | M | 16 | 3.3 | 11 | 9 | 6 | 8 | 19 |
| mean ± stdev | 65.8 ± 8.5 | 8 M, 7F | 16.5 ± 1.8 | 3.8 ± 3.4 | 32.1 ± 17.6 | 27.1 ± 17.8 | 18.1 ± 19.9 | |||
| published norms | > 309 | > 2610 | ||||||||
| Case | P&PT, 3 Pictures/52 3 | Word–picture match/48 4 | NAT/10 5 | BDAE articulatory agility/7 6 | BDAE phrase length/7 6 | BDAE embedded sentences/10 6 | BDAE sentence repetition/10 6 | Pseudoword repetition/10 7 | Reading (irregular minus regular) 8 | Spelling (irregular minus regular) 8 |
| 1 | 47 | 48 | 3 | 7 | 7 | 5 | 8 | 7 | 0 | 0 |
| 2 | 51 | 48 | 9 | 7 | 7 | 10 | 7 | 9 | n/a | n/a |
| 3 | 51 | 48 | 5 | 7 | 7 | 6 | 7 | 0 | −1 | 2 |
| 4 | 48 | 47 | 7 | 6 | 7 | 3 | 2 | 0 | −4 | n/a |
| 5 | 49 | 44 | 6 | 7 | 7 | 4 | 3 | 7 | 0 | −7 |
| 6 | 50 | 48 | 7 | 7 | 7 | 10 | 6 | n/a | −2 | 0 |
| 7 | 48 | 48 | 3 | 2 | 2 | 9 | 1 | 0 | 2 | 2 |
| 8 | 51 | 45 | 5 | 6 | 4 | 7 | 6 | 4 | 1 | 0 |
| 9 | 48 | 48 | 5 | 4 | 7 | 10 | 8 | 7 | 1 | 1 |
| 10 | 51 | 47 | 0 | 6 | 7 | 7 | 8 | 6 | 0 | n/a |
| 11 | 50 | 48 | 8 | 6 | 7 | 10 | 9 | 8 | 0 | 0 |
| 12 | 49 | 48 | 9 | 7 | 6 | 10 | 9 | 10 | −1 | −1 |
| 13 | 22 | 39 | 0 | 7 | 6 | 1 | 3 | 4 | −2 | n/a |
| 14 | 41 | 33 | 9 | 7 | 7 | 8 | 9 | 10 | −7 | −6 |
| 15 | 45 | 43 | 9 | 7 | 7 | 10 | 10 | 10 | −4 | −7 |
| mean ± stdev | ||||||||||
| published norms | > 4711 | n/a | 9.4–9.712 | 713 | 713 | 1013 | 1013 | n/a | n/a | n/a |
/maximum score
n/a: not available
Acronyms and references for the tests:
FBI = modified Frontal Behavioral Inventory (FBI) (Kertesz, A., Davidson, W., & Fox, H. (1997). Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Canadian Journal of Neurological Sciences/Journal Canadien des Sciences Neurologiques, 24, 29–36.), three questions omitted: Concreteness, Verbal Apraxia, and Alien Hand.
MOCA = Montreal Cognitive Assessment (MoCA) (Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., … Chertkow, H. (2005). The Montreal cognitive assessment, MoCA: A brief screening tool For mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699.)
P&PT = Three-Picture version of the Pyramids and Palm Trees test (Howard, D., & Patterson, K. (1992). The pyramids and palm trees test: A test of semantic access from words and pictures. Bury St. Edmunds, UK: Thames Valley Test Company.)
Word–picture matching (Rogers, S. L., & Friedman, R. B. (2008). The underlying mechanisms of semantic memory loss in Alzheimer’s disease and semantic dementia. Neuropsychologia, 46, 12–21)
NAT = subject and object Wh-questions from the Northwestern Anagram Test (NAT) (Weintraub, S., Mesulam, M. M., Wieneke, C., Rademaker, A., Rogalski, E. J., & Thompson, C. K. (2009). The northwestern anagram test: Measuring sentence production in primary progressive aphasia. American Journal of Alzheimer’s Disease & Other Dementias, 24, 408–416)
BDAE = Boston Diagnostic Aphasia Examination (Goodglass, H., Kaplan, E., & Barresi, B. (2001). Boston diagnostic aphasia examination (3rd ed.). Austin: Pro-Ed)
Repetition of five-syllable pseudowords (Meyer, A. M., Snider, S. F., Campbell, R. E., & Friedman, R. B. (2015). Phonological short-term memory in logopenic variant primary progressive aphasia and mild Alzheimer’s disease. Cortex, 71, 183–189.)
