Abstract
Background
The management of severe and recalcitrant diabetic foot ulcers is challenging. Distraction osteogenesis is accompanied by vascularization and regeneration of the surrounding tissues. Longitudinal distraction of the proximal tibia stimulates increased and prolonged blood flow to the distal tibia. However, the effects of transverse distraction of the proximal tibia cortex on severe and recalcitrant diabetic foot ulcers are largely unknown.
Questions/purposes
(1) Does tibial cortex transverse distraction increase healing and decrease major amputation and recurrence of severe and recalcitrant diabetic foot ulcers compared with routine management (which generally included débridement, revascularization, negative pressure wound therapy, local or free flaps, or skin grafts as indicated)? (2) Does neovascularization and perfusion increase at the foot after the procedure? (3) What are the complications of tibial cortex transverse distraction in patients with severe and recalcitrant diabetic foot ulcers?
Methods
Between July 2014 and March 2017, we treated 136 patients with diabetes mellitus and University of Texas Grade 2B to 3D ulcers (wound penetrating to the tendon, capsule, bone, or joint with infection and/or ischemia). The patients had failed to respond to treatment for at least 6 months, and their ulcers had a mean ± SD area of 44 cm2 ± 10 cm2. All 136 patients underwent tibial cortex transverse distraction (partial corticotomy of the upper tibia, which was in normal condition, followed by 4 weeks of transverse distraction medially then laterally). We compared these patients with the last 137 consecutive patients we treated with standard surgical treatment, consisting of débridement, revascularization, local or free flap or skin equivalent, or graft reconstruction along with negative-pressure wound therapy between May 2011 and June 2013; there was a 1-year period during which both treatments were in use, and we did not include patients whose procedures were performed during this time in either group. Patients in both groups received standard off-loading and wound care. The patients lost to follow-up by 2 years (0.7% of the treatment group [one of 137] and 1.4% of the control group [two of 139]; p = 0.57) were excluded. The patients in the treatment and control groups had a mean age of 61 years and 60 years, respectively, and they were predominantly men in both groups (70% [95 of 136] versus 64% [88 of 137]; p = 0.32). There were no differences with respect to parameters associated with the likelihood of ulcer healing, such as diabetes and ulcer duration, ulcer grades and area, smoking, and arterial status. We compared the groups with respect to ulcer healing (complete epithelialization without discharge, maintained for at least 2 weeks, which was determined by an assessor not involved with clinical care) in a 2-year follow-up, the proportion of ulcers that healed by 6 months, major amputation, recurrence, and complications in the 2-year follow-up. Foot arterial status and perfusion were assessed in the tibial cortex transverse distraction group using CT angiography and perfusion imaging.
Results
The tibial cortex transverse distraction group had a higher proportion of ulcers that healed in the 2-year follow-up than the control group (96% [131 of 136] versus 68% [98 of 137]; odds ratio 10.40 [95% confidence interval 3.96 to 27.43]; p < 0.001). By 6 months, a higher proportion of ulcers healed in the tibia cortex transverse distraction group than the control group (93% [126 of 136] versus 41% [56 of 137]; OR 18.2 [95% CI 8.80 to 37.76]; p < 0.001). Lower proportions of patients in the tibia cortex transverse distraction group underwent major amputation (2.9% [four of 136] versus 23% [31 of 137], OR 0.10 [95% CI 0.04 to 0.30]; p < 0.001) or had recurrences 2.9% (4 of 136) versus 17% (23 of 137), OR 0.20 [95% CI 0.05 to 0.45]; p < 0.001) than the control group in 2-year follow-up. In the feet of the patients in the tibial cortex transverse distraction group, there was a higher density of small vessels (19 ± 2.1/mm2 versus 9 ± 1.9/mm2; mean difference 10/mm2; p = 0.010), higher blood flow (24 ± 5 mL/100 g/min versus 8 ± 2.4 mL/100 g/min, mean difference 16 mL/100 g/min; p = 0.004) and blood volume (2.5 ± 0.29 mL/100 g versus 1.3 ± 0.33 mL/100 g, mean difference 1.2 mL/100 g; p = 0.03) 12 weeks postoperatively than preoperatively. Complications included closed fractures at the corticotomy site (in 1.5% of patients; two of 136), which were treated with closed reduction and healed, as well as pin-site infections (in 2.2% of patients; three of 136), which were treated with dressing changes and they resolved without osteomyelitis.
Conclusions
Proximal tibial cortex transverse distraction substantially facilitated healing and limb salvage and decreased the recurrence of severe and recalcitrant diabetic foot ulcers. The surgical techniques were relatively straightforward although the treatment was unorthodox, and the complications were few and minor. These findings suggest that tibial cortex transverse distraction is an effective procedure to treat severe and recalcitrant diabetic foot ulcers compared with standard surgical therapy. Randomized controlled trials are required to confirm these findings.
Level of Evidence
Level II, therapeutic study.
Introduction
Diabetes mellitus is a leading cause of chronic disease, affecting 425 million people globally [27]. One of the major complications of diabetes mellitus is diabetic foot ulcers, with an annual worldwide incidence of 6.3% [52]. After a diabetic foot ulcer has occurred, complications such as gangrene and infection can develop, sometimes leading to amputation. Even when ulcers heal, the risk of recurrence remains high [1]. There are many causes for these foot ulcers, with known risk factors including peripheral neuropathy and vascular disease [48]. Chronic neuropathy leads to insensitive and deformed feet, resulting in high pressure on some foot areas and eventual ulceration [10]. Peripheral artery disease leads to limb ischemia and contributes to ischemic ulceration [48].
Nonoperative treatments of diabetic foot ulcers (such as footwear and wound care) often are not effective, and some patients have difficulty adhering to the treatment regimen [12, 13]. Surgical therapies such as débridement, bone procedures (metatarsal head resection and metatarsophalangeal joint arthroplasty) [4, 9, 12, 45] and tendon-balancing interventions (Achilles tendon lengthening and digital flexor tendon tenotomy) [12, 40, 46] are more effective for treating localized ulcers. However, these procedures are less effective for more diffuse and severe ulcers, and are often associated with high complication rates, including violation of the metatarsophalangeal joint. Revascularization increases distal perfusion and creates favorable conditions for ulcer healing [20]. However, some ulcers do not heal because of small-artery occlusion and impaired foot perfusion [6, 18]. Furthermore, the reconstruction of soft-tissue defects in diabetic foot ulcers using microsurgical flaps is frequently unsuccessful because of postoperative complications such as flap loss [44]. The treatment of diabetic foot ulcers, particularly severe and recalcitrant ulcers, therefore, remains challenging.
Because diabetic foot ulcers involve bone, nerve, vascular tissues, muscle, and skin, treatments aim to regenerate multiple tissues. Distraction osteogenesis induces large volumes of new bone [23-26] that is accompanied by neovascularization and increased perfusion to the bone and surrounding soft tissues [8, 19, 33, 38, 39, 41]. Moreover, longitudinal distraction of the proximal tibia has stimulated increased and prolonged perfusion to the distal tibia [5]. Additionally, transverse distraction of tibial cortex was suggested for treatment of local ischemic diseases [23]. However, whether proximal tibial cortex transverse distraction stimulates neovascularization and improves perfusion of the foot and healing of diabetic foot ulcers remains unknown.
We therefore asked the following questions: (1) Does tibial cortex transverse distraction increase healing and decrease major amputation and recurrence of severe and recalcitrant diabetic foot ulcers compared with routine management (which generally included débridement, revascularization, negative pressure wound therapy, local or free flaps, or skin grafts as indicated)? (2) Does neovascularization and perfusion increase at the foot after the procedure? (3) What are the complications of tibial cortex transverse distraction in patients with severe and recalcitrant diabetic foot ulcers?
