Abstract
Background
RTS,S, the most advanced vaccine against malaria, is now undergoing pilot implementation in Malawi, Ghana, and Kenya where an estimated 360,000 children will be vaccinated each year. In this study we evaluate RTS,S alongside bed net use and estimate cost-effectiveness.
Methods
RTS,S phase III trial and bed net prevalence data were used to determine the effect of vaccination in the urban/periurban and rural areas of Lilongwe, Malawi. Cost data were used to calculate the cost-effectiveness of various interventions over three years.
Findings
Since bed nets reduce malaria incidence and homogeneous vaccine efficacy was assumed, participants without bed nets received greater relative benefit from vaccination with RTS,S/AS01 than participants with bed nets. Similarly, since malaria incidence in rural Lilongwe is higher than in urban Lilongwe, the impact and cost-effectiveness of vaccine interventions is increased in rural areas. In rural Lilongwe, we estimated that vaccinating one child without a bed net would prevent 2·59 (1·62 to 3·38) cases of malaria over three years, corresponding to a cost of $10·08 (7·71 to 16·13) per case averted. Alternatively, vaccinating one child with a bed net would prevent 1·59 (0·87 to 2·57) cases, corresponding to $16·43 (10·16 to 30·06) per case averted. Providing RTS,S/AS01 to 30,000 children in rural Lilongwe was estimated to cost $782,400 and to prevent 58,611 (35,778 to 82,932) cases of malaria over a three-year period. Joint interventions providing both vaccination and bed nets (to those without them) were estimated to prevent additional cases of malaria and to be similarly cost-effective, compared to vaccine-only interventions.
Interpretation
To maximize malaria prevention, vaccination and bed net distribution programs could be integrated.
Keywords: Malaria, Malawi, Africa, Vaccine, Bed nets, Cost-effectiveness
BACKGROUND
In 2017, there were an estimated 219 (95% CI 203 to 262) million cases of malaria, most of which were in Africa, and an estimated 435 (95% CI 401 to 470) thousand malaria related deaths, disproportionately in African children under five years of age.1 This is an improvement over 2010 estimates, but progress has stalled since 2015, particularly in high burden countries.1
The RTS,S malaria vaccine underwent a phase III clinical trial, spanning 11 study sites across seven countries, from 2009–2014.2 The vaccine consists of three baseline doses, with a fourth dose given 18 months after the third dose.2 Protective efficacy, as calculated by one minus the incidence rate ratio (1-IRR), of the vaccine among children 5–17 months old averaged 36·3% across sites and ranged from 22·0% to 74·6% depending on geographic area.3 The World Health Organization is selectively implementing the vaccine at sites in Malawi, Ghana, and Kenya where 360,000 children will be vaccinated per year, in total.2
To evaluate whether large-scale implementation of RTS,S in sub-Saharan Africa would be an effective use of resources, eight critical questions were laid out, in an effort to guide future research.4 This analysis focuses on one of these critical questions: whether RTS,S/AS01 is a cost-effective preventative measure in conjunction with bed nets.
Bed nets, often treated with insecticides, are one of the key interventions used to prevent malaria and represent the principal vector control strategy in Malawi.5,6 Bed net use was “optimized” in the phase III trial, meaning each participant had access to a bed net as part of the study design.3
We used Phase III data to estimate the expected three-year absolute reduction in malaria cases due to the four-dose RTS,S vaccine, for a child living in Lilongwe, Malawi. We also compared the cost-effectiveness of various bed net/vaccine intervention combinations. Though the phase III trial occurred primarily in Lilongwe urban and periurban areas, we calculated the effect of interventions in both urban and rural settings, as current pilot implementation efforts are occurring in the rural areas of Lilongwe district. These estimates complement existing literature on RTS,S vaccine efficacy, with estimates of the potential impact and cost-effectiveness of vaccine interventions against malaria in implementation settings.
METHODS
Data
The phase III RTS,S trial in Lilongwe enrolled children aged 5–17 months (n=783) and passively surveilled them for malaria for 18 months, beginning after the administration of the three baseline doses of RTS,S or a control vaccine.3 On the 18th month of follow-up, a fourth dose was given to half of those who received the three-dose baseline RTS,S vaccine and a control vaccine was given to the rest of the participants.3 We considered follow-up time for up to an additional 18 months (up to three years total) post-fourth-dose. All participants were tested for malaria in months 18 and 30 of follow-up but were otherwise passively surveilled.3 An episode of clinical malaria was defined as illness accompanied by an axillary temperature of at least 37.5°C and Plasmodium falciparum asexual parasitemia >5000 parasites per mm³ measured by microscopy.3 Insecticide-treated bed nets were distributed by study staff to children at the time of screening.3 Bed net use data were collected using a concurrent household ecological survey, unique to the Malawi trial site, where a field worker observed whether a net was on a child’s bed up to four times during the follow-up period. These visits were scheduled for every six months of follow-up during the first two years but had great variability in terms of when they were conducted.
The outcome of interest was the count of malaria cases post bed net measurement and before the subsequent measurement. We only counted cases after bed net use was determined through the ecological survey to mitigate the potential for reverse causation.7 An exception was made for the time between baseline and the first bed net measurement, where malaria cases were paired with the first bed net measurement. This was done to avoid excluding the beginning of follow-up.
Because up to four bed net measurements were taken, one can think of each participant having up to four periods of follow-up, corresponding to each bed net measurement. Additionally, participants assigned to the four-dose vaccine group had the same treatment as the three-dose vaccine group in the first 18 months, but a different treatment in the second 18 months, as the fourth dose was not given until the 18-month mark. The unit of analysis in this study is each time interval corresponding to the same participant, most recent ecological survey, and 18-month period (first vs. second). Each participant can experience one of ten possible combinations of exposures (first/second 18-month period AND control/three-dose/four-dose vaccine AND yes/no bed net use) in each time interval (Figure 1). For example, one potential exposure is: no bed net and the three-dose vaccine in the first 18 months. Note that participants could not be exposed to the fourth dose of the vaccine if the time interval occurred in the first 18 months.
