Abstract
Two largely distinct bodies of research have demonstrated age-related alterations and disease-specific aberrations in both local gamma oscillations and patterns of cortical thickness. However, seldom has the relationship between gamma activity and cortical thickness been investigated. Herein, we combine the spatiotemporal precision of magnetoencephalography (MEG) with high-resolution magnetic resonance imaging and surface-based morphometry to characterize the relationships between somatosensory gamma oscillations and the thickness of the cortical tissue generating the oscillations in 94 healthy adults (age range: 22–72). Specifically, a series of regressions were computed to assess the relationships between thickness of the primary somatosensory cortex (S1), S1 gamma response power, peak gamma frequency, and somatosensory gating of identical stimuli. Our results indicated that increased S1 thickness significantly predicted greater S1 gamma response power, reduced peak gamma frequency, and improved somatosensory gating. Furthermore, peak gamma frequency significantly and partially mediated the relationship between S1 thickness and the magnitude of the S1 gamma response. Finally, advancing age significantly predicted reduced S1 thickness and decreased gating of redundant somatosensory stimuli. Notably, this is the first study to directly link somatosensory gamma oscillations to local cortical thickness. Our results demonstrate a multi-faceted relationship between structure and function, and have important implications for understanding age- and disease-related deficits in basic sensory processing and higher-order inhibitory function.
Keywords: magnetoencephalography (MEG), surface-based morphometry (SBM), magnetic resonance imaging (MRI), primary somatosensory cortex (S1), paired pulse
1. Introduction
A growing body of research suggests that gamma oscillations (≥ 30 Hz) are critical to animal behavior, from the most basic to the most complex.1–23 These high-frequency oscillations are known to be susceptible to age-related change,13,24,25 and are aberrant in many clinical populations,26–32 with such abnormalities often being directly linked to symptom severity. Interestingly, a largely separate body of research has demonstrated that healthy aging is accompanied by widespread cortical thinning,33–35 and atypical patterns of cortical thinning have been observed in the aforementioned clinical populations as well.36–40 Importantly, when these two bodies of research are taken together, a pattern emerges such that many of the neural regions demonstrating the most robust aging effects are spatially coincident with those that exhibit age-related gamma alterations. Within a similar vein, the neural regions which display structural aberrations within the aforementioned clinical populations are oftentimes spatially concordant with the regions characterized by aberrant gamma in each respective population.
While the pattern outlined above suggests that cortical thickness may be directly linked to gamma oscillations, surprisingly few studies have investigated this relationship. The few exceptions have most frequently probed the association between visual gamma metrics, elicited by the presentation of a visual grating stimulus, and thickness of the occipital cortex in healthy adults, but these studies have reported discrepant findings. For example, some studies have demonstrated a positive association between the peak frequency of the gamma response in primary visual cortex (V1) and the local cortical thickness,41–43 while an equal number of studies have found no such relationship.44–46 Although it is hard to draw definitive links between visual gamma and occipital volumetrics at this point, it is important to note that the aforementioned studies differed with regards to the age range of enrollees. The studies also differed on whether age was controlled for when assessing gamma/cortical thickness relationships, the visual task utilized, the specific occipital region submitted to cortical thickness computations (e.g., whole occipital lobe, pericalcarine cortex), and the gamma frequency range considered (e.g., 30–70 Hz, 30–80 Hz). Thus, methodological inconsistencies likely contributed to the conflicting reports.
Beyond visual gamma, the relationship between auditory gamma and cortical thickness has been assessed by Edgar and colleagues.47 This experiment utilized a 40 Hz steady-state auditory task and magnetoencephalography (MEG) in patients with schizophrenia and healthy controls. Their findings indicated that the magnitude of the 40 Hz steady-state response within the left superior temporal gyrus was positively correlated with the local cortical thickness, while additionally being negatively associated with age. Interestingly, these relationships were specific to healthy controls, suggesting that patients with schizophrenia have disrupted gamma/structure relationships.47
Taken together, the body of research mentioned above suggests that investigating the link between cortical gamma oscillations and the thickness of the region generating such oscillations may further inform our understanding of how brain structure impacts local brain function. The goal of the present study was to extend this focus into the somatosensory realm. Specifically, we applied paired-pulse electrical stimulation to the median nerve during MEG in a large sample of healthy adults spanning a wide age range (22–72 years old). Previous research utilizing a similar methodological design has demonstrated a robust increase in gamma activity within the primary somatosensory cortex (S1) nearly instantaneously after stimulation, and this response is believed to reflect the initial bottom-up processing of the stimulus.48–50 Additionally, when two identical stimulations are presented in short succession (i.e., paired pulse) a sensory gating phenomenon is observed, such that the gamma response to the second stimulation (Stim2) is strongly reduced relative to that elicited by the first stimulation (Stim1).51–53 Such gating is believed to reflect the filtering out or inhibition of redundant information, so as to optimize the neural resources available for behaviorally relevant neural computations.54 Importantly, evidence suggests that these somatosensory-related gamma metrics are modulated by age,5,55 and present atypically in clinical populations.6,50 Thus, determining the relationship between somatosensory-related gamma oscillations and cortical thickness may provide new insight on the mechanisms underlying age-related gamma alterations and declines in both lower- and higher-order cognitive function.
In the current multimodal imaging study, we report the first data linking somatosensory gamma oscillations and the thickness of the cortical tissue generating the oscillations. Specifically, we combined high-resolution structural magnetic resonance imaging (sMRI) and surface-based morphometry (SBM) with the spatiotemporal precision of MEG and a paired-pulse somatosensory paradigm. We hypothesized that the strength of the S1 gamma response to Stim1 would be positively correlated with the cortical thickness of the generating tissue. Additionally, substantial inter-individual variability in somatosensory, motor, and visual peak gamma frequency has been reported,5,9,24,41,42,56 and our laboratory has previously observed an inverse relationship between gamma power and peak gamma frequency within both the somatosensory and visual systems.6,57 Thus, we anticipated that the peak frequency of the S1 gamma response would be negatively correlated with S1 gamma power and S1 thickness. Finally, as previous research has demonstrated that age is inversely related to both somatosensory gating and S1 thickness,33,35 we anticipated a negative relationship between the gating ratio and S1 thickness. Herein, we directly probed these inter-relationships while controlling for age, as warranted.
2. Materials and Methods
2.1. Participants
We studied 94 healthy adults (43 females; M age: 45.82, SD: 15.15, range: 22–72) who were recruited from the local community. Exclusion criteria included any medical illness affecting central nervous system function, neurological or psychiatric disorder, history of head trauma, current substance abuse, and ferromagnetic implants. After providing a complete description of the study, written informed consent was obtained from participants following the guidelines of the University of Nebraska Medical Center’s Institutional Review Board, which approved the study protocol.
2.2. MEG Experimental Paradigm
During MEG recording, participants sat in a nonmagnetic chair within a magnetically-shielded room while electrical stimulation was applied to the right median nerve using external cutaneous stimulators connected to a Digitimer DS7A constant-current stimulator system (Digitimer Ltd, Garden City, UK). At least 80 paired-pulse trials were collected per participant with an inter-stimulus interval of 500 ms and an inter-pair interval that randomly varied between 4500 and 4800 ms. Each pulse generated a 0.2 ms constant-current square wave that was set to 10% above the motor threshold required to elicit a subtle twitch of the thumb.