Reading and spelling of irregular and regular words (developed at the Center for Aphasia Research and Rehabilitation at Georgetown University Medical Center).
Kertesz, A., Davidson, W., & Fox, H. (1997). Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Canadian Journal of Neurological Sciences, 24, 29–36.
Z. Nasreddine, wwwmocatest.org 2004
Callahan, B.L. et al., (2010). Normative Data for the Pyramids and Palm Trees Test in the Quebec-French Population. Archives of Clinical Neuropsychology, 25, 212–217.
Weintraub S., Mesulam N-M., Wieneke C., Rademaker, A., Rogalski, E.J., & Thompson, C.K. (2009). The Northwestern Anagram Test: Measuring Sentence Production in Primary Progressive Aphasia. American Journal of Alzheimer’s Disease & Other Dementias, 24, 408–416
Borod et al, Normative data on the Boston Diagnostic Aphasia Examination, Parietal Lobe Battery, and the Boston Naming Test in the Journal of Clinical Neuropsychology (1980); the journal is not available online
2.2. Imaging acquisition and processing
At the same time points, the participants had brain MRI in a 3T scanner, including T1 high-resolution-weighted images (T1-WI), diffusion tensor images (DTI), and resting state functional MRI (rs-fMRI). The image parameters were: (1) T1-WI: sagittal orientation, original matrix 170 × 170, 256 slices, voxel size 1 × 1 × 1.2 mm, TR/TE 6700/3.1 ms; (2) DTI: axial orientation, original matrix 128 × 128, 70 slices, voxel size 0.83 × 0.83 × 2.2 mm, TR/TE 8500/61 ms, 32 gradients, b factor 1000 s/mm2; (3) rs-fMRI: axial orientation, original matrix 80 × 80, 36 slices, voxel size 3×3×4 mm, TR/TE 2000/ 30 ms, 210 dynamics. Associated focal lesions and visually detectable artifacts were excluded by visual inspection. Sub-voxel artefacts (e.g., DTI and motion related) were corrected by regular post-processing steps, as described below. White matter “hyperintensities” (related to vascular disease) were minimal, considering the age range. In addition, the MRI modalities studied (T1-WI, FA/MD, rsfMRI) are either not or mildly affected by such lesions.
The images were automatically segmented and post-processed in a public web-based service for multi-contrast imaging segmentation and quantification, the MRICloud (http://www.MRICloud.org) (Mori et al., 2016). Briefly, in MRICloud, the process for segmenting the T1-WI, used for volumetric analysis, involves orientation and homogeneity correction; two-level brain segmentation (skull-stripping, then whole brain); image mapping based on a sequence of linear, non-linear algorithms, and Large Deformation Diffeomorphic Mapping (LDDMM); and a final step of multi-atlas labeling fusion (MALF), adjusted by PICSL (Tang et al., 2013). For the DTI, the tensor reconstruction and quality control followed the algorithm used by DtiStudio (http://www.MRIStudio.org). The automated DTI segmentation was similar to that used for T1-WIs, except for the use of complementary contrasts (mean diffusivity [MD], fractional anisotropy [FA], and eigenvector [fiber orientation]) and a diffeomorphic likelihood fusion algorithm (Tang et al., 2014) for multi-atlas mapping. For the rsfMRI post-processing (Faria et al., 2012), the T1-WI and the respective segmentations obtained as described above are co-registered to the motion and slice timing-corrected, resting-state dynamics. Time courses are extracted from all the cortical and subcortical gray matter regions defined in the atlases and regressed for physiological nuisance; intensity and motion “outliers” are extracted with ART (SPM toolbox). Seed-by-seed correlation matrices are obtained from the “nuisance-corrected” time courses, and z-transformed by the Fisher’s method. After the multi-modal brain segmentation and quantification, each individual is represented by a vector of image features: 56 structural volumes, 168 FA and 168 MD measures, and 3003 pairwise z-correlations (between 78 cortical and subcortical gray matter areas).
