Abstract
Background
Children with cerebral palsy have impaired muscle growth and muscular contractures that limit their ROM. Contractures have a decreased number of serial sarcomeres and overstretched lengths, suggesting an association with a reduced ability to add the serial sarcomeres required for normal postnatal growth. Contractures also show a markedly reduced number of satellite cells—the muscle stem cells that are indispensable for postnatal muscle growth, repair, and regeneration. The potential role of the reduced number of muscle stem cells in impaired sarcomere addition leading to contractures must be evaluated.
Questions/purposes
(1) Does a reduced satellite cell number impair the addition of serial sarcomeres during recovery from an immobilization-induced contracture? (2) Is the severity of contracture due to the decreased number of serial sarcomeres or increased collagen content?
Methods
The hindlimbs of satellite cell-specific Cre-inducible mice (Pax7CreER/+; Rosa26DTA/+; n = 10) were maintained in plantarflexion with plaster casts for 2 weeks so that the soleus was chronically shortened and the number of its serial sarcomeres was reduced by approximately 20%. Subsequently, mice were treated with either tamoxifen to reduce the number of satellite cells or a vehicle (an injection and handling control). The transgenic mouse model with satellite cell ablation combined with a casting model to reduce serial sarcomere number recreates two features observed in muscular contractures in children with cerebral palsy. After 30 days, the casts were removed, the mice ankles were in plantarflexion, and the mice’s ability to recover its ankle ROM by cage remobilization for 30 days were evaluated. We quantified the number of serial sarcomeres, myofiber area, and collagen content of the soleus muscle as well as maximal ankle dorsiflexion at the end of the recovery period.
Results
Mice with reduced satellite cell numbers did not regain normal ankle ROM in dorsiflexion; that is, the muscles remained in plantarflexion contracture (-16° ± 13° versus 31° ± 39° for the control group, -47 [95% confidence interval -89 to -5]; p = 0.03). Serial sarcomere number of the soleus was lower on the casted side than the contralateral side of the mice with a reduced number of satellite cells (2214 ± 333 versus 2543 ± 206, -329 [95% CI -650 to -9]; p = 0.04) but not different in the control group (2644 ± 194 versus 2729 ± 249, -85 [95% CI -406 to 236]; p = 0.97). The degree of contracture was strongly associated with the number of sarcomeres and myofiber area (r2 =0.80; P < 0.01) rather than collagen content. No differences were seen between groups in terms of collagen content and the fraction of muscle area.
Conclusions
We found that a reduced number of muscle stem cells in a transgenic mouse model impaired the muscle’s ability to add sarcomeres in series and thus to recover from an immobilization-induced contracture.
Clinical Relevance
The results of our study in transgenic mouse muscle suggests there may be a mechanistic relationship between a reduced number of satellite cells and a reduced number of serial sarcomeres. Contracture development, secondary to impaired sarcomere addition in muscles in children with cerebral palsy may be due to a reduced number of muscle stem cells.
Introduction
Cerebral palsy, which is caused by a nonprogressive brain injury during the perinatal period, is the most common movement disability in children [57]. Although the primary injury is nonprogressive, the growing musculoskeletal system is progressively impaired. Muscle weakness, impaired longitudinal muscular growth, increased extracellular matrix components [37, 53], and contractures that limit joint ROM are observed [23]. Cross-sectional muscle growth is also lower in children with cerebral palsy than in children with typical development [4, 5, 45]. The natural progression of walking leads to a gradual reduction in total joint excursion [7, 30]. Correspondingly, passive ROM of the lower limb decreases from early childhood to adolescence [46], suggesting that longitudinal muscle growth is unable to align with bone growth. Most rehabilitation approaches for children with cerebral palsy, such as serial casting, stretching, strengthening, and splinting, focus on promoting appropriate muscle growth and preventing or treating muscular contractures [23]. Neural changes because of a brain injury, such as spasticity, are thought to contribute to the development of contractures [27]. Long-term studies show that dorsal rhizotomies and botulinum toxin reduce spasticity but do not prevent contractures [55, 56]. Even lengthening of muscles surgically to correct contractures does not prevent the recurrence of contractures [48]. The biological basis of contracture development, which affects these children’s ability to move [49], is thus poorly understood.
During the postnatal development of mammals, skeletal muscle growth leads to increases in the length of myofiber and area of existing myofibers [21]. Longitudinal growth occurs by the addition of sarcomeres (the basic skeletal muscle force-generating unit) in series so that there is a several-fold increase in the number of serial sarcomeres [61]. This presumably maintains an appropriate sarcomere length and generates optimal forces [22]. This growth, in response to increasing bone length [8], ensures the muscle’s ability to function efficiently over the appropriate joint ROM as the body’s size changes [15]. Even in adulthood, skeletal muscles are able to add or subtract sarcomeres in response to chronic stretching or shortening [62] and to recover to prestretching or shortening conditions. In contrast, during postnatal growth, children with cerebral palsy show impaired muscle growth and routinely develop muscular contractures [46]. These contractures demonstrate a decrease in the number of serial sarcomeres [38], over-stretched sarcomeres [35, 53], and reduced [14, 37] and heterogeneous [11] myofiber areas, suggesting that an inability to appropriately add sarcomeres during growth might lead to contracture development.
During postnatal muscle growth with an increasing number of serial sarcomeres, multinucleated myofibers add myonuclei along the length of the fiber [18, 61]. Because myonuclei are postmitotic (that is, they are unable to divide), these new myonuclei come from satellite cells [42]. These cells, situated between the basal lamina and sarcolemma, are resident muscle stem cells [13] that are essential for repair and regeneration of skeletal muscles after an injury [26, 34, 58, 64]. Muscle stem cells are indispensable for muscle growth during postnatal development [2, 33, 47] and their number (pool size) dictates myogenic potential [51]. While it is not entirely clear how the original brain injury can affect the muscle stem cells, surprisingly muscle contractures in children with cerebral palsy have 60% to 70% fewer satellite cells (that is, a decreased cell pool) [14, 52, 59]. Consequently, muscle contractures may be caused by an impaired addition of sarcomeres because of a reduced number of satellite cells.
Understanding the potential role of reduced satellite cell number in contracture development and impaired sarcomere addition may be valuable for the development of therapeutic strategies for contracture prevention and treatment. Currently, it is not possible to measure sarcomere addition in humans noninvasively and the biological basis for contractures is poorly understood. Current animal models of cerebral palsy are useful for certain research questions focused on brain repair and development [12, 16, 31] but are limited in their ability to test such a hypothesis for the biological basis of contractures. This is primarily because either they do not capture the full clinical picture due to fatalities that limit them to the immediate neonatal time period or they exhibit mild symptoms [12, 16, 31]. Consequently, one option is to focus only on the muscular system by combining a previously established muscle stem cell ablation-capable transgenic mouse model (Pax7CreER/+;Rosa26DTA/+) [20, 28, 32, 39] with properties of sarcomeres to create a clinical contracture-like state, that is, a reduced serial sarcomere number, increased extra-cellular matrix, and reduced muscle stem cells and then to evaluate recovery. Previously, we used this transgenic mice (Pax7CreER/+;Rosa26DTA/+) model, in which the number of satellite cells was experimentally reduced with tamoxifen, and showed that even with a reduced number of satellite cells, a limited addition of serial sarcomeres in response to chronic stretching may occur, but this is accompanied by a marked alteration in muscle morphology and in the extracellular matrix [32]. This suggested that a reduced number of satellite cells negatively affected muscle homeostasis.
