Abstract
Stable carbon and oxygen isotope ratios of raw pollen sampled from nine abundant tree species growing in natural habitats of central and northern Europe were investigated to understand the intra- and inter-specific variability of pollen-isotope values. All species yielded specific δ13Cpollen and δ18Opollen values and patterns, which can be ascribed to their physiology and habitat preferences. Broad-leaved trees flowering early in the year before leaf proliferation (Alnus glutinosa and Corylus avellana) exhibited on average 2.6‰ lower δ13Cpollen and 3.1‰ lower δ18Opollen values than broad-leaved and coniferous trees flowering during mid and late spring (Acer pseudoplatanus, Betula pendula, Carpinus betulus, Fagus sylvatica, Picea abies, Pinus sylvestris and Quercus robur). Mean species-specific δ13Cpollen values did not change markedly over time, whereas δ18Opollen values of two consecutive years were often statistically distinct. An intra-annual analysis of B. pendula and P. sylvestris pollen revealed increasing δ18Opollen values during the final weeks of pollen development. However, the δ13Cpollen values remained consistent throughout the pollen-maturation process. Detailed intra-individual analysis yielded circumferential and height-dependent variations within carbon and oxygen pollen-isotopes and the sampling position on a tree accounted for differences of up to 3.5‰ for δ13Cpollen and 2.1‰ for δ18Opollen. A comparison of isotope ranges from different geographic settings revealed gradients between maritime and continental as well as between high and low altitudinal study sites. The results of stepwise regression analysis demonstrated, that carbon and oxygen pollen-isotopes also reflect local non-climate environmental conditions. A detailed understanding of isotope patterns and ranges in modern pollen is necessary to enhance the accuracy of palaeoclimate investigations on δ13C and δ18O of fossil pollen. Furthermore, pollen-isotope values are species-specific and the analysis of species growing during different phenophases may be valuable for palaeoweather reconstructions of different seasons.
Introduction
Stable carbon and oxygen isotope ratios of plant material are generally determined to understand the relationship between plants and their surrounding environment [1]. The continuously deepening knowledge of stable isotope patterns in plants of natural habitats finds applications in, for example, plant ecology, phytochemistry, genetic research and reconstructions of past environmental changes [2–6]. Plant physiological reactions to environmental factors, such as temperature, moisture availability and density of the surrounding vegetation, are known to affect the stable carbon and oxygen isotope composition of plant material [1, 7, 8]. Carbon isotope ratios (δ13C) of plant material are mostly determined by the factor-dependent amount of discrimination against 13C during CO2 uptake and by subsequent photosynthetic processes [e.g. 9, 10], while oxygen isotope ratios (δ18O) are often linked with the isotopic composition of environmental source water [11–13].
In general, the stable carbon isotope composition of stem material, leaves and pollen of the same plant individual highly correlate with one another [13–15]. Research on stable carbon isotope ratios of modern pollen focused mainly on species-specific patterns and ranges [15–18] and has been applied to investigate predominant photosynthetic pathways within grasslands [19–21]. Loader and Hemming [22] analysed δ13Cpollen of Pinus sylvestris from 28 sites across Europe and identified a positive linear correlation between δ13C values and the prevailing temperature during pollen formation. Also, Jahren [14] detected positive correlations of δ13Cpollen values with temperature for nine out of 14 plant species. Studies focussing on the determination of influencing climate factors on the isotope values of modern pollen include Bell et al. [15], who showed that δ13Cpollen of Cedurs atlantica (Atlas cedar) correlates with precipitation and a long-term annual and summer scPDSI (self-calibrating Palmer Drought Severity Index). Schwarz [13] suspected a relationship between δ13Cpollen and relative humidity, but no significant correlation of δ13Cpollen of Pinus retinosa (Red pine), Pinus sylvestris (Scots pine) and Quercus rubra (Northern red oak) could be detected at North American sampling sites. However, all correlations were highly species-dependent and several plants strongly reacted to other untested environmental factors, superimposing the climate signal archived in the pollen [14].