2.3. Statistical analyses
In order to select image features, at the first visit, that potentially correlated with later performance on BNT we used the Least absolute shrinkage and selection operator (Lasso). Lasso is a regression method that performs both variable selection and regularization to enhance the prediction accuracy and interpretability of the model it produces (Santosa & Symes, 1986; Tibshirani, 1996). Similarly to the ridge regression, it forces the sum of the absolute value of the regression coefficients to be less than a fixed value. In the case of Lasso, it forces certain coefficients to be set to zero, effectively choosing a simplest model that does not include those coefficients. Lasso is well suited for scenarios where the number of predictors is large compared to the sample size, and traditional variable selection methodologies may overfit random error or noise.
Lasso was performed with the “cv.glmnet” (R package “glmnet”), which does automatic cross-validation (5-fold, in the present study) on a grid of X values used for 11-penalized regression problems. The X was chosen as “lambda.1se”, for which the performance in terms of estimated expected generalization error is within one standard error of the minimum. Independent Lasso regressions were performed for each modality (volumes, FA, MD, rsfMRI) in order to minimize the dominance of modalities with large number of features (in this case, rsfMRI).
Linear correlation was employed in order to better evaluate the direct relationship between each selected image feature, with later BNT scores. Age, gender, education, and symptom duration were covariates in the model. We report the correlation coefficient, p-value, and standard error of the linear correlations between each selected image feature and the later BNT, as well as the p-values corrected by 1000-fold permutation and multiple comparison correction using False Discovery Rate (FDR).
The code for the statistical analysis (written in R), for the figure (created with BrainNet Viewer (Xia, Wang, & He, 2013)), and the data used in this study are archived in a publicly accessible repository (uploaded in the Mendeley Data for this journal).
3. Results
Studies of neurodegenerative disease often indicate that outcome is best predicted by severity of baseline performance (O’Connor et al., 2016; Woolf et al., 2016). Consistent with this observation, the initial BNT score explained 96% of the variance in the nine-month BNT score. However, the initial BNT explained only 77% of the variance of the 21- month BNT score; some patients with initial high scores showed substantial decline by 21 months. Demographic covariates (age, gender, education, symptom duration) had no significant association with BNT scores.
The baseline imaging features selected by Lasso as strongly correlated to the 21-months BNT (Fig. 1) were all pairwise rsfMRI correlations; no volumetric or DTI feature was automatically selected by the algorithm. The direct correlation between each of these features and the 21-monhts BNT is reported in Table 2. Most of the selected areas are part of what is often defined as the “language network”, such as inferior frontal, middle and inferior temporal, temporal pole, superior parietal, and fusiform gyrus; and “default network”, such as medial frontal and cingulate (table 2). As proof of concept, a multivariable model created with these features explained 97% of the 21-month BNT variability, 80% after correction for “optimism” (Effron, 2004). The addition of the initial BNT did not improve the model.
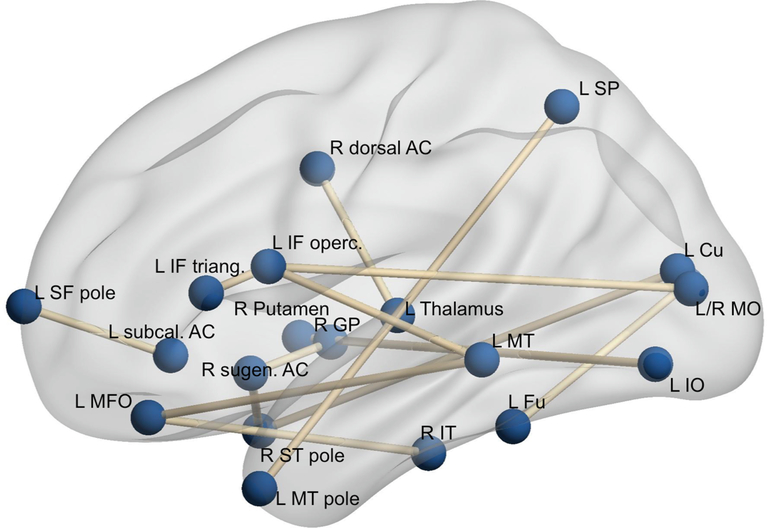
Fig. 1.