In this study we asked: (1) Does a reduced number of satellite cells impair the addition of serial sarcomeres during recovery from an immobilization-induced contracture? (2) Is the severity of contracture due to the decreased number of serial sarcomeres or increased collagen content?
Materials and Methods
Study Design and Study Outcomes
We used a two-factorial study design to evaluate the role of satellite cells in sarcomere addition during recovery from an immobilization-induced contracture in a transgenic mouse model. First, we created a plantarflexion contracture model by casting one hindlimb in ankle plantarflexion to reduce the number of sarcomeres in the soleus and reduce ankle ROM (Fig. 1). Second, we pharmacologically reduced muscle stem cell number by using the transgenic construct and assessed the ability of the muscle to recover ankle range of motion by sarcomere addition after cast removal (Fig. 1). Our two-factorial design involved comparing the tamoxifen group (treatment group) to a vehicle group (control group) and the casted limb with the contralateral limb. We believe this model is relevant to muscles in children with cerebral palsy since we are recreating, at a muscular level, two specific deficits that are seen in these children—contractures with a reduced number of sarcomeres along the length of the muscle and a reduced number of muscle stem cells. Additionally, we assessed the ability of these muscles to recover from this state as way of potentially understanding the cause of contractures.
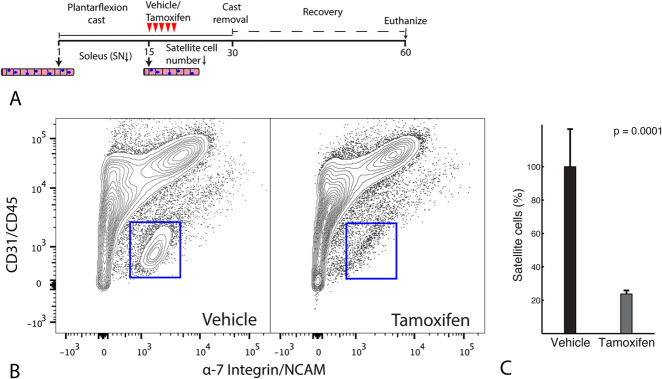
Fig. 1.
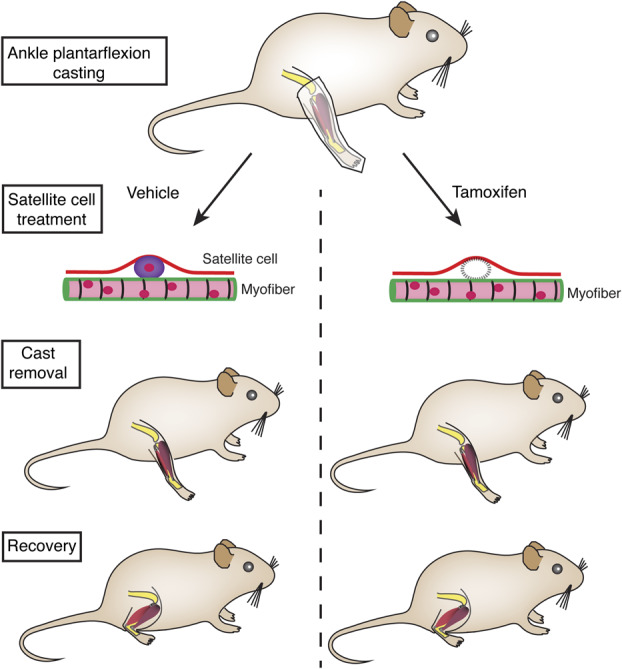
This figure shows an overview of the experiments. The right ankle of the transgenic Pax7-DTA mice were casted in plantarflexion such that the soleus fibers would shorten in length, while the tibialis anterior will add sarcomeres and increase in length. The contralateral leg served as a casting-control. After this, the mice underwent treatment for satellite cells (muscle stem cells) with tamoxifen inducing the Cre-Lox system to systemically ablate satellite cells from all muscles (right). The casts were then removed with all mice demonstrating resting plantarflexion angle. The tamoxifen (right) and vehicle-control (left) groups were allowed to recover their ankle ROM over 30 days.
Our primary study outcome of interest was the ability to add basic muscle contractile proteins—sarcomeres—during the process of recovery from a contracture-like state using a transgenic mouse model that had a reduced number of muscle stem cells. We quantified serial sarcomere number (the number of sarcomeres longitudinally), myofiber area (sarcomeres in a muscle cross-section) and extracellular matrix components from the muscles. Our secondary study outcome of interest was the severity of ankle contracture, that is, maximal ankle dorsiflexion angle, as a measure of recovery from the plantarflexion cast. We assessed whether maximal ankle angle was different in the group with reduced number of stem cells and if ankle contractures were associated with sarcomere addition or extracellular components.
Ethical Approval
All procedures were conducted in accordance with institutional guidelines for the care and use of laboratory animals as approved by the Institutional Animal Care and Use Committee at the University of California, San Diego.
Animals
Ten Pax7CreER/+; Rosa26DTA/+ mice (systemic satellite cell-specific, conditional ablation-capable mice, referred to in this paper as Pax7-DTA), aged 8 weeks, were used for the experiments [39]. They were crossed from individual homozygous Pax7CreER/CreER and Rosa26DTA/DTA strains (Jackson Laboratories, stock numbers 010530 and 010527, Bar Harbor, ME, USA) and divided into two groups: the Pax7-DTA control (vehicle) group (n = 5; four male mice and one female) and Pax7-DTA treatment (tamoxifen) group (n = 5; three male and two female mice). Pax7CreER/+; Rosa26DTA/+ mice treated with tamoxifen underwent ablation and reduction of their number of satellite cells by the diphtheria toxin via Cre-Lox recombination but not with a vehicle control [39]. The efficacy of this knockdown is 70% to 90%, systemic, and permanent. Mice were housed in a temperature- and humidity-controlled environment and maintained on a 12:12-hour light-dark cycle with free access to food and water. Briefly, the experimental protocol was initial immobilization of the hindfoot in plantarflexion for 14 days to reduce serial sarcomeres in the soleus, followed by tamoxifen or vehicle treatment for 5 days, washout for 10 days, removal of the cast, and recovery with cage remobilization for 30 days (Fig. 2A). We chose the minimum number of mice to allow us to assess this based on the anticipated large effect of 70% to 90% depletion of satellite cells (treatment group), 40% to 50% reduction in myofiber area (contralateral leg group) and 20% to 30% reduction in serial sarcomere number (contralateral leg group) at the end of the casting period, as reported in other studies [39, 50, 62]. Furthermore, we divided the muscles in half longitudinally to conduct analyses on both frozen and fixed tissue from the same mice to better answer questions of association.
Fig. 2.
This figure shows (A) the experimental casting protocol in which the hindlimb of Pax7-DTA mice were immobilized in plantarflexion for 2 weeks to reduce the number of serial sarcomeres in the soleus muscle. The mice were treated with oral gavage for 5 days with tamoxifen or a vehicle control. Subsequently, the mice were allowed to recover for 1 month and their muscles were harvested. (B) This is a representative image from the fluorescence-activated cell sorting analysis of the gastrocnemius and quadriceps muscles to quantify satellite cells; that is, α-7 integrin+/neural cell adhesion molecule+/CD31-/CD45- cells in the tamoxifen-treated (right) and vehicle-treated groups (left). The blue boxes indicate the representative cell populations. (C) The number of satellite cells was quantified with a fluorescence-activated cell sorting analysis, and this image shows substantial knockdown in satellite cells in mice in the tamoxifen-treated group (right) compared with those in the vehicle-treated control group. Data are shown as the mean ± SD.