Figure 1:
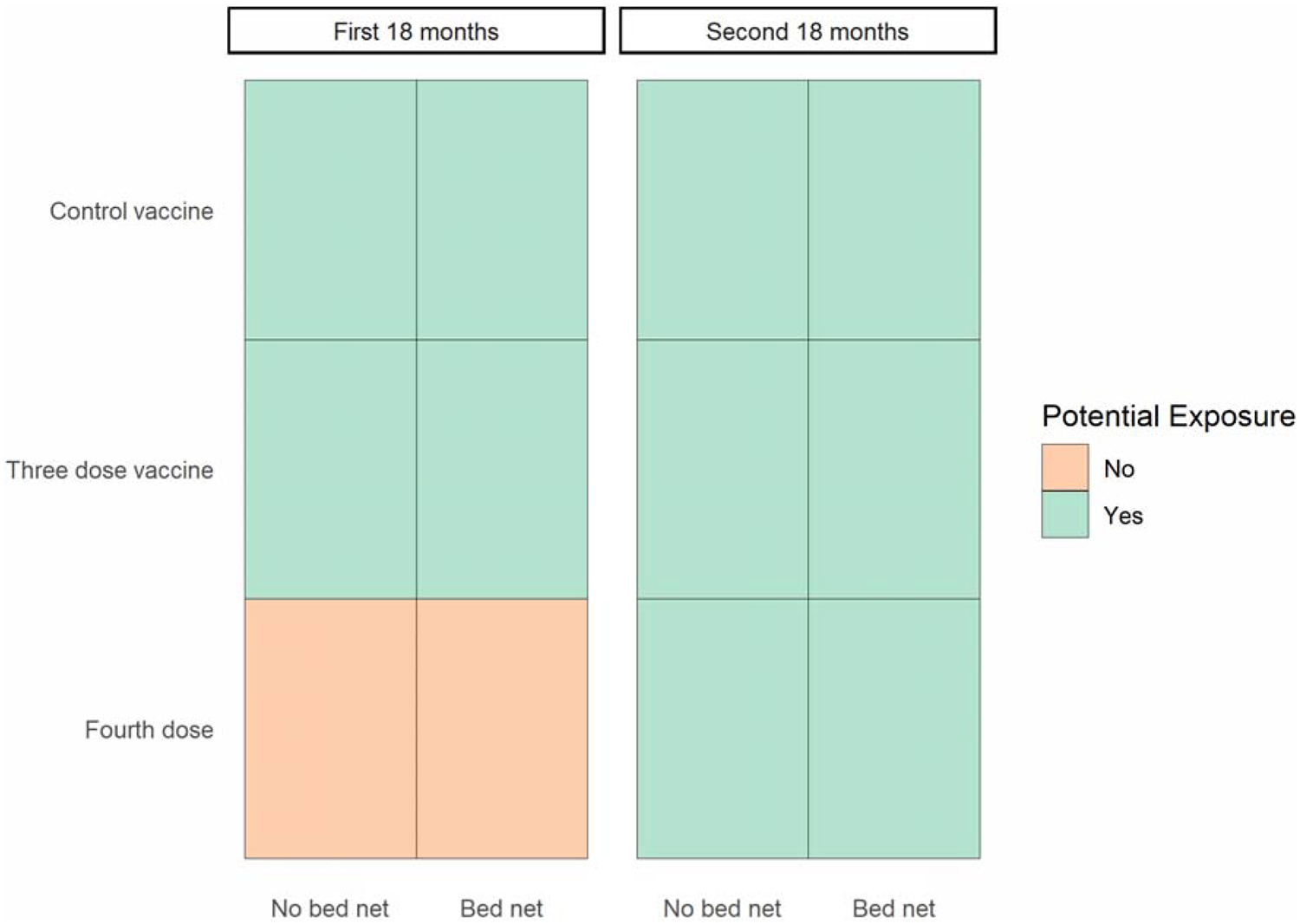
Potential Exposure Combinations in Each Time Interval of Follow-up During the First and Second 18 Months of the Phase III Trial of RTS,S
An estimate of bed net use prevalence, from outside this study, is necessary to calculate the vaccine average effect in the general population. The 2011–2013 malaria transmission intensity (MTI) study was an annual cross-sectional survey of P. falciparum prevalence administered concurrently with the phase III RTS,S trial in each study site.8 The survey was implemented during the peak malaria season and enrolled 400 under-five-year-olds annually, from the same catchment area of the phase III trial.8 Subjects in the RTS,S vaccine trial were excluded from participation.8 The survey included individual-level bed net use for the previous night. The most recent estimate of bed net use prevalence (82·5%) from 2013 was used in this analysis to generalize to modern interventions in Lilongwe city.8 The 2017 Malaria Indicator Survey (MIS) was used to obtain an estimate of bed net use prevalence in under-fives in rural Lilongwe (63·4%) in order to generalize interventions to rural areas.4
An analysis of data from the phase III trial, using a 2-dimensional spatial spline, showed transmission intensities up to 1·52 cases per year in a population with no vaccination and a 95% bed net coverage in urban Lilongwe, with estimates tending to be higher out the outskirts of the study area (Supplemental Figure 1).9 In our calculations, we used a transmission estimate of 2·0 cases per year in rural Lilongwe for a population without vaccination and bed net coverage. We expect this estimate to be conservative, given the protective nature of bed nets and increased rurality, but we also included graphs allowing transmission intensity to vary between 1·0 to 3·0 cases per year in the no bed net, no vaccine (NBNV) group.
The RTS,S vaccine was assumed to cost US$26.08 for the four-dose regimen and bed nets were assumed to cost $2·20 per net per year, based on estimates used for prior cost-effectiveness analyses.10,11 The cost estimate for the four-dose vaccine includes the direct cost of the vaccine doses ($20) and indirect costs such as wastage, freight, and insurance.10
Bednets and Generalizability of the Vaccine Effect
Whether or not an individual used an owned bed net can be said to be determined by a set of unknown variables, U. Since children vaccinated with RTS,S represent a random sample of children usually attending Expanded Programme on Immunization (EPI) visits, the distribution of U should be the same in the study and target populations.12 By conditioning on bed net use in the trial, we assumed that we accounted for the increased bed net access and thus for being enrolled in the trial.13
Model
We required a model which incorporated all ten of the possible vaccine and bed net usage combinations (Figure 1) while excluding the two which could not have occurred. Furthermore, the three-doseand four-dose vaccine groups must have the same treatment effect in the first 18 months and different effects in the second 18 months. The desired model is specified below:
We fit a generalized mixed effects Poisson model in R version 3.5.1 using the “lme4” package.14,15 We used a log-link, a random effect for each participant (bi) to account for within-subject correlation due to repeated measures, and a time (days) offset, Tij. Yij is the number of malaria cases experienced by participant i, during period j. Wij is one if participant i used a bed net during period j and zero otherwise. Ai is one if participant i received the three-dose RTS,S vaccine at baseline and a control vaccine at 18 months and zero otherwise. Bi is one if participant i received the three-dose RTS,S vaccine at baseline and the fourth dose at 18 months and zero otherwise. Pij is zero if period j occurred during the first 18 months of follow-up and one if period j occurred during the second 18 months.