Patients and Methods
Study Design and Setting
Between July 2014 and March 2017, we treated 145 patients with diabetes mellitus and University of Texas Grade 2B to 3D ulcers (wound penetrating to the tendon, capsule, bone, or joint with infection and/or ischemia) [3]. During that time, all patients with that diagnosis were treated with tibial cortex transverse distraction. In this prospective, observational study of the new treatment, we compared this group to a historical control group of patients who underwent standard surgical treatment (consisting of serial débridement [14], revascularization [21, 22], local or free flap or skin equivalent or graft reconstruction [15, 44], and negative-pressure wound therapy [2, 15]) performed by the same surgeons (YC, QH) during a preceding period (May 2011 to June 2013); the endpoints of interest were ulcer healing, limb salvage, ulcer recurrence, foot neovascularization and perfusion, and complications. During the 1-year transition period, our surgical protocol evolved and patients treated during this time were excluded. Data were collected as part of routine patient follow-up examinations. The study was approved by the institutional review board at the First Affiliated Hospital of Guangxi Medical University. All participants provided informed consent before entering the study.
Participants
Patient Inclusion and Exclusion Criteria
During the period in question, we saw 145 patients who were at least 18 years old, with diagnosis of diabetes mellitus based on the American Diabetes Association criteria [7], and who had nonhealing or recurrent ulcers in the lower limbs for at least 6 months [28]. Previous nonoperative treatments included local wound care, footwear modifications, infection and glycemic control, and negative-pressure wound therapy [2, 15]. Previous operative treatments included serial débridement [14], revascularization [21, 22], skin equivalents or grafting, and local or free flap transplantation [20, 44]. We also included patients with ulcers classified as University of Texas Grade 2B to 3D (wounds with infection and/or ischemia involving the tendon, capsules, bone, or joints) [3] and those with a 2-year follow-up. All 145 patients underwent tibial cortex transverse distraction. Patients were excluded if they had local signs of infection that presented as cellulitis or suppuration in the surgical area of the calf; severe peripheral vascular disease (popliteal arteries with occlusion ≥ 80% of the lumen and unable to receive vascular reconstruction); malignant disease in the ulcers; foot ulcers without the presence of diabetes; active Charcot arthropathy of the foot; stroke or myocardial infarction in the past 3 months, or with a history of cardiac failure, cancer, or renal failure; treatment with corticosteroids, immunosuppressive drugs, and/or chemotherapy; and death of unrelated causes before the end of the 2-year follow-up. If patients had stenosis ≥ 80% of the lumen of popliteal arteries but could be treated using revascularization, they were also candidates for tibial cortex transverse distraction.
Clinical and Imaging Evaluation
The position and duration of the diabetic foot ulcers were registered. The presence and severity of infection was evaluated using the International Working Group on the Diabetic Foot/Infectious Diseases Society of America classification system [35, 36]. If an infection was suspected or proven, wound culturing was performed to identify causative organisms and their antibiotic sensitivities. For an infected open wound, a probe-to-bone test was performed, and plain radiographs of the foot were taken to detect diabetic foot osteomyelitis [35]. Specific oral or parenteral antibiotic agents were used according to the International Working Group on the Diabetic Foot/Infectious Diseases Society of America guidelines [35, 36]. Peripheral sensory neuropathy was defined as an inability to feel a 10 g Semmes Weinstein monofilament [32]. Peripheral arterial disease of the lower extremities was defined as the absence of palpable dorsalis pedis and posterior tibial arteries and/or an ankle-brachial index less than 0.9 [11, 43].
The vascular status of the lower limbs was evaluated with color duplex ultrasound and CT angiography. Patients with severe artery stenosis (more than 50% of diameter reduction and/or presence of monophasic Doppler ultrasonography) [16] and occlusion caused by atherosclerosis and/or calcification were referred to a vascular surgeon for further evaluation and revascularization, when indicated. To assess perfusion in the ulcerated feet of the patients, CT perfusion imaging was performed as previously described [51]. Laboratory parameters included the glycated hemoglobin A1c value, which was measured with standard methods.
Demographics, Description of Study Population
Patient Characteristics and Follow-up
A total of 145 patients were eligible for tibial cortex transverse distraction, and 165 in the control group were eligible. During follow-up, 6% of the patients (eight of 145) in the tibial cortex transverse distraction group and 16% of patients (26 of 165) in the control group died. The deaths were all because of atherosclerotic vascular disease (myocardial infarction or stroke). Among the remaining patients, 0.7% in the treatment group (one of 137) and 1.4% in the control group (two of 139) were lost to follow-up after ulcer healing, leaving 136 patients in the treatment group and 137 in the control group for analysis (Table 1). There were no differences in factors related to the likelihood of ulcer healing such as age, sex, BMI, smoking status, diabetes mellitus duration, duration of ulcers, or ulcer area (see Table 1, Supplemental Digital Content 1, http://links.lww.com/CORR/A269).
Table 1.
Patient descriptive characteristics
Description of Experiment, Treatment, or Surgery
Surgical Techniques
Patients underwent tibial cortex transverse distraction under spinal anesthesia or a femoral nerve block in the supine position without a tourniquet (Fig. 1). The corticotomy window was a vertical rectangle (5 cm in height with a width of 1.5 cm) located on the upper tibia which was in normal condition and located below the tibial tuberosity. This site was selected because of its proximity to the neurovascular bundles and high perfusion. Generally, perfusion at the distal 1/3 of the tibia is poor, and fractures of this area frequently lead to nonunion. Additionally, the diaphyseal circumference of the proximal tibia is larger than the middle and distal thirds and reduces the risk of fracture at the surgical site. The reasons for selecting corticotomy on the anteromedial rather than the lateral surface of the tibia were that the lateral approach to the tibia could cause injury to the common peroneal nerve and that the anteromedial surface of the tibia is almost flat, making the corticotomy procedure easier to perform (Fig. 2).
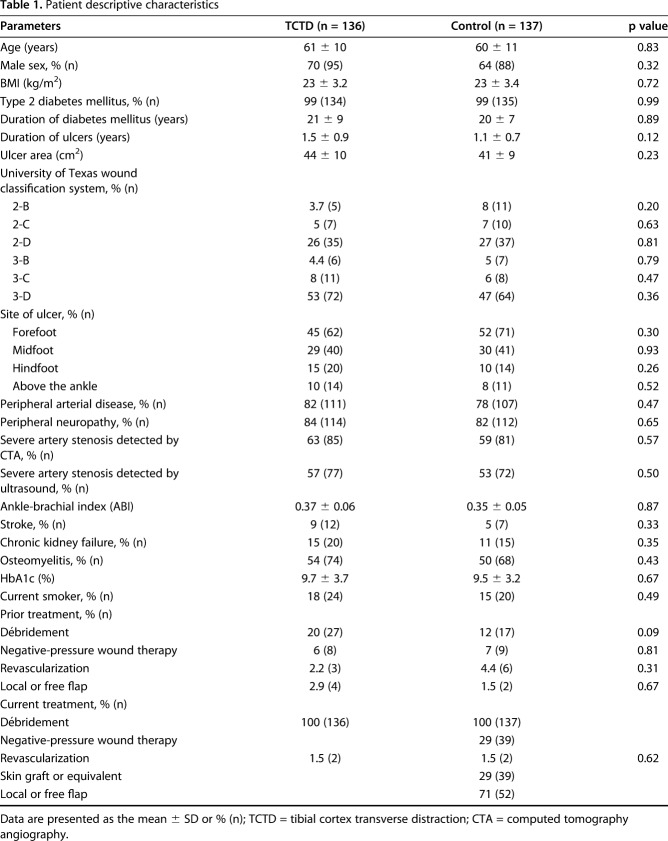
Fig. 1A-G.
This schematic shows tibial cortex transverse distraction. (A) The corticotomy window was a vertical rectangle located on the anteromedial tibia of a lower limb with diabetic foot ulcer (U), with the proximal end 1.5 cm below the tibial tuberosity (T) and the lateral end 2 cm next to the tibial crest. (B and C) The rectangular corticotomy was 5 cm in height with a width of 1.5 cm. Two pins 2 cm away were screwed into the cortex fragment for distraction, and two more pins were screwed into the tibial shaft to anchor the external fixator. (D and E) By turning the nuts, the surgeon could distract the cortex medially and then laterally to return it to its original position. (F) The external fixator was removed and the corticotomized cortex would unite, with (G) the foot ulcer healing gradually.