Hindlimb Immobilization
The right hindlimbs were immobilized in maximal plantarflexion for 30 days in a plaster cast created with plaster strips (BSN Medical, Rutherford College, NC, USA) combined with Webril undercast padding (Covidien, Mansfield, MA, USA) and self-adherent bandage (3M, St. Paul, MN, USA) (Fig. 2A). The first 2 weeks were sufficient to create muscle shortening and an approximately 20% loss of serial sarcomeres in the soleus, similar to previous reports [50]. The contralateral noncasted hindlimb served as an internal control for all the mice. Casts were routinely inspected and repaired/replaced if they broke down. Casting and cast repairs were performed with mice under anesthesia via inhalation of 2% isoflurane (Butler Schein, Dublin, OH, USA) delivered by an Ohmeda anesthesia system (model VMA; Amersham, UK). Overall, the casts held up for the whole period and were repaired only once or twice during the experiment.
Satellite Cell Ablation
After the first 2-weeks in a cast, the mice in the treatment group (tamoxifen group) were administered tamoxifen (2 mg/100 uL per day, dissolved in 10% ethanol in peanut oil; MP Biomedicals, Solon, OH, USA) via oral gavage for 5 consecutive days to use the CreLox system and activate diphtheria toxin expression within the satellite (muscle stem) cells leading to targeted cell death. Mice in the Pax7-DTA control group were administered a vehicle treatment (10% ethanol in peanut oil), which does not affect the CreLox system and does not kill the satellite cells via diphtheria toxin. We used the oral gavage, then had a 10-day washout period, as used previously [32, 39], before removing the casts (Fig. 2A). The casts were maintained during this period to ensure there was no confounding of partial response by sarcomere addition before satellite cells depletion.
Recovery Period and Ankle ROM
After 30 days in total, we removed the casts under anesthesia, and the animals were allowed normal cage activity for 30 days (Fig. 2A). After cast removal, all mice had a completely plantarflexed ankle consistent with the casting procedure. A previous study showed that 30 days is sufficient to restore the normal number of serial sarcomeres and ankle ROM [62]. After 30 days, under anesthesia, the mouse was positioned on its side and a blunt forceps was used to move the foot passively into the maximal permissible dorsiflexion using steady force while stabilizing the leg, and pictures were obtained in the sagittal plane. The same person (MCK) performed the tests on all mice using the same technique. The maximal dorsiflexion angle was then calculated from the images using the angle between the shank and the proximal foot using ImageJ (National Institutes of Health, Bethesda, MD, USA), and this angle was used as a measure of recovery from plantarflexion. After the recovery period, animals were euthanized under anesthesia using cervical dislocation.
Passive Torque Measurement
Immediately after euthanizing the mice, we positioned their ankles in a specially designed footplate (Aurora Scientific, Ontario, Canada) that allowed us to measure ankle torque and control of the ankle’s position, as previously described [3]. Briefly, the immobilized ankle was secured in a foot jig with strips of bandage to align the center of the ankle joint with the jig’s center of rotation, and the ankle was firmly held in place. A custom program allowed us to position the ankle in 30° of plantarflexion from either neutral (0°) or the minimal plantarflexion angle allowed, in case of persistent contractures. The ankle was passively moved into dorsiflexion at 30°, followed by movement back into plantarflexion at the rate of 10° per second, during which passive ankle torque was measured. During passive dorsiflexion, the peak torque was calculated using a custom Matlab program (Mathworks, Natick, MA, USA) and this parameter was used as the primary outcome variable. We were unable to measure this value in one mouse from the tamoxifen group because it had severe contractures and was thus not included in our analysis of the peak passive torque.
Muscle Harvesting and Fixation
After euthanizing the animals, hindlimbs were skinned and under a dissecting microscope, we measured the lengths of the soleus myotendinous and distal tendon in vivo with the ankle close to neutral (in approximately 0° of dorsiflexion or minimal plantarflexion) using a digital caliper. We carefully dissected the tibialis anterior, extensor digitorum longus, and soleus muscles from the hindlimb. The soleus and tibialis anterior muscles were divided longitudinally to provide two specimens: one that was chemically fixed and one that was flash frozen. For one mouse in the tamoxifen group, the tibialis anterior, extensor digitorum longus, and soleus muscles were only fixed due to severe contractures. In addition, the gastrocnemius and quadriceps muscles were used to evaluate the efficacy of tamoxifen treatment inducing the Cre-Lox system to reduce satellite cell number with flow cytometry. Muscle specimens were pinned at an in vivo length on a piece of cork and flash frozen in liquid nitrogen-cooled isopentane (-159° C) and stored in a -80° C freezer until subsequent analysis.
The soleus, tibialis anterior, and extensor digitorum longus muscles were pinned on a piece of cork near their in vivo lengths and fixed for 48 hours in 10% formalin (Fisher Scientific, Fair Lawn, NJ, USA). After fixation, the muscles were rinsed three times in phosphate-buffered saline to remove any residual fixative and were stored in phosphate-buffered saline for subsequent analysis.
Flow Cytometry
We used previously used procedures for flow cytometry [32] to quantify global ablation of muscle stem cells-satellite cells in the quadriceps and gastrocnemius muscles. Briefly, the muscles were incubated in a digestion buffer consisting of collagenase and dispase at 37° C, tissues were mechanically broken down with surgical tools and filtered with a 40-µm mesh. This was centrifuged at 1300 rpm at 4° C for 10 minutes to create a pellet containing all the mononuclear cells from the sample and then resuspended in fluorescence-activated cell sorting buffer (1 mM of EDTA, 2.5% goat serum in phosphate-buffered saline, and pH of 7.4). Fluorophore-conjugated antibodies were used for α7-integrin (AbLab, Vancouver, Canada, 1:200), CD31 (eBiosciences, San Diego, CA, USA, 1:200), and CD45 (eBiosciences, San Diego, CA, USA, 1:200), respectively. An unconjugated antibody for the neural cell adhesion molecule (BD Biosciences, San Jose, CA, USA, 1:200) was used with a secondary fluorescent antibody (Goat anti-mouse Alexafluor 488, Invitrogen, Waltham, MA, USA).
We performed cell sorting using a Canto instrument (BD Biosciences, San Jose, CA, USA). R-phycoerythrin fluorochromes were used for α7-integrin and the neural cell adhesion molecule, Pacific blue was used for CD31 and CD45 (endothelial cells and inflammatory cells), and data for 60,000-100,000 cells per sample were obtained. We performed primary gating to exclude cellular debris and performed secondary gating analysis using FlowJo software version 10 (BD Biosciences, San Jose, CA, USA). In our samples, we defined satellite cells as α7-integrin+/ neural cell adhesion molecule+/CD31-/CD45- cells. Unstained controls and gating controls (α7-integrin/neural cell adhesion molecule-/CD31+/CD45+) ensured specificity of satellite cell quantification. In tamoxifen-treated Pax7-DTA mice, 0.58% ± 0.32% of all mononuclear cells were α7-integrin+/neural cell adhesion molecule+/CD31-/CD45- satellite cells compared with 3.05% ± 1.75% of cells in the vehicle-treated Pax7-DTA mice (Fig. 2B). A 76% reduction (range 74%-79%) in the number of satellite cells was observed in the treatment group (p = 0.001) (Fig. 2C).