Little is known about the variability within modern oxygen pollen-isotope values. Nonetheless, they have already been successfully applied to determine the provenance of honey [e.g. 23, 24]. Loader and Hemming [25] identified a negative relationship of δ18Opollen values with the δ18O values of precipitation during pollen formation, contrasting δ18O of wood and leaves that is typically positively related to the δ18O of precipitation [e.g. 26, 27]. Even if the δ18Opollen is highly determined by the δ18O of local precipitation, the degree of dependence seems to vary with plant type and physiology [13]. Hence, δ13Cpollen and δ18Opollen values are influenced by local climate conditions during pollen formation [15, 22], but not all variability within pollen-isotope values can be ascribed to climate-related environmental factors alone.
Morphology-based analysis of fossil pollen is frequently used to reconstruct palaeovegetation, since fossil pollen are often well preserved, widespread and abundant in various Cenozoic archives [28–34]. A combination of traditional pollen analysis and stable isotope analysis of fossil pollen might enhance environmental reconstructions in a high spatio-temporal resolution. Due to plant-specific timings in pollen production and pollen shedding, even intra-annual weather signals may be recorded in the δ13Cpollen and δ18Opollen [13]. Some studies have already applied δ13C analysis to fossil pollen in an attempt to reconstruct past environmental changes [35–37]. A fossil δ13Cpollen record of C. atlantica from Morocco revealed the feasibility of reconstructing a long-term trend of increasing aridity by analysing species-specific pollen-isotopes [38]. Also, fossil Nothofagus (Southern beech) δ13Cpollen indicated different moisture availability in Antarctica during the early and middle Eocene [39].
However, interpretation of fossil pollen-isotopes are based on observations of modern δ13Cpollen and δ18Opollen patterns and ranges. In addition to climate conditions during pollen formation, non-climate impact factors may need to be considered when interpreting the δ13Cpollen and δ18Opollen values. These factors include site-specific environmental parameters such as type of soil, plant associations, position on slopes, and the proximity of the individual tree to perennial waterbodies. A comparison of several abundant tree species growing at different sites under the same environmental conditions, intra-tree differences and fluctuations over several vegetation periods helps to assess the impact of non-climate environmental factors on the pollen-isotope values.
In the present study, we address the species-specific natural variability of pollen-isotopes of nine abundant tree species across seven European sites ranging from Belgium to Poland and Finland to Italy, sampled during the years 2015 and 2016. The species have been chosen based on their widespread abundance in natural European forests and the frequency of their pollen in fossil records.
Inter- and intra-tree δ13Cpollen and δ18Opollen isotope variabilities were assessed and tested for relationships with non-climate environmental factors by stepwise regression analysis. In doing so, we aimed to advance our understanding of pollen stable isotope signals for future palaeoclimate reconstructions. In detail we investigated:
Species-specific δ13Cpollen and δ18Opollen patterns of selected tree taxa and the variability of their pollen-isotopes between two consecutive vegetation periods (2015/2016).
δ13Cpollen and δ18Opollen at different stages of the pollen maturation process.
Variability of δ13Cpollen and δ18Opollen at different heights and cardinal directions of individual trees.
δ13Cpollen and δ18Opollen along a gradient of continentality (W–E transect) and along a gradient in day length (N–S transect).
