Abstract
A high incidence of thrombotic events, particularly deep vein thrombosis and pulmonary embolism, has been clearly documented in COVID-19 patients. In addition, small series of patients with coronary, cerebrovascular and peripheral arterial thrombotic events have also been reported, but their true incidence and consequences are not well described, and constitute the objective of this study. From February 1st to April 21st, 2020, 2115 COVID-19 patients were treated at Hospital Universitario Fundación Alcorcón (Madrid, Spain), and 1419 were eventually admitted. Patient characteristics and outcomes were collected by reviewing their electronic medical records. Fourteen patients had a systemic arterial thrombotic event, which represents a 1% incidence in relation to the total number of hospitalized patients. Three patients suffered an acute coronary syndrome, two with persistent ST-segment elevation, one of whom was treated invasively, and one with transient ST-segment elevation. Eight patients had a cerebrovascular event. Six suffered an acute ischemic stroke and two a transient ischemic attack, 50% of them had a Rankin score ≥ 3 at discharge. Three additional patients had a limb thrombotic event, all of them infrapopliteal, and were managed conservatively. All three cases developed necrosis of the toes, two of them with bilateral involvement. The hospitalization death rate of patients with an arterial event was 28.6%. Although COVID-19 may favor the occurrence of thrombotic events, the destabilization and thrombosis of arterial atherosclerotic plaques do not seem to be a frequent mechanism which warrants the need for specific systematic preventive measures.
Keywords: Thrombosis, Acute coronary syndrome, Stroke, Peripheral arterial disease, COVID-19
Highlights
Evidence of thrombosis is a common finding in severe COVID-19 patients.
There is very little data on the incidence and consequences of coronary, cerebrovascular and peripheral vascular thrombotic events.
In a large cohort of 1419 COVID-19 patients we observed a 1% incidence of systemic arterial thrombotic events, with a death rate of 28.6%.
Although SARS-CoV2 infection may favor arterial thrombotic events, with grave consequences, it does not seem to be a frequent enough phenomena to warrant the need for specific systematic preventive measures.
Introduction
In patients with SARS-CoV2 (Severe Acute Respiratory Syndrome Coronavirus 2) infection it has been recently emphasized that thrombotic events contribute to the severity [1] of the disease and antithrombotic treatment has been gradually scaled up worldwide [2]. An increased incidence of pulmonary embolism has been reported [3] and there is also evidence of cardiac involvement with elevated markers of myocardial damage in 10–20% of cases [4, 5]. Small series of patients with coronary [6, 7], cerebrovascular [8] and peripheral arterial events [9] have been reported, but their true incidence and clinical consequences are not well established. Knowing such incidence will help to prioritize or de-escalate the focus on the diagnosis of these complications as well as to implement specific preventive measures if their frequency warrants it.
Methods
The city of Madrid has been the epicenter of the COVID-19 epidemic in Spain. From February 1st up until April 21st, 2020, a total of 2115 COVID-19 patients were treated at Hospital Universitario Fundación Alcorcón (Madrid, Spain), of which 251 have died (11.9%). Of these 2115 patients, 1419 were eventually admitted and constitute the cohort for this analysis. All patients with clinically suspected cerebral, coronary or peripheral acute vascular events were evaluated by a member of the Neurology, Cardiology or Vascular Surgery Department. Cases were prospectively collected by the three subspecialties and entered into a specific database. Their detailed clinical characteristics and laboratory data were gathered by retrospectively reviewing their electronic medical records. A descriptive analysis of these variables was carried out, calculating the incidence over the total number of hospitalized patients. The study was approved by the center’s Research Ethics Committee.
Results
During the period studied 14 COVID-19 patients had a systemic arterial event, which represents an incidence of 1% in relation to the total number of hospitalized patients. Patient characteristics and outcome are summarized in Table 1. Vascular events occurred along the whole course of the disease but they tended to cluster during the second week. Their specific time distribution is depicted in Fig. 1.
Table 1.