Muscle Architecture Measurements
Methods for measuring the architecture of the muscles were modified from a protocol previously described in detail [9]. Briefly, the muscles were dissected with sharp forceps and spring scissors longitudinally into numerous segments starting from the proximal and distal part of the muscles. From these segments, three to six fiber bundles of 10 to 15 fibers were dissected from the proximal and distal segments and lengths measured under a dissecting microscope with digital calipers. Fiber lengths were measured from three fiber bundles whenever possible (in two specimens, there was insufficient tissue and only two measurements were obtained) and were averaged to provide the mean fiber length for each sample. These fiber bundles were mounted on slides and cover slipped to measure sarcomere lengths (Ls) to calculate serial sarcomere number for each sample. Due to structural changes measurement of Ls using the gold-standard laser diffraction method [36] did not work. Consequently, the lengths were measured optically by bright-field microscopy at 40x magnification (Leica DM3000, Buffalo Grove, IL, USA). The distance between 10 to 15 serial Z-bands was measured using the computerized caliper and divided by the corresponding number of sarcomeres to calculate the average sarcomere length for each sample bundle. We made measurements for three to five bundles, which we averaged to provide the mean sarcomere length for each muscle. Then, we calculated the number of serial sarcomeres by dividing the mean fiber length (Lf) by the mean sarcomere length (Ls). This measurement was our main outcome measure for muscle architecture since it accounts for any variability in the pinning and fixation process that would affect both the fiber lengths and sarcomere lengths.
Muscle Histology and Immunohistochemistry
We created slides from serial cross-sections (10 µm) of flash-frozen soleus and tibialis anterior muscles embedded in an optimal cutting temperature compound were created on a cryostat at -25° C (Microm HM500, Walldorf, Germany) and stored in a -20° C freezer. Hematoxylin and eosin staining were used to evaluate the general morphology and area fraction (the fraction of muscle area) of the soleus muscle, as described previously [32]. We obtained images of three to four sections per specimen under 10x objective and ImageJ (National Institutes of Health) was used to calculate this. Color thresholding, based on the hue and saturation of the hematoxylin and eosin-stained sections, were used to differentiate between the pink (that is, the muscle area), and the white (that is, the extracellular matrix area) regions. Measurement values of the muscle area were normalized to the area of the whole tissue section (100%), which we referred to as the fraction of muscle area [32, 40]. For myofiber area calculation using Laminin immunohistochemistry, the slides were washed with phosphate-buffered saline and covered with a blocking solution (2% bovine serum albumin, 5% fetal bovine serum, 0.2% Triton X-100, 2% goat serum, and 1 x phosphate-buffered saline) for 30 minutes. A primary unconjugated antibody (Laminin 9393, rabbit polyclonal, Sigma, St. Louis, MO, USA) for the basal lamina of the myofibers and a secondary fluorescent antibody (Alexafluor 488 goat anti-rabbit IgG, Invitrogen, Waltham, MA, USA) were used. A fluorescent mounting medium containing 4',6-diamidino-2-phenylindole (DAPI) to stain nuclei was used, and the slides were sealed with a cover slip. Fluorescence microscopy was used to capture images of the sections at 10 X magnification and myofiber areas were calculated from the slides for 439 ± 163 fibers per sample using ImageJ (National Institutes of Health), as described previously [41].
Hydroxyproline Content
Collagen content was determined with a spectrophotomeric analysis for hydroxyproline content using our standardized protocol [17, 53]. Briefly, muscle samples were hydrolyzed in 6 N of hydrochloric acid for 24 hours at 110° C. Samples were pipetted into 96-well plates and treated with 37.5 µl or chloramine T solution for 20 minutes at room temperature, followed by a 37.5-µL solution of p-diaminobenzaldehyde for 30 minutes at 60° C. Subsequently, sample absorbance was read with a spectrophotometer at 550 nm in triplicate and compared against a standard curve to determine the hydroxyproline content. The hydroxyproline content, normalized to the wet weight of tissue, was converted to the collagen content using a constant (7.46) that defines the number of hydroxyproline residues per collagen molecule.
Statistical Analysis
Satellite cells were expressed as the percentage of all mononuclear cells in both the tamoxifen and vehicle groups. Values for samples in the tamoxifen group were normalized to the average value of the vehicle group as a measure of satellite cell knockdown. Each flow cytometry session included both samples from the tamoxifen and vehicle groups to correct for any differences in gating for cell selection across sessions. We used ANOVA to compare the percentage of satellite cells between groups. The significance level (α) for all tests was set at 0.05 (Prism 6.0d; GraphPad Software Inc, La Jolla, CA, USA). We used two-way ANOVA (cast x treatment) to compare the number of serial sarcomeres, fiber length, distal tendon length, myotendinous length, myofiber area, fraction of muscle area, and collagen content between immobilized and nonimmobilized hindlimbs and between vehicle-control and tamoxifen groups, and multiple-comparisons were made using Sidak’s post hoc tests. Maximal dorsiflexion angle of the immobilized limbs was assessed with a one-way ANOVA. Linear regression analysis was performed to quantify the association among the number of serial sarcomeres and maximal dorsiflexion angle. We used analysis of covariance (ANCOVA) to evaluate the difference in these associations between the tamoxifen and vehicle groups. Univariate and multivariate regression analyses were used to evaluate the associations among collagen content, number of sarcomeres, maximal dorsiflexion angle, and peak passive torque. In addition, ANCOVA was used to evaluate the difference in these associations between the tamoxifen and vehicle groups. Data are presented as the mean ± SD (mean difference [95% CI]; p value), unless otherwise noted.
Results
Reduced Satellite Cell Number Impairs Serial Sarcomere Addition During Contracture Recovery
Our results showed that recovery from an immobilization-induced contracture by addition of sarcomeres was impaired with a reduced number of satellite cells but muscle morphology and collagen content was not altered. The number of serial sarcomeres in the soleus muscle was lower on the tamoxifen-casted side (2214 ± 333) than on the contralateral side (2543 ± 206, -329 [95% confidence interval -650 to -9]; p = 0.04), while these were not different between the vehicle control-casted side (2644 ± 194) and the contralateral side (2729 ± 249, -85 [95% CI -406 to 236]; p = 0.97) (Fig. 3A). Correspondingly, the number of serial sarcomeres in the antagonistic extensor digitorum longus muscle was higher on the tamoxifen-casted side (2732 ± 416) than on the contralateral side (2009 ± 210, 723 [95% CI 377 to 1068]; p = 0.001), but there were no differences in the number of serial sarcomeres in the extensor digitorum longus between the casted (2479 ± 285) and contralateral sides (2179 ± 245, 300 [95% CI -86 to 686]; p = 0.12) for the vehicle control group (Fig. 3B). No differences were observed in the number of serial sarcomeres in the tibialis anterior in the tamoxifen-treated group between the casted (3204 ± 387) and noncasted sides (3127 ± 100, 77 [95% CI -362 to 516]; p = 0.87) or with the vehicle-treated group (3255 ± 391, 3497 ± 228, respectively, -153 [95% CI -592 to 287]; p = 0.60) (Fig. 3C).
Fig. 3.