2.3. MEG data acquisition
Recordings occurred in a one-layer magnetically-shielded room with active shielding engaged, and neuromagnetic responses were sampled continuously at 1 kHz using an acquisition bandwidth of 0.1–330 Hz and a 306-sensor Elekta system (Elekta, Helsinki, Finland). MEG data from each participant were individually corrected for head motion and noise reduced using the signal space separation method with a temporal extension.58,59
2.4. MEG Coregistration
Preceding MEG measurement, four coils were attached to the participant’s head and localized, together with the three fiducial points and scalp surface, with a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). During MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each coil, inducing a measurable magnetic field which allowed each coil to be localized in reference to the sensors throughout the recording session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system, each participant’s MEG data were coregistered with their structural T1-weighted neuroanatomical data before source space analyses using BESA MRI (Version 2.0; BESA GmbH, Gräfelfing, Germany). Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space, along with the functional images, after beamforming (see below).
2.5. MEG Time-Frequency Transformation and Statistics
A high-pass filter of 0.5 Hz, low-pass filter of 200 Hz, and notch filter of 60 Hz (width: 2 Hz) were applied to the MEG time series data. Cardiac artifacts were then removed using signal-space projection (SSP), which was accounted for during source reconstruction.60 The continuous magnetic time series was divided into epochs of 3700 ms duration, from −800 to 2900 ms, with the onset of the first stimulation being defined as 0 ms, and the baseline being the −700 to −300 ms window. Epochs contaminated with artifacts were rejected based on a fixed threshold method, supplemented with visual inspection. The artifact-free epochs were transformed into the time-frequency domain using complex demodulation with a resolution of 5 Hz and 10 ms (range: 10–100 Hz). The resulting spectral power estimations per sensor were averaged across trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized per time-frequency bin using the baseline power per frequency bin (i.e., mean power during the −700 to −300 ms time period).
The time-frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across all participants and all gradiometers. Each data point in the spectrogram was first evaluated using a mass univariate approach based on the general linear model (GLM). To reduce the risk of false positive results while maintaining reasonable sensitivity, a two-stage procedure was adopted. In the first stage, one-sample t-tests were conducted on each data point and the output spectrogram of t-values was thresholded at p < .05 to identify time-frequency bins containing potentially significant oscillatory activity across all participants. In stage two, the time-frequency bins that survived this threshold were clustered with temporally and/or spectrally neighboring bins that were also significant. A cluster value was then computed by summing the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster-values and the significance level of the observed clusters (from stage one) were tested directly using this distribution.61,62 For each comparison, at least 10,000 permutations were computed. Based on these analyses, only the time-frequency windows that contained significant oscillatory events across all participants were subjected to the beamforming (i.e., imaging) analysis. Thus, a data-driven approach was utilized for determining the time-frequency windows that were entered into the source reconstruction.
2.6. MEG Source Imaging
Cortical networks were imaged using the Dynamic Imaging of Coherent Sources (DICS) beamformer,63–65 which calculates source power for the entire brain volume by employing spatial filters in the time-frequency domain. The single images were derived from the cross spectral densities of all combinations of MEG gradiometers averaged over the time-frequency range of interest,63 and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images was normalized per participant using a separately averaged pre-stimulus noise period (i.e., baseline) of equal duration and bandwidth.64 Such images are typically referred to as pseudo-t maps, with units (pseudo-t) that reflect noise-normalized power differences (i.e., active vs. passive) per voxel. Thus, the normalized source power was computed for the statistically-determined time-frequency bands over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. Each participant’s functional images were then transformed into standardized space using the transform that was previously applied to the structural images and spatially resampled to 1 mm isotropic voxels. MEG pre-processing and imaging used the Brain Electrical Source Analysis (version 6.1) software.
The resulting 3D maps of brain activity were averaged across all participants to assess the neuroanatomical basis of the significant oscillatory responses identified through the sensor-level analysis. Using the grand-averaged beamformer image the peak voxel of the oscillatory response was identified, and voxel time series data (i.e., virtual sensors) were extracted from the peak voxel, per individual. To compute the virtual sensors, we applied the sensor weighting matrix derived from the forward solution to the preprocessed signal vector, which yielded a time series for the specific coordinate. Note that the time series then underwent complex demodulation with a resolution of 5 Hz and 10 ms to identify the peak power of the response following Stim1 and Stim2, per individual. These values were further used to compute the gating ratio per participant, by dividing the peak power following Stim2 by that following Stim1. The time series were also convolved at a resolution of 1 Hz and 50 ms to identify the peak frequency of the oscillatory response following Stim1, per participant.
2.7. Structural MRI Acquisition and Processing
All participants underwent high-resolution T1-weighted MRI on a Philips Achieva 3T X-series scanner using an eight-channel head coil and a 3D fast-field echo sequence (TR: 8.09 ms; TE: 3.7 ms; field of view: 240 mm; slice thickness: 1.0 mm with no gap; in-plane resolution: 1.0 × 1.0 mm). The structural MRI data were processed using the standard pipeline in the CAT12 toolbox (http://dbm.neuro.uni-jena.de/cat/, version 12.6) at a resolution of 1 mm3 within SPM12 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/) using MATLAB (2017b) software (MathWorks, Natick, Massachusetts, USA). The surface-based morphometry pipeline in CAT12 is fully automated and utilizes a projection-based thickness (PBT) approach to estimate cortical thickness and reconstruct the central surface in one step.66 Essentially, following tissue segmentation67 the white matter (WM) distance is estimated, and the local maxima (which is equal to the cortical thickness) are projected onto other gray matter voxels using a neighboring relationship described by the WM distance. PBT accounts for partial volume correction, sulcal blurring, and sulcal asymmetries without sulcus reconstruction. To rectify topological defects, a correction based on spherical harmonics was employed,68 and the cortical surface mesh was reparameterized into a common coordinate system via an algorithm that reduces area distortion.69 Finally, the resulting maps were resampled and smoothed using a 15 mm FWHM Gaussian kernel.
For quality assurance, a two-step process was adopted. First, prior to segmentation, data were visually inspected for artifacts. Second, the quality control measures incorporated in the CAT12 processing pipeline were utilized to identify the most deviant data following segmentation. These data were inspected further for the presence of newly introduced artifacts.
2.8. Region of Interest Analysis
Utilizing the peak voxel coordinates identified in the grand-averaged beamformer image (see Section 2.6 MEG Source Imaging), a mask was constructed for the cortical surface mesh. Specifically, a 4 mm cortically-constrained sphere centered on the peak voxel coordinates was generated using the WFU Pickatlas (version 3.0).70,71 This mask was spatially resampled to 1 mm isotropic voxels to align with the processed structural MRI and MEG data. Finally, the normalized volume mask was transformed into the surface template space using the transform provided in CAT12. Cortical thickness values were then extracted per participant utilizing this ROI mask. Thus, the attained values reflect the average cortical thickness within the estimated group-wise peak voxel of the gamma oscillatory response. Figure 1 illustrates our methodological pipeline. Of note, the same procedure was applied to derive the average cortical thickness across spheres of varying sizes (2 mm, 4 mm, 8 mm, and 12 mm), and in all cases all statistically significant findings held. We report on the data using the 4 mm cortical thickness mask as it aligned with the 4 mm native resolution of the beamformer source images.