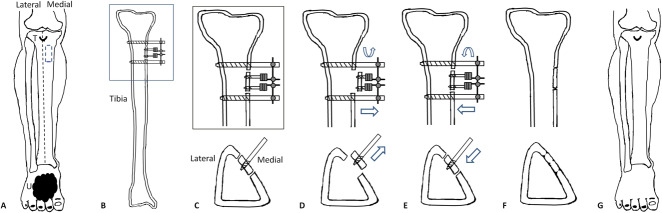
Fig. 2A-M.
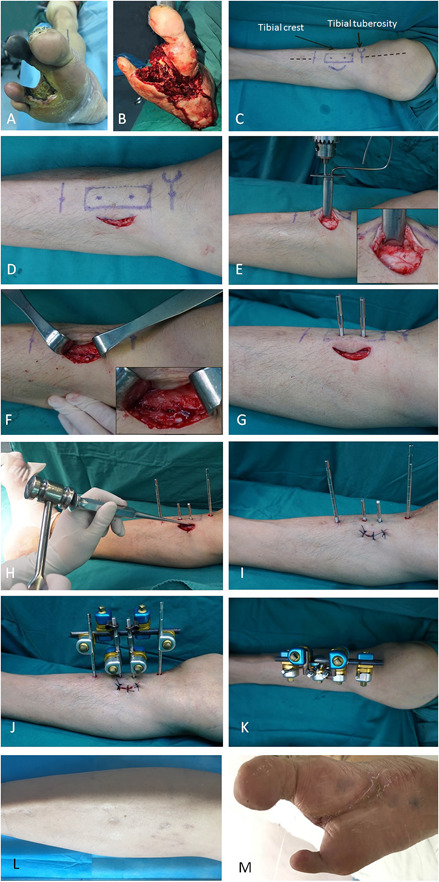
This figure shows the tibial cortex transverse distraction procedure. (A-C) The surgery was performed on the upper 1/3 of the ipsilateral tibia of a foot with a diabetic foot ulcer. (C) The positions of incision, corticotomy, and nailing are shown with markings as part of preoperative planning. (D) A 4-cm medially curved incision was made 1 cm from the tibial crest and 2 cm distal to the tibial tubercle. (E) The soft tissue was retracted with blunt dissection and the periosteum was exposed, which was not removed from the cortex. Corticotomy was performed by drilling multiple holes in a rectangle (1.5 cm × 5.0 cm) in the cortex. (F) Drilling was limited to the ipsilateral cortex, avoiding penetration beyond the depth of the cortex. (G) After osteotomy, two 3-mm drill holes were made in the osteotomized cortex (not extending to the contralateral cortex) followed by the insertion of two pins for distraction. Then, two 4-mm nailing holes extending to the contralateral cortex were made, followed by the insertion of two pins to stabilize the external frame (see Fig. 1, Supplemental Digital Content 7, http://links.lww.com/CORR/A275). (H) The holes were connected using an osteotome to separate the cortex from the tibial shaft. (I) The incision was closed in layers with proper sutures. (J-K) The pins were attached to the fixator frame, which had two screws for distraction. Aggressive débridement was performed to remove nonviable tissues surrounding the ulcer, and minor amputation was performed if necessary. (L-M) The distraction maintained for 4 weeks and then the external fixator was removed. The incision would heal with the ulcer healed gradually.
After surgery, aggressive débridement was performed based on international guidelines [15, 22, 34] (Fig. 2). Digital photographs were taken and the diabetic foot ulcer area was evaluated by measuring the maximum length and width by an observer who was unaware of the treatments. Minor amputations (resections through or distal to the ankle) [28] were performed when necessary. All wounds after incisions and minor amputations were left for complete healing without the use of skin equivalents or grafts or flaps. After débridement, tissue samples were sent for a microbiological assessment and histopathologic examination. Patients who had infections received empiric antibiotic treatment postoperatively, which was modified according to antibiograms. Infected bone was surgically removed and antibiotic therapy was administered [35].
In the control group, all the patients underwent early and aggressive débridement [14, 34] (Table 1), and 72% of them (99 of 137) required more than one. Revascularization [21, 22] was applied to 1.5% of patients (2 of 137) who had severe stenosis (≥ 80% of the lumen) of popliteal and anterior and posterior tibial arteries to improve blood supply to the feet (percutaneous transluminal angioplasty for one and percutaneous transluminal angioplasty and bypass surgery for the other). This was followed by further débridement and free flap reconstruction. Percutaneous transluminal angioplasty was performed twice for one patient. In conjunction with débridement, negative-pressure wound therapy [2, 15] was applied to 29% of patients (39 of 137) for promotion of wound healing and wound bed preparation; and 24% of the patients (33 of 137) required more than one. Local or free flaps were transferred in 52% of patients (71 of 137) [20, 44]. Free flaps were used for 21% of patients (29 of 137), and two patients underwent secondary flap reconstruction. Skin equivalents or grafts were applied to 29% (39 of 137) of patients, and more than once to 20% of them (27 of 137). Patients received free-flap reconstruction had complete bed rest for 7 days postoperatively. Wound care and off-loading were applied in an identical manner [15, 22] in patients in each group.
Aftercare
Postoperative Wound Care and Tibial Cortex Transverse Distraction
In the tibial cortex transverse distraction group, pin care was applied with daily dressing changes. Postoperative radiographs were taken to confirm the position of the corticotomy site and pins of the external fixator. After a 4-day latent period, tibial cortex transverse distraction was initiated at a rate of 0.25 mm every 6 hours. Patients were then discharged and instructed to finish bone distraction at home; that is, 14 days of medial distraction followed by 14 days of lateral distraction (Fig. 1). Radiography was performed 2 and 4 weeks after bone distraction to confirm the position of the cortex (Fig. 3). Standard daily wound care and off-loading casts were applied to patients in both groups. Because the external fixator provided excellent stability, early partial weightbearing with crutches was allowed. After 4 weeks of distraction, the external fixator was removed in the outpatient department.
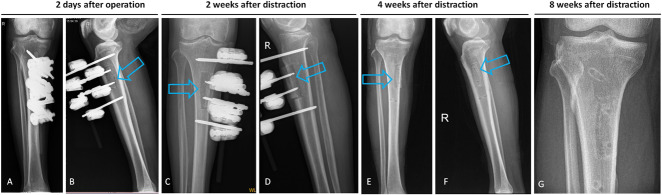
Fig. 3A-G.
These postoperative radiographs show a tibia that underwent tibial cortex transverse distraction. (A-B) The corticotomy and external fixator sites were confirmed on AP and lateral radiographs 2 days postoperatively. (C-D) After 2 weeks of medial distraction, the cortex fragment transported medially, splitting at the tibia shaft. (E-F) This was followed by 2 weeks of lateral distraction, after which the external fixator was removed. (G) The cortex fragment was completely united 8 weeks after distraction (4 weeks after removal of the external fixator).
Description of Follow-up Routine
In the tibial cortex transverse distraction group, patients were followed weekly during the first 12 weeks postoperatively in the outpatient department for dressing changes. Four weeks after removal of the external fixator, full weightbearing ambulation was allowed. Further follow-up occurred at 2-month intervals until the final 2-year follow-up examination. If the ulcers were healed, off-loading shoes and/or soles were applied.
