Abstract
Introduction
Current guidelines do not routinely recommend adjuvant therapy for resected stage I large cell lung neuroendocrine cancer (LCNEC). However, data regarding the role of adjuvant therapy in early LCNEC are limited. This National Cancer Database (NCDB) analysis was performed to improve the evidence guiding adjuvant therapy for early LCNEC.
Methods
Overall survival (OS) of patients with pathologic T1-2aN0M0 LCNEC who underwent surgery in the NCDB from 2003 to 2015 was evaluated with Kaplan-Meier and multivariable Cox proportional hazards analyses. Patients who died within 30 days of surgery and with >R0 resection were excluded.
Results
Of 2642 patients meeting study criteria, 481 (18%) received adjuvant therapy. Adjuvant chemotherapy in stage IB patients was associated with a significant increase in OS (hazard ratio [HR] 0.67; 95% confidence interval [CI] 0.50, 0.90). However, there was no significant difference in survival between adjuvant chemotherapy and no adjuvant therapy for stage IA LCNEC (HR 0.92; 95%CI 0.75, 1.11). Adjuvant radiation, whether alone or in combination with chemotherapy, was not associated with a change in OS. In subgroup analysis, patients receiving adjuvant chemotherapy following lobar resection for stage IB LCNEC had a significant survival benefit compared to patients not receiving adjuvant therapy.
Conclusion
In early stage LCNEC, adjuvant chemotherapy appears to confer an additional overall survival advantage only in patients with completely resected stage IB LCNEC and not for patients with completely resected stage IA LCNEC.
Introduction:
Large cell lung neuroendocrine cancer (LCNEC) is a subset of non-small cell lung cancer (NSCLC), and comprises about 2-4% of all lung cancer, with an estimated 4000 to 8000 cases a year [1,2]. LCNEC carries a poor prognosis, with an overall five-year survival of approximately 35% and disease-free survival of 25% [2–4]. There is a poor understanding of the biology of LCNEC, and consequently no consensus on the best treatment strategy [5].
The role of adjuvant therapy is unclear in early LCNEC. There are limited data from primarily small retrospective analyses demonstrating that following surgical resection of stage I to III LCNEC, use of adjuvant chemotherapy is associated with an improved 5-year survival ranging from 51–59% [6–9]. In addition, a small prospective trial of 15 patients who received adjuvant platinum and etoposide chemotherapy compared to a historical control group of 23 patients who received no adjuvant chemotherapy demonstrated a clear survival benefit across stage I to III with use of adjuvant chemotherapy [10]. The current National Comprehensive Cancer Network guidelines recommend consideration of adjuvant chemotherapy for high-risk stage IB NSCLC including poorly differentiated neuroendocrine tumors, but do not explicitly recommend adjuvant therapy for stage IA or stage IB LCNEC.
We performed a large nationwide retrospective cohort study using the National Cancer Database (NCDB) to evaluate the impact of adjuvant therapy on survival in patients with completely resected stage I LCNEC.
Methods:
National Cancer Database
The NCDB is a combined endeavor of the American Cancer Society and the American College of Surgeons Commission, and includes about 80% of newly diagnosed cancers annually across the United States and Puerto Rico collected by certified tumor registrars in 1500 hospitals nationwide [11,12].
Study Protocol
This study was approved by our Institutional Review Board. From the NCDB, all patients diagnosed with pathologic T1-2aN0M0 LCNEC from January 1, 2003 through December 31, 2015 were identified using International Classification of Diseases for Oncology, Third Edition (ICD-0-3) histology and topography codes. ICD-0-3 histology codes 8012/3 (large cell carcinoma, NOS), 8013/3 (large cell neuroendocrine carcinoma), and 8014/3 (large cell carcinoma with rhabdoid phenotype) were used. The cohort was limited to patients with pathologic T1-2a LCNEC who underwent a surgical intervention in accordance with the newest AJCC’s eighth edition guidelines on lung cancer staging. Patients with node-positive disease (>N0), metastatic disease (M1), margin-positive resection (R1, R2, or unknown), unknown type of operation, and who died within 30 days of surgery were excluded (Fig. 1). The primary outcome of interest was overall survival (OS).
Figure 1.