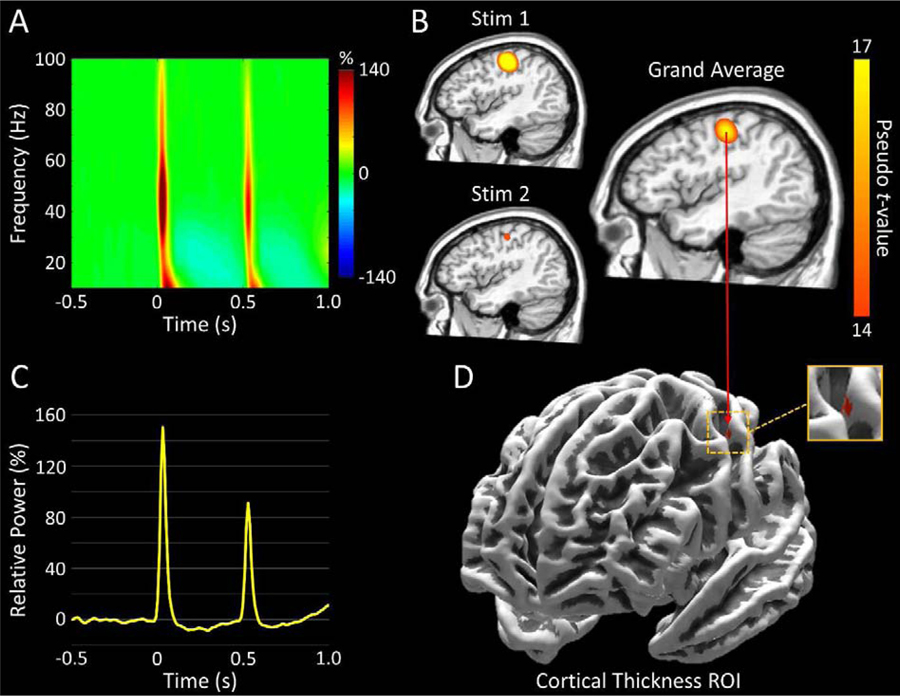
Figure 1.
Multimodal Analysis Pipeline. (A): Time-frequency spectrogram with time (ms) shown on the x-axis and frequency (Hz) denoted on the y-axis. Percent power change was computed for each time-frequency bin relative to the respective bin’s baseline power (−700 to −300 ms). The onset of the first stimulation (Stim1) began at 0 ms, while that of the second stimulation (Stim2) occurred at 500 ms. Data represent a peak sensor, averaged across all participants, located near the sensorimotor cortices, with the color legend displayed to the right. As can be discerned, there was a strong oscillatory response following each stimulation, with the higher frequency components being strongest during the first 50 ms after stimulation onset in the 30–75 Hz range. (B): Group-averaged gamma (30–75 Hz) beamformer images (pseudo-t) for Stim1 (0–50 ms; top left), Stim2 (500–550 ms; bottom left), and the grand-average (right). Following each stimulation, strong increases in gamma activity were observed in virtually identical areas of the contralateral hand region of the primary somatosensory cortex, with the response following Stim2 being visibly attenuated. The peak voxel within the grand-averaged beamformer image was identified, and time series (i.e., virtual sensor) data was extracted, per participant, from this location. (C): Group-averaged baseline-normalized (i.e., relative) 30–75 Hz power envelope from the peak voxel depicted in panel B. The data clearly depict a weaker gamma response following Stim2. (D): Map displaying the region-of-interest (ROI) from which cortical thickness values were extracted. The ROI was a 4 mm cortically-constrained sphere centered on the peak voxel from the grand-averaged beamformer image depicted in panel B.
2.9. Statistical Analyses
The primary relationships of interest were those between S1 cortical thickness and the magnitude of the somatosensory-related gamma oscillatory response, the peak frequency of the response, and somatosensory gating. Following convention, we indexed somatosensory gating by computing the gating ratio (i.e., gamma response power to Stim2/gamma response power to Stim1), in which a lower gating ratio is indicative of stronger gating (i.e., better suppression of redundant stimuli). Thus, three simple regressions were computed in which the peak power and peak frequency of the oscillatory response following Stim1, as well as the gating ratio, were independently regressed on ROI-based cortical thickness. As previous research has demonstrated age-related cortical thinning,33,35 as well as age-related differences in the oscillatory metrics of interest,5 we independently regressed ROI-based cortical thickness, peak power, peak frequency, and gating ratio on age. All regressions were computed in SPSS Statistics (version 25).
3. Results
All 94 participants were able to successfully complete the MEG and MRI aspects of the study. However, 9 participants were excluded from the final analyses due to excessive artifacts in their MEG data or having oscillatory response magnitudes that were beyond 3 standard deviations away from the mean. The remaining 85 participants (40 females) had a mean age of 46.77 years (SD = 14.75, range: 22–72).
3.1. Sensor-Level Analysis
Statistical analyses of the time-frequency spectrograms revealed significant increases in 10–90 Hz oscillatory activity during the first 100 ms following the onset of Stim1 and Stim2 in many sensors near the sensorimotor and parietal regions (p < .001, corrected; Figure 1A). As shown in Figure 1A, the majority of the spectral power was contained within the 30–75 Hz band for a duration of 50 ms after each stimulation, and this was especially true for the Stim2 response. Thus, we applied a beamformer to the following windows: 30–75 Hz from 0 to 50 ms, and 30–75 Hz from 500–550 ms.
3.2. Beamformer Analysis
As anticipated, the increase in gamma (30–75 Hz) oscillatory activity following both Stim1 and Stim2 were localized to the contralateral S1 hand region, with virtually identical peak voxel locations (Figure 1B). Thus, to extract virtual sensors, we first averaged the beamformer images across both stimulations and all participants. We then utilized the resulting peak voxel in the grand-averaged map to extract baseline-normalized voxel time series data for each participant, and Figure 1C depicts the 30–75 Hz time series envelope collapsed across all participants. Additionally, this same peak voxel was at the center of the 4 mm cortically-constrained ROI that was constructed, and from which cortical thickness values were extracted per participant (Figure 1D).
3.3. Cortical Thickness Predicts Gamma Response Power
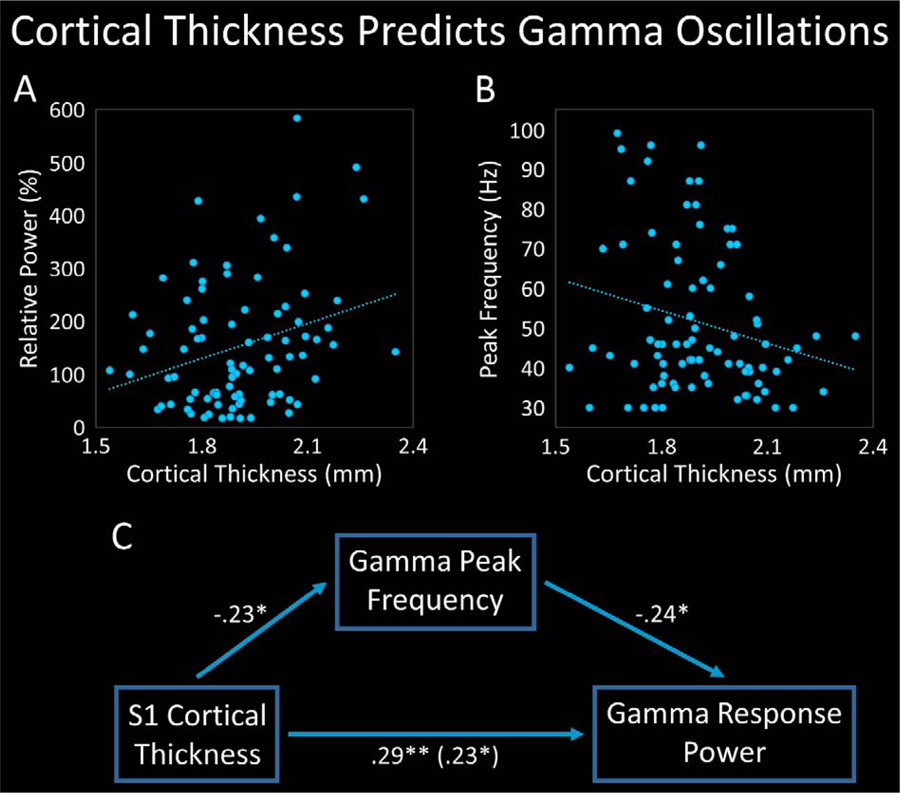
To investigate the relationship between the magnitude of the somatosensory-related gamma response and the cortical thickness of the tissue generating the response, we regressed the peak power of the S1 gamma response on S1 thickness. As hypothesized, S1 thickness significantly predicted the strength of the gamma response within the same region, such that for every 1 mm increase in thickness, a 220.4% increase in gamma response power would be expected, R2 = .083, p = .007 (Figure 2A; Table 1).