Outcomes
The primary outcomes included the proportion of ulcers that healed by 2 years and 6 months, the proportion of patients with successful limb salvage (without major amputations) [28] and recurrence by 2 years. Ulcers were considered healed when complete epithelialization with no drainage was observed and maintained for at least 2 weeks [28]. This was recorded by an assessor (GL) who did not participate in the operations or the daily wound care. In the tibial cortex transverse distraction group, secondary outcomes included changes in the lower-limb small arteries, as evaluated by CT angiography, and blood flow and blood volume in the muscle of ulcerated feet, as evaluated by CT perfusion preoperatively to 12 weeks postoperatively. The numbers and kinds of complications were recorded for the study group and obtained from a chart review in the historical control group
Statistical Analysis
Demographic and clinical data were tested for normality using the Shapiro-Wilk test. Data were compared between groups using a t-test for normally distributed variables, Mann-Whitney U tests for nonparametric variables, and the chi-square test or Fisher's exact test (if the expected count was less than 5 for any contingency cell) for categorical data, as appropriate. Data are presented as the mean ± SD for continuous variables and as numbers and percentages for categorical measures. Paired t-tests were used to compare preoperative and postoperative small-vessel density and blood volume and flow in the tibial cortex transverse distraction group. Statistical significance was set at an alpha level < 0.05. SPSS version 20.0 (IBM Corp, Chicago, IL, USA) was used for all statistical analyses.
Results
Healing
A higher proportion of ulcers healed by 2 years in the tibial cortex transverse distraction group than in the control group (96% [131 of 136] versus 72% [98 of 137]; odds ratio 10.40 [95% CI 3.96 to 27.43]; p < 0.001) (Table 2). By 6 months, a higher proportion of ulcers healed in the tibial cortex transverse distraction group (93% [126 of 136] versus 41% [56 of 137]; OR 18.2 [95% CI 8.80 to 37.76]; p < 0.001) (Figs. 4-6; see Videos 1-5, Supplemental Digital Content 2, http://links.lww.com/CORR/A270; Supplemental Digital Content 3, http://links.lww.com/CORR/A271; Supplemental Digital Content 4, http://links.lww.com/CORR/A272; Supplemental Digital Content 5, http://links.lww.com/CORR/A273; and Supplemental Digital Content 6, http://links.lww.com/CORR/A274). After ulcer healing, the patients in the tibial cortex transverse distraction group could wear shoes and walk. A lower proportion of patients in the tibial cortex transverse distraction group underwent major amputation than in the control group (2.9% [four of 136] versus 23% [31 of 137], OR 0.10 [95% CI 0.04 to 0.30]; p < 0.001). Of patients in the tibial cortex transverse distraction group, 1.5% (two of 136) underwent major amputations because of massive thromboses in the popliteal artery and tibial posterior and anterior arteries 2 and 3 months postoperatively, respectively. A further 1.5% (two of 136) of patients displayed occlusion of the popliteal arteries (≥ 80% of the lumen) because of arterial calcification but did not undergo revascularization before tibial cortex transverse distraction because vascular surgery was contraindicated. Their wounds showed improvement after tibial cortex transverse distraction but did not heal completely until 3 months postoperatively; these patients finally underwent major amputations. In the control group, the reasons for major amputations were flap failure (n = 7; five had congestion and two had arterial insufficiency), persistent, severe systemic infection (n = 4), a thrombosed vascular graft (n = 4), persistent ulceration (n = 2), and pain that caused disability (n = 1). The amputations were all below the knee as of latest follow-up except for a single transfemoral amputation in the control group. The residual limbs in all patients who underwent major amputation healed.
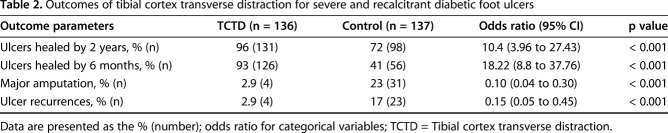
Table 2.
Outcomes of tibial cortex transverse distraction for severe and recalcitrant diabetic foot ulcers
Fig. 4A-G.
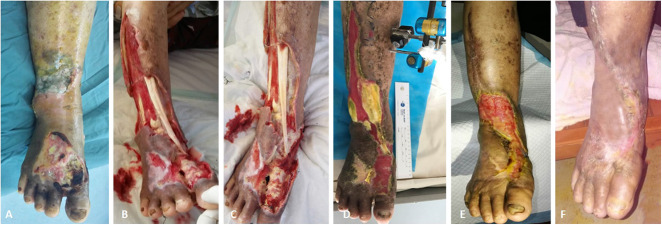
This figure shows the effects of tibial cortex transverse distraction in a 67-year-old man with severe and resistant plantar diabetic foot ulcer. (A-C) These images show ulcers before surgery. Almost all planta were involved, and purulent secretion, cacosmia, and swelling were obvious. The foot muscles, bone, and tendon were exposed. The first toe had been amputated and gangrene of the second and third toes was evident. Foot swelling was apparent. After débridement, the second and third toes were removed. (D) Four weeks postoperatively, the wound was much smaller, with epithelization at the edges, without pain or infection and with minimal swelling. The wound bed was clean and covered by robust granulation tissue. (E-F) These images show the foot at 8 weeks and 10 weeks postoperatively, respectively. (G) The ulcer was completely healed at 12 weeks postoperatively.
Fig. 6.
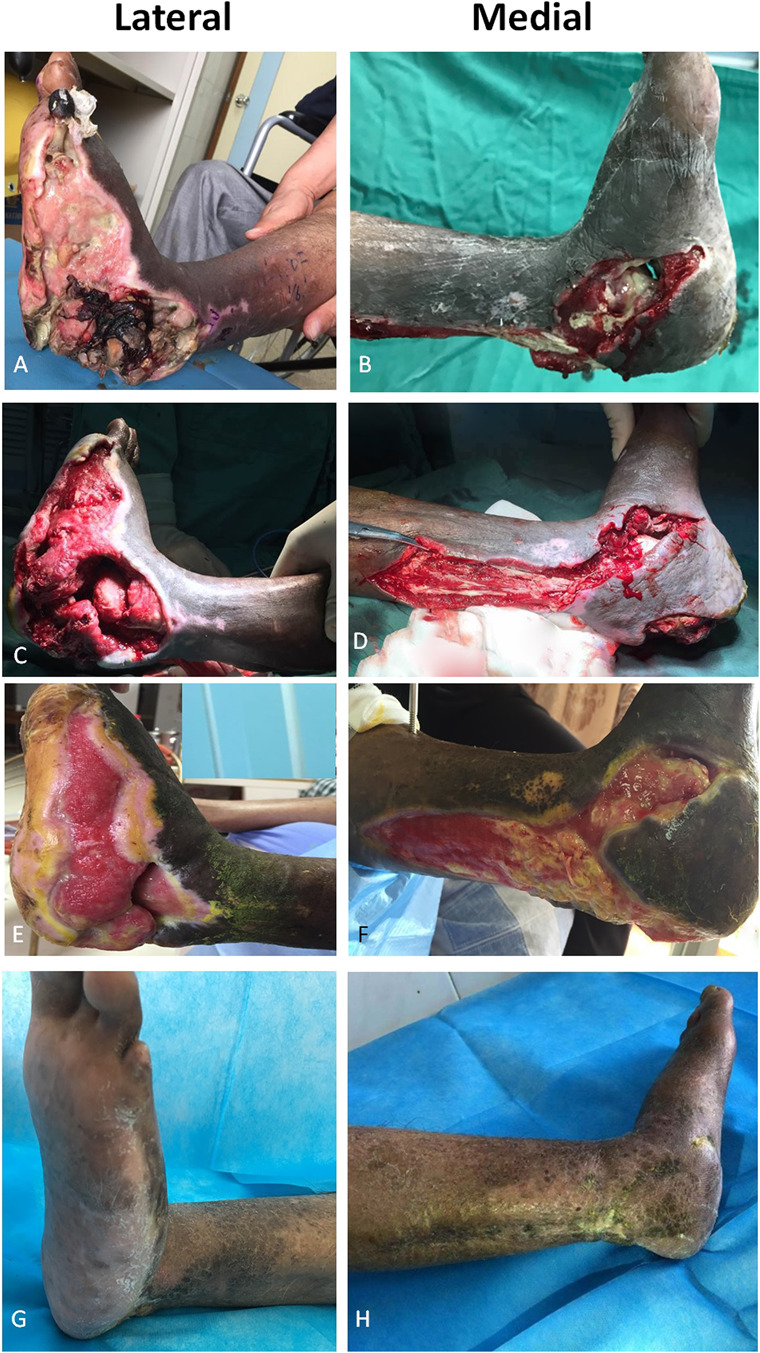
This figure shows the effect of tibial cortex transverse distraction in a 68-year-old woman with severe and resistant diabetic foot ulcers in the dorsum of the right foot and anterolateral aspect of the lower limb. (A) This image shows ulcers before surgery. Swelling was present. (B-C) Two weeks postoperatively, the necrotic tissues had been removed during débridement and the tendon, muscle, and periosteum were exposed. (D) Four weeks postoperatively, the wound bed was red and clean. An Ilizarov frame was attached. (E) Eight weeks postoperatively, re-epithelization occurred progressively from the periphery to the center of the wound. (F) Twelve weeks postoperatively, the ulcer was completely healed.