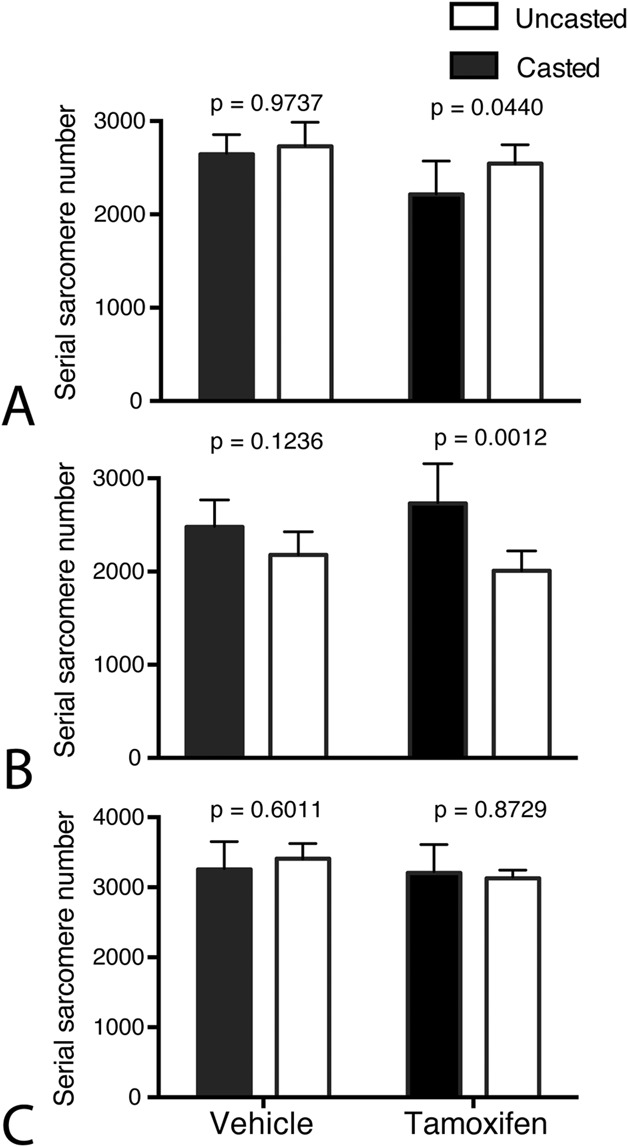
This figure shows the number of serial sarcomeres after 1 month of recovery in (A) the soleus, (B) extensor digitorum longus, and (C) tibialis anterior muscles in tamoxifen-treated mice and vehicle-treated mice compared with the contralateral, uncasted side (open bar graphs). Data are presented as the mean ± SD.
At the level of fiber lengths, similar results were observed; the soleus fiber length was shorter on the tamoxifen casted side (6.73 mm ± 1.36 mm) compared with the contralateral side (7.91 mm ± 0.61 mm, -1.19 [95% CI -2.35 to -0.02]; p = 0.046), while the vehicle control casted side (8.10 mm ± 0.77 mm) was not different compared with the contralateral side (8.52 mm ± 0.90 mm, -0.43 [95% CI -1.59 to 0.74]; p = 0.57). Correspondingly, the extensor digitorum longus fiber length was greater on the tamoxifen-treated casted side (7.11 mm ± 1.21 mm) compared with the contralateral side (5.53 mm ± 0.50 mm, 1.58 [95% CI 0.69 to 2.47]; p = 0.003), while the vehicle control casted side and the contralateral side were not different (6.60 mm ± 0.39 mm and 6.29 mm ± 0.25 mm, respectively, 0.31 [95% CI -0.69 to 1.31]; p = 0.65). The distal tendon of the soleus was longer on the tamoxifen-casted side (7.05 mm ± 0.84 mm) than on the contralateral side (5.96 mm ± 0.29 mm, 1.08 [95% CI 0.32 to 1.85]; p = 0.01), while it was not different between the vehicle control-casted side and the contralateral side (6.04 mm ± 0.60 mm and 6.03 mm ± 0.53 mm, respectively, (0.006 [95% CI -0.66 to 0.69]; p >0.99).
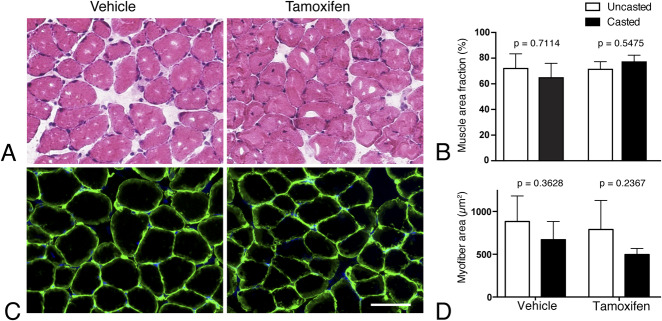
The muscle area fraction in the soleus was not different across the groups and sides: 77% ± 5% on the tamoxifen-casted side, 71% ± 6% on the contralateral side (-7.67 [95% CI -27.83 to 12.49]; p = 0.55), 64% ± 11% on the vehicle control-casted side, and 72% ± 12% on the contralateral side (5.67 [95% CI -14.49 to 25.83]; p = 0.71) (Fig. 4A-B).
Fig. 4.
This figure shows the morphology and myofiber area of the soleus muscle in vehicle-treated (left) and tamoxifen-treated mice (right) along with their contralateral uncasted soleii (open bars). The representative images show (A) hematoxylin and eosin staining and (B) quantification of the percentage area of hematoxylin and eosin-stained images as muscle versus extracellular matrix-related. These images show (C) laminin antibody labeling of the basal lamina and (D) the myofiber area from laminin-labeled images (scale bar = 50 µm for all images).
Similarly, the collagen content of the soleus was not different: 9.66 µg/mg ± 10.30 µg/mg on the tamoxifen-casted side, 3.96 µg/mg ± 1.10 µg/mg on the contralateral side (-5.71 [95% CI -22.96 to 11.55]; p = 0.62), 11.36 µg/mg ± 13.70 µg/mg on the vehicle control-casted side, and 7.68 µg/mg ± 3.56 µg/mg on the contralateral side (-3.68 [95% CI -19.12 to 11.76]; p = 0.77).
The myofiber area of the soleus was not different between the groups across sides. On the tamoxifen-casted side it was 496 µm2 ± 69 µm2 compared with 789 µm2 ± 341 µm2 on the contralateral, uncasted side (293 [95% CI -185 to 771]; p = 0.23). In the vehicle control-treated mice, the casted side had a myofiber area of 671 µm2 ± 202 µm2 compared with 883 µm2 ± 296 µm2 on the contralateral side (213 [95% CI -215 to 640]; p = 0.36) (Fig. 4C-D). Similarly, the myofiber area of the antagonist tibialis anterior muscle was similar across the groups across the sides; 858 µm2 ± 134 µm2 on the tamoxifen-casted side, 1192 µm2 ± 364 µm2 on the contralateral side (334 [95% CI -163 to 831]; p = 0.19), 1006 µm2 ± 407 µm2 on the vehicle control-casted side, and 1276 µm2 ± 580 µm2 on the contralateral side (270 [95% CI -227 to 767]; p = 0.3183).