Patients characteristics and outcome
| Stroke/TIA (n = 8) | ACS (n = 3) | Acute limb ischemia (n = 3) | Total (n = 14) | |
|---|---|---|---|---|
| Age (years) | 76.4 ± 7.1 | 69 ± 7 | 69.3 ± 5.6 | 73.2 ± 7.3 |
| Female sex | 1 (12.5%) | 1 (33.3%) | 1 (33.3%) | 3 (21.4%) |
| Past medical history | – | – | – | – |
| Hypertension | 8 (100%) | 2 (66.7%) | 3 (100%) | 13 (92.9%) |
| Diabetes | 4 (50%) | 0 | 1 (33.3%) | 5 (35.7%) |
| Hypercholesterolemia | 7 (87.5%) | 1 (33.3%) | 1 (33.3%) | 9 (64.3%) |
| Smoking | 6 (75%) | 1 (33.3%) | 0 | 7 (50%) |
| Previous ACS | 3 (37.5%) | 0 | 0 | 3 (21.4%) |
| AF | 2 (25%) | 0 | 0 | 2 (14.8%) |
| Previous stroke/TIA | 2 (25%) | 0 | 0 | 2 (14.2%) |
| DVT/PE | 0 | 1 (33.3%) | 0 | 1 (7.1%) |
| History of malignancy | 5 (62.5%) | 0 | 0 | 5 (35.7%) |
| Previous medication | – | – | – | – |
| Antiagreggation | 3 (37.5%) | 1 (33.3%) | 0 | 4 (28.5%) |
| Oral anticoagulation | 2 (25%) | 0 | 0 | 2 (14.2%) |
| Severe COVID-19 | 6 (75%) | 2 (66.7%) | 3 (100%) | 12 (85.7%) |
| Laboratory findings at vascular event | – | – | – | – |
| C-reactive protein (mg/L) | 100.5 (27–206) | 92.5 N/A | 335 N/A | 176 (43–253) |
| d-dimer (ng/L) | 2589 (735–8156) | 3035 N/A | 5380 N/A | 3334 (932–7462) |
| Creatine kinase (U/L) | 62 (41–68) | 132 N/A | 689 N/A | 66.5 (46–376) |
| Lactate dehydrogenase (U/L) | 296 (192–384) | 436 N/A | 434 N/A | 338 (258–440) |
| Ferritine (ng/L) | 318 (213–768) | 1084 N/A | 1398 N/A | 672 (277–1398) |
| Pneumonia | 7 (87.5%) | 3 (100%) | 2 (66.7%) | 12 (85.7%) |
| Thromboprophylaxis with heparine | 5 (62.5%) | 2 (66.7%) | 3 (100%) | 10 (71.4%) |
| Prophylactic | 3 (37.5%) | 0 | 2 (66.7%) | 5 (35.7%) |
| Therapeutic | 2 (25%) | 2 (66.7%) | 1 (33.3%) | 5 (35.7%) |
| Days from onset of symptoms to vascular event | 6.3 ± 5.4 | 10.7 ± 8.1 | 12.3 ± 2.5 | 8.5 ± 5.8 |
| Death | 2 (25%) | 1 (33.3%) | 1 (33.3%) | 4 (28.6%) |
Data are presented as mean ± standard deviation or median (interquartile range) for continuous measures and number (%) for categorical measures
ACS acute coronary syndrome, TIA transient ischemic attack, DVT deep vein thrombosis, PE pulmonary embolism, COVID-19 Coronavirus disease 2019
Fig. 1.
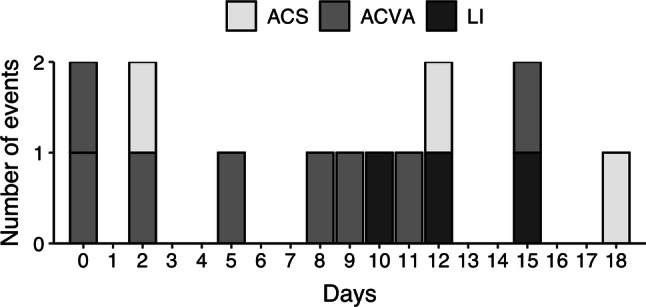
Days from onset of symptoms to vascular event (n = 14). ACS acute coronary syndrome, ACVA acute cerebrovascular accident, LI limb ischemia
Three patients suffered an acute coronary syndrome (ACS), two with persistent ST-segment elevation. The first was a 66-year-old woman with mild pneumonia. An emergency coronary angiogram was performed, and an acute occlusion of the circumflex artery was stented with good outcome. The other patient was a 77-year-old male with very severe acute respiratory distress and poor life prognosis. Conservative management for an inferior infarction was opted for, with death occurring 8 days later from respiratory causes. The third patient was a 64-year-old male with severe respiratory distress on non-invasive mechanical ventilation who developed chest pain with transient ST elevation but no troponin elevation and was managed medically. Once his respiratory status resolved, a coronary angiography showed no significant coronary disease and the patient was successfully discharged. As a matter of comparison, in the same time period in 2019 four patients were treated in our hospital who developed an ACS whilst hospitalized for another reason.
Eight patients had an ischemic stroke (IS) or a transient ischemic attack (TIA). Most patients had multiple cardiovascular risk factors and comorbidities (Table 2). The first patient, a 77-year-old male with severe bilateral pneumonia, suffered a right carotid IS, treated with intravenous thrombolysis, presenting a new IS in the context of hemodynamic instability due to severe respiratory failure, with Rankin score of 5 at discharge. An 86-year-old male, with a history of TIA who was admitted due to severe bilateral pneumonia, presented an IS in the left carotid region and died within 24 h. A 69-year-old male with severe pneumonia, a history of atrial fibrillation (AF) on acenocoumarol and IS with right carotid occlusion, presented a new right carotid IS in the context of hemodynamic instability, and recovered spontaneously. Two patients with non-severe pneumonia suffered a TIA and were managed conservatively. Two patients admitted with severe pneumonia, suffered an IS due to intracranial arterial occlusion and were treated conservatively. The last patient, a 66-year-old male with AF on edoxaban, active lung cancer and severe bilateral pneumonia, presented bilateral IS, culture-negative aortic valve endocarditis and subsequently died from respiratory decline. Patients with severe COVID criteria [4] were more likely to suffer IS than TIA and had worse Rankin scores at discharge.