Fig. 5A-H.
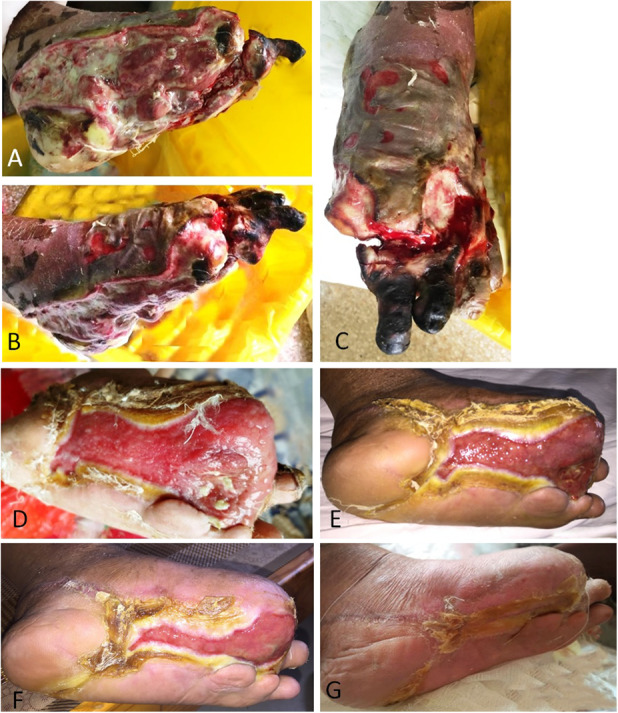
This figure shows the effect of tibial cortex transverse distraction in a 49-year-old man with a severe and resistant diabetic foot ulcer in the left foot. (A-B) Before débridement, a large wound was present at the lateral side, heel, and medial side. The medial malleolus was exposed and a channel connecting the medial and lateral foot was observed. (C-D) After débridement, large wounds were present. (E-F) Six weeks postoperatively, the lateral and medial parts of the wound and part of the heel were much smaller, with a marked callus at the edge. The medial malleolus was partially covered by granulation tissue. (G-H) When the wound was almost healed and no infections were present, internal fixation (Kirschner wires) was used to achieve ankle joint fusion and which further provided hindfoot stability (see Video 1, Supplemental Digital Content 2, http://links.lww.com/CORR/A270). At 12 weeks postoperatively, the diabetic foot ulcer was completely healed, and the patient was able to walk with the healed foot.
A lower proportion of patients in the tibial cortex transverse distraction group had recurrences than the control group in the 2-year follow-up (2.9% [4 of 136] versus 17% [23 of 137], OR 0.20 [95% confidence interval 0.05 to 0.45]; p < 0.001). In the tibial cortex transverse distraction group, 2.9% (four of 136) patients had recurrences. Three of the lesions were treated with general drainage and healed after 2 weeks. The other lesion was treated with tibial cortex transverse distraction again and healed 6 weeks postoperatively. In the control group, two patients with persistent recurrent ulcers underwent major amputations. The other 13 recurrent ulcers healed after standard wound care.
Blood Flow
Neovascularization and perfusion increased after tibial cortex transverse distraction. The ulcerated feet of patients in the tibial cortex transverse distraction group displayed arteries earlier and had a higher density of small vessel 12 weeks postoperatively than they did preoperatively (19 ± 2.1/mm2 versus 9 ± 1.9/mm2; mean difference 10/mm2, 95% CI [9.52 to 10.48]; p = 0.010) (Fig. 7A-B). CT perfusion showed that patients in the tibial cortex transverse distraction group had increased blood flow (24 ± 5 mL/100 g/min versus 8 ± 2.4 mL/100 g/min; mean difference 16 mL/100 g/min, 95% CI [15.07 to 16.93]; p = 0.004) and volume (2.5 ± 0. 33 mL/100 g versus 1.3 ± 0.29 mL/100 g; mean difference 1.2 mL/100 g/min, 95% CI [1.13 to 1.27]; p = 0.03) 12 weeks postoperatively compared with preoperatively (Fig. 8A-D).
Fig. 7A-B.
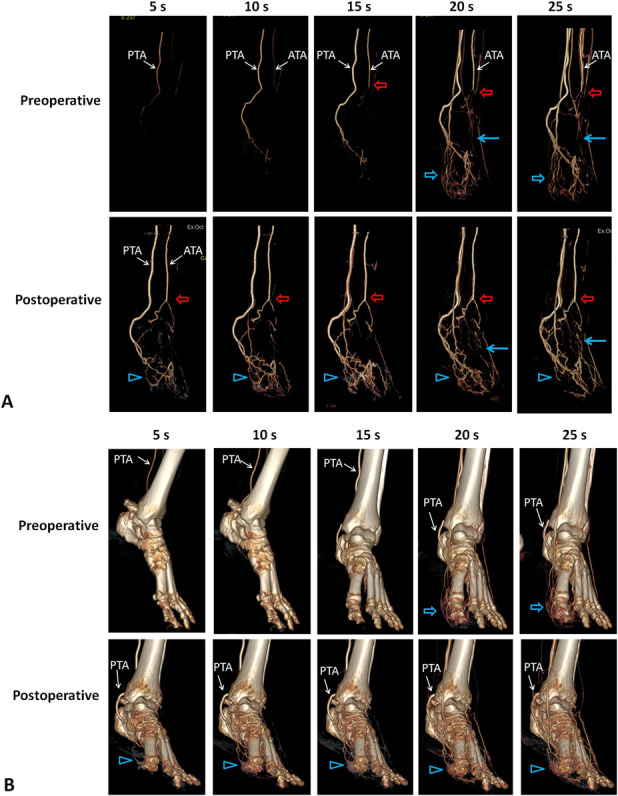
This representative CT angiography image is from a patient with diabetic foot ulcer treated with tibial cortex transverse distraction. (A) Sequential CT images show diabetic foot ulcer at the planta and dorsum of the left foot, which was completely healed 8 weeks preoperatively. The ulcerated feet displayed the anterior tibial artery and posterior tibial artery at 12 weeks postoperatively earlier than they did preoperatively. Some small arteries became visible postoperatively compared with preoperatively (red arrows), suggesting patency after artery occlusion. The foot had more small vessels at the planta (blue solid arrows) and forefoot (blue hollow arrows). (B) This image shows the presence of the corresponding vessels and their anatomic relationship with bone.
Fig. 8A-D.
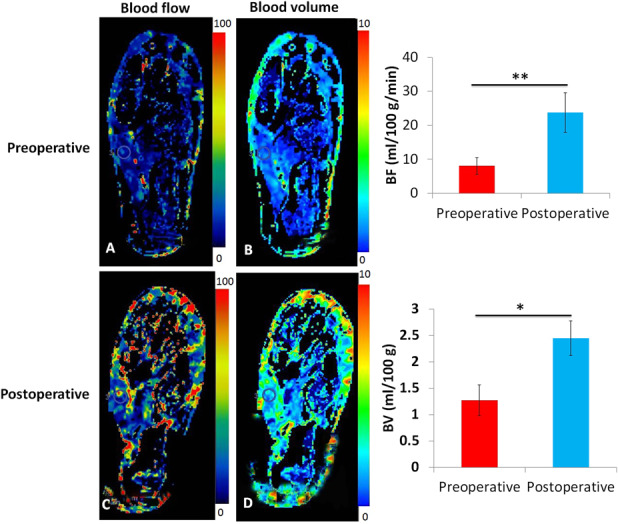
These (A and B) preoperative and (C and D) postoperative perfusion maps show blood flow and volume in feet with diabetic foot ulcers treated with tibial cortex transverse distraction. Ulcerated feet tended to have increased blood flow and volume 3 months postoperatively compared with preoperatively. This observation was confirmed by the statistical analysis. The circles indicate the region of interests selected at the abductor hallucis muscle.