Calculations
In calculations, the intercept taken from the model odel was adjusted by , where σ2 is the variance of the random intercept.16 Given that the model produces expected malaria counts per day, 18-month counts for each bed net and treatment group were calculated by multiplying the per-day estimate by 365·25*1·5. Estimates were obtained for the first and second 18 months, which were combined to estimate malaria counts over three years in each bed net and vaccine group.
From these group means, we calculated the reduction in malaria cases per person due to vaccination, conditional upon whether the subject possessed a bed net or not. Using a cost estimate, we calculated a cost per case averted by the vaccine for each bed net group in urban Lilongwe. By altering the baseline transmission intensity in the NBNV group, we estimated the expected cases averted and the cost per case averted by the vaccine for each bed net group in rural Lilongwe. Using bed net prevalence estimates, we then determined the number of malaria cases averted in the first three years under a variety of interventions. Finally, we calculated the cost-effectiveness of these interventions.
We calculated confidence intervals with simulation-based inference using the “arm” package by sampling 10,000 replicates from the joint distribution of the parameter estimates (multivariate normal) and selecting the 2·5th and 97·5th percentiles of sampled estimates.17
Sensitivity Analysis
Malaria incidence at baseline may influence both bed net use and malaria incidence over follow-up, introducing confounding bias into the estimated effect of bed net use on malaria incidence. Because some calculations use the estimated effect of bed net use to determine the efficacy of bed nets, we evaluated background malaria incidence as a confounder on the relationship between bed net use and malaria. We applied the “mgcv” package, fitting a generalized additive model with a two-dimensional spatial spline of malaria incidence as a random intercept.9
FINDINGS
Data Characteristics and Model Results
Of the enrolled 783 children, 693 (89%) had bed net data from at least one ecological survey and were included in this analysis. There were 2,128 bed net measurements in total over the four periods of follow-up corresponding to the four ecological surveys (Table 1). A bed net was not observed in 97 instances, or 4·56% of observations. Bed net use remained consistent in periods 1 to 3, but in period 4 every single participant was observed with a bed net. Participants had data from an average of 3·07 of the four ecological surveys and 39·54% had data from all four, with similar distributions in each treatment group. A large proportion of each treatment group was retained for the entire three-year period and there was less loss to follow-up before the 18-month mark in the four-dose vaccine group compared to the other two groups (Figure 2).
Table 1:
Frequency of Outcomes and Exposures over the Specified Periods
| Period 1 (N=693) | Period 2 (N=639) | Period 3 (N=522) | Period 4 (N=274) | Overall (N=2128) | |
|---|---|---|---|---|---|
| Vaccine Group, N (proportion) | |||||
| Control | 227 (0·33) | 210 (0·33) | 180 (0·34) | 91 (0·34) | 708 (0·33) |
| Three-dose vaccine | 241 (0·35) | 215 (0·34) | 164 (0·31) | 91 (0·33) | 711 (0·33) |
| Four-dose vaccine | 225 (0·32) | 214 (0·33) | 178 (0·34) | 92 (0·33) | 709 (0·33) |
| Bed net use, N (proportion) | |||||
| Yes | 664 (0·96) | 596 (0·93) | 497 (0·95) | 274 (1·00) | 2031 (0·95) |
| No | 29 (0·04) | 43 (0 ·07) | 25 (0·05) | 0 (0·00) | 97 (0·05) |
| Cases / time (days) | 208 / 238,512 | 147 / 166,938 | 192 / 189,396 | 97 / 122,391 | 644 / 717,236 |
| Cases per 18 months | 0·48 | 0·48 | 0·56 | 0·43 | 0·49 |
Figure 2:
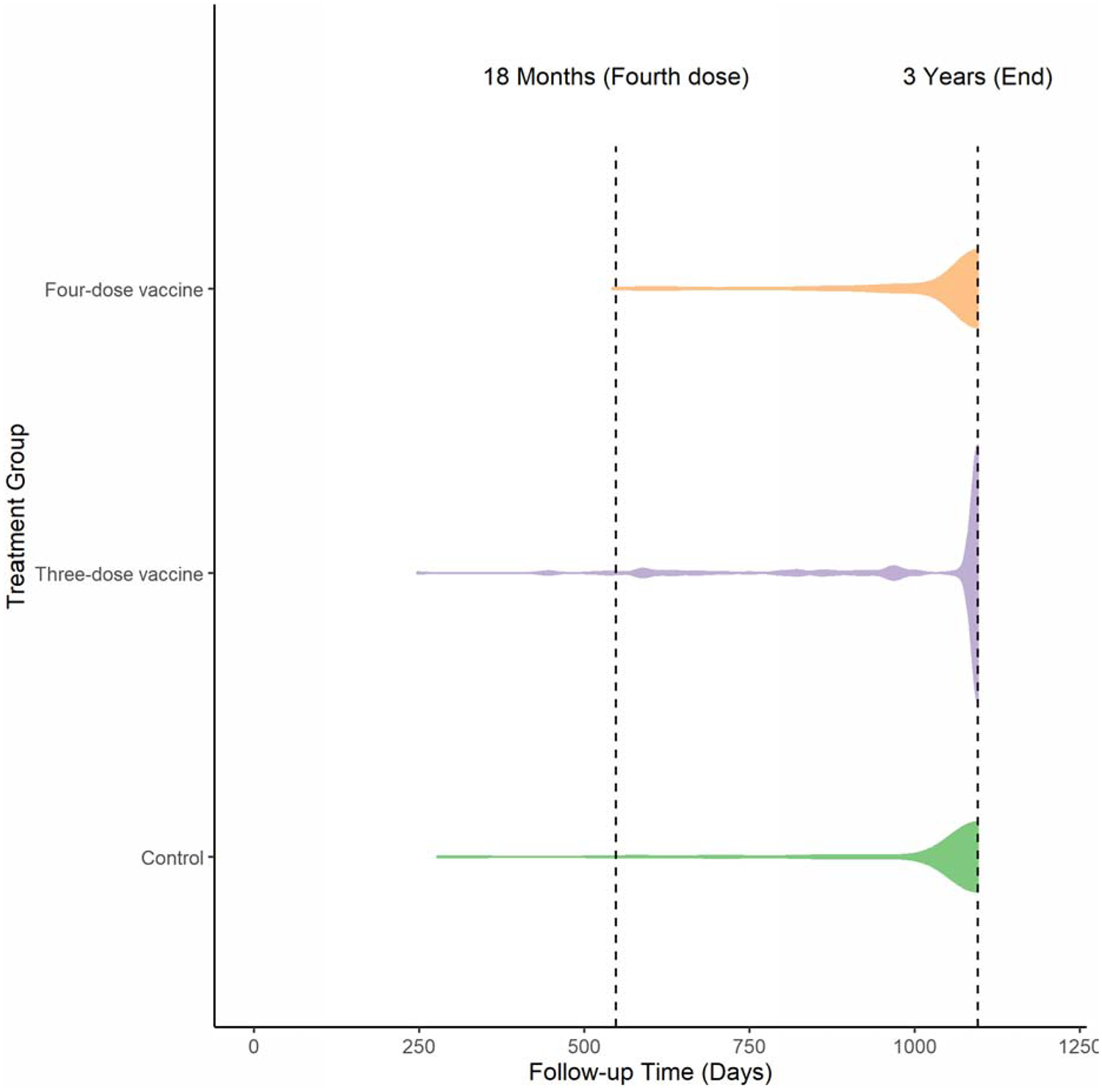
Violin Plots of Follow-up Time by Treatment Group