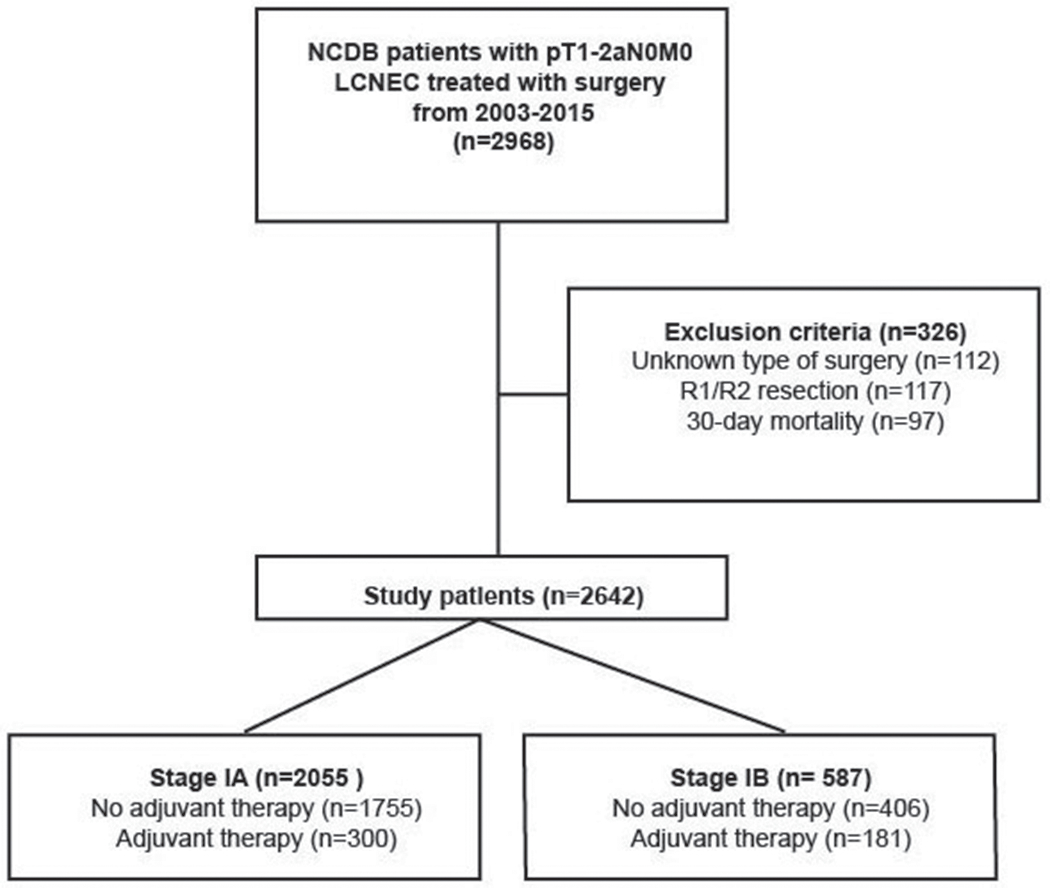
Scheme of patient selection for this study.
Statistical Analysis
Patients were stratified by type of adjuvant therapy received (surgery alone, chemotherapy alone, radiation alone, or chemotherapy with radiation). Comparisons between groups were performed with the Wilcoxon rank sum test for continuous variables and Pearson’s chi-squared test for categorical and ordinal variables. Differences in median survival and five-year survival were evaluated with the Kaplan-Meier product-limit and log-rank tests. A Cox proportional hazards regression model was used to further analyze survival. Variables included in the Cox model were determined to be clinically relevant a priori, and included type of adjuvant therapy, type of operation, age, sex, race, insurance type, and Charlson/Deyo comorbidity condition score (CDCC). A 1:1 propensity-score matched analysis was performed using the nearest-neighbor algorithm based on patient and tumor variables, limiting the analysis to patients who had been treated with a lobectomy or pneumonectomy, and stratifying patients by pathologic stage (stage IA and IB). All statistical analyses were performed with SPSS version 25 for Mac (IBM, Armonk, NY) and R version 3.5 for Mac (Vienna, Austria). A p value less than or equal to 0.05 was considered statistically significant.
Results:
A total of 2642 patients met study criteria (Fig. 1; Table 1). Of this cohort, 481 patients (18%) underwent adjuvant therapy of any kind, with the majority of patients (n=381; 79%) receiving chemotherapy alone. The characteristics of patients in the entire cohort are summarized in Table 1. The majority of patients were Caucasian, had government insurance, underwent a lobectomy, and had pathologic T1b or T1c disease. Patients undergoing adjuvant therapy were more likely to be younger, privately insured, and have a more advanced pathological T stage.
Table 1.
Demographic characteristics of study patients
| No Adjuvant Therapy (n= 2161) | Adjuvant Therapy (n= 481) | p value | |
|---|---|---|---|
| Age (years, median) | 67.1 | 62.5 | <0.001 |
| Sex (female) | 1067 (49.4%) | 237 (49.3%) | 0.967 |
| Race | 0.916 | ||
| White | 1935 (90.2%) | 431 (90.2%) | |
| Black | 179 (8.3%) | 41 (8.6%) | |
| Other | 32 (1.5%) | 6 (1.3%) | |
| Year of diagnosis, median (inter-quartile range) | 2008 (2005-2011) | 2008 (2005-2010) | 0.175 |
| CDCC Score | 0.287 | ||
| 0 | 923 (42.7%) | 221 (45.9%) | |
| 1 | 879 (40.7%) | 192 (39.9%) | |
| 2+ | 359 (16.6%) | 68 (14.1%) | |
| Insurance status | <0.001 | ||
| Private | 628 (29.4%) | 196 (41.0%) | |
| Government | 1468 (68.8%) | 270 (56.5%) | |
| None | 37 (1.7%) | 12 (2.5%) | |
| Facility location | 0.694 | ||
| Metro | 1666 (77.1%) | 374 (77.8%) | |
| Urban | 351 (16.2%) | 80 (16.6%) | |
| Rural | 144 (6.7%) | 27 (5.6%) | |
| Facility type | <0.001 | ||
| Community cancer program | 135 (6.3%) | 53 (11.1%) | |
| Comprehensive community cancer program | 972 (45.0%) | 228 (47.7%) | |
| Academic/research program | 795 (36.8%) | 147 (30.8%) | |
| Integrated network cancer program | 256 (11.9%) | 50 (10.5%) | |
| Surgery | 0.172 | ||
| Wedge resection | 357 (16.5%) | 74 (15.4%) | |
| Segmental resection | 81 (3.7%) | 11 (2.3%) | |
| Lobectomy | 1700 (78.7%) | 387 (80.5%) | |
| Pneumonectomy | 23 (1.1%) | 9 (1.9%) | |
| Pathologic T stage | <0.001 | ||
| Ia | 156 (7.2%) | 24 (5.0%) | |
| Ib | 846 (39.1%) | 126 (26.2%) | |
| Ic | 753 (34.8%) | 150 (31.2%) | |
| 2a | 406 (18.8%) | 181 (37.6%) | |
| Unplanned 30-day readmission | 95 (4.5%) | 14 (3.0%) | 0.151 |
| Adjuvant therapy | |||
| None | 2161 (100%) | ||
| Chemotherapy | 381 (79.2%) | ||
| Chemoradiation | 54 (11.2%) | ||
| Radiation | 46 (9.6%) |
The median survival for patients in the cohort was 68 months (95% confidence interval [CI] 63, 72); patients who underwent adjuvant therapy had a median survival of 81 months (95%CI 68, 94), while those who did not had a survival of 65 months (95%CI 60, 70). Five-year OS was 53% (95%CI 51, 55) for the entire cohort. Univariable analysis demonstrated a significant increase in OS with the use of adjuvant therapy for stage I LCNEC compared to no adjuvant therapy (log-rank p=0.002).