Graphic representation of the resting state fMRI correlations, at baseline, that correlated with Boston Naming Test 21 months later. Fu: fusiform; Cu: Cuneus; SP: superior parietal; GP: globus pallidus; ACC: anterior cingulate; IT, MT, ST: superior, middle, and inferior temporal; SF, IF: superior and inferior frontal; MFO: middle fronto-orbital gyrus; IO, MO: inferior and middle orbital. The brain networks were visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv/) (Xia et al., 2013).
Table 2.
Pairwise resting state fMRI correlations at first visit that correlated with BNT at 21 weeks.
| Estimate (SE) | R2 adjusted | p-value | p-permutation | p-FDR | |
|---|---|---|---|---|---|
| L superior frontal vs. L subcallosal anterior cingulate | 69.023 | 0.345 | 0.013 | 0.013 | 0.013 |
| L inferior frontal pars opercularis vs. pars triangularis | 51.605 | 0.501 | 0.002 | 0.005 | 0.008 |
| L inferior frontal pars opercularis vs. L middle temporal | 63.016 | 0.485 | 0.002 | 0.002 | 0.008 |
| L inferior frontal pars opercularis vs. L middle occipital | 59.199 | 0.399 | 0.007 | 0.009 | 0.009 |
| L medium fronto-orbital vs. L middle temporal | 73.666 | 0.448 | 0.004 | 0.005 | 0.009 |
| L medium fronto-orbital vs. L inferior temporal | 82.821 | 0.519 | 0.001 | 0.002 | 0.008 |
| L superior parietal vs. L middle temporal pole | 63.798 | 0.442 | 0.004 | 0.004 | 0.009 |
| R superior temporal pole vs. L cuneus | −49.652 | 0.386 | 0.008 | 0.006 | 0.009 |
| R superior temporal pole vs. R subgenual anterior cingulate | −89.569 | 0.593 | 0.000 | 0.001 | 0.006 |
| L fusiform vs. R middle occipital | 68.480 | 0.392 | 0.007 | 0.012 | 0.009 |
| L inferior orbital vs. R putamen | −78.285 | 0.399 | 0.007 | 0.016 | 0.009 |
| L subgenual anterior cingulate vs. R globus pallidus | 65.374 | 0.339 | 0.013 | 0.012 | 0.013 |
| R dorsal anterior cingulate vs L thalamus | −72.540 | 0.392 | 0.007 | 0.006 | 0.009 |
R2 adjusted is the correlation coefficient and Estimate (SE) is the standard error of the linear correlations between each selected image feature and the later BNT; p-permutation is the p-value after 1000-folds permutation; p-FDR is the p-value corrected by False Discovery Rate.
4. Discussion
A wealth of rsfMRI studies have shown that one or more networks become progressively impaired in different neurodegenerative diseases, such as Alzheimer’s disease and frontotemporal lobar degeneration, the most common underlying diseases in PPA. Studies have demonstrated that connectivity measured by rsfMRI at a given period of time correlates with function in multiple sclerosis, dementia, depression, traumatic brain injury (Palacios et al., 2013) and other neurological diseases. Furthermore, changes in connectivity correlate with changes in function in both healthy controls (Salami, Wahlin, Kaboodvand, Lundquist, & Nyberg, 2016) and neurologically impaired individuals (Goveas et al., 2011), indicating that rsfMRI may provide a good biomarker for recovery. For example, changes in the default mode network correlated with cognitive recovery at three months after stroke (Dacosta-Aguayo et al., 2015). Likewise, changes in connectivity in the default mode network correlated with improvement in cognitive scores and depression in patients with multiple sclerosis who received cognitive rehabilitation, while changes in executive networks correlated with improvement in quality of life in the same patients (Parisi et al., 2014).