Where efficacy is defined as 1-IRR, we calculate that the RTS,S three-dose baseline vaccine is 53·08% (95% CI 36·19% to 65·50%) effective over an 18-month period and that bed net users have a 38·68% (95% CI 9·27% to 58·56%) lower rate of malaria (Table 2). We also find that the fourth dose of RTS,S is 43·08% (95% CI 15·98% to 61·44%) effective over 18 months. Without the fourth dose, the three-dose RTS,S vaccine had an efficacy of 17·48% (95% CI −18·08%, to 42·33%) in the second 18 months of follow-up. If the observed reduction due to bed net use can be considered to be an unbiased estimate of the true causal effect, a combined intervention giving a bed net and the four-dose RTS,S vaccine is 71·23% (95% CI 52·66% to 82·51%) effective over the first 18 months and 65·10% (95% CI 39·36% to 79·91%) effective over the second 18-months.
Table 2:
Model Output and Interpretation
| Variance Estimate | ||||
|---|---|---|---|---|
| Random Effects | ||||
| Participant ID (σ2) | 1·39 | |||
| Parameter Estimate | Std. Error | Exp of Parameter Estimate | Interpretation of Exp | |
| Fixed Effects | ||||
| Intercept (α) | −6·77 | 0·23 | 0·0012 | No relevant interpretation16 |
| Bed net (β1) | −0·49 | 0·20 | 0·61 | The rate ratio of malaria cases per unit time in the bed net group versus the no bed net group |
| Base vaccine, first 18 months (β2) | −0·76 | 0·16 | 0·47 | The rate ratio of malaria cases per unit time in the three- and four-dose vaccine groups versus the control group in the first 18 months |
| Second 18 months (β3) | −0·26 | 0·12 | 0·77 | The rate ratio of malaria cases per unit time in the second 18 months versus the first 18 months |
| Base vaccine, second 18 months (β4) | −0·19 | 0·18 | 0·83 | The rate ratio of malaria cases per unit time in the three-dose vaccine group versus the control group in the second 18 months |
| Fourth dose, second 18 months (β5) | −0·56 | 0·20 | 0·57 | The rate ratio of malaria cases per unit time in the four-dose vaccine group versus the control group in the second 18 months |
Calculations
The “Lilongwe Urban” estimates (Table 3) are computed using the model parameters and describe single-participant, three-year interventions. The “Lilongwe Rural” estimates are computed in a similar manner, however, the transmission intensity in the NBNV group was chosen to be fixed at 2·0 cases per year at baseline. Thus, the confidence intervals in the “Lilongwe Rural” do not account for uncertainty in this value. Over three years, in Lilongwe city, vaccinating a child without a bed net prevented 1·09 (95% CI 0·53 to 1·93) cases of malaria corresponding to $23·86 (13·48 to 48·95) per case averted. Vaccinating a child with a bed net prevented 0·67 (95% CI 0·36 to 1·02) cases of malaria, corresponding to $38·91 (25·55 to 72·90) per case averted. More cases were prevented by the vaccine in children without bed nets because children without bed nets are expected to experience a higher incidence of malaria. We are assuming the vaccine has the same relative efficacy in all children, which will lead to different numbers of cases averted if baseline incidence differs between two groups (i.e., children with and without bed nets). Due to higher incidence of malaria, compared to urban areas, vaccination in rural areas would prevent additional cases of malaria and would be more cost-effective: $10·08 (7·71 to 16·13) and $16·43 (10·16 to 30·06) in children with and without bed nets, respectively.
Table 3:
Cost-Effectiveness in Urban and Rural Settings, First Three Years
| Cases per 3 person-years (95% CI) | Cases averted per 3 person-years (95% CI) | Cost per case averted (95% CI) | |
|---|---|---|---|
| Lilongwe Urban | |||
| No bed net | |||
| No vaccine | 2·24 (1·45 to 3·46) | NA | NA |
| Four-dose vaccine | 1·15 (0·75 to 1·78) | 1·09 (0·53 to 1·93) | $23·86 (13·48 to 48·95) |
| Bed net | |||
| No vaccine | 1·38 (1·11 to 1·71) | NA | NA |
| Four-dose vaccine | 0·71 (0·57 to 0·88) | 0·67 (0·36 to 1·02) | $38·91 (25·55 to 72·90) |
| Lilongwe Rural | |||
| No bed net | |||
| No vaccine | *5·31 (4·81 to 5·96) | NA | NA |
| Four-dose vaccine | 2·72 (2·01 to 3·74) | 2·59 (1·62 to 3·38) | $10·08 (7·71 to 16·13) |
| Bed net | |||
| No vaccine | 3·26 (2·15 to 4·91) | NA | NA |
| Four-dose vaccine | 1·67 (1·01 to 2·77) | 1·59 (0·87 to 2·57) | $16·43 (10·16 to 30·06) |
The confidence interval only takes into account uncertainty in the reduction of the rate of malaria cases from the first 18 months to the second 18 months, not uncertainty in the base rate in the first 18 months.