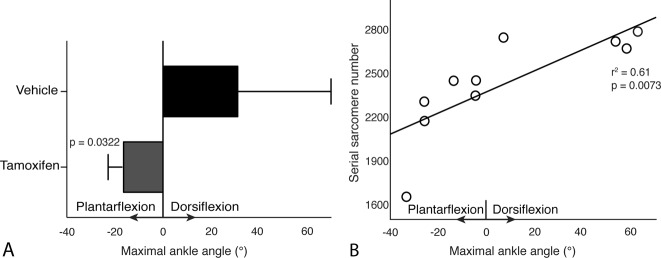
Severity of Contracture Related to Sarcomere Addition Rather Than Increased Collagen Content
A reduced number of satellite cells hampered the recovery from the immobilization-induced contractures and the severity of the residual contractures appeared to be due to insufficient sarcomere addition rather than an increase in extracellular matrix. The maximal ankle dorsiflexion angle in the tamoxifen-treated casted group was lower than that in the vehicle-treated casted group (-16° ± 13° versus 31° ± 39°, -47 [95% CI -89 to -5]; p = 0.03) (Fig. 5A) (positive values indicate dorsiflexion). The maximal ankle angle (the extent of recovery from contracture) was strongly associated with the number of serial sarcomeres in the soleus (r2 = 0.61; p = 0.007) (Fig. 5B). This association was greater for the tamoxifen-treated group (slope = 23 sarcomeres/°) than the vehicle-treated group (slope = 3.92 sarcomeres/°; p = 0.035).
Fig. 5.
This figure shows the severity of contracture 1 month after mobilization. (A) This figure shows the maximal dorsiflexion angle after 1 month of cage mobilization following the plantarflexion casting in tamoxifen-treated mice and vehicle-treated control mice (positive values indicate dorsiflexion, negative values indicate plantarflexion). (B) This figure shows the association between the maximal dorsiflexion angle (that is, the degree of recovery) and the number of serial sarcomeres in the soleus muscle. Data are shown as the mean ± SD.
Maximal ankle angle was positively associated with the number of sarcomeres; that is, serial sarcomere number of the soleus (r2 = 0.73; p = 0.0035) and myofiber area of the soleus (r2 = 0.78, p = 0.002) but was not associated with collagen content (r2 =0.27; p = 0.15). The number of sarcomeres in series (serial sarcomere number) and in parallel (myofiber area) explained 80% of variance in the maximal ankle angle, that is, degree of recovery from contractures (r2 =0.80; p = 0.008). Correspondingly, peak passive torque was negatively associated with serial sarcomere number (r2 = 0.47, p = 0.0404) but was not associated with collagen content (r2 = 0.43; p = 0.057).
Discussion
Children with cerebral palsy have impaired muscle growth leading to development of contractures, which are associated with a reduced serial sarcomere number and a reduced number of satellite cells, that is, muscle stem cells. Here, we used a transgenic mouse model to create an immobilization-induced contracture that had reduced ROM, reduced serial sarcomere number; then we pharmacologically reduced the number of satellite cells and evaluated whether recovery was affected. Our results clearly demonstrate that a reduced number of satellite cells hindered the ability of mice to recover from a contracture-like state and their muscles all remained in plantarflexion compared with the muscles of mice with a normal complement of satellite cells that regained ROM in dorsiflexion. Maximal ankle angle was negatively associated with the number of serial sarcomeres in the soleus muscle. The slope of this association was steeper in mice with a reduced number of satellite cells, suggesting an increased requirement for the addition of serial sarcomeres during recovery from maximal plantarflexion to dorsiflexion. We found that the morphology and collagen content of the soleus muscle were not altered in mice, although there was an increase in the length of the distal tendon in mice with a reduced number of satellite cells. Importantly, we showed that the maximal dorsiflexion angle was positively associated with the number of serial sarcomeres and myofiber area; that is, sarcomeres in series and parallel but not collagen content. The peak passive torque during dorsiflexion was negatively associated with the number of sarcomeres but not associated with the collagen content of the soleus muscle.
Limitations
This study has a number of limitations. First, the clinical question of interest for this study was whether there is a role for reduced satellite cell number in impaired muscle growth, specifically sarcomere addition in children with cerebral palsy. However, currently it is not possible to measure this in humans; animal models thus provide an alternative. Current animal models for cerebral palsy do not recreate the complex muscular picture observed clinically [12]. Our transgenic mouse model is not a model of cerebral palsy but a satellite cell ablation model. We chose it to recreate two of the key features observed in muscle contractures: reduced serial sarcomere number and reduced number of satellite cells to evaluate a relationship between them. Furthermore, mammalian skeletal muscle physiology is fairly conserved across species [10] and mouse muscles are accepted as a surrogate for human muscles [6]. Consequently, although this work was performed in mice and is not a brain injury model, we believe that the results from this study are applicable and relevant to the potential mechanism of development of contractures in children with cerebral palsy.
We did not directly measure the number of serial sarcomeres at the end of the casting phase because of the limited number of transgenic animals. However, previous studies in our laboratory and others showed that the number of serial sarcomeres in the soleus muscle was reduced by approximately 20% when the muscle was maintained in a shortened position [50, 61, 62]. Mice in the control group recovered most of their serial sarcomeres and this number was only 3% lower than on the contralateral side, whereas mice in the treatment groups had 13% fewer serial sarcomeres than on the contralateral side. In addition, our sarcomere lengths were longer than expected, which could be because we pinned the muscles at a longer length than in vivo. To overcome this potential confounder, our main outcome variable was serial sarcomere number, which is calculated by normalizing fiber lengths to sarcomere lengths and is not affected by variations in muscle lengths that occur during the fixation process [19]. We did not directly measure cage activity or use any specific strategy to increase mobility in the mice during the recovery period. Mice were mobile during both the casting and recovery phases and did not seem impaired in their ability to eat food, drink water, and move around. Finally, we had a mixed group of male and female mice. Because tamoxifen is an estrogen blocker, it may have other nonspecific effects. Consistent with the methods of previous studies [20, 29, 32, 39], we followed a washout period of 10 days after the treatment to minimize this confounder.
Reduced Satellite Cell Number Impairs Serial Sarcomere Addition During Contracture Recovery
Our results demonstrate that a reduced number of satellite cells impaired the addition of serial sarcomeres during recovery from an immobilization-induced contracture. The addition of sarcomeres is believed to occur at the ends of fibers [61], where satellite cells are in higher concentrations during the postnatal period [1] and during recovery from a shortened position, the addition of sarcomeres has been shown to occur at the end of myofibers [61]. Some authors [61] posited that satellite cells play a role in this, but they never directly tested the idea. Our results support this hypothesis. In skeletally mature mice, satellite cell ablation does not prevent regrowth, at least after unloading-induced atrophy [29]; however, if present, these cells are activated [25]. Similar results have been observed in adult mice with obligatory hypertrophic processes, such as those seen in synergistic ablation models [39] but satellite cells might be required in the long-term for hypertrophy and maintaining the extracellular matrix [20]. Our results suggest that regrowth after immobilization in a shortened position is associated with the addition of sarcomeres in series (serial sarcomere number) and in parallel (myofiber area), and these sarcomeres were reduced in the satellite cell-ablated group. The age of the mice used for our experiments was 8 to 10 weeks as opposed to 16 to 20 weeks in previous studies [39]. A recent study using an obligatory hypertrophy model has shown that satellite cell ablation in growing mice (8 weeks old) leads to a reduced muscle hypertrophy, although this is not seen in adult mice (16 weeks old) [43]. This is consistent with our results.