When limiting the analysis to only patients with stage IA LCNEC, there was no significant survival benefit with adjuvant therapy compared to no adjuvant therapy (Fig. 2). However, there was a significant survival benefit associated with adjuvant therapy for stage IB (Fig. 3). The median survival and five-year OS for stage IA patients with adjuvant therapy was 73 months (95%CI 58, 88) and 56% (95%CI 50, 62), compared to 68 months (95%CI 63, 73) and 54% (95%CI 51, 56) for patients without adjuvant therapy, respectively. Stage IB patients with adjuvant therapy had a median and five-year OS of 86 months (95%CI 67, 104) and 62% (95%CI 54, 70), compared to 46 months (95%CI 37, 56) and 43% (95%CI 38, 48) for those without adjuvant therapy, respectively.
Figure 2.
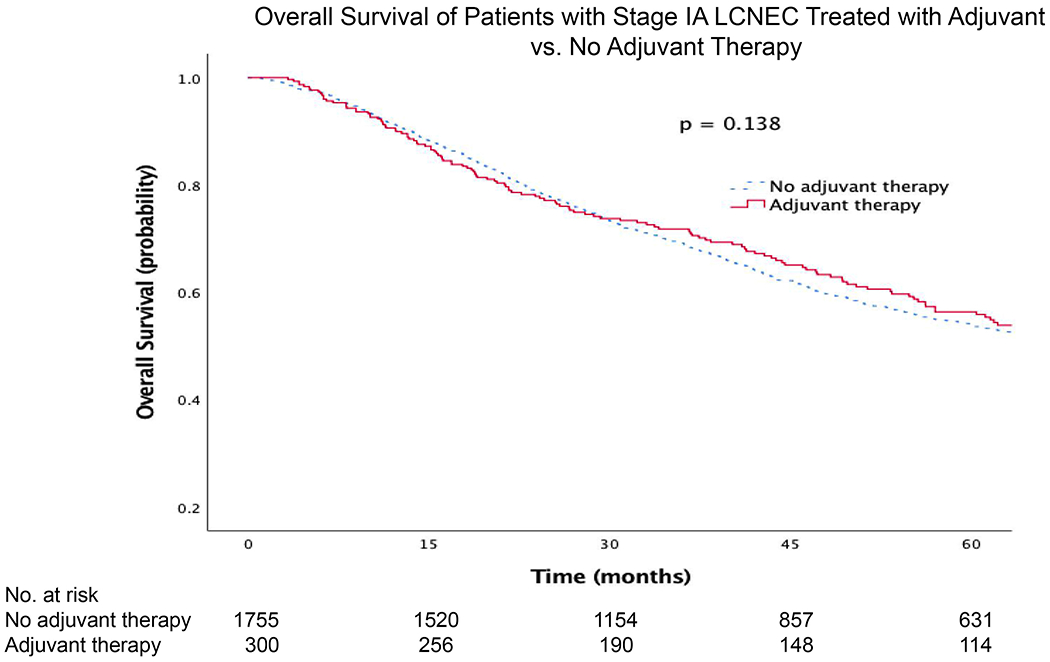
Kaplan-Meier survival curves for patients with stage IA LCNEC treated with adjuvant vs. no adjuvant therapy
Figure 3.