Material and methods
Sampling locations, sample collection and preparation
We sampled modern pollen from 658 individual trees of nine selected tree species growing in seven national and nature parks, which we consider natural habitats (Fig 1; Tables 1 and 2). None of the species sampled in this study is endangered or protected and sampling followed generally a non-invasive scheme of few inflorescences per individual tree. Therefore, after contacting and consulting with the national park authorities of each sampling site, no specific permissions were required for these locations and activities. Pollen were collected during two consecutive vegetation periods (February to June of 2015 and 2016) within the species-specific flowering periods (Fig 2; Table 2). The schedule for sampling followed individual phenology and thus roughly the geographic distribution and climate conditions of west-east and south-north gradients in Europe [40]. All selected tree species use the C3 photosynthetic pathway. In principle, 20 flowering individuals were sampled per site and per species (Table 2). In case of dense forests, trees close to hiking trails, forestry roads or glades were sampled because sunlight illuminating the full height of tree crowns allows the development of lower branches with inflorescences. Samples were taken with a pruning device and an extendable stick from branches at positions of one metre up to seven metres above ground. Male inflorescences were cut off and placed in plastic bags. Bulk -samples of an individual tree were composed of several inflorescences of different branches from various heights. In the field, the samples were kept in a cooling box. Later, they were stored in a refrigerator at 6 °C to prevent mould infestation. In the laboratory, the samples were dried in a drying oven at a maximum temperature of 45 °C for seven to nine days. Dry samples were kept in a freezer at -16 °C until further processing. The separation of pollen from other flower tissue was achieved by thorough rinsing with deionised water using sieves with mesh sizes from 10 μm to 200 μm. Following rinsing and sieving the pollen were freeze-dried for 48–72 hours until fully dehydrated, transferred into Eppendorf (2 ml) safe lock tubes and frozen at -16 °C for preservation.
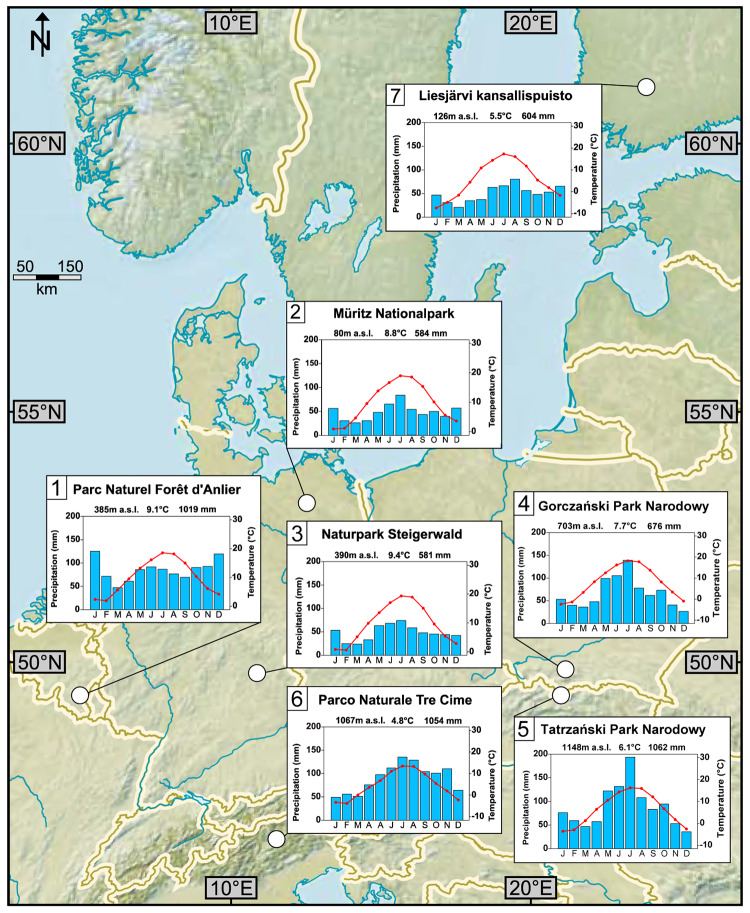
Fig 1. Study region (46.6°N– 53.3°N/5.7°E– 23.7°E).
Topographic map of central Europe showing the sites (Nature Parks or National Parks) for pollen sampling (white dots) and respective climate diagrams with average seasonal temperature and precipitation. Refer to Table 1 for sampling site numbers and further details. Map modified from https://www.cia.gov/library/publications/the-world-factbook/index.html.