As we cannot be confident that the chosen rate of 2·0 cases per year in the NBNV group is the true baseline rate, we relaxed this assumption, allowing the transmission intensity (cases per year) in the NBNV group to vary between 1·0 and 3·0 cases and recalculating estimated cases averted due to vaccination and cost per case averted (Figure 3). Naturally, more cases are averted and the cost per case averted decreases as transmission intensity rises. We observe that the cost-effectiveness curves (bed net and no bed net) trend towards convergence, as transmission intensity increases.
Figure 3: Cases per Year (Transmission Intensity) in the No Bed Net, No Vaccine (NBNV) Group at Baseline versus Cases Averted and Cost per Case Averted.
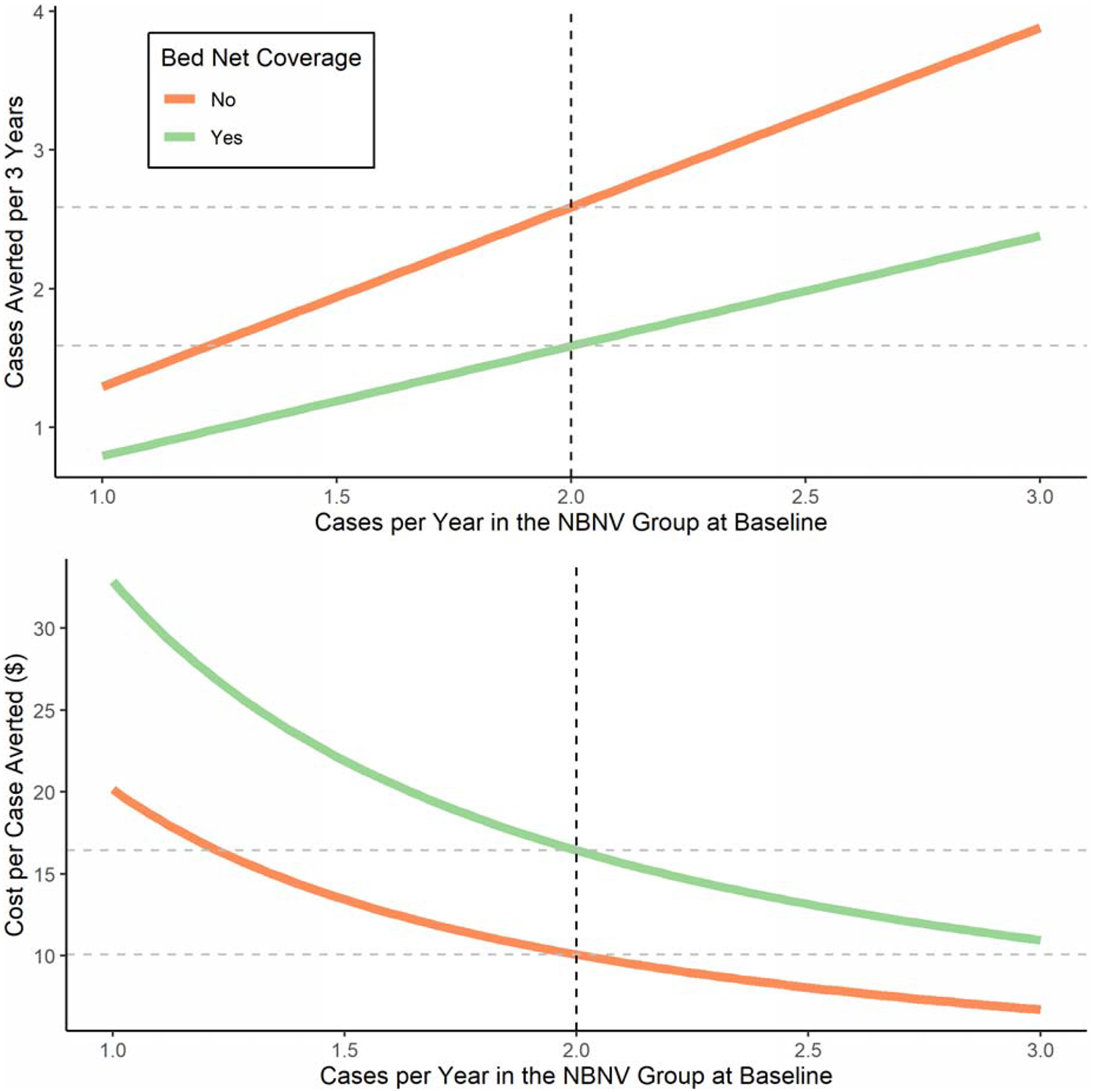
The dotted vertical line represents the assumed rural incidence of 2 cases per year
We calculate the cost and impact of a variety of interventions over the first three years of implementation (Table 4). These calculations assume bed net prevalences of 82·5% and 63·4% in urban and rural Lilongwe.5,8 Intervention 1 applies the vaccine effect, weighted by bed net prevalence, from the trial to 30,000 people in urban Lilongwe. In interventions 2 and 3, bed nets are administered to vaccinated children who lack them, with assumed usage rates of 100% and 50%, respectively. Interventions 4, 5, and 6 correspond to interventions 1, 2, and 3 in rural areas. The urban interventions carry a cost per case averted between $33–35 while the rural interventions carry a cost per case averted between $11·50–13·50. The cost per case averted does not increase by providing bed nets to those who lack them, even at 50% usage. We relaxed the assumption of 2·0 cases per year in the NBNV group, allowing the transmission intensity (cases per year) to vary between 1·0 and 3·0 cases recalculating estimated cases averted due to each intervention and cost per case averted (Figure 4). Here we see that adding bed nets to an intervention prevents additional cases of malaria and remains similarly cost-effective, regardless of transmission intensity.