Tamoxifen treatment induced a 74% to 79% reduction in the number of satellite cells in Pax7-DTA mice, slightly lower than the 80% to 90% reduction reported previously [39]. However, it is higher than a 60% to 70% reduction in satellite cells in children with cerebral palsy [14, 52], allowing us to test our hypothesis as it applies to the clinical population. There was no correlation between the number of satellite cells and number of serial sarcomeres. However, this is not surprising, given that the level of reduction was similar across the groups (74%-79%). Although serial sarcomeres were reduced by -13% at the end of the recovery period in the satellite cell-reduced group, clinically, in children with cerebral palsy, this reduction is much greater (-46%), suggesting there is much greater impairment in the addition of serial sarcomeres because of cerebral palsy.
Severity of Contracture Related to Sarcomere Addition Rather Than Increased Collagen Content
We also found that the mice with reduced number of satellite cells had residual plantarflexion contractures at the end of the recovery period. The severity of these contractures was associated with the ability to add sarcomeres in series and in parallel rather than increased collagen content. Because there is an increase in intramuscular collagen content in the first few weeks of muscle immobilization in a shortened position [60, 63], we evaluated whether this explained the residual contractures we observed in our satellite cell-reduced group. Our results showed that at the end of the 30-day recovery period collagen content did not dictate the severity of contracture in the treatment group. Furthermore, the results of the multiple regression analysis suggested that the maximal ankle angle was primarily a function of increased sarcomeres in series and parallel rather than a function of decreased collagen content. The stepwise regression model showed that the collagen content did not contribute to an increase in the ankle angle. Satellite cells negatively regulate the extracellular matrix [20, 32, 44] and play a role in the reorganization of increased collagen content during the recovery period. In our previous work [32], we reported that with chronic stretching, limited addition of serial sarcomeres was possible if there was a lower number of satellite cells. However, there was a considerable increase in the extracellular matrix and a reduction of myofiber areas. Importantly, those muscles had a normal number of serial sarcomeres before stretching, which is different from the clinical situation of a reduced number of serial sarcomeres and gradual addition of sarcomeres. Consequently, our current study tested the clinically relevant hypothesis of whether an adequate number of satellite cells is required for a muscle to add serial sarcomeres and recover from a contracture. Our results showed that an inadequate number of satellite cells hinders recovery from a muscular contracture. Stretching-induced activation of satellite cells [54] may be affected if a contracture is present, and an adequate number is needed for an appropriate response.
Conclusions
In this transgenic mouse model, we showed that sarcomere addition in series and in parallel during recovery from skeletal muscle contractures are dependent on the presence of an adequate number of satellite cells (muscle stem cells). The severity of the residual contractures in the presence of reduced number of satellite cells are related to the number of serial sarcomeres, fiber length, and myofiber area, rather than an increase in collagen content. We believe these data are relevant to children with cerebral palsy, who have substantial limitations in their joint ROM because of muscular contractures [49]. These muscles have both a reduced serial sarcomere number and a reduced number of satellite cells [14, 52]. Rehabilitation focuses on preventing contractures and facilitating muscle growth with serial casting, botulinum toxin injections, and stretching [24], to increase the number of serial sarcomeres. From our results in a model system, we speculate that in children with cerebral palsy, longitudinal muscle growth by the addition of sarcomeres is potentially impaired because of a reduced number of satellite cells, leading to the development of contractures. Future work needs to evaluate whether increasing or maintaining the number of satellite cells before development of contractures by targeted exercise training, such as endurance training that has a positive impact on their number and function, or by pharmacological treatment of satellite cells themselves, can promote muscle growth and prevent development of contractures.
Acknowledgments
We thank John J. McCarthy PhD, and Charlotte Peterson PhD, of the University of Kentucky, Center for Muscle Biology, for donating the Pax7 and Rosa26DTA mice; Gretchen Meyer PhD, for her assistance with flow cytometry; and Mary Esparza BS for her assistance with the experimental procedures.
Footnotes
The institution of one or more of the authors (SD, MCK, RLL) has received, during the study period, funding from National Institutes of Health (P30AR061303, HD050837, and HD094602), Department of Veterans Affairs (A9028-R) and Orthopaedic Research and Education Foundation (funding provided by DePuy Synthes Joint Reconstruction). This work was supported (or supported in part) by Research Career Scientist Award Number IK6 RX003351 from the United States Department of Veterans Affairs Rehabilitation R&D (Rehab RD) Service.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the animal protocol of this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at University of California, San Diego, CA, USA, and Shirley Ryan AbilityLab, Chicago, IL, USA.
References
- 1.Allouh MZ, Yablonka-Reuveni Z, Rosser BWC. Pax7 reveals a greater frequency and concentration of satellite cells at the ends of growing skeletal muscle fibers. J Histochem Cytochem. 2008;56:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachman JF, Klose A, Liu W, Paris ND, Blanc RS, Schmalz M, Knapp E, Chakkalakal JV. Prepubertal skeletal muscle growth requires Pax7-expressing satellite cell-derived myonuclear contribution. Development. 2018;145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol. 2004;286:C355-364. [DOI] [PubMed] [Google Scholar]
- 4.Barber LA, Read F, Lovatt Stern J, Lichtwark G, Boyd RN. Medial gastrocnemius muscle volume in ambulant children with unilateral and bilateral cerebral palsy aged 2 to 9 years. Dev Med Child Neurol. 2016;58:1146-1152. [DOI] [PubMed] [Google Scholar]
- 5.Barber LEE, Hastings-Ison T, Baker R, Barrett ROD, Lichtwark G. Medial gastrocnemius muscle volume and fascicle length in children aged 2 to 5 years with cerebral palsy. Dev Med Child Neurol. 2011;53:543-548. [DOI] [PubMed] [Google Scholar]
- 6.Bareja A, Billin A. Satellite cell therapy - from mice to men. Skelet Muscle. 2013;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell KJ, Ounpuu S, DeLuca PA, Romness MJ. Natural progression of gait in children with cerebral palsy. J Pediatr Orthop. 2002;22:677-682. [PubMed] [Google Scholar]
- 8.Boakes JL, Foran J, Ward SR, Lieber RL. Muscle adaptation by serial sarcomere addition 1 year after femoral lengthening. Clin Orthop Relat Res. 2007;456:250-253. [DOI] [PubMed] [Google Scholar]
- 9.Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221:177-190. [DOI] [PubMed] [Google Scholar]
- 10.Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol. 2001;204:1529-1536. [DOI] [PubMed] [Google Scholar]
- 11.Castle ME, Reyman TA, Schneider M. Pathology of spastic muscle in cerebral palsy. Clin Orthop Relat Res. 1979:223-232. [PubMed] [Google Scholar]
- 12.Clowry GJ, Basuodan R, Chan F. What are the best animal models for testing early intervention in cerebral palsy? Front Neurol. 2014;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289-301. [DOI] [PubMed] [Google Scholar]
- 14.Dayanidhi S, Dykstra PB, Lyubasyuk V, McKay BR, Chambers HG, Lieber RL. Reduced satellite cell number in situ in muscular contractures from children with cerebral palsy. J Orthop Res. 2015;33:1039-1045. [DOI] [PubMed] [Google Scholar]