Table 1. Sampling locations and site characteristics.
| Site | Site name | Park authority | Coordinates | Forest type and location characteristics | MAT (°C; min., max.), |
|---|---|---|---|---|---|
| No. | (country) | address | (altitude) | (soil type after WRB-FULL) | MAP |
| 1 | Parc naturel Forêt d’Anlier (Belgium) | Fédération des Parcs naturels de Wallonie Rue de Coppin, 20 5100 Jambes Tel. +32 81 30 21 81 e-mail: info@fpnw.be | 49.7899° N 5.6829° E (385 m a.s.l.) | Mesophytic deciduous broad-leaved and mixed coniferous-broad-leaved forest; Beech and mixed beech forest, montane to altimontane type, partly with fir and spruce. (Dystric Cambisol) | 9.1 (-0.1 to 16.1), 1019 mm |
| 2 | Müritz-Nationalpark (Germany) | Nationalparkamt Müritz Schlossplatz 3 17237 Hohenzieritz Tel. 039824/252-0 e-mail: poststelle@npa-mueritz.mvnet.de | 53.3268° N 13.1925° E (80 m a.s.l.) | Mesophytic deciduous broad-leaved and mixed coniferous-broad-leaved forest; Beech and mixed beech forest, lowland to submontane type. (Haplic Luvisol) | 8.8 (-0.9 to 17.2), 584 mm |
| 3 | Naturpark Steigerwald (Germany) | Naturpark Steigerwald e.V. Hauptstraße 1 91443 Scheinfeld Tel. 09161/92-1523 e-Mail: info@steigerwald-naturpark.de | 49.8616° N 10.5241° E (390 m a.s.l.) | Mesophytic deciduous broad-leaved and mixed coniferous-broad-leaved forest; Mixed oak-hornbeam forest. (Dystric Cambisol) | 9.4 (-1.2 to 17.5), 581 mm |
| 4 | Gorczański Park Narodowy (Poland) | Gorczański Park Narodowy Poręba Wielka 590 34–735 Niedźwiedź Tel. +48 33 17 207 e-mail: gpn@gorcepn.pl | 49.5608° N 20.1614° E (703 m a.s.l.) | Mesophytic deciduous broad-leaved and mixed coniferous-broad-leaved forest; Beech and mixed beech forest, montane to altimontane type, partly with fir and spruce. (Haplic Leptosol/ Dystric Cambisol) | 7.7 (-3.8 to 17.5), 676 mm |
| 5 | Tatrzański Park Narodowy (Poland) | Tatrzański Park Narodowy Kuźnice 1 34–500 Zakopane Tel. +48 18 20 23 200 e-mail: sekretariat@tpn.pl | 49.2571° N 19.9691° E (1148 m a.s.l.) | Mesophytic and hygromesophytic coniferous and mixed broad-leaved-coniferous forest; Montane to altimontane, partly submontane fir and spruce forests in the nemoral zone. (Calcaric Leptosol/ Dystric Leptosol) | 6.1 (-5.3 to 15.3), 1062 mm |
| 6 | Parco Naturale Tre Cime (Italy) | Amt für Natur Landhaus 11 Rittner Straße 4 39100 Bozen Tel. +39 0471 41 77 70 e-mail: natur.bozen@provinz.bz.it | 46.6412° N 12.3374° E (1067 m a.s.l.) | Mesophytic deciduous broad-leaved and mixed coniferous-broad-leaved forest; Beech and mixed beech forest, montane to altimontane type, partly with fir and spruce. (Rendzic Leptosol) | 4.8 (-5.8 to 15.0), 1054 mm |
| 7 | Liesjärvi kansallispuisto (Finland) | Metsähallitus P.O. Box 94 (Ratatie 11) FI-01301 Vantaa Tel. +358 206 39 4000 | 60.6633° N 23.8797° E (126 m a.s.l.) | Mesophytic and hygromesophytic coniferous and mixed broad-leaved-coniferous forest; Southern boreal type. (Haplic Podzol) | 5.5 (-7.1 to 16), 604 mm |
Sampling site overview including park authority address with contact details for sampling permissions, geographic coordinates, average elevation above sea level, forest and soil classification, average temperatures and mean annual precipitation. Forest classifications follow the “General Map of the Natural Vegetation of Europe” (Federal Agency for Nature Conservation, Bonn 2001). Soil classifications according to the European Soil Data Centre [41]; MAP (mean annual precipitation) and MAT (mean annual temperature; including the mean temperature of the coldest and warmest month) have been calculated based on the high-resolution gridded dataset CRU TS at http://www.cru.uea.ac.uk/data.