Figure 2.
Cortical Thickness Predicts Gamma Response Power and Peak Frequency. (A): Scatterplot displaying the relationship between primary somatosensory cortex (S1) thickness (x-axis) and the strength of the gamma response following the first somatosensory stimulation (Stim1; y-axis). S1 thickness significantly predicted the strength of the gamma response. (B): Scatter-plot depicting the relationship between S1 thickness (x-axis) and the peak frequency of the gamma response following Stim1 (y-axis). S1 thickness significantly predicted peak gamma frequency. (C): Mediation diagram in which S1 thickness is the causal variable, gamma response power is the outcome variable, and peak gamma frequency is the mediator. Peak frequency significantly and partially mediated the relationship between S1 thickness and the magnitude of the gamma response, and both the direct and indirect effects were significant. Standardized regression coefficients are displayed. *p < .05. **p < .01.
Table 1.
Hierarchical Multiple Regression Results for Gamma Response Power on Cortical Thickness (CT) and Peak Gamma Frequency
| Model | b | SE | t | β | F | R2 | ∆F | ∆R2 | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Simple regression of gamma response power on cortical thickness | |||||||||
| Intercept | −2.67 | 1.53 | −1.74 | 7.56** | .08 | (−5.72, 0.38) | |||
| CT | 2.20 | 0.80 | 2.75** | 0.29 | (0.61, 3.80) | ||||
| Simple regression of peak gamma frequency on cortical thickness | |||||||||
| Intercept | 103.46 | 24.11 | 4.29**** | 4.68* | .05 | (55.51, 151.41) | |||
| CT | −27.25 | 12.60 | −2.16* | −0.23 | (−52.32, −2.19) | ||||
| Simple regression of gamma response power on peak gamma frequency | |||||||||
| Intercept | 2.52 | 0.37 | 6.80**** | 8.09** | .09 | (1.79, 3.26) | |||
| Frequency | −0.02 | 0.01 | −2.84** | −0.30 | (−0.03, −0.01) | ||||
| Hierarchical regression of gamma response power on cortical thickness and peak gamma frequency | |||||||||
| Intercept | −1.04 | 1.65 | −0.63 | 6.67*** | .14 | (−4.32, 2.25) | |||
| CT | 1.77 | 0.80 | 2.21* | 0.23 | (0.18, 3.37) | ||||
| Frequency | −0.02 | 0.01 | −2.32* | −0.24 | 5.39* | .06 | (−0.03, −0.002) | ||
Note. N = 85. b = unstandardized regression coefficient. SE = standard error. β = standardized regression coefficient. ΔF = F change. ΔR2 = R2 change. CI = confidence interval.
p < .05.
p < .01.
p < .005.
p < .001.
3.4. Cortical Thickness Predicts Peak Gamma Frequency
To characterize the relationship between the peak frequency of the somatosensory-related gamma response to Stim1 and the cortical thickness of the tissue generating the response, we regressed S1 peak gamma frequency on S1 thickness. As hypothesized, S1 thickness significantly predicted the peak frequency of the S1 gamma response, such that for every 1 mm increase in S1 thickness, a 27.25 Hz decrease in peak frequency would be anticipated, R2 = .053, p = .033 (Figure 2B; Table 1).
3.5. Peak Frequency Predicts Gamma Response Power
As previous research using a median-nerve stimulation paradigm has demonstrated a negative relationship between S1 gamma response power and the peak frequency of the response,6 we regressed gamma response power on peak frequency to investigate whether a similar relationship was present in our data. Corroborating previous research, peak frequency significantly predicted the magnitude of the somatosensory gamma response, such that for every 1 Hz increase in peak frequency, the strength of the gamma response would be expected to decrease by 1.9%, R2 = .089, p = .006 (Table 1).
3.6. Peak Frequency Mediates the Relationship Between Cortical Thickness and Gamma Response Power
Taking the aforementioned relationships into account, we next investigated whether peak frequency mediated the relationship between S1 thickness and S1 gamma response power by performing a mediation analysis in accordance with the method described by Baron and Kenny.72 Specifically, a hierarchical regression was computed in which the power of the S1 gamma response following Stim1 was regressed on cortical thickness in the first step, and additionally regressed on the peak frequency of the response in the second step. Interestingly, S1 thickness significantly predicted gamma response power above-and-beyond the effect of peak frequency, p = .030, and peak frequency significantly and uniquely predicted gamma response power above-and-beyond the effect of cortical thickness, ∆R2 = .056, p = .023 (Figure 2C; Table 1). Thus, the hierarchical regression suggested that peak frequency partially mediated the relationship between S1 thickness and S1 gamma response power. To determine the significance of the indirect effect, a statistically stringent nonparametric bootstrapping analysis with 10,000 simulations was employed.73,74 The results demonstrated that both the direct and indirect effects were significant, b = 1.773, p = .029, CI [0.16, 3.49], and b = .430, p = .023, CI [.042, .990], respectively, and peak frequency significantly and partially mediated the relationship between S1 thickness and the strength of the gamma response.