- 15.Dayanidhi S, Lieber RL. Skeletal muscle satellite cells: Mediators of muscle growth during development and implications for developmental disorders. Muscle Nerve. 2014;50:723-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drobyshevsky A, Cotten CM, Shi Z, Luo K, Jiang R, Derrick M, Tracy ET, Gentry T, Goldberg RN, Kurtzberg J, Tan S. Human umbilical cord blood cells ameliorate motor deficits in rabbits in a cerebral palsy model. Dev Neurosci. 2015;37:349-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards CA, O'Brien WD. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161-167. [DOI] [PubMed] [Google Scholar]
- 18.Enesco M, Puddy D. Increase in the number of nuclei and weight in skeletal muscle of rats of various ages. Am J Anat. 1964;114:235-244. [DOI] [PubMed] [Google Scholar]
- 19.Felder A, Ward SR, Lieber RL. Sarcomere length measurement permits high resolution normalization of muscle fiber length in architectural studies. J Exp Biol. 2005;208:3275. [DOI] [PubMed] [Google Scholar]
- 20.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014;28:1654-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gokhin DS, Ward SR, Bremner SN, Lieber RL. Quantitative analysis of neonatal skeletal muscle functional improvement in the mouse. J Exp Biol. 2008;211:837-843. [DOI] [PubMed] [Google Scholar]
- 22.Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham HK, Rosenbaum P, Paneth N, Dan B, Lin JP, Damiano DL, Becher JG, Gaebler-Spira D, Colver A, Reddihough DS, Crompton KE, Lieber RL. Cerebral palsy. Nat Rev Dis Primers. 2016;2:15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham HK, Selber P. Musculoskeletal aspects of cerebral palsy. J Bone Joint Surg Br. 2003;85:157-166. [DOI] [PubMed] [Google Scholar]
- 25.Guitart M, Lloreta J, Manas-Garcia L, Barreiro E. Muscle regeneration potential and satellite cell activation profile during recovery following hindlimb immobilization in mice. J Cell Physiol. 2018;233:4360-4372. [DOI] [PubMed] [Google Scholar]
- 26.Günther S, Kim J, Kostin S, Lepper C, Fan C-M, Braun T. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell. 2013;13:590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hof AL. Changes in muscles and tendons due to neural motor disorders: implications for therapeutic intervention. Neural Plast. 2001;8:71-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson JR, Kirby TJ, Fry CS, Cooper RL, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Reduced voluntary running performance is associated with impaired coordination as a result of muscle satellite cell depletion in adult mice. Skeletal Muscle. 2015;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol. 2012;303:C854-C861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DC, Damiano DL, Abel MF. The evolution of gait in childhood and adolescent cerebral palsy. J Pediatr Orthop. 1997;17:392-396. [PubMed] [Google Scholar]
- 31.Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4:130ra146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinney MC, Dayanidhi S, Dykstra PB, McCarthy JJ, Peterson CA, Lieber RL. Reduced skeletal muscle satellite cell number alters muscle morphology after chronic stretch but allows limited serial sarcomere addition. Muscle Nerve. 2017;55:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieber RL, Fridén J. Spasticity causes a fundamental rearrangement of muscle-joint interaction. Muscle Nerve. 2002;25:265-270. [DOI] [PubMed] [Google Scholar]
- 36.Lieber RL, Yeh Y, Baskin RJ. Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter. Biophys J. 1984;45:1007-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathewson MA, Chambers HG, Girard PJ, Tenenhaus M, Schwartz AK, Lieber RL. Stiff muscle fibers in calf muscles of patients with cerebral palsy lead to high passive muscle stiffness. J Orthop Res. 2014;32:1667-1674. [DOI] [PubMed] [Google Scholar]
- 38.Mathewson MA, Ward SR, Chambers HG, Lieber RL. High resolution muscle measurements provide insights into equinus contractures in patients with cerebral palsy. J Orthop Res. 2015;33:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer GA, Lieber RL. Skeletal muscle fibrosis develops in response to desmin deletion. Am J Physiol Cell Physiol. 2012;302:C1609-C1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minamoto VB, Hulst JB, Lim M, Peace WJ, Bremner SN, Ward SR, Lieber RL. Increased efficacy and decreased systemic-effects of botulinum toxin A injection after active or passive muscle manipulation. Dev Med Child Neurol. 2007;49:907-914. [DOI] [PubMed] [Google Scholar]
- 42.Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421-435. [DOI] [PubMed] [Google Scholar]
- 43.Murach KA, White SH, Wen Y, Ho A, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skeletal Muscle. 2017;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noble JJ, Fry NR, Lewis AP, Keevil SF, Gough M, Shortland AP. Lower limb muscle volumes in bilateral spastic cerebral palsy. Brain Dev. 2014;36:294-300. [DOI] [PubMed] [Google Scholar]
- 46.Nordmark E, Hagglund G, Lauge-Pedersen H, Wagner P, Westbom L. Development of lower limb range of motion from early childhood to adolescence in cerebral palsy: a population-based study. BMC Med. 2009;7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23:3430-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rethlefsen SA, Yasmeh S, Wren TA, Kay RM. Repeat hamstring lengthening for crouch gait in children with cerebral palsy. J Pediatr Orthop. 2013;33:501-504. [DOI] [PubMed] [Google Scholar]
- 49.Rodda JM, Graham HK, Carson L, Galea MP, Wolfe R. Sagittal gait patterns in spastic diplegia. J Bone Joint Surg Br. 2004;86:251-258. [DOI] [PubMed] [Google Scholar]
- 50.Shah SB, Peters D, Jordan KA, Milner DJ, Friden J, Capetanaki Y, Lieber RL. Sarcomere number regulation maintained after immobilization in desmin-null mouse skeletal muscle. J Exp Biol. 2001;204:1703-1710. [DOI] [PubMed] [Google Scholar]
- 51.Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith LR, Chambers HG, Lieber RL. Reduced satellite cell population may lead to contractures in children with cerebral palsy. Dev Med Child Neurol. 2013;55:264-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. 2011;589:2625-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol. 2006;290:C1487-C1494. [DOI] [PubMed] [Google Scholar]
- 55.Tedroff K, Granath F, Forssberg H, Haglund-Akerlind Y. Long-term effects of botulinum toxin A in children with cerebral palsy. Dev Med Child Neurol. 2009;51:120-127. [DOI] [PubMed] [Google Scholar]
- 56.Tedroff K, Löwing K, Jacobson D, Åström E. Does loss of spasticity matter? A 10-year follow-up after selective dorsal rhizotomy in cerebral palsy. Dev Med Child Neurol. 2011;53:724-729. [DOI] [PubMed] [Google Scholar]
- 57.Van Naarden Braun K, Doernberg N, Schieve L, Christensen D, Goodman A, Yeargin-Allsopp M. Birth prevalence of cerebral palsy: a population-based study. Pediatrics. 2016;137:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci U S A. 2013;110:16474-16479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.von Walden F, Jalaleddini K, Evertsson B, Friberg J, Valero-Cuevas FJ, Pontén E. Forearm flexor muscles in children with cerebral palsy are weak, thin and stiff. Front Comput Neurosci. 2017;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams PE, Catanese T, Lucey EG, Goldspink G. The importance of stretch and contractile activity in the prevention of connective tissue accumulation in muscle. J Anat. 1988;158:109-114. [PMC free article] [PubMed] [Google Scholar]
- 61.Williams PE, Goldspink G. Longitudinal growth of striated muscle fibres. J Cell Sci. 1971;9:751-767. [DOI] [PubMed] [Google Scholar]
- 62.Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973;116:45-55. [PMC free article] [PubMed] [Google Scholar]
- 63.Williams PE, Goldspink G. Connective tissue changes in immobilised muscle. J Anat. 1984;138 (Pt 2):343-350. [PMC free article] [PubMed] [Google Scholar]
- 64.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23-67. [DOI] [PMC free article] [PubMed] [Google Scholar]