Complications
Complications after tibial cortex transverse distraction were few and minor. All patients in the tibial cortex transverse distraction group achieved good union of the osteotomized cortex (Fig. 3). In the tibial cortex transverse distraction group, 1.5% of patients (two of 136) sustained closed tibial fractures at the corticotomy site within 1 week of external fixator removal. These fractures were treated with closed reduction and external fixation and achieved union after 4 weeks. A total of 2.2% of patients (three of 136) had pin tract infections and were treated with dressing changes and healed uneventfully.
Discussion
Distraction osteogenesis is accompanied by angiogenesis and neovascularization in the surrounding tissues [23, 24, 38, 41], and longitudinal distraction of the proximal tibia stimulates increased and prolonged blood flow to the distal tibia [5]. However, whether tibial cortex transverse distraction is effective in the treatment of diabetic foot ulcers had, to our knowledge, not been analyzed. In this study, we assessed the efficacy of tibial cortex transverse distraction in the treatment of severe and recalcitrant ulcer diabetic foot ulcers. We found that tibial cortex transverse distraction resulted in a greater likelihood of healing and lower proportions of patients who had major amputations and recurrences than patients undergoing standard surgical treatment did in a 2-year follow-up. Furthermore, healed feet showed greater perfusion in the muscle during follow-up, together with a higher density of small vessels, compared with preoperatively. The complications were few and minor. These results suggest that tibial cortex transverse distraction is an effective procedure to treat severe and recalcitrant ulcer diabetic foot ulcers compared with standard surgical therapy.
This study had a number of limitations. First, the study was nonrandomized and selection bias was a concern. However, we believe that selection bias was not a serious limitation here because during the control period, all patients were treated using similar approaches. Likewise, during the prospective, observational portion of the study when tibial cortex transverse distraction was used, we applied the same indications throughout: All patients with University of Texas Grade 2B to 3D and recalcitrant diabetic foot ulcers were treated with tibial cortex transverse distraction. No other interventions were used for patients who met those indications. Even so, our findings should be confirmed in randomized trials.
Second, historical controls were used. This raises concerns about whether the treatment and control groups were similar enough to compare, and whether co-treatment bias or variations in treatment during the historical control period may have influenced our study’s results. We believe that these limitations are not severe because in general throughout the period the control group was treated, the surgeons, débridement, wound care, and off-loading protocols did not change. Therefore, we believe the historical controls serve as reasonable comparisons.
Third, this is a nonrandomized study and so assessment bias is a concern. However, offsetting this concern is the fact that ulcer size, healing, and recurrence were determined using digital photographs of the feet by assessors who were unaware of the treatment allocation. Furthermore, the decision of major amputation was confirmed by independent investigators who were blinded to the treatments. Thus, we believe that this limitation is not severe.
Fourth, we focused on severe and recalcitrant diabetic foot ulcers; thus, our data may not generalize well to patients with milder ulcers. Our own clinical experience with tibial cortex transverse distraction for milder ulcers (data not shown) suggests that the decision should depend upon the comprehensive evaluation of the factors affecting ulcer healing such as ulcer grade, area, site and duration, infection, glycemic control, nutritional status, and vascular perfusion. If the wound is still not healing well after other treatments, we would consider using tibial cortex transverse distraction.
Fifth, the followup duration is 2 years. This raises the concern over transfer bias. Indeed, patients whose ulcers have healed are generally less likely to return for visit because they believe they do not need to see a doctor again. To minimize loss to followup, we offered convenient office hours, individualized patient contact via phone or email, and physician visits to the patient's home for those who missed clinic appointments (see videos). Consequently, the percentages of patients lost to followup were low in both groups and not differential between the groups. Therefore, we believe that the loss to followup did not have had a major impact on the results. Sixth, we found that the patients in the tibial cortex transverse distraction group were able to walk after ulcer healing (see videos), but functional outcomes concerning health-related quality of life were not evaluated. This raises the question of whether this approach, which involves a lengthy period of convalescence, would be preferable over major amputation; the question is important because major amputations together with prostheses can allow a patient to regain the ability to walk comfortably reasonably quickly by comparison. We note, though, that this may not be as true among older patients with multiple comorbidities such as we treated in this study, and in patients with diabetes and foot ulcers (again, such as we treated here), in whom ipsilateral re-amputation and contralateral amputation are relatively high [17, 31]. Additionally, some groups of patients (perhaps including Asians) prefer to keep their limbs at all costs [50]. Thus, limb salvage using tibial cortex transverse distraction may be preferable to major amputation for these patients. Comparing functional outcomes and quality of life after tibial cortex transverse distraction to amputation will call for further study.
Last, the percentage of patients undergoing vascularization was relatively low compared with international guidelines that recommend considering revascularization when ulcer healing is not observed within 6 weeks [21, 22]. These discrepancies may have been caused by a number of factors including the expertise of vascularization specialists, the cost of vascularization surgery, and a lack of patient acceptance. Additionally, most patients with severe diabetic foot ulcers were contraindicated for revascularization because of multiple comorbidities and poor general health. Studies reported that 30% to 60% of patients were referred to a specialist (foot team) when the foot ulcer duration exceeded 3 months, and 30% to 50% of them were considered unsuitable for revascularization [20]. Consistent with this, in a cohort study of 669 patients from 15 Grade III-A hospitals in China, 89.5% (619) had diabetic foot ulcers of grade 2 or higher according to the Wagner grading system [49] but only 7.6% (50) underwent revascularization [29]. Despite the low percentage of vascularization, we achieved excellent outcomes (that is, ulcer healing and recurrence and major amputation) using tibial cortex transverse distraction. Thus, the effectiveness of the tibial cortex transverse distraction was underestimated compared with those with higher percentages of revascularization [17, 47].
Healing
Previous surgical procedures to treat diabetic foot ulcers showed 60% to 100% of ulcers healed at 4 months to 2 years of follow-up [20, 40]. However, these techniques were applied to select patients such as those with metatarsal head or midfoot plantar ulcers [12, 40]. Furthermore, even when the ulcers healed, the recurrence risk was high, with reported rates of 30% to 40% within the first year of ulcer healing [1, 48]. In this study, we did not limit our technique to patients with these ulceration characteristics and attained excellent rates of ulcer healing and limb salvage and low recurrence rates at a mean follow-up period of 2 years. Previous studies reported surgical protocols (including extensive surgical débridement, peripheral percutaneous angioplasty, and intravenous antibiotic therapy) to treat patients with more severe diabetic foot ulcers (Texas University Grade 2-3 and Class C-D) [3], and reported healing rates of 60.8%, major amputation rates of 15.7%, and deaths in 16.25% of patients after a follow-up duration of 20 months ± 13 months [17, 47]. In this study, the severity of diabetic foot ulcers in the tibial cortex transverse distraction group was much higher, as indicated by the ulcer area (44 cm2 ± 10 cm2) and position (10% above the ankle), as well as comorbidities (15% with chronic kidney failure and 54% with osteomyelitis). However, patients in the tibial cortex transverse distraction group had improved clinical outcomes (ulcer healing and recurrence and major amputation), highlighting tibial cortex transverse distraction as a therapy for severe and recalcitrant ulcer diabetic foot ulcers.