Table 4:
Vaccine Implementation in 30,000 Children, First Three Years
| Intervention | Bed nets given | Number Vaccinated | Total Cost ($) | Cases Averted | Cost per case averted ($) | ||
|---|---|---|---|---|---|---|---|
| With net | No net | ||||||
| 1) | Lilongwe urban | 0 | 24,750 | 5,250 | 782,400 | 22,325 (11,956 to 34,474) | 35·05 (22·69 to 65·40) |
| 2) | Lilongwe urban + bed nets (100% adoption) | 5,250 | 30,000 | 0 | 817,050 | 24,661 (14,022 to 37,466) | 33·13 (21·80 to 58·26) |
| 3) | Lilongwe urban+ bed nets (50% adoption) | 5,250 | 27,375 | 2,625 | 817,050 | 23,492 (13,147 to 35,961) | 34·78 (22·71 to 62·09) |
| 4) | Lilongwe rural | 0 | 19020 | 10,980 | 782,400 | 58,611 (35,778 to 82,932) | 13·35 (9·43 to 21·87) |
| 5) | Lilongwe rural + bed nets (100% adoption) | 10,980 | 30,000 | 0 | 818,634 | 70,178 (51,765 to 88,662) | 11·67 (9·23 to 15·81) |
| 6) | Lilongwe rural + bed nets (50% adoption) | 10,980 | 24,525 | 5,490 | 818,634 | 64,395 (44,292 to 85,740) | 12·71 (9·54 to 18·46) |
Figure 4: Cases per Years (Transmission Intensity) in the No Bed Net, No Vaccine (NBNV) Group at Baseline versus Total Cases Averted and Cost per Case Averted in Each Three-Year Rural Intervention.
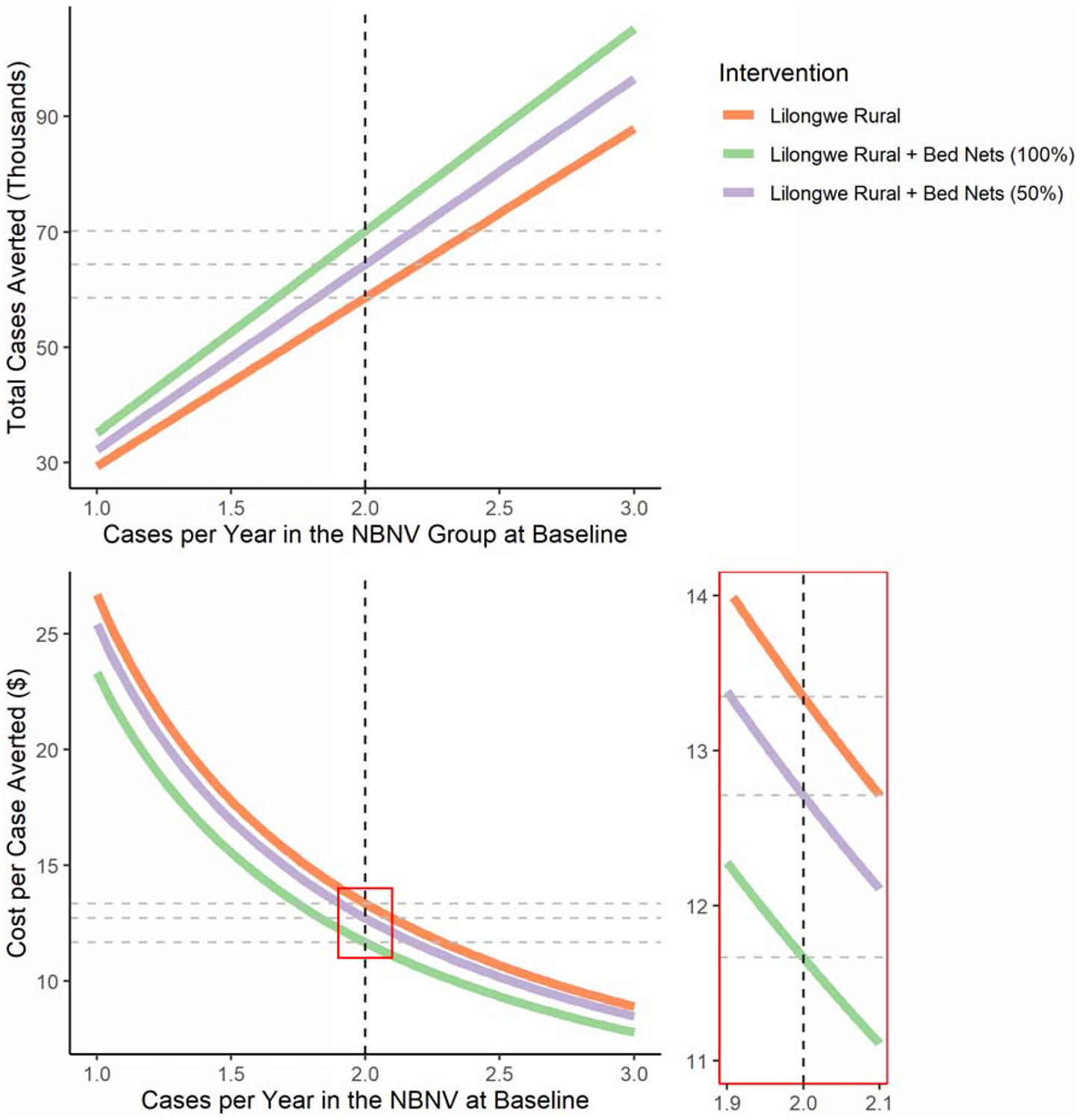
The dotted vertical line represents the assumed rural incidence of 2 cases per year in the control group
Sensitivity Analysis
We fit a generalized additive model, identical in structure to the model specified earlier in the methods section, except for the addition of a two-dimensional spatial spline as a random intercept. Our main analysis found that bed net use was associated with a 38·68% (95% CI 9·27% to 58·56%) reduction of malaria incidence while the sensitivity analysis found that bed net use was associated with a 34·64% (95% CI −0·87% to 57·64%) reduction of malaria incidence. This model finds a combined intervention of the vaccine and bed net use to be 66·90% (95% CI 44·80% to 80·15%) effective in the first 18 months and 65·68% (95% CI 38·26% to 80·93%) effective in the second 18 months, comparable to the original estimates of 71·23% (95% CI 52·66% to 82·51%) and 65·10% (95% CI 39·36%, to 79·91%), respectively.
INTERPRETATION
By combining RTS,S trial, bed net usage, bed net prevalence, and cost data, we estimated the effects of vaccination with the RTS,S vaccine alongside bed net use in Lilongwe, Malawi. Assuming homogeneity of vaccine efficacy, the protective nature of bed nets implies that vaccinating an individual with a bed net is less cost-effective than vaccinating an individual without a bed net. This is not to say that bed nets necessarily reduce the importance of the RTS,S vaccine, or vice versa. In fact, our analysis suggests that there is great potential to pair the two interventions. An intervention pairing the RTS,S vaccine and bed net distribution is estimated to be 71·23% (95% CI 42·10% to 85·70%) effective against clinical malaria in the first 18 months and 65·10% (95% CI 23·77% to 84·02%) effective in the second 18 months.