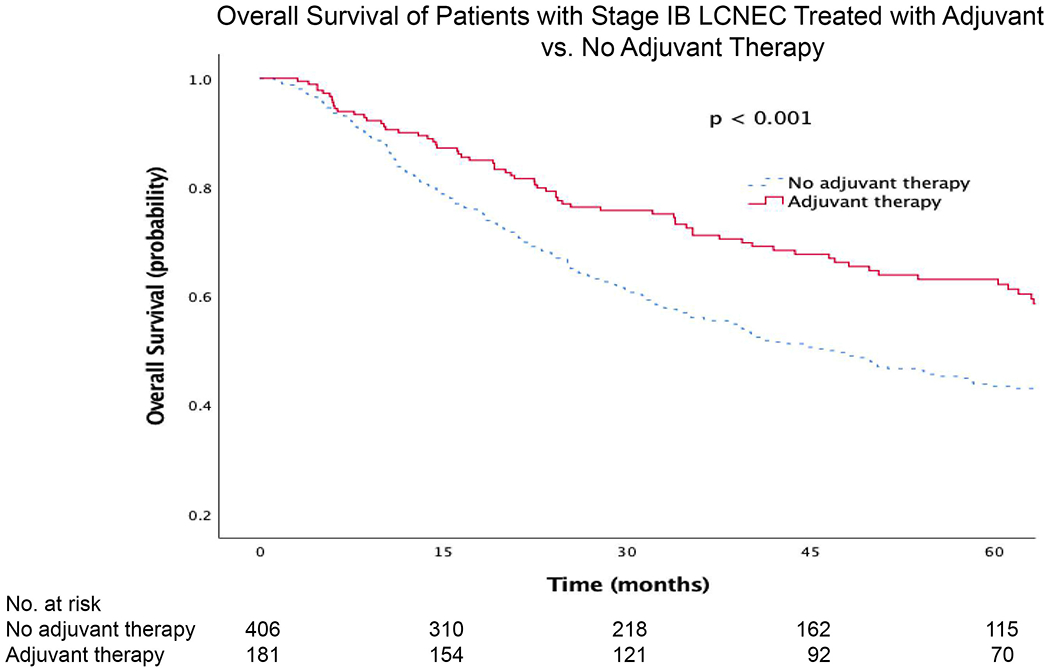
Kaplan-Meier survival curves for patients with stage IB LCNEC treated with adjuvant vs. no adjuvant therapy
Multivariable Cox modeling demonstrated no significant survival advantage for adjuvant chemotherapy in stage IA (Table 2), but demonstrated a survival benefit for adjuvant chemotherapy in stage IB (Table 3). Notably, adjuvant radiation therapy alone and adjuvant chemoradiation were not associated with improved survival when compared to no therapy in the overall cohort and in stage IA; however, radiation alone was a weak independent predictor of mortality in stage IB (Tables 2–3).
Table 2.
Cox multivariable regression of independent predictors of survival in patients with stage IA LCNEC treated with or without adjuvant therapy
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.02 | 1.02 | 1.03 | < 0.001 |
| Female sex | 0.86 | 0.76 | 0.97 | 0.013 |
| Race | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 0.79 | 0.62 | 1.02 | 0.066 |
| Other | 0.91 | 0.54 | 1.51 | 0.707 |
| Charleson-Deyo Comorbidity Index | ||||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 1.14 | 1.00 | 1.30 | 0.058 |
| 2+ | 1.52 | 1.29 | 1.80 | < 0.001 |
| Year of diagnosis (per year) | 0.99 | 0.96 | 1.01 | 0.183 |
| Insurance status | ||||
| Private | Ref | Ref | Ref | Ref |
| Government | 1.21 | 1.03 | 1.42 | 0.022 |
| None | 1.50 | 0.86 | 2.64 | 0.157 |
| Facility location | ||||
| Metro | Ref | Ref | Ref | Ref |
| Urban | 0.96 | 0.81 | 1.13 | 0.605 |
| Rural | 1.28 | 1.01 | 1.63 | 0.041 |
| Facility type | ||||
| Community Cancer Program | Ref | Ref | Ref | Ref |
| Comprehensive Community Cancer Program | 1.10 | 0.86 | 1.40 | 0.466 |
| Academic/Research Program | 0.99 | 0.77 | 1.27 | 0.913 |
| Integrated Network Cancer Program | 1.29 | 0.97 | 1.71 | 0.086 |
| Surgery | ||||
| Wedge resection | Ref | Ref | Ref | Ref |
| Segmental resection | 1.24 | 0.91 | 1.69 | 0.178 |
| Lobectomy | 0.72 | 0.62 | 0.83 | < 0.001 |
| Pneumonectomy | 1.08 | 0.61 | 1.92 | 0.785 |
| Adjuvant therapy | ||||
| None | Ref | Ref | Ref | Ref |
| Chemotherapy | 0.92 | 0.75 | 1.13 | 0.429 |
| Chemoradiation | 1.34 | 0.88 | 2.05 | 0.169 |
| Radiation | 1.21 | 0.78 | 1.88 | 0.387 |
Table 3.