Blood Flow
Studies reported that longitudinal distraction of the tibial metaphysis resulted in a seven-to-eightfold increase in blood flow in the distal tibia during distraction and the blood flow persisted at levels twofold to threefold higher for up to 17 weeks preoperatively [5]. In line with this, we found that ulcerated feet showed increased neovascularization and perfusion for up to 3 months postoperatively compared with preoperatively, consistent with granulation tissue growth during ulcer healing. It has been shown that small-artery occlusion and impaired foot perfusion (micro-perfusion) plays an important role in the pathogenesis of diabetic foot ulcers [6, 18]. Furthermore, although the blood flow in the large arteries of lower extremity was restored after revascularization, the foot micro-perfusion was not improved completely [6]. Consequently, some ulcers failed to heal or healed slowly or recurred [6]. Thus, the increased neovascularization and perfusion at the foot has probably led to better clinical outcomes (ulcer healing and recurrence and major amputation) in the tibial cortex transverse distraction group compared with the control and those with higher percentages of revascularization [17, 47]. Since the surgical site (proximal tibia) was in a normal condition and distant to the target treatment site (foot ulcer) and the distraction was maintained for only 4 weeks, the findings suggest that tibial cortex transverse distraction has a distant and prolonged curative effect on diabetic foot ulcers. However, data on neovascularization and perfusion from the control group was lacking as CT perfusion was unavailable in our hospital in the control period. We therefore could not conclude that the increased neovascularization and perfusion was caused by tibial cortex transverse distraction. Furthermore, tibial fractures also increase blood flow to the foot skin [30], but a group receiving only tibial corticotomy but no transverse distraction was not assessed because we were concerned this approach would be ineffective. Thus we could not exclude the possibility that tibial corticotomy alone could increase foot perfusion. However, the prolonged duration of blood flow in fracture is lower than in distraction osteogenesis [5, 30]. Furthermore, the corticotomy of the tibial cortex transverse distraction comprises only 1/5 of the circumference of the tibia. Thus, the effects of corticotomy on foot perfusion should be minimal. The underlying mechanism for the effect of the procedure requires future investigation.
Complications
Complications after tibial cortex transverse distraction were few and minor. Previous studies used lateral distraction of the tibial segment to treat thromboangiitis obliterans [37, 42]. However, the osteotomized segment was unable to return to its original position after distraction, which gave the tibia a thicker deformity [37, 42]. Moreover, in four patients, the osteotomized cortex was pulled and penetrated the soft tissue [42]. This led to osteomyelitis and infection of the cortex fragment, ultimately leading to bone section [42]. In the current study, after medial distraction, we performed lateral distraction to return the cortex to its original position, thus avoiding these problems. The cortex segment in an earlier study had a longitudinal length of 12 cm [37], which was much longer than that of this study (5 cm). This may have led to the two intraoperative tibial shaft fractures in their study [42] that did not occur in our study. A two-ring frame was also employed for 4 months (122 days ± 23 days) [37, 42]. This duration was much longer than that used in our study and was inconvenient for the patients. In our preliminary study, we performed corticotomy at two tibial sites with a smaller area (3.5 cm × 1.5 cm) for each to avoid tibial fracture intraoperatively in patients with less severe diabetic foot ulcers (58.3% [seven of 12 patients] with an area less than 25 cm2) [51]. We found no tibial fracture intraoperatively and a proportion of 92% of patients achieved ulcer healing [51]. Based on this, we adjusted the protocol to the current one to simplify the procedure.
Conclusions
In conclusion, we found tibial cortex transverse distraction facilitates healing and limb salvage and decreases recurrence in the treatment of severe and recalcitrant diabetic foot ulcers. Furthermore, tibial cortex transverse distraction is associated with relatively few and minor complications. These findings suggest that tibial cortex transverse distraction is an effective procedure to treat severe and recalcitrant diabetic foot ulcers compared with standard surgical therapy. Large population-based trials are needed to confirm the effectiveness and safety of the procedure in the future.
Supplementary Material
Acknowledgments
We thank the veteran participants who made this research possible. We thank Zhandong Bo MD, and Gaobin Luo MD, of the Department of Bone and Joint Surgery, and Wei Su MD, of the Department of Orthopedic Trauma and Hand Surgery at Guangxi Medical University, for their assistance in data collection.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
The institution of the authors (YC, QH) has received, during the study period, funding from the National Natural Science Foundation of China (81601930), Natural Science Foundation of Guangxi (2017GXNSFAA198318, 2016GXNSFBA380007, and 2017GXNSFAA198293), and Postdoctoral Science Foundation of China (223363), Science Foundation for the Excellent Young Scholars of Guangxi Collaborative Innovation Center for Biomedicine (GCICB-TC-2017003).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
The work was performed at the First Affiliated Hospital of Guangxi Medical University, Nanning, China.
References
- 1.Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med . 2017;376:2367-2375. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DG, Lavery LA, Diabetic Foot Study C. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet . 2005;366:1704-1710. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care . 1998;21:855-859. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DG, Lavery LA, Vazquez JR, Short B, Kimbriel HR, Nixon BP, Boulton AJ. Clinical efficacy of the first metatarsophalangeal joint arthroplasty as a curative procedure for hallux interphalangeal joint wounds in patients with diabetes. Diabetes Care . 2003;26:3284-3287. [DOI] [PubMed] [Google Scholar]
- 5.Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin Orthop Relat Res . 1994:124-131. [PubMed] [Google Scholar]
- 6.Arora S, Pomposelli F, LoGerfo FW, Veves A. Cutaneous microcirculation in the neuropathic diabetic foot improves significantly but not completely after successful lower extremity revascularization. Journal of Vascular Surgery . 2002;35:501-505. [DOI] [PubMed] [Google Scholar]
- 7.Association AD. Executive summary: Standards of medical care in diabetes--2014. Diabetes Care . 2014;37 Suppl 1:S5-13. [DOI] [PubMed] [Google Scholar]
- 8.Aston JWJ, Williams SA, Allard RN, Sawamura S, Carollo JJ. A new canine cruciate ligament formed through distraction histogenesis. Report of a pilot study. Clin Orthop Relat Res . 1992:30-36. [PubMed] [Google Scholar]
- 9.Biz C, Gastaldo S, Dalmau-Pastor M, Corradin M, Volpin A, Ruggieri P. Minimally Invasive Distal Metatarsal Diaphyseal Osteotomy (DMDO) for Chronic Plantar Diabetic Foot Ulcers. Foot Ankle Int . 2018;39:83-92. [DOI] [PubMed] [Google Scholar]
- 10.Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med . 2004;351:48-55. [DOI] [PubMed] [Google Scholar]
- 11.Brownrigg JR, Hinchliffe RJ, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, Reekers J, Shearman CP, Zierler RE, Schaper NC, International Working Group on the Diabetic F. Effectiveness of bedside investigations to diagnose peripheral artery disease among people with diabetes mellitus: a systematic review. Diabetes Metab Res Rev . 2016;32 Suppl 1:119-127. [DOI] [PubMed] [Google Scholar]
- 12.Bus SA, van Deursen RW, Armstrong DG, Lewis JE, Caravaggi CF, Cavanagh PR, International Working Group on the Diabetic F. Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes Metab Res Rev . 2016;32 Suppl 1:99-118. [DOI] [PubMed] [Google Scholar]
- 13.Crews RT, Candela J. Decreasing an Offloading Device's Size and Offsetting Its Imposed Limb-Length Discrepancy Lead to Improved Comfort and Gait. Diabetes Care . 2018;41:1400-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev . 2010:CD003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Game FL, Attinger C, Hartemann A, Hinchliffe RJ, Londahl M, Price PE, Jeffcoate WJ, International Working Group on the Diabetic F. IWGDF guidance on use of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev . 2016;32 Suppl 1:75-83. [DOI] [PubMed] [Google Scholar]
- 16.Gerhard-Herman M, Gardin JM, Jaff M, Mohler E, Roman M, Naqvi TZ, American Society of E, Society of Vascular M, Biology. Guidelines for noninvasive vascular laboratory testing: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr . 2006;19:955-972. [DOI] [PubMed] [Google Scholar]
- 17.Giurato L, Vainieri E, Meloni M, Izzo V, Ruotolo V, Fabiano S, Pampana E, Lipsky B, Gandini R, Uccioli L. Limb salvage in patients with diabetes is not a temporary solution but a life-changing procedure. Diabetes Care . 2015;38:e156-157. [DOI] [PubMed] [Google Scholar]
- 18.Greenman RL, Panasyuk S, Wang X, Lyons TE, Dinh T, Longoria L, Giurini JM, Freeman J, Khaodhiar L, Veves A. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet . 2005;366:1711-1717. [DOI] [PubMed] [Google Scholar]
- 19.Gubin AV, Borzunov DY, Marchenkova LO, Malkova TA, Smirnova IL. Contribution of G.A. Ilizarov to bone reconstruction: historical achievements and state of the art. Strategies Trauma Limb Reconstr . 2016;11:145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinchliffe RJ, Brownrigg JR, Andros G, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, Reekers J, Shearman CP, Zierler RE, Schaper NC, International Working Group on the Diabetic F. Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: a systematic review. Diabetes Metab Res Rev . 2016;32 Suppl 1:136-144. [DOI] [PubMed] [Google Scholar]
- 21.Hinchliffe RJ, Brownrigg JR, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, Reekers J, Shearman CP, Zierler RE, Schaper NC, International Working Group on the Diabetic F. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev . 2016;32 Suppl 1:37-44. [DOI] [PubMed] [Google Scholar]
- 22.Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, Driver VR, Frykberg R, Carman TL, Marston W, Mills JL, Sr, Murad MH. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg . 2016;63:3S-21S. [DOI] [PubMed] [Google Scholar]
- 23.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res . 1989:249-281. [PubMed] [Google Scholar]
- 24.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res . 1989:263-285. [PubMed] [Google Scholar]
- 25.Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res . 1990:8-26. [PubMed] [Google Scholar]
- 26.Ilizarov GA, Ledyaev VI. The replacement of long tubular bone defects by lengthening distraction osteotomy of one of the fragments. 1969. Clin Orthop Relat Res . 1992:7-10. [PubMed] [Google Scholar]
- 27.International Diabetes Federation. IDF Diabetes Atlas, 8th edition Available at: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html. Accessed October 1, 2017. [Google Scholar]
- 28.International Working Group on the Diabetic Foot. Definitions and criteria of diabetic foot. Available at: https://iwgdfguidelines.org/guidelines/guidelines/. Accessed Oct 19, 2015.