In a population of 30,000 with 63·4% bed net coverage and 2 malaria cases per year at baseline, we estimate pairing bed nets with vaccine administration would prevent an additional 11,567 cases of malaria (20% increase over vaccination alone) over three years and reduce the cost per case prevented. Though it is not considered in this analysis, averting additional cases of malaria reduces other costs: a systematic review found the median cost of diagnosing a case of malaria was $4.32 and the median financial cost of treating an episode of uncomplicated malaria was $5.84.11 This median cost per uncomplicated case, $10.16, is only a few dollars less than the cost per case averted of the rural interventions considered. These result warrants investigation into the feasibility of stockpiling bed nets at vaccination sites and distributing them to uncovered individuals upon vaccination.
Even in a population where bed net coverage is high, the RTS,S vaccine has utility. Alone, the four-dose schedule prevents 53·08% (95% CI 36·19% to 65·50%) of malaria cases over the first 18 months and 43·08% (95% CI 15·98% to 61·44%) over the second 18 months. In children with bed nets, these point estimates correspond to per-person estimates of 0·67 cases prevented over three years in urban areas and 1·59 cases in rural areas.
Limitations
One major limitation is that the phase III trial was not a factorial design and thus we cannot assume the estimated effect of bed net use on malaria incidence to be the true causal effect. We assumed this when we estimated the effect of combined bed net and vaccine interventions using this data, thus those results (efficacies of the combined interventions and interventions 2, 3, 5, and 6) must be interpreted with caution. However, our sensitivity analysis found little change in the reduction of malaria incidence due to bed net use upon controlling for background incidence. Additionally, randomized trials and other observational studies of bed nets have found similar effects of bed nets on the incidence of malaria.18–23 Additionally, the indirect (herd) effects of the potential interventions were not considered. In the presence of an indirect effect, these interventions would prevent additional cases of malaria and cost less per case averted.
Furthermore, by omitting an interaction term between vaccination and bed net use and generalizing to rural settings, we have assumed that the efficacy of RTS,S/AS01 does not vary by transmission intensity. A previous analysis done in Lilongwe, Malawi found no evidence that vaccine efficacy varied by rainfall, which suggests that transmission intensity does not influence the efficacy of RTS,S.24
An additional limitation is our measurement of bed net use. Though we include multiple bed net use measurements throughout follow-up, each measurement serves as an imperfect proxy for bed net use over a time period spanning multiple months and thus could be subject to exposure misclassification.
Other Relevant Studies
Penny and Verity et al. predicts the median cost-effectiveness to be $25 per case averted for the four-dose vaccine.10 This is somewhat different from our urban Lilongwe estimate of $35·05 (22·69 to 65·40), though our interval contains their estimate. We included 36 months of follow-up, where they included 32, and our analysis was limited to Lilongwe where their analysis was across all eleven phase III trial sites.10 Furthermore, our analysis accounted for bed net coverage.
Winskill et al. focused on the order in which a variety of interventions should be introduced.25 Their model found that scaling up bed net use to very high coverage was prioritized relative to RTS,S implementation.25 They did not consider that the two interventions could be implemented together, and that RTS,S vaccination might be a vehicle to achieving higher bed net coverages. Another paper by Penny et al. found that vaccination of 100,000 children with the three-dose vaccine would prevent 45,000 to 80,000 clinical cases of malaria in the first ten years of implementation.26 Galactionova et al estimated that between 66491 and 104933 cases would be averted in Malawi due to the vaccination of 100,000 children.27 Finally, Seo et al. followed a “hypothetical Malawian birth cohort” and compared bed nets and RTS,S interventions.28 Their Markov model found that the RTS,S vaccine was more cost effective, however, they did not consider vaccine efficacies below 49·6%.28
These modeling studies have some advantages over our analysis because they addressed waning vaccine efficacy as well as seasonality or other factors which might influence the transmission intensity of malaria. However, our study used longitudinal data including individual-level bed net use, which separates our analysis from the previous analyses. Additionally, our analysis focused on a single country and therefore our analysis may provide a more accurate picture for Malawi.
Conclusion
The current pilot implementation of RTS,S will reduce malaria cases across Malawi, Ghana, and Kenya and eventually in other countries, if implemented. The Malawi site of the RTS,S trial was set in urban and periurban Lilongwe which has lower malaria incidence than in rural areas. Thus, the present implementation in rural areas should theoretically have a larger per-person impact than the trial. Future programs could pair bed net distribution with vaccine implementation, as the cost per case averted remains stable at 50% adoption. Since children are already traveling to clinics for vaccination, providing bed nets to vaccinated children who lack them could be a logical and cost-effective way to capitalize on the structure of vaccine implementation to prevent additional cases of malaria. Furthermore, provision of bed nets together with RTS,S might avoid a reduction of bed net use after vaccination due to parents believing their kids are protected. A factorial trial would help to build evidence for this type of intervention.
Supplementary Material
Role of the Funding Source
Griffin J Bell is funded on Impacts of Environment, Host Genetics and Antigen Diversity on Malaria Vaccine Efficacy (1R01AI137410-01), awarded to Jeffrey A Bailey and Michael Emch.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate
The trial protocol was approved by both the institutional review board (IRB) at UNC Chapel Hill and by the Malawi National Health Sciences Research Committee.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Griffin J. Bell, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC 27599, USA
Matthew Loop, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC 27599, USA.
Hillary Topazian, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC 27599, USA.
Michael Hudgens, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC 27599, USA.
Tisungane Mvalo, University of North Carolina, Chapel Hill, NC 27599, USA; University of North Carolina Project, Lilongwe, Malawi.
Jonathan J. Juliano, Division of Infectious Diseases, School of Medicine, University of North Carolina, Chapel Hill, NC 27599, USA
Portia Kamthunzi, University of North Carolina, Chapel Hill, NC 27599, USA; University of North Carolina Project, Lilongwe, Malawi.
Gerald Tegha, University of North Carolina Project, Lilongwe, Malawi.
Innocent Mofolo, University of North Carolina, Chapel Hill, NC 27599, USA; University of North Carolina Project, Lilongwe, Malawi.
Irving Hoffman, University of North Carolina, Chapel Hill, NC 27599, USA; University of North Carolina Project, Lilongwe, Malawi.