Cox multivariable regression of independent predictors of survival in patients with stage IB LCNEC treated with or without adjuvant therapy
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.01 | 1.00 | 1.03 | 0.056 |
| Female sex | 0.92 | 0.73 | 1.15 | 0.445 |
| Race | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 1.02 | 0.68 | 1.54 | 0.907 |
| Other | 0.39 | 0.12 | 1.29 | 0.124 |
| Charleson-Deyo Comorbidity Index | ||||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 0.85 | 0.66 | 1.10 | 0.222 |
| 2+ | 1.26 | 0.93 | 1.71 | 0.131 |
| Year of diagnosis (per year) | 0.98 | 0.94 | 1.02 | 0.226 |
| Insurance status | ||||
| Private | Ref | Ref | Ref | Ref |
| Government | 1.81 | 1.34 | 2.45 | < 0.001 |
| None | 1.04 | 0.44 | 2.46 | 0.925 |
| Facility location | ||||
| Metro | Ref | Ref | Ref | Ref |
| Urban | 1.07 | 0.81 | 1.41 | 0.653 |
| Rural | 0.95 | 0.61 | 1.50 | 0.835 |
| Facility type | ||||
| Community Cancer Program | Ref | Ref | Ref | Ref |
| Comprehensive Community Cancer Program | 0.76 | 0.49 | 1.18 | 0.216 |
| Academic/Research Program | 0.96 | 0.61 | 1.53 | 0.869 |
| Integrated Network Cancer Program | 0.99 | 0.59 | 1.65 | 0.963 |
| Surgery | ||||
| Wedge resection | Ref | Ref | Ref | Ref |
| Segmental resection | 1.38 | 0.70 | 2.71 | 0.355 |
| Lobectomy | 0.63 | 0.43 | 0.93 | 0.020 |
| Pneumonectomy | 0.53 | 0.20 | 1.38 | 0.191 |
| Adjuvant therapy | ||||
| None | Ref | Ref | Ref | Ref |
| Chemotherapy | 0.67 | 0.5 | 0.9 | 0.007 |
| Chemoradiation | 1.55 | 0.84 | 2.85 | 0.163 |
| Radiation | 2.05 | 1.03 | 4.07 | 0.041 |
Additionally, compared to wedge resections, lobectomy was a strong independent predictor of survival in both stages IA and IB (Tables 2–3). Of 431 patients undergoing wedge resections, only 17% received adjuvant therapy. Of 2087 patients undergoing lobectomy, 19% received adjuvant therapy. Of stage IB patients, only 23% of patients with wedge resections and 30% of patients with lobectomy received adjuvant chemotherapy (p=0.325). Given the significant survival advantage experienced by patients undergoing lobectomy compared to wedge resections, we performed a subgroup analysis of patients undergoing wedge resection alone, stratified by stage and administration of adjuvant therapy. In a multivariable Cox model, patients with stage IB LCNEC receiving a wedge resection and adjuvant chemotherapy experienced improved survival compared to those not receiving adjuvant therapy (HR 0.36; 95%CI 0.14, 0.94) (Table 5). In contrast, patients with stage IA LCNEC undergoing wedge resection with adjuvant therapy experienced no survival benefit compared to those who did not receive adjuvant therapy (HR 0.97; 95%CI 0.66, 1.41) (Table 4).
Table 5.
Cox multivariable regression of independent predictors of survival in patients with stage IB LCNEC undergoing wedge resection
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Hazard Ratio | Lower | Upper | p-value | |
| Age (years, median) | 0.97 | 0.92 | 1.02 | 0.24 |
| Sex (female) | 1.07 | 0.52 | 2.20 | 0.85 |
| Year of diagnosis, median (inter-quartile range) | 0.87 | 0.73 | 1.03 | 0.10 |
| CDCC Score (reference: 0) | ||||
| 1 | 0.38 | 0.14 | 1.01 | 0.05 |
| 2+ | 0.77 | 0.30 | 1.97 | 0.58 |
| Facility type (reference: non-academic) | ||||
| Academic/research program | 1.27 | 0.58 | 2.77 | 0.55 |
| Tumor grade (reference: well differentiated) | ||||
| Poorly differentiated | 0.13 | 0.01 | 1.51 | 0.10 |
| Undifferentiated | 0.10 | 0.009 | 1.15 | 0.06 |
| Adjuvant therapy (reference: none) | ||||
| Chemotherapy | 0.36 | 0.14 | 0.94 | 0.04 |
Table 4.
Cox multivariable regression of independent predictors of survival in patients with stage IA LCNEC undergoing wedge resection
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Hazard Ratio | Lower | Upper | p-value | |
| Age (years, median) | 1.03 | 1.01 | 1.04 | 0.001 |
| Sex (female) | 0.80 | 0.64 | 1.01 | 0.05 |
| Year of diagnosis, median (inter-quartile range) | 1.00 | 0.96 | 1.04 | 0.82 |
| CDCC Score (reference: 0) | ||||
| 1 | 0.96 | 0.75 | 1.24 | 0.75 |
| 2+ | 1.51 | 1.11 | 2.04 | 0.008 |
| Facility type (reference: non-academic) | ||||
| Academic/research program | 0.95 | 0.74 | 1.21 | 0.65 |
| Insurance type (reference: private) | ||||
| Government | 1.00 | 0.72 | 1.37 | 0.98 |
| No insurance | 2.35 | 0.82 | 6.78 | 0.11 |
| Tumor grade (reference: well differentiated) | ||||
| Poorly differentiated | 0.43 | 0.10 | 1.85 | 0.26 |
| Undifferentiated | 0.34 | 0.08 | 1.48 | 0.15 |
| Adjuvant therapy (reference: none) | ||||
| Chemotherapy | 0.97 | 0.66 | 1.41 | 0.86 |
Another subgroup analysis was performed with patients receiving lobar resections alone, stratified by stage and receipt of adjuvant therapy. In multivariable analysis for patients receiving lobar resections for stage IA LCNEC, there was no difference in survival between patients who received adjuvant therapy and those who did not (HR 0.88; 95%CI 0.68, 1.13). In contrast, stage IB patients who underwent lobar resection experienced a significant survival benefit with adjuvant chemotherapy compared to no adjuvant therapy (HR 0.73; 95%CI 0.51, 1.05).