- 29.Jiang Y, Ran X, Jia L, Yang C, Wang P, Ma J, Chen B, Yu Y, Feng B, Chen L, Yin H, Cheng Z, Yan Z, Yang Y, Liu F, Xu Z. Epidemiology of type 2 diabetic foot problems and predictive factors for amputation in China. Int J Low Extrem Wounds . 2015;14:19-27. [DOI] [PubMed] [Google Scholar]
- 30.Kellerova E, Delius W, Olerud S, Strom G. Changes in the muscle and skin blood flow following lower leg fracture in man. Acta Orthop Scand . 1970;41:249-260. [DOI] [PubMed] [Google Scholar]
- 31.Kono Y, Muder RR. Identifying the incidence of and risk factors for reamputation among patients who underwent foot amputation. Ann Vasc Surg . 2012;26:1120-1126. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Fernando DJS, Veves A, Knowles EA, Young MJ, Boulton AJM. Semmes-Weinstein monofilaments: a simple, effective and inexpensive screening device for identifying diabetic patients at risk of foot ulceration. Diabetes Research and Clinical Practice . 1991;13:63-67. [DOI] [PubMed] [Google Scholar]
- 33.Kuo KN, Qureshi A, Bush-Joseph CA, Templeton A. Ilizarov distraction histogenesis to reconstruct massive posttraumatic osteoarticular defects: a case report. J Bone Joint Surg Am . 2003;85:1125-1128. [DOI] [PubMed] [Google Scholar]
- 34.Lavery LA, Davis KE, Berriman SJ, Braun L, Nichols A, Kim PJ, Margolis D, Peters EJ, Attinger C. WHS guidelines update: Diabetic foot ulcer treatment guidelines. Wound Repair Regen . 2016;24:112-126. [DOI] [PubMed] [Google Scholar]
- 35.Lipsky BA, Aragon-Sanchez J, Diggle M, Embil J, Kono S, Lavery L, Senneville E, Urbancic-Rovan V, Van Asten S, International Working Group on the Diabetic F, Peters EJ. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev . 2016;32 Suppl 1:45-74. [DOI] [PubMed] [Google Scholar]
- 36.Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E, Infectious Diseases Society of A. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis . 2012;54:e132-173. [DOI] [PubMed] [Google Scholar]
- 37.Marwah V. Management of thromboangiitis obliterans using distraction osteogenesis: A retrospective study. Indian J Orthop . 2012;46:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuyama J, Ohnishi I, Kageyama T, Oshida H, Suwabe T, Nakamura K. Osteogenesis and angiogenesis in regenerating bone during transverse distraction: quantitative evaluation using a canine model. Clin Orthop Relat Res . 2005:243-250. [DOI] [PubMed] [Google Scholar]
- 39.Minematsu K, Tsuchiya H, Taki J, Tomita K. Blood flow measurement during distraction osteogenesis. Clin Orthop Relat Res . 1998:229-235. [PubMed] [Google Scholar]
- 40.Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of Achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. J Bone Joint Surg Am . 2003;85:1436-1445. [PubMed] [Google Scholar]
- 41.Ohashi S, Ohnishi I, Kageyama T, Imai K, Nakamura K. Distraction osteogenesis promotes angiogenesis in the surrounding muscles. Clin Orthop Relat Res . 2007;454:223-229. [DOI] [PubMed] [Google Scholar]
- 42.Patwa JJ, Krishnan A. Buerger's Disease (Thromboangiitis Obliterans)- Management by Ilizarov's Technique of Horizontal Distraction. A Retrospective Study of 60 Cases. Indian J Surg . 2011;73:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg . 2011;41:110-116. [DOI] [PubMed] [Google Scholar]
- 44.Suh HS, Oh TS, Hong JP. Innovations in diabetic foot reconstruction using supermicrosurgery. Diabetes Metab Res Rev . 2016;32 Suppl 1:275-280. [DOI] [PubMed] [Google Scholar]
- 45.Tamir E, Tamir J, Beer Y, Kosashvili Y, Finestone AS. Resection Arthroplasty for Resistant Ulcers Underlying the Hallux in Insensate Diabetics. Foot Ankle Int . 2015;36:969-975. [DOI] [PubMed] [Google Scholar]
- 46.Tamir E, Vigler M, Avisar E, Finestone AS. Percutaneous tenotomy for the treatment of diabetic toe ulcers. Foot Ankle Int . 2014;35:38-43. [DOI] [PubMed] [Google Scholar]
- 47.Uccioli L, Gandini R, Giurato L, Fabiano S, Pampana E, Spallone V, Vainieri E, Simonetti G. Long-term outcomes of diabetic patients with critical limb ischemia followed in a tertiary referral diabetic foot clinic. Diabetes Care . 2010;33:977-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Netten JJ, Price PE, Lavery LA, Monteiro-Soares M, Rasmussen A, Jubiz Y, Bus SA, International Working Group on the Diabetic F. Prevention of foot ulcers in the at-risk patient with diabetes: a systematic review. Diabetes Metab Res Rev . 2016;32 Suppl 1:84-98. [DOI] [PubMed] [Google Scholar]
- 49.Wagner FW., Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle . 1981;2:64-122. [DOI] [PubMed] [Google Scholar]
- 50.Young BA, Maynard C, Reiber G, Boyko EJ. Effects of ethnicity and nephropathy on lower-extremity amputation risk among diabetic veterans. Diabetes Care . 2003;26:495-501. [DOI] [PubMed] [Google Scholar]
- 51.Zeng Z, Dong Y, Hua Q, Kuang X, Li K, Deng X, Qiu S. Computed tomography perfusion study evaluating the curative effect of tibial transverse transport in patients with severe diabetic foot. Journal of Orthopaedic Translation . 2019;04:5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (dagger). Ann Med . 2017;49:106-116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.