Jeffrey A Bailey, Department of Pathology and Laboratory Medicine, Brown University, Providence, RI 02903, USA.
Michael Emch, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC 27599, USA.
REFERENCES
- 1.World Malaria Report 2018. S.l.: World Health Organization; 2019. [Google Scholar]
- 2.First malaria vaccine in Africa: A potential new tool for child health and improved malaria control. World Health Organization; p. 4. [Google Scholar]
- 3.Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. The Lancet. 2015. July 4;386(9988):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moorthy VS, Hutubessy R, Newman RD, Hombach J. Decision-making on malaria vaccine introduction: the role of cost-effectiveness analyses. Bull World Health Organ. 2012. November 1;90(11):864–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Malaria Control Programme - NMCP/Malawi and ICF. 2018. Malawi Malaria Indicator Survey 2017. Lilongwe, Malawi: NMCP/Malawi and ICF; Available at http://dhsprogram.com/pubs/pdf/MIS28/MIS28.pdf. [Google Scholar]
- 6.Janko MM, Churcher TS, Emch ME, Meshnick SR. Strengthening long-lasting insecticidal nets effectiveness monitoring using retrospective analysis of cross-sectional, population-based surveys across sub-Saharan Africa. Sci Rep. 2018. November 20;8(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothman K, Greenland S. Causation and Causal Inference in Epidemiology. American Journal of Public Health. 2005;95(S1):S144–S150. [DOI] [PubMed] [Google Scholar]
- 8.Escamilla V, Alker A, Dandalo L, Juliano JJ, Miller WC, Kamthuza P, et al. Effects of community-level bed net coverage on malaria morbidity in Lilongwe, Malawi. Malaria Journal. 2017. April 7;16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood SN. Royal Statistical Society Publications [Internet]. [cited 2018 Dec 25]. Available from: https://rss.onlinelibrary.wiley.com/doi/abs/10.1111/1467-9868.00374
- 10.Penny MA, Verity R, Bever CA, Sauboin C, Galactionova K, Flasche S, et al. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. The Lancet. 2016. January 23;387(10016):367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White MT, Conteh L, Cibulskis R, Ghani AC. Costs and cost-effectiveness of malaria control interventions - a systematic review. Malaria Journal. 2011. November 3;10(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leach A, Vekemans J, Lievens M, Ofori-Anyinam O, Cahill C, Owusu-Agyei S, et al. Design of a phase III multicenter trial to evaluate the efficacy of the RTS,S/AS01 malaria vaccine in children across diverse transmission settings in Africa. Malaria Journal. 2011. August 4;10(1):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesko CR, Buchanan AL, Westreich D, Edwards JK, Hudgens MG, Cole SR. Generalizing study results: a potential outcomes perspective. Epidemiology. 2017. July;28(4):553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 15.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015. October 7;67(1):1–48. [Google Scholar]
- 16.Fitzmaurice GM, Laird NM, Ware JH Contrasting Marginal and Mixed Effects Models In Applied Longitudinal Analysis. 2nd ed Hoboken: Wiley; 2012. [Google Scholar]
- 17.Gelman Andrew and Su Yu-Sung (2018). arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. R package version 1.10–1. https://CRAN.R-project.org/package=arm
- 18.Lengeler C, Snow RW. From efficacy to effectiveness: insecticide-treated bednets in Africa. Bull World Health Organ. 1996;74(3):325–32. [PMC free article] [PubMed] [Google Scholar]
- 19.Choi HW, Breman JG, Teutsch SM, Liu S, Hightower AW, Sexton JD. The Effectiveness of Insecticide-Impregnated Bed Nets in Reducing Cases of Malaria Infection: A Meta-Analysis of Published Results. The American Journal of Tropical Medicine and Hygiene. 1995. May 1;52(5):377–82. [DOI] [PubMed] [Google Scholar]
- 20.Sexton JD, Ii TKR, Brandling-Bennett AD, Breman JG, Roberts JM, Odera JS, et al. Permethrin-Impregnated Curtains and Bed-Nets Prevent Malaria in Western Kenya. The American Journal of Tropical Medicine and Hygiene. 1990. July 1;43(1):11–8. [DOI] [PubMed] [Google Scholar]
- 21.Lindblade KA, Mwandama D, Mzilahowa T, Steinhardt L, Gimnig J, Shah M, et al. A cohort study of the effectiveness of insecticide-treated bed nets to prevent malaria in an area of moderate pyrethroid resistance, Malawi. Malaria Journal. 2015. January 28;14(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fullman N, Burstein R, Lim SS, Medlin C, Gakidou E. Nets, spray or both? The effectiveness of insecticide-treated nets and indoor residual spraying in reducing malaria morbidity and child mortality in sub-Saharan Africa. Malaria Journal. 2013. February 13;12(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Protopopoff N, Mosha JF, Lukole E, Charlwood JD, Wright A, Mwalimu CD, et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. The Lancet. 2018. April 21;391(10130):1577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han L, Hudgens MG, Emch ME, Juliano JJ, Keeler C, Martinson F, et al. RTS,S/AS01 Malaria Vaccine Efficacy is Not Modified by Seasonal Precipitation: Results from a Phase 3 Randomized Controlled Trial in Malawi. Sci Rep [Internet]. 2017. August 3 [cited 2019 Feb 26];7 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5543056/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winskill P, Walker PG, Griffin JT, Ghani AC. Modelling the cost-effectiveness of introducing the RTS,S malaria vaccine relative to scaling up other malaria interventions in sub-Saharan Africa. BMJ Global Health. 2017. January 1;2(1):e000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penny MA, Galactionova K, Tarantino M, Tanner M, Smith TA. The public health impact of malaria vaccine RTS,S in malaria endemic Africa: country-specific predictions using 18 month follow-up Phase III data and simulation models. BMC Medicine. 2015. July 29;13(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galactionova K, Tediosi F, Camponovo F, Smith TA, Gething PW, Penny MA. Country specific predictions of the cost-effectiveness of malaria vaccine RTS,S/AS01 in endemic Africa. Vaccine. 2017. January 3;35(1):53–60. [DOI] [PubMed] [Google Scholar]
- 28.Seo MK, Baker P, Ngo KN-L. Cost-effectiveness analysis of vaccinating children in Malawi with RTS,S vaccines in comparison with long-lasting insecticide-treated nets. Malaria Journal. 2014. February 24;13(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