To minimize the imbalance between the groups of patients, a 1:1 propensity score-matched analysis was performed exclusively of patients undergoing lobar resections, stratified by pathologic stage. In 147 propensity score-matched pairs of patients with stage IA disease, adjuvant chemotherapy was not associated with a survival benefit compared to no adjuvant therapy in multivariable regression (Table 6). However, in 131 pairs of patients with stage IB disease, adjuvant chemotherapy was associated with an improvement in survival compared to no adjuvant therapy (HR 0.58; 95%CI 0.40, 0.84) (Table 7).
Table 6.
Cox multivariable regression of independent predictors of survival in propensity score-matched pairs of patients with stage IA LCNEC undergoing lobar resection
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Hazard Ratio | Lower | Upper | p-value | |
| Age (years, median) | 1.02 | 1.00 | 1.05 | 0.05 |
| Sex (female) | 0.88 | 0.61 | 1.26 | 0.48 |
| Year of diagnosis, median (inter-quartile range) | 0.96 | 0.90 | 1.03 | 0.25 |
| CDCC Score (reference: 0) | ||||
| 1 | 0.85 | 0.55 | 1.31 | 0.46 |
| 2+ | 1.36 | 0.84 | 2.20 | 0.22 |
| Facility type (reference: non-academic) | ||||
| Academic/research program | 0.73 | 0.48 | 1.10 | 0.13 |
| Insurance type (reference: private) | ||||
| Government | 1.46 | 0.95 | 2.25 | 0.09 |
| No insurance | 1.60 | 0.37 | 6.90 | 0.53 |
| Tumor grade (reference: well differentiated) | ||||
| Poorly differentiated | 1.15 | 0.15 | 8.67 | 0.89 |
| Undifferentiated | 0.91 | 0.12 | 6.89 | 0.92 |
| Adjuvant therapy (reference: none) | ||||
| Chemotherapy | 0.89 | 0.63 | 1.26 | 0.50 |
Table 7.
Cox multivariable regression of independent predictors of survival in propensity score-matched pairs of patients with stage IB LCNEC undergoing lobar resection
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Hazard Ratio | Lower | Upper | p-value | |
| Age (years, median) | 1.04 | 1.01 | 1.07 | 0.005 |
| Sex (female) | 0.99 | 0.67 | 1.45 | 0.95 |
| Year of diagnosis, median (inter-quartile range) | 0.99 | 0.92 | 1.06 | 0.69 |
| CDCC Score (reference: 0) | ||||
| 1 | 0.78 | 0.51 | 1.20 | 0.26 |
| 2+ | 1.10 | 0.64 | 1.92 | 0.73 |
| Facility type (reference: non-academic) | ||||
| Academic/research program | 0.71 | 0.44 | 1.14 | 0.15 |
| Insurance type (reference: private) | ||||
| Government | 1.15 | 0.71 | 1.86 | 0.57 |
| No insurance | 0.95 | 0.33 | 2.71 | 0.92 |
| Tumor grade (reference: well differentiated) | ||||
| Poorly differentiated | 2.09 | 0.27 | 16.4 | 0.48 |
| Undifferentiated | 2.75 | 0.35 | 21.7 | 0.34 |
| Adjuvant therapy (reference: none) | ||||
| Chemotherapy | 0.58 | 0.40 | 0.84 | 0.004 |
Comment:
We present the largest analysis, to our knowledge, of the effect of adjuvant therapy on survival in stage I LCNEC. In our NCDB study, the use of adjuvant chemotherapy was associated with improved survival overall in pathologic stage I LCNEC; however, the OS benefit was only significant in stage IB but not stage IA LCNEC. There was no survival advantage associated with receipt of adjuvant radiation, whether alone or in combination with chemotherapy.
Our study suggests a role for adjuvant chemotherapy in resected stage IB LCNEC, despite the conflicting and limited literature. The current NCCN guidelines [13] recommend consideration of adjuvant therapy in patients with ‘high risk’ features including poorly differentiated neuroendocrine histology and pathologic stage IB NSCLC, but do not explicitly recommend routine adjuvant therapy for LCNEC. Iyoda and colleagues report the only prospective trial comparing 15 patients who received cisplatin-etoposide for mostly stage I LCNEC with a historic control group, finding an 89% five-year OS with adjuvant chemotherapy compared to 47% in the control group [14]. Several smaller retrospective cohort analyses have shown conflicting trends, with the largest European study in 2017 revealing no OS benefit with adjuvant chemotherapy including stage I LCNEC (adjusted HR 0.71; 95%CI 0.36, 1.41) [15], while other studies have demonstrated either a trend towards improved survival or no survival benefit in stage I disease [7,8,15–19]. A recent analysis of the NCDB by Kujtan and colleagues demonstrated a significant OS advantage for even stage IA LCNEC treated with adjuvant chemotherapy, although their study used a smaller population in an older version of the database, and also used the AJCC sixth and seventh edition staging coded by NCDB, which limit the interpretation of their findings [20]. Specifically, the sample size of stage IA patients in their study was approximately half the sample size of our analysis of patients with stage IA disease. Our analysis included a much larger number of patients and used the newest eighth edition of AJCC staging.