Table 2. Overview of species.
| Taxonomy | Common name | Flowering period |
Individuals | Individuals |
|---|---|---|---|---|
| 2015 | 2016 | |||
| Coniferophyta | ||||
| Pinaceae | ||||
| Picea abies (L.) H. KARST. | Norway spruce | May/June | 63 | 65 |
| Pinus sylvestris (L.) | Scots pine | May/June | 69 | 59 |
| Magnoliopsida | ||||
| Sapindaceae | ||||
| Acer pseudoplatanus (L.) | Sycamore | April/May | 9 | 24 |
| Betulaceae | ||||
| Alnus glutinosa (L.) GAERTN. | Black alder | January/March | 43 | 49 |
| Betula pendula ROTH | Silver birch | April/May | 33 | 38 |
| Carpinus betulus (L.) | European hornbeam | April/May | 1 | 18 |
| Corylus avellana (L.) | Common hazel | January/March | 43 | 49 |
| Fagaceae | ||||
| Fagus sylvatica (L.) | European beech | May | 11 | 46 |
| Quercus robur (L.) | Pendunculate oak | May | 16 | 22 |
Taxonomic classification of the nine investigated tree species including their common names and specific flowering periods. The number of individuals sampled represents the sum of trees sampled at all sites in 2015 and 2016, respectively.
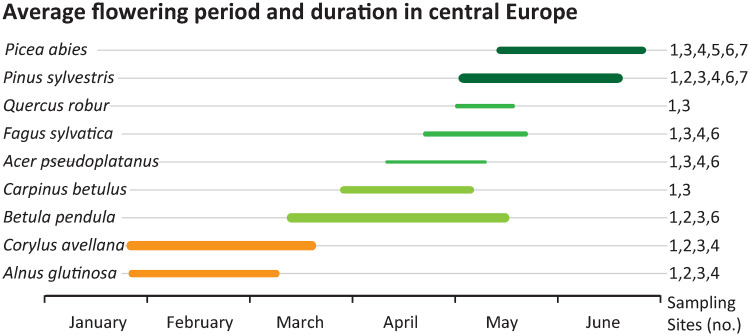
Fig 2. Average seasonal timing and duration of flowering periods in central Europe.
Relative amount of pollen released by the nine examined species of this study indicated by line thickness in three steps (summarised from http://www.pollenstiftung.de and personal observation). Colours classify species according to their average blossoming time (orange: early blossoming, January to March; green colour saturation level indicates spring to early summer). The duration is an estimated average of species from central European locations. Sampling site (no.) indicates the sites where the various species have been sampled according to Table 1 and Fig 1.