3.7. Cortical Thickness Predicts Somatosensory Gating
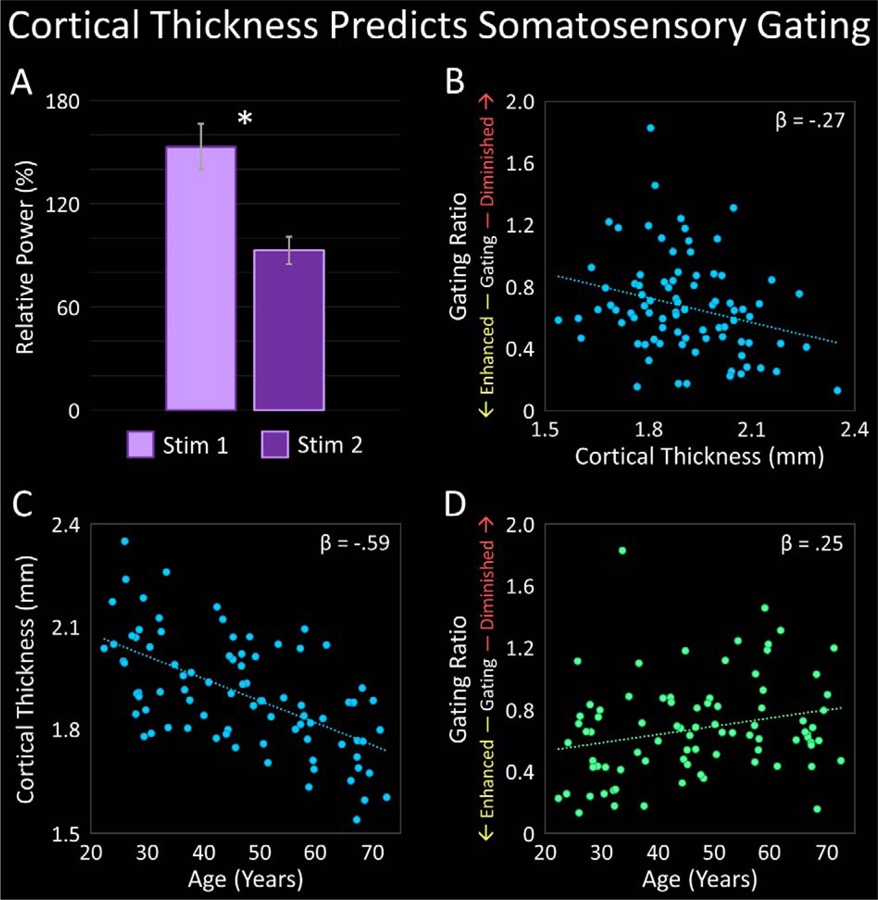
We first investigated whether significant gating was observed in the present study using a paired-samples t-test, which confirmed that the power of the gamma response following Stim1 was significantly greater than the power of the gamma response following Stim2, t(84) = 7.43, p < .001 (Figure 3A). Thus, significant somatosensory gating was observed. To assess the relationship between such gating and the thickness of the cortical tissue generating the response, we regressed the gating ratio on S1 thickness. As expected, S1 thickness significantly predicted the gating ratio, such that for every 1 mm increase in S1 thickness, the gating ratio would be expected to decrease by .53, R2 = .072, p = .013 (Figure 3B; Table 2). In other words, participants who had thicker S1 cortices were anticipated to have greater suppression of redundant information (i.e., lower gating ratios).
Figure 3.
Cortical Thickness and Age Predict Somatosensory Gating. (A): Bar graph depicting the average strength of the 30–75 Hz gamma response within the primary somatosensory cortex (S1) following the first stimulation (Stim1; lavender) and second stimulation (Stim2; violet). The response following Stim2 was significantly weaker than that following Stim1, indicating significant somatosensory gating. *(p < .001). (B): Scatterplot displaying the relationship between S1 thickness (x-axis) and the gating ratio (Stim2/Stim1; y-axis). S1 thickness significantly predicted somatosensory gating (p < .05). (C): Scatterplot depicting the relationship between age (x-axis) and S1 thickness (y-axis). Age significantly predicted S1 thickness (p < .001). (D): Scatterplot demonstrating the relationship between age (x-axis) and the gating ratio (y-axis). Age significantly predicted somatosensory gating (p < .05). Standardized regression coefficients are displayed in the upper right corner of each scatterplot.
Table 2.
Hierarchical Multiple Regression Results for Gating Ratio on Cortical Thickness (CT) and Age
| Model | b | SE | t | β | F | R2 | ΔF | ΔR2 | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Simple regression of gating ratio on cortical thickness | |||||||||
| Intercept | 1.68 | 0.40 | 4.23**** | 6.47* | .07 | (0.89, 2.47) | |||
| CT | −0.53 | 0.21 | −2.54* | −0.27 | (−0.94, −0.12) | ||||
| Simple regression of cortical thickness on age | |||||||||
| Intercept | 2.21 | 0.05 | 47.01**** | 45.26**** | .35 | (2.11, 2.30) | |||
| Age | −0.01 | 0.001 | −6.73**** | −0.59 | (−0.008, −0.005) | ||||
| Simple regression of gating ratio on age | |||||||||
| Intercept | 0.43 | 0.11 | 3.82**** | 5.53* | .06 | (0.21, 0.65) | |||
| Age | 0.01 | 0.002 | 2.35* | 0.25 | (0.001, 0.01) | ||||
| Hierarchical regression of gating ratio on age and cortical thickness | |||||||||
| Intercept | 1.23 | 0.58 | 2.12* | 3.80* | .09 | (0.08, 2.39) | |||
| Age | 0.003 | 0.003 | 1.06 | 0.14 | (−0.003, 0.009) | ||||
| CT | −0.37 | 0.26 | −1.42 | −0.19 | 2.01 | .02 | (−0.88, 0.15) | ||
Note. N = 85. b = unstandardized regression coefficient. SE = standard error. β = standardized regression coefficient. ΔF = F change. ΔR2 = R2 change. CI = confidence interval.
p < .05.
p < .01.
p < .005.
p < .001.
3.8. Age Predicts Cortical Thickness and Somatosensory Gating
As cortical thickness has been shown to decrease with advancing age,33,35 we also assessed the relationship between these two variables by regressing S1 thickness on age. In line with previous studies, age significantly predicted S1 thickness, such that for every 1 year increase in age, the thickness of S1 would be expected to decrease by .006 mm, R2 = .353, p < .001 (Figure 3C; Table 2). Additionally, simple regressions between each gamma-related metric and age revealed that age significantly predicted gating ratio, such that for every 1 year increase in age, the gating ratio would be expected to increase by .005, R2 = .062, p = .021 (Figure 3D; Table 2). Stated differently, age was negatively associated with somatosensory gating, such that individuals generally had reduced suppression of redundant information with increasing age. Given these relationships, we next probed whether S1 thickness mediated the relationship between age and somatosensory gating, again using the Baron and Kenny method.72 That is, a hierarchical regression was performed in which gating ratio was regressed on age in the first step, and additionally regressed on S1 thickness in the second step. The results indicated that while the overall model significantly predicted the gating ratio, R2 = .085, p = .026, cortical thickness did not significantly relate to the gating ratio above-and-beyond the effect of age, p = .160, nor did age significantly relate to the gating ratio above-and-beyond the effect of cortical thickness, p = .291 (Table 2). This pattern of results suggests that while both age and the thickness of S1 were related to somatosensory gating, the collinearity between age and cortical thickness made it difficult to parse apart the independent contributions of both using the present model. Finally, age did not significantly predict S1 gamma response power, R2 = .035, p = .088, nor the peak frequency of the gamma response, R2 = .009, p = .387.
For completeness we also probed whether somatosensory gating was related to gamma response power and peak gamma frequency, and these analyses are described in detail in the supplementary information. Briefly, peak gamma frequency was not related to somatosensory gating. Gamma response power was related to somatosensory gating, but this relationship could not be parsed apart from that of cortical thickness.
4. Discussion
Herein, we used a multimodal neuroimaging approach to characterize the relationships between somatosensory-related gamma oscillations and the thickness of the cortical tissue generating those oscillations. Our results portrayed a multi-faceted relationship between structure and function, as cortical thickness was predictive of both the strength and the peak frequency of the S1 gamma response, as well as somatosensory gating. Furthermore, peak frequency partially mediated the relationship between cortical thickness and the magnitude of the response. These findings and their implications are discussed in greater detail below.
Cortical thinning is believed to primarily reflect a reduction in neuronal size via declines in dendritic arborization and synaptic density,75 and evidence suggests that cortical oscillatory activity, as detected by MEG, chiefly reflects the population-level synchronized post-synaptic dendritic currents of pyramidal neurons.76 As such, the positive association that we observed between gamma response strength following somatosensory stimulation and the thickness of the cortical patch generating those oscillations is not altogether surprising. Essentially, if a population of neurons is comprised of pyramidal cells that contain fewer synapses and truncated dendrites, this would presumably lead to an overall reduction in the amount of net electrical activity within the region, as there would physically be fewer synaptic sites to generate synaptic potentials, as well as reduced dendritic structure through which current could flow. This in turn would lead to weaker oscillatory responses when this more structurally-sparse population becomes synchronized.