The genetic and histologic heterogeneity of LCNEC combined with its rarity likely accounts for the conflicting findings of the benefits of adjuvant therapy for this patient population. LCNEC has many histological variants and is often difficult to separate from SCLC or NSCLC, which presents a diagnostic challenge for pathologists [21]. Genomic analysis of LCNEC demonstrates transcription often similar to SCLC, with a high frequency of TP53 and RB1 mutations, but also reveals NSCLC-like populations expressing STK1, KRAS, and KEAP1 mutations [22–24]. In Rekhtman and colleagues’ targeted genetic profile of LCNEC tumors, all patients who responded to adjuvant platinum-backbone chemotherapy had SCLC-like tumors, while those with NSCLC-like cancer did not respond to chemotherapy [22]. Prospective trials have not demonstrated a survival benefit for stage IB NSCLC treated with adjuvant platinum-based therapy [25–27], while adjuvant therapy has been associated with a survival benefit in small cell lung cancer [28]. Our analysis along with the existing literature on LCNEC therefore suggests that the most effective management for early LCNEC may be a customized approach to adjuvant therapy based on the genetic characteristics of individual patients aligning to SCLC-like or NSCLC-like behavior.
Our study has several limitations. One, it is a retrospective analysis and is therefore subject to confounding not adjusted for in our study. Two, the NCDB provides incomplete information, and does not provide data about type of chemotherapy, dosage of therapy, and postoperative complications. The NCDB also does not supply information about recurrence, which would confound survival. It also only reports overall rather than disease-free survival, which is an important limitation in the external validity of the study. In a cancer that is difficult to diagnose based on pathologic features, the NCDB data likely also represent some tumors mis-diagnosed as LCNEC, and excludes others that should have been categorized as LCNEC. Finally, there is substantial selection bias that remains unaccounted for in this large nationwide analysis, because we are not privy to the reasons for important management decisions like choice of surgery, administration of adjuvant therapy, and type of therapy.
Our NCDB study demonstrates a significant improvement in overall survival for stage IB LCNEC treated with adjuvant chemotherapy compared to no adjuvant therapy. In patients with stage IA LCNEC, adjuvant therapy was not associated with a significant survival benefit. While prospective, randomized data are needed to validate the findings of this study, the rarity of this cancer presents a challenge to conduct of prospective trials.
Acknowledgements:
Dr. Jawitz was supported by a National Institutes of Health T32 grant in cardiovascular research.
Footnotes
This paper was presented at the World Conference on Lung Cancer in Toronto, September 2018
References
- [1].Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol Off J Am Soc Clin Oncol 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- [2].Fasano M, Della Corte CM, Papaccio F, Ciardiello F, Morgillo F. Pulmonary Large-Cell Neuroendocrine Carcinoma. J Thorac Oncol 2015;10:1133–41. doi: 10.1097/JTO.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Iyoda A, Hiroshima K, Moriya Y, Sekine Y, Shibuya K, Iizasa T, et al. Prognostic impact of large cell neuroendocrine histology in patients with pathologic stage Ia pulmonary non–small cell carcinoma. J Thorac Cardiovasc Surg 2006;132:312–5. doi: 10.1016/j.jtcvs.2006.02.046. [DOI] [PubMed] [Google Scholar]
- [4].Battafarano RJ, Fernandez FG, Ritter J, Meyers BF, Guthrie TJ, Cooper JD, et al. Large cell neuroendocrine carcinoma: An aggressive form of non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;130:166–72. doi: 10.1016/j.jtcvs.2005.02.064. [DOI] [PubMed] [Google Scholar]
- [5].Russo GL, Pusceddu S, Proto C, Macerelli M, Signorelli D, Vitali M, et al. Treatment of lung large cell neuroendocrine carcinoma. Tumor Biol 2016;37:7047–57. doi: 10.1007/s13277-016-5003-4. [DOI] [PubMed] [Google Scholar]
- [6].Filosso PL, Guerrera F, Evangelista A, Galassi C, Welter S, Rendina EA, et al. Adjuvant chemotherapy for large-cell neuroendocrine lung carcinoma: results from the European Society for Thoracic Surgeons Lung Neuroendocrine Tumours Retrospective Database. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg 2017;52:339–45. doi: 10.1093/ejcts/ezx101. [DOI] [PubMed] [Google Scholar]
- [7].Sarkaria IS, Iyoda A, Roh MS, Sica G, Kuk D, Sima CS, et al. Neoadjuvant and Adjuvant Chemotherapy in Resected Pulmonary Large Cell Neuroendocrine Carcinomas: A Single Institution Experience. Ann Thorac Surg 2011;92:1180–7. doi: 10.1016/j.athoracsur.2011.05.027. [DOI] [PubMed] [Google Scholar]