Table 2.
Cerebrovascular events characteristics (n = 8)
| NIHSS scale initial | 4 (1.5–4.75) |
|---|---|
| Neuroimaging | — |
| CT—acute ischemic lesions | 2 (25%) |
| CT—white matter lesions | 3 (37.5%) |
| CT—chronic stroke | 3 (37.5%) |
| Angio-CT—large vessel occlusion | 4 (50%) |
| Diagnosis | – |
| Stroke | 7 (87.5%) |
| TIA | 1 (12.5%) |
| Localization of the stroke | – |
| Carotid | 5 (62.5%) |
| Vertebrobasilar | 2 (25%) |
| Lacunar | 1 (12.5%) |
| Etiology of stroke | — |
| Atherothrombotic | 3 (37.5%) |
| Cardioembolic | 2 (25%) |
| Cryptogenic stroke | 2 (25%) |
| Undetermined etiology due to two or more causes | 1 (12.5%) |
| Ranking at discharge | — |
| 0–2 | 4 (50%) |
| 3–5 | 2 (25%) |
| 6 | 2 (25%) |
Data are presented as median (interquartile range) for continuous measures and number (%) for categorical measures
NIHSS National Institute of Health Stroke Scale, CT computed tomography, TIA transitory ischemic attack
During the same time period three patients developed an acute lower limb ischemic event. All cases presented necrosis of the toes, two of them with bilateral involvement, and another with extension to the forefoot. The first patient, a 74-year-old man admitted with severe bilateral pneumonia developed an infrapopliteal arterial occlusion, and subsequently died within two days due to her respiratory condition. A 71-year-old woman admitted with bilateral pulmonary embolism developed a bilateral infrapopliteal thrombotic event 2 days after admission and as of today she is still hospitalized. Finally, a 63-year-old diabetic man, while critically ill in the ICU with severe bilateral pneumonia, developed foot ischemia 7 days after admission. He was eventually discharged from the hospital but is scheduled for minor toe or transmetatarsal amputation. Due to their poor general condition all patients received only conservative treatment with full anticoagulation. Interestingly, the two patients that were awake and conscious showed no or minimal pain, with no sensory loss.
Discussion
The relationship between viral respiratory infections and arterial thrombosis, especially ACS, is clearly described [10]. However, there is very little data regarding the incidence of systemic arterial events in patients with SARS-CoV-2 infection. In a series of 18 patients with COVID-19 and ST elevation, in more than 50% of them the origin was considered to be non-coronary [6] analogously to one of our patients. In a study of 214 patients in Wuhan, China, 5 patients (2.3%) presented IS and 1 had a cerebral hemorrhage [8], a higher incidence than in our study. Acute limb ischemia has been reported in two young COVID-19 patients with occlusion of major arteries of the upper and lower limbs [9]. However, our cases seem to be slightly different as they experienced more distal vessel thromboses and occurred in older patients and with more comorbidities.
Although in our study vascular events were distributed along the first 3 weeks of the disease, there were more cases, particularly limb thrombosis, during the second week possibly reflecting the hypercoagulable state that has been described in this condition [2]. The mortality rate of COVID-19 patients that suffer an arterial event seems to be very high, 28.6% in our study, and they appear to happen in patients with a particularly severe clinical course of the infection.
There is convincing analytical and clinical data on the cardiac [4–7] and cerebral [8] involvement in patients with COVID-19. Although the incidence of thrombotic events, at least for cerebrovascular, seems to be higher than expected and their consequences are certainly very serious, the overall incidence of systemic arterial events is low, 1%. Our data suggest that, although COVID-19 may favor the occurrence of thrombotic events, the destabilization and thrombosis of atherosclerotic plaques do not seem to be a frequent mechanism which warrants the need for specific systematic preventive measures. Nevertheless a high level of suspicion and clinical surveillance should certainly be maintained.
A limitation of this study would be the potential underestimation of cases if the first manifestation of the thrombotic event had been sudden death. However, we believe it’s unlikely many cases will have had no other symptoms before death, as they were hospitalized and under close medical supervision.
Author contributions
LV and JB: designed the study and reviewed the results. EC, PS, VE, LF and LdB: collected and analyzed the data. AN and JB: wrote and revised the manuscript.
Funding
None.
Data availability
Yes.
Code availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the center’s Research Ethics Committee.
Consent to participate
Granted exemption by the center’s Research Ethics Committee.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 4.Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020 doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevation in patients with COVID-19—a case series. N Engl J Med. 2020 doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stefanini GG, Montorfano M, Trabattoni D, et al. ST-elevation myocardial infarction in patients with COVID-19. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perini P, Nabulsi B, Bianccini C, Azzarone M, Freyrie A. Acute limb ischemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020;395:1546. doi: 10.1016/S0140-6736(20)31051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Yes.
Not applicable.