While our findings, as well as those of Edgar and colleagues,47 appear to lend support to the aforementioned interpretation, our results also suggest that this is likely an oversimplification of the structure-function relationship. That is, while cortical thickness exhibited a direct effect on gamma response power, it also demonstrated an indirect effect through peak gamma frequency. Importantly, cortical thinning likely reflects changes to the structural properties of both pyramidal cells and the inhibitory interneurons that are essential for the generation of gamma oscillations. Extensive evidence suggests that gamma oscillations are generated by local networks of fast-spiking parvalbumin-expressing interneurons that precisely time γ-aminobutyric acidA (GABAA) mediated inhibition.77 As such, the relationship between gamma response power and cortical thickness that we observed may be mediated by GABAA receptor density and/or GABA concentration. Interestingly, numerous visual and sensorimotor studies have reported a positive association between gamma frequency and GABA concentration within the same cortical regions.24,44,56,78 As GABA is the most copious inhibitory neurotransmitter in the cortex, it has been postulated that gamma frequency serves as an oscillatory marker of local inhibition in sensorimotor regions,5 with higher gamma frequencies indicative of blunted bottom-up processing of the stimulus. On the flip side of this, lower gamma frequency may then be denotive of lower local GABA concentration, and thus, an accentuated initial processing of the stimulus. In the present study, such enhancement would presumably facilitate the processing of the somatosensory stimulus, and a similar interpretation has been put forth by Cheyne concerning the motor-related gamma response.79 Taken together, while a stronger somatosensory gamma response may partially owe to structural components including greater synaptic density and dendritic arborizations, an enhancement of the response via lower gamma frequency may also be at play. However, it is important to acknowledge that these interpretations are speculative, and further research is needed to directly test these relationships.
Thus far, the discussion has centered on the relationship between cortical thickness and the gamma response induced by Stim1, but an equally interesting discovery was the relationship between structure and somatosensory gating. As previously mentioned, sensory gating is believed to have a beneficial higher-order inhibitory function by which the brain inhibits the processing of redundant stimuli, and protects the organism from being flooded with irrelevant information.54 Thus, decreased sensory gating likely reflects deficient inhibitory function.52 In agreement with previous research,5,35,80,81 our results suggest that with older age comes impaired somatosensory neural inhibition, as well as thinning of S1. We extend this literature by demonstrating that S1 thinning is also predictive of degraded higher-order somatosensory inhibition, but at present the tight relationship between age and S1 thickness renders it difficult to disentangle the independent effect of either in relation to sensory gating.
Perhaps with aging and cortical thinning the structural loss of key intercellular circuit-level connections renders the local network of neurons less dynamic in function, and thus more susceptible to irrelevant information. This interpretation ties into an influential theory in neurocognitive aging: the dedifferentiation hypothesis.82 The theory posits that healthy aging is accompanied by neural dedifferentiation, in which the function of a given neural region becomes less distinct and/or less specialized with age.82,83 Applying this to the present study, in younger adults, the presumably abundant synapses and dendritic arbors within S1 may permit more dynamic and selective oscillatory responses to differentially process Stim1 and Stim2. However, in older adults, the presumably sparser structural connections within S1 may hinder the plasticity of these oscillatory mechanisms, thereby increasing similarity among the processing of Stim1 and Stim2. Again, this interpretation is speculative in nature and is likely overly simplistic, as a combination of neurobiological factors likely underlie the pattern of results we observed. For example, age-related reductions in sensorimotor cortex GABA levels have been reported, and evidence suggests that such alterations contribute to age-related neural dedifferentiation.84 Thus, GABA-mediated inhibition may be a critical component in further elucidating the relationships between age, cortical thickness, and gamma-based somatosensory gating, and future research should investigate these variables simultaneously.
Taken together, the novel relationships unveiled in the present experiment represent a substantial step forward in our understanding of the complex linkage between gamma oscillations and the structural integrity of the cortical tissue generating those oscillations. However, the study was not without limitations. For example, our study was cross-sectional in design, and as such we were unable to assess changes in cortical thickness and gamma-related metrics over time. A longitudinal approach would further enhance our understanding of the structure-function relationships, as cortical thinning and changes in somatosensory gamma oscillations may progress at different rates. Additionally, tracking longitudinal change may aid in causal interpretation, if certain alterations are observed to precede others in time. A second limitation of the present study was our exclusive focus on somatosensory gamma oscillations. It is widely recognized that somatosensory stimulation induces oscillatory responses in other frequency bands, including alpha and beta, which are temporally distinct from the gamma response and may reflect higher-order perceptual and attentional processes.85–88 Additionally, a large body of literature has characterized the evoked responses following somatosensory stimulation. Herein we focused on gamma oscillations, as their tight link to both bottom-up sensory processing and higher-order inhibitory function may be particularly relevant for various clinical disorders characterized by sensory deficits and/or disinhibition,4,47,89,90 as well as for advancing the understanding of age-related reductions in such functions. However, future studies should assess the relationships between cortical thickness and both lower-frequency somatosensory oscillations and evoked responses.
5. Conclusions
In summary, the present study was the first to merge sMRI and MEG to characterize the relationships between somatosensory-related gamma oscillations and the local thickness of the cortical tissue generating those oscillations. Our data indicate a rich connection between structure and function, and suggest that cortical thickness is tied to both basic sensory processing, as well as higher-order inhibitory function. Further, these results encourage the future use of multimodal neuroimaging in the study of clinical disorders that are characterized by atypical gamma oscillations,3,11,91,92 aberrant sensory function, and/or disinhibition, as well as in investigations of age-related sensory and inhibitory decline.
Supplementary Material
Highlights.
Possible links between local cortical thickness and gamma-band activity are unclear
Adults underwent structural MRI and a somatosensory gating task during MEG
Advanced oscillatory and surface-based morphometry analysis methods were combined
Local cortical thickness predicted gamma response power, frequency, and gating
Peak gamma frequency mediated the relationship between thickness and response power
Acknowledgements and Financial Disclosures
Funding: This work was supported by the National Institutes of Health [grants R01 MH103220, R01 MH116782, R01 MH118013 (TWW); F31 AG055332 (AIW)] and the National Science Foundation [grant #1539067 (TWW)]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Proskovec, Ms. Spooner, Mr. Wiesman, and Dr. Wilson report no competing financial or other interests.