- [8].Iyoda A, Hiroshima K, Toyozaki T, Haga Y, Baba M, Fujisawa T, et al. Adjuvant chemotherapy for large cell carcinoma with neuroendocrine features. Cancer 2001;92:1108–12. doi: [DOI] [PubMed] [Google Scholar]
- [9].Iyoda A, Hiroshima K, Moriya Y, Iwadate Y, Takiguchi Y, Uno T, et al. Postoperative recurrence and the role of adjuvant chemotherapy in patients with pulmonary large-cell neuroendocrine carcinoma. J Thorac Cardiovasc Surg 2009;138:446–53. doi: 10.1016/j.jtcvs.2008.12.037. [DOI] [PubMed] [Google Scholar]
- [10].Iyoda A, Hiroshima K, Moriya Y, Takiguchi Y, Sekine Y, Shibuya K, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg 2006;82:1802–7. doi: 10.1016/j.athoracsur.2006.05.109. [DOI] [PubMed] [Google Scholar]
- [11].National Cancer Database. Am Coll Surg n.d https://www.facs.org/quality-programs/cancer/ncdb (accessed August 25, 2018). [Google Scholar]
- [12].Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 5.2018 n.d http://www.jnccn.org/content/16/7/807.full (accessed August 26, 2018). [Google Scholar]
- [14].Iyoda A, Hiroshima K, Moriya Y, Takiguchi Y, Sekine Y, Shibuya K, et al. Prospective Study of Adjuvant Chemotherapy for Pulmonary Large Cell Neuroendocrine Carcinoma. Ann Thorac Surg 2006;82:1802–7. doi: 10.1016/j.athoracsur.2006.05.109. [DOI] [PubMed] [Google Scholar]
- [15].Veronesi G, Morandi U, Alloisio M, Terzi A, Cardillo G, Filosso P, et al. Large cell neuroendocrine carcinoma of the lung: a retrospective analysis of 144 surgical cases. Lung Cancer Amst Neth 2006;53:111–5. doi: 10.1016/j.lungcan.2006.03.007. [DOI] [PubMed] [Google Scholar]
- [16].Kim KW, Kim HK, Kim J, Shim YM, Ahn M- J, Choi Y- L. Outcomes of Curative-Intent Surgery and Adjuvant Treatment for Pulmonary Large Cell Neuroendocrine Carcinoma. World J Surg 2017;41:1820–7. doi: 10.1007/s00268-017-3908-8. [DOI] [PubMed] [Google Scholar]
- [17].Rossi G, Cavazza A, Marchioni A, Longo L, Migaldi M, Sartori G, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol Off J Am Soc Clin Oncol 2005;23:8774–85. doi: 10.1200/JCO.2005.02.8233. [DOI] [PubMed] [Google Scholar]
- [18].Iyoda A, Hiroshima K, Nakatani Y, Fujisawa T. Pulmonary Large Cell Neuroendocrine Carcinoma: Its Place in the Spectrum of Pulmonary Carcinoma A part of this report was presented by Iyoda A et al. Pulmonary large cell neuroendocrine carcinoma. JJLC 2006;46:315–20 [in Japanese with English abstract]. [Google Scholar]; Ann Thorac Surg 2007;84:702–7. doi: 10.1016/j.athoracsur.2007.03.093. [DOI] [PubMed] [Google Scholar]
- [19].Saji H, Tsuboi M, Matsubayashi J, Miyajima K, Shimada Y, Imai K, et al. Clinical response of large cell neuroendocrine carcinoma of the lung to perioperative adjuvant chemotherapy. Anticancer Drugs 2010;21:89. doi: 10.1097/CAD.0b013e328330fd79. [DOI] [PubMed] [Google Scholar]
- [20].The Role of Systemic Therapy in the Management of Stage I Large Cell Neuroendocrine Carcinoma of the Lung - ScienceDirect n.d https://www.sciencedirect.com/science/article/pii/S1556086418300819 (accessed August 26, 2018). [DOI] [PubMed]
- [21].Pelosi G, Sonzogni A, Harari S, Albini A, Bresaola E, Marchiò C, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res 2017;6:513–29. doi: 10.21037/tlcr.2017.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Cancer Res Off J Am Assoc Cancer Res 2016;22:3618–29. doi: 10.1158/1078-0432.CCR-15-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Clinical Lung Cancer Genome Project (CLCGP), Network Genomic Medicine (NGM). A genomics-based classification of human lung tumors. Sci Transl Med 2013;5:209ra153. doi: 10.1126/scitranslmed.3006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miyoshi T, Umemura S, Matsumura Y, Mimaki S, Tada S, Makinoshima H, et al. Genomic Profiling of Large-Cell Neuroendocrine Carcinoma of the Lung. Clin Cancer Res Off J Am Assoc Cancer Res 2017;23:757–65. doi: 10.1158/1078-0432.CCR-16-0355. [DOI] [PubMed] [Google Scholar]
- [25].Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589–97. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- [26].Douillard J-Y, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- [27].Scagliotti GV, Fossati R, Torri V, Crinò L, Giaccone G, Silvano G, et al. Randomized Study of Adjuvant Chemotherapy for Completely Resected Stage I, II, or IIIA Non–Small-Cell Lung Cancer. JNCI J Natl Cancer Inst 2003;95:1453–61. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- [28].Yang C- FJ, Chan DY, Speicher PJ, Gulack BC, Wang X, Hartwig MG, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol Off J Am Soc Clin Oncol 2016;34:1057–64. doi: 10.1200/JCO.2015.63.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Burdett S, Stewart L, PORT Meta-analysis Group. Postoperative radiotherapy in non-small-cell lung cancer: update of an individual patient data meta-analysis. Lung Cancer Amst Neth 2005;47:81–3. doi: 10.1016/j.lungcan.2004.09.010. [DOI] [PubMed] [Google Scholar]