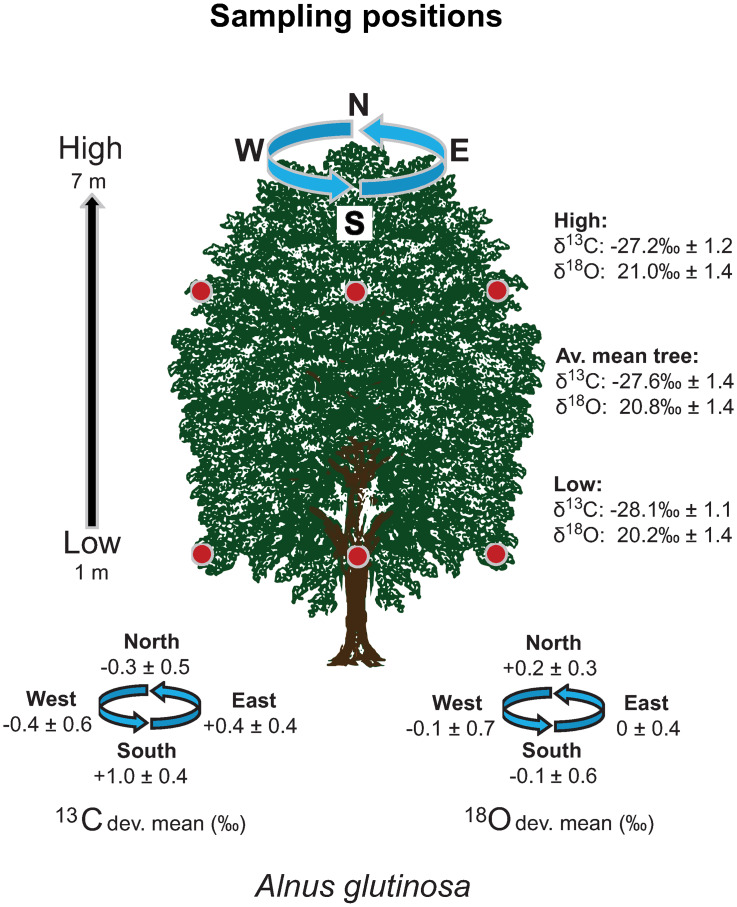
To investigate intra-tree isotope patterns, an additional 152 intra-tree sub-samples were taken at different heights and cardinal directions from 22 trees of eight species (Fig 3). In addition, individual inflorescences were collected separately in plastic bags to allow a high-resolution intra-tree variance analysis.
Fig 3. Intra-tree pollen-isotope variability of Alnus glutinosa.
The sampling scheme for intra-tree pollen-isotope analysis comprises the sampling of pollen from each cardinal direction at a low and a high position in a tree (1 m and 7 m above ground; red dots). Average isotope values (Av. mean tree) as well as the values of both the high and low positions are given for one exemplary Alnus glutinosa tree. 13C and 18O dev. mean = average deviation from the mean isotope value of the tree.
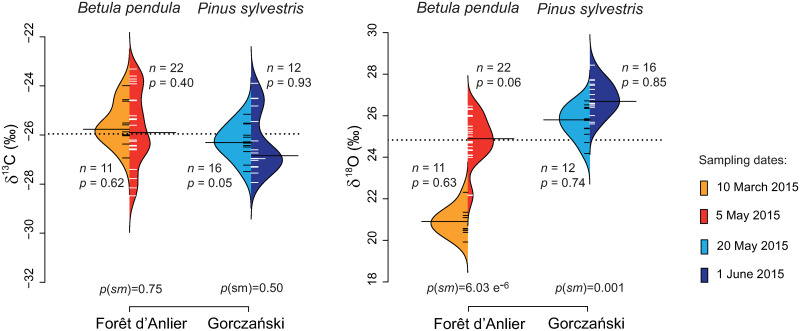
Intra-annual analyses at different stages of the pollen maturation process were carried out for B. pendula and P. sylvestris, which were sampled twice at the same location within one vegetation period. Betula pendula was collected at Forêt d’Anlier on 10 March and 5 May 2015, whereas P. sylvestris was sampled twice in Gorczański National Park on the 20 May and 1 June 2015. The individual trees sampled for the intra-annual analysis grew within a small assessable area with similar habitat conditions. Due to minimal individual offsets in flower development and senescence, only some tree individuals could be sampled and analysed twice. Seven of the P. sylvestris individuals were identical (total number of samples at first sampling: 12; total number of samples at second sampling: 16) and eight B. pendula trees were samples twice (total number of samples at first sampling: 11; total number of samples at second sampling: 22). An elevation transect in the Tatrzański Mountains National Park extends from 1053 m a.s.l. to 1345 m a.s.l. on a north-facing slope. Along the gradient of roughly 300 m, 15 individual trees of Picea abies were sampled at five different elevations in 2015 and 2016.