References
- 1.Hoogenboom N, Schoffelen JM, Oostenveld R, Parkes LM & Fries P Localizing human visual gamma-band activity in frequency, time and space. Neuroimage 29, 764–773, 10.1016/j.neuroimage.2005.08.043 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Muthukumaraswamy SD & Singh KD Visual gamma oscillations: the effects of stimulus type, visual field coverage and stimulus motion on MEG and EEG recordings. Neuroimage 69, 223–230, 10.1016/j.neuroimage.2012.12.038 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Wilson TW, Wetzel MW, White ML & Knott NL Gamma-frequency neuronal activity is diminished in adults with attention-deficit/hyperactivity disorder: a pharmaco-MEG study. J Psychopharmacol 26, 771–777, 10.1177/0269881111430731 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson TW et al. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex 18, 371–378, 10.1093/cercor/bhm062 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spooner RK, Wiesman AI, Proskovec AL, Heinrichs-Graham E & Wilson TW Rhythmic Spontaneous Activity Mediates the Age-Related Decline in Somatosensory Function. Cereb [DOI] [PMC free article] [PubMed]
- 6.Spooner RK et al. Aberrant oscillatory dynamics during somatosensory processing in HIV-infected adults. Neuroimage Clin 20, 85–91, 10.1016/j.nicl.2018.07.009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fries P Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annual review of neuroscience 32, 209–224, 10.1146/annurev.neuro.051508.135603 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Trevarrow MP et al. The developmental trajectory of sensorimotor cortical oscillations. Neuroimage 184, 455–461, 10.1016/j.neuroimage.2018.09.018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinrichs-Graham E, Hoburg JM & Wilson TW The peak frequency of motor-related gamma oscillations is modulated by response competition. Neuroimage 165, 27–34, 10.1016/j.neuroimage.2017.09.059 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurz MJ, Becker KM, Heinrichs-Graham E & Wilson TW Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Developmental medicine and child neurology 56, 1072–1077, 10.1111/dmcn.12513 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson TW et al. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol 36, 596–613, 10.1080/87565641.2011.555573 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson TW et al. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain and cognition 73, 75–84, 10.1016/j.bandc.2010.03.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiesman AI & Wilson TW The impact of age and sex on the oscillatory dynamics of visuospatial processing. Neuroimage 185, 513–520, 10.1016/j.neuroimage.2018.10.036 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magazzini L & Singh KD Spatial attention modulates visual gamma oscillations across the human ventral stream. Neuroimage 166, 219–229, 10.1016/j.neuroimage.2017.10.069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel M, Donner TH, Oostenveld R, Fries P & Engel AK Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron 60, 709–719, 10.1016/j.neuron.2008.09.010 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Howard MW et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex 13, 1369–1374, 10.1093/cercor/bhg084 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Tallon-Baudry C, Bertrand O, Peronnet F & Pernier J Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci 18, 4244–4254 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sederberg PB et al. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex 17, 1190–1196, 10.1093/cercor/bhl030 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Osipova D et al. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci 26, 7523–7531, 10.1523/JNEUROSCI.1948-06.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proskovec AL, Wiesman AI & Wilson TW The strength of alpha and gamma oscillations predicts behavioral switch costs. Neuroimage 188, 274–281, 10.1016/j.neuroimage.2018.12.016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho RY, Konecky RO & Carter CS Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America 103, 19878–19883, 10.1073/pnas.0609440103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakai Y et al. Four-dimensional functional cortical maps of visual and auditory language: Intracranial recording. Epilepsia 60, 255–267, 10.1111/epi.14648 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnefond M, Kastner S & Jensen O Communication between Brain Areas Based on Nested Oscillations. eNeuro 4, 10.1523/ENEURO.0153-16.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaetz W, Edgar JC, Wang DJ & Roberts TP Relating MEG measured motor cortical oscillations to resting gamma-aminobutyric acid (GABA) concentration. Neuroimage 55, 616–621, 10.1016/j.neuroimage.2010.12.077 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heideman SG et al. Anticipatory neural dynamics of spatial-temporal orienting of attention in younger and older adults. Neuroimage 178, 46–56, 10.1016/j.neuroimage.2018.05.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An KM et al. Altered Gamma Oscillations during Motor Control in Children with Autism Spectrum Disorder. J Neurosci 38, 7878–7886, 10.1523/JNEUROSCI.1229-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L et al. Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: evidence for dysfunctional network activity in frontal-posterior cortices. J Neurosci 32, 9563–9573, 10.1523/JNEUROSCI.1073-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirano Y et al. Spontaneous Gamma Activity in Schizophrenia. JAMA Psychiatry 72, 813–821, 10.1001/jamapsychiatry.2014.2642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu TY, Chen YS, Su TP, Hsieh JC & Chen LF Abnormal early gamma responses to emotional faces differentiate unipolar from bipolar disorder patients. Biomed Res Int 2014, 906104, 10.1155/2014/906104 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barratt EL et al. Abnormal task driven neural oscillations in multiple sclerosis: A visuomotor MEG study. Hum Brain Mapp 38, 2441–2453, 10.1002/hbm.23531 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barry RJ et al. Resting-state EEG gamma activity in children with attention-deficit/hyperactivity disorder. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 121, 1871–1877, 10.1016/j.clinph.2010.04.022 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Wilson TW, Heinrichs-Graham E, White ML, Knott NL & Wetzel MW Estimating the passage of minutes: deviant oscillatory frontal activity in medicated and unmedicated ADHD. Neuropsychology 27, 654–665, 10.1037/a0034032 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fjell AM et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex 19, 2001–2012, 10.1093/cercor/bhn232 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fjell AM & Walhovd KB Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci 21, 187–221 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Salat DH et al. Thinning of the cerebral cortex in aging. Cereb Cortex 14, 721–730, 10.1093/cercor/bhh032 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Zielinski BA et al. Longitudinal changes in cortical thickness in autism and typical development. Brain : a journal of neurology 137, 1799–1812, 10.1093/brain/awu083 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X et al. Reduced cortical thickness in right Heschl’s gyrus associated with auditory verbal hallucinations severity in first-episode schizophrenia. BMC Psychiatry 15, 152, 10.1186/s12888-015-0546-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Eijndhoven P et al. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. The American journal of psychiatry 170, 1477–1486, 10.1176/appi.ajp.2013.12121504 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Righart R et al. Volume versus surface-based cortical thickness measurements: A comparative study with healthy controls and multiple sclerosis patients. PloS one 12, e0179590, 10.1371/journal.pone.0179590 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narr KL et al. Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 48, 1014–1022, 10.1097/CHI.0b013e3181b395c0 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muthukumaraswamy SD, Singh KD, Swettenham JB & Jones DK Visual gamma oscillations and evoked responses: variability, repeatability and structural MRI correlates. Neuroimage 49, 3349–3357, 10.1016/j.neuroimage.2009.11.045 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Gaetz W, Roberts TP, Singh KD & Muthukumaraswamy SD Functional and structural correlates of the aging brain: relating visual cortex (V1) gamma band responses to age-related structural change. Hum Brain Mapp 33, 2035–2046, 10.1002/hbm.21339 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Pelt S, Shumskaya E & Fries P Cortical volume and sex influence visual gamma. Neuroimage 178, 702–712, 10.1016/j.neuroimage.2018.06.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edden RA, Muthukumaraswamy SD, Freeman TC & Singh KD Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci 29, 15721–15726, 10.1523/JNEUROSCI.4426-09.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robson SE et al. Structural and neurochemical correlates of individual differences in gamma frequency oscillations in human visual cortex. J Anat 227, 409–417, 10.1111/joa.12339 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarzkopf DS, Robertson DJ, Song C, Barnes GR & Rees G The frequency of visually induced gamma-band oscillations depends on the size of early human visual cortex. J Neurosci 32, 1507–1512, 10.1523/JNEUROSCI.4771-11.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edgar JC et al. Cortical thickness as a contributor to abnormal oscillations in schizophrenia? Neuroimage Clin 4, 122–129, 10.1016/j.nicl.2013.11.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaetz WC & Cheyne DO Localization of human somatosensory cortex using spatially filtered magnetoencephalography. Neuroscience letters 340, 161–164 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Ihara A et al. Neuromagnetic gamma-band activity in the primary and secondary somatosensory areas. Neuroreport 14, 273–277, 10.1097/01.wnr.0000053663.14657.5b (2003). [DOI] [PubMed] [Google Scholar]