In this study, we found that the strength of rsfMRI correlations among frontal (inferior frontal, in particular), temporal cortex, fusiform, superior parietal (part of the “language network”), cuneus, inferior and middle occipital, subcortical gray matter, and cingulate (part of default network) relates to later naming impairment in PPA patients. These findings are compatible with the previous knowledge about the atrophy pattern in these patients. However, it is interesting that the rsfMRI variables correlated with naming outcome more strongly and more reliably than the volumes of the same areas.
The “holy grail” of neuroimaging is to identify imaging markers at baseline that can predict later outcomes. Clinically, prognosis at baseline is the most salient goal to patients and their families. From all the tested image modalities (volumes and diverse DTI indices), rsfMRI was the one that provided the features that most strongly correlated with later naming performance. A handful of studies have shown that rsfMRI can identify patients most likely to respond to treatment, in a variety of disease states, including depression (Andreescu et al., 2013) and epilepsy (Negishi, Martuzzi, Novotny, Spencer, & Constable, 2011). Similarly, baseline amplitude of low frequency fluctuations in right middle temporal gyrus correlated with greater response to a phonological treatment for naming impairment in post-stroke aphasia (van Hees et al., 2014). Likewise, density of grey matter (Cotelli et al., 2016) predicted improvements in response to transcranial direct current stimulation plus language therapy in PPA. Here we have shown that connectivity between specific gray matter regions at baseline solidly correlates with naming performance 21 months later. A model created with the initial rsfMRI features accounted for 80% of variance of later naming performance in this sample, more accurately than baseline naming scores alone (which explained 77%). Note, however, that any model created with such limited sample has to be confirmed in an independent sample. The present study is essentially an exploratory correlational analysis of factors that may be relevant for creating further predictive models.
We recognize that the main limitation of this study is the small sample size, mainly due to the longitudinal design and the low prevalence of PPA. Although we cross-validated the feature selection, the modest sample size did not allow us to train and test predictive models in independent samples, neither allowed us to determine if results vary by PPA variant. Although the rate of decline varies across variants, with most rapid decline in naming in svPPA, there are slow and rapid de- cliners within each variant. Furthermore, it is often difficult to identify the variant early on, especially if impaired naming (and perhaps spelling) are the only symptoms. Likewise, it is difficult to distinguish among variants late in the course, when all language domains are often impaired (Rogalski et al., 2011). Despite these limitations, it is reasonable to infer that initial imaging features (specifically, rsfMRI correlations) carry information about later performance in naming, and are potentially useful for refining prognosis.
Acknowledgements
We are grateful to the individuals who participated in this research. The research reported in this paper was supported by the National Institutes of Health (National Institute of Deafness and Communication Disorders) through awards R01 DC011317, R01 DC05375 and P50 DC014664. The content is solely the responsibility of the authors and does not necessarily represent the views the National Institutes of Health.