Stable isotope analysis
For each measurement, the amount of 220 μg ± 10% of chemically untreated pollen material was weighed directly into silver capsules using a high-precision scale (Mettler Toledo AX 26 Delta Range). δ13C and δ18O were determined using a DELTA V isotope ratio mass spectrometer (IRMS; Thermo Fisher Scientific™, Bremen) at the dendrochronological laboratory, section 4.3, GFZ Potsdam, Germany. To exclude potential water contamination from air humidity, all samples were vacuum dried at 100 °C for at least 12 hours in a Thermo Scientific Heraeus VT 6060 P prior to measurement. The pollen material was reduced to CO for simultaneous IRMS analysis of carbon and oxygen isotope ratios in a High Temperature Conversion Elemental Analyzer (TC/EA; 1400 °C; Thermo Fisher Scientific™, Bremen) coupled to the IRMS. All isotope ratios are expressed relative to VPDB for δ13C and VSMOW for δ18O. Isotope data were compared against international and lab-internal reference material (IAEA-CH3, IAEA-CH6 and IAEA 601 and 602) using two reference standards with widespread isotopic compositions for a single-point normalisation [42]. Most of the 809 individual pollen samples were weighed and measured with two or three repetitions. In total, we conducted 2132 measurements of stable isotopes. The pollen-isotope dataset is deposited at Pangea Database (https://doi.org/10.1594/PANGAEA.910977).
Statistical analysis
All calculations and graphics were done using programmes R [43] and RStudio [44]. Value distributions for each site and year with indicated average are shown as bean plots [45]. Many parametric tests assume a normal distribution. Hence, we tested whether the pollen-isotope values in a given sample of a species from each location and year were normally distributed using the Shapiro-Wilk test. The minimum sample size analysed was three. The null hypothesis of normal distribution was rejected, if the probability p was smaller than the significance level (range: 0–1; significance level: p = 0.05). As several distributions were non-normal, we used the non-parametric Mann-Whitney U test for equality of medians (p(sm)) to compare inter-annual distribution patterns of pollen-isotopes (range: 0–1; significance level: p(sm) = 0.05). A possible relationship between isotope values and elevation at Tatrańzki Park Narodowy (Poland) was investigated using linear correlation. Inter- and intra-tree variability of sub-samples taken at different tree heights and cardinal directions was characterised by calculating respective standard deviations (1σ).
Stepwise regression analysis
Variables influencing the stable isotope composition in pollen (δ13C, δ18O) were explored by means of stepwise regression as implemented by the JMP Pro 13.1.0 software. Stepwise regression reduces variance to a linear model by eliminating insignificant predictors and was performed (1) by species (to investigate the most important influencing factors for each species over several sites) and (2) by site (to evaluate possible location-dependent environmental factors for the pollen-isotopes). For the analysis by species eight potential predictors entered our models, including the categorical variables year (of sampling: 2015 and 2016), month (of sampling: February to June), maturity (of the pollen at the time of sampling: -1 = immature, 0 = mature, 1 = withered inflorescence), slope (steepness: 0 = flat, 1 = minimal incline, up to 10°, 2 = moderate incline, more than 10°), water (proximity to water body: 0 = none in the direct vicinity, 1 = one between 10 and 20 m away, 2 = one up to ten metres away), water classification (type of water body, e.g. river, lake, wetland), soil (type of soil; Table 1) and the predictor site as a site-specific combination of latitude, longitude and altitude. For the second analysis (by site) the continuous variable altitude was additionally included to the categorical variables year, month, maturity, slope, water, water classification, soil and species and thus, nine potential predictors entered the second model. The factors were noted for each individual tree during