- 50.Hagiwara K et al. Oscillatory gamma synchronization binds the primary and secondary somatosensory areas in humans. Neuroimage 51, 412–420, 10.1016/j.neuroimage.2010.02.001 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Hsiao FJ, Cheng CH, Chen WT & Lin YY Neural correlates of somatosensory paired-pulse suppression: a MEG study using distributed source modeling and dynamic spectral power analysis. Neuroimage 72, 133–142, 10.1016/j.neuroimage.2013.01.041 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Cheng CH et al. Sensory gating, inhibition control and gamma oscillations in the human somatosensory cortex. Scientific reports 6, 20437, 10.1038/srep20437 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiesman AI et al. Oscillatory dynamics and functional connectivity during gating of primary somatosensory responses. J Physiol 595, 1365–1375, 10.1113/JP273192 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cromwell HC, Mears RP, Wan L & Boutros NN Sensory gating: a translational effort from basic to clinical science. Clin EEG Neurosci 39, 69–72, 10.1177/155005940803900209 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagiwara K et al. Age-related changes across the primary and secondary somatosensory areas: an analysis of neuromagnetic oscillatory activities. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 125, 1021–1029, 10.1016/j.clinph.2013.10.005 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB & Singh KD Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proceedings of the National Academy of Sciences of the United States of America 106, 8356–8361, 10.1073/pnas.0900728106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson TW, McDermott TJ, Mills MS, Coolidge NM & Heinrichs-Graham E tDCS Modulates Visual Gamma Oscillations and Basal Alpha Activity in Occipital Cortices: Evidence from MEG. Cereb Cortex 28, 1597–1609, 10.1093/cercor/bhx055 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taulu S & Simola J Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in medicine and biology 51, 1759–1768, 10.1088/0031-9155/51/7/008 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Taulu S, Simola J & Kajola M Applications of the signal space separation method. IEEE Transactions on Signal Processing 53, 3359–3372, 10.1109/TSP.2005.853302 (2005). [DOI] [Google Scholar]
- 60.Uusitalo MA & Ilmoniemi RJ Signal-space projection method for separating MEG or EEG into components. Medical & biological engineering & computing 35, 135–140 (1997). [DOI] [PubMed] [Google Scholar]
- 61.Ernst MD Permutation Methods: A Basis for Exact Inference. Statistical Science 19, 676–685, 10.1214/088342304000000396 (2004). [DOI] [Google Scholar]
- 62.Maris E & Oostenveld R Nonparametric statistical testing of EEG- and MEG-data. Journal of neuroscience methods 164, 177–190, 10.1016/j.jneumeth.2007.03.024 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Gross J et al. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proceedings of the National Academy of Sciences of the United States of America 98, 694–699, 10.1073/pnas.98.2.694 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hillebrand A, Singh KD, Holliday IE, Furlong PL & Barnes GR A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25, 199–211, 10.1002/hbm.20102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Veen BD, van Drongelen W, Yuchtman M & Suzuki A Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE transactions on bio-medical engineering 44, 867–880, 10.1109/10.623056 (1997). [DOI] [PubMed] [Google Scholar]
- 66.Dahnke R, Yotter RA & Gaser C Cortical thickness and central surface estimation. Neuroimage 65, 336–348, 10.1016/j.neuroimage.2012.09.050 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Ashburner J & Friston KJ Unified segmentation. Neuroimage 26, 839–851, 10.1016/j.neuroimage.2005.02.018 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Yotter RA, Dahnke R, Thompson PM & Gaser C Topological correction of brain surface meshes using spherical harmonics. Hum Brain Mapp 32, 1109–1124, 10.1002/hbm.21095 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yotter RA, Thompson PM & Gaser C Algorithms to improve the reparameterization of spherical mappings of brain surface meshes. J Neuroimaging 21, e134–147, 10.1111/j.1552-6569.2010.00484.x (2011). [DOI] [PubMed] [Google Scholar]
- 70.Maldjian JA, Laurienti PJ, Kraft RA & Burdette JH An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003). [DOI] [PubMed] [Google Scholar]
- 71.Maldjian JA, Laurienti PJ & Burdette JH Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21, 450–455 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Baron RM & Kenny DA The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51, 1173–1182 (1986). [DOI] [PubMed] [Google Scholar]
- 73.RC, T. R: A language and environment for statistical computing. (2013).
- 74.Tingley D, Yamamoto T, Hirose K, Keele L & Imai K Mediation: R package for causal mediation analysis. Journal of Statistical Software 59, 1–38 (2014).26917999 [Google Scholar]
- 75.Esiri MM Ageing and the brain. J Pathol 211, 181–187, 10.1002/path.2089 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Lopes da Silva FH in MEG: An Introduction to Methods (eds Hansen PC, Kringelbach ML, & Salmelin R) Ch. 1, 1–23 (Oxford University Press, 2010). [Google Scholar]
- 77.Bartos M, Vida I & Jonas P Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature reviews. Neuroscience 8, 45–56, 10.1038/nrn2044 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Kujala J et al. Gamma oscillations in V1 are correlated with GABA(A) receptor density: A multimodal MEG and Flumazenil-PET study. Scientific reports 5, 16347, 10.1038/srep16347 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheyne DO MEG studies of sensorimotor rhythms: a review. Exp Neurol 245, 27–39, 10.1016/j.expneurol.2012.08.030 (2013). [DOI] [PubMed] [Google Scholar]
- 80.Cheng CH & Lin YY Aging-related decline in somatosensory inhibition of the human cerebral cortex. Experimental brain research 226, 145–152, 10.1007/s00221-013-3420-9 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Lenz M et al. Increased excitability of somatosensory cortex in aged humans is associated with impaired tactile acuity. J Neurosci 32, 1811–1816, 10.1523/JNEUROSCI.2722-11.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park DC, Polk TA, Mikels JA, Taylor SF & Marshuetz C Cerebral aging: integration of brain and behavioral models of cognitive function. Dialogues in clinical neuroscience 3, 151–165 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grady C The cognitive neuroscience of ageing. Nature reviews. Neuroscience 13, 491–505, 10.1038/nrn3256 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cassady K et al. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage 186, 234–244, 10.1016/j.neuroimage.2018.11.008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng CH, Chan PY, Baillet S & Lin YY Age-Related Reduced Somatosensory Gating Is Associated with Altered Alpha Frequency Desynchronization. Neural plasticity 2015, 302878, 10.1155/2015/302878 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukuda M, Juhasz C, Hoechstetter K, Sood S & Asano E Somatosensory-related gamma-, beta- and alpha-augmentation precedes alpha- and beta-attenuation in humans. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 121, 366–375, 10.1016/j.clinph.2009.10.036 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bauer M, Oostenveld R, Peeters M & Fries P Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci 26, 490–501, 10.1523/JNEUROSCI.5228-04.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palva S, Linkenkaer-Hansen K, Naatanen R & Palva JM Early neural correlates of conscious somatosensory perception. J Neurosci 25, 5248–5258, 10.1523/JNEUROSCI.0141-05.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilson TW, Rojas DC, Reite ML, Teale PD & Rogers SJ Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biological psychiatry 62, 192–197, 10.1016/j.biopsych.2006.07.002 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wiesman AI et al. Aberrant occipital dynamics differentiate HIV-infected patients with and without cognitive impairment. Brain : a journal of neurology 141, 1678–1690, 10.1093/brain/awy097 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilson TW et al. Broadband neurophysiological abnormalities in the medial prefrontal region of the default-mode network in adults with ADHD. Hum Brain Mapp 34, 566–574, 10.1002/hbm.21459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilson TW et al. Aberrant high-frequency desynchronization of cerebellar cortices in early-onset psychosis. Psychiatry Res 174, 47–56, 10.1016/j.pscychresns.2009.03.009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.