References
- Andreescu C, Tudorascu DL, Butters MA, Tamburo E, Patel M, Price J, et al. (2013). Resting state functional connectivity and treatment response in late-life depression. Psychiatry Research, 214(3), 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotelli M, Manenti R, Paternico D, Cosseddu M, Brambilla M, Petesi M, et al. (2016). Grey matter density predicts the improvement of naming abilities after tDCS intervention in agrammatic variant of primary progressive aphasia. Brain Topography, 29(5), 738–751. [DOI] [PubMed] [Google Scholar]
- Dacosta-Aguayo R, Grana M, Iturria-Medina Y, Fernandez-Andujar M, Lopez-Cancio E, Caceres C, et al. (2015). Impairment of functional integration of the default mode network correlates with cognitive outcome at three months after stroke. Hum Brain Mapping, 36(2), 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effron B (2004). The estimation of prediction error: Covariance penalties and cross-validation. Journal of the American Statistical Association, 99(467), 14. [Google Scholar]
- Faria AV, Joel SE, Zhang YJ, Oishi K, van Zjil PCM, Miller MI, et al. (2012). Atlas-based analysis of resting-state functional connectivity: Evaluation for reproducibility and multi-modal anatomy-function correlation studies. Neuroimage, 61(3), 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola KA, Thorne A, Veldhuisen LD, Felix CM, Hankinson S, Pham J, et al. (2015) Neural substrates of spontaneous narrative production in focal neurodegenerative disease. Neuropsychologia, 79(Pt A), 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveas JS, Xie C, Ward BD, Wu Z, Li W, Franczak M, et al. (2011). Recovery of hippocampal network connectivity correlates with cognitive improvement in mild Alzheimer’s disease patients treated with donepezil assessed by resting-state fMRI. J Magn Reson Imaging, 34(4), 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wu D, Ceritoglu C, Li Y, Kolasny A, Valliant MA, et al. (2016). MRICloud: Delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Computing in Science & Engineering 18(21), 15. [Google Scholar]
- Negishi M, Martuzzi R, Novotny EJ, Spencer DD, & Constable RT (2011). Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia, 52(9), 1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor CM, Clemson L, Flanagan E, Kaizik C, Brodaty H, Hodges JR, et al. (2016). The relationship between behavioural changes, cognitive symptoms, and functional disability in primary progressive aphasia: A longitudinal study. Dement Geriatr Cogn Disord, 42(3–4), 215–226. [DOI] [PubMed] [Google Scholar]
- Palacios EM, Sala-Llonch R, Junque C, Roig T, Tormos JM, Bargallo N, et al. 2013et al. Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMANeurol, 70(7), 845–851. [DOI] [PubMed] [Google Scholar]
- Parisi L, Rocca MA, Valsasina P, Panicari L, Mattioli F, & Filippi M (2014). Cognitive rehabilitation correlates with the functional connectivity of the anterior cingulate cortex in patients with multiple sclerosis. Brain Imaging and Behaviour, 8(3), 387–393. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, & Mesulam MM (2011). Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology, 76(21), 1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, & Rosen HJ (2013). Neuroimaging in frontotemporal dementia. International Review of Psychiatry, 25(2), 221–229. [DOI] [PubMed] [Google Scholar]
- Salami A, Wahlin A, Kaboodvand N, Lundquist A, & Nyberg L (2016). Longitudinal evidence for dissociation of anterior and posterior MTL resting-state connectivity in aging: Links to perfusion and memory. Cerebral Cortex, 26(10), 3953–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosa F, & Symes WW (1986). Linear inversion of band-limited reflection seismograms. SIAM Journal on Scientific and Statistical Computing 7(4), 1307–1330. [Google Scholar]
- Sebastian R, Thompson CB, Wang NY, Wright A, Meyer A, Friedman RB, et al. (2018). Patterns of decline in naming and semantic knowledge in primary progressive aphasia. Aphasiology, 32(9), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Weintraub S, Johnson NA, Parrish TB,& Gitelman DR (2007). Altered effective connectivity within the language network in primary progressive aphasia. Journal of Neurosciences, 27(6), 1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XY, Oishi K, Faria AV, Hillis AE, Albert MS, Mori S, et al. (2013). Bayesian parameter estimation and segmentation in the multi-atlas random orbit model. PLoS ONE, 8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XY, Yoshida S, Hsu J, Huisman T, Faria AV, Oishi K, et al. (2014). Multicontrast multi-atlas parcellation of diffusion tensor imaging of the human brain. PLoS ONE, 9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R (1996). Regression shrinkage and selection via the Lasso. Journal of the Royal Statistical Society. Series B (Methodological), 58(1), 267–288 [Google Scholar]
- van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, & Copland DA (2014). Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabiliation and Neural Repair, 28(4), 325–334. [DOI] [PubMed] [Google Scholar]
- Woolf C, Slavin MJ, Draper B, Thomassen F, Kochan NA, Reppermund S, et al. (2016). Can the clinical dementia rating scale identify mild cognitive impairment and predict cognitive and functional decline? Dementia and Geriatric Cognitive Disorders, 41(5–6), 292–302. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, & He Y (2013). BrainNet viewer: A network visualization tool for human brain connectomics. PLoS One, 8(7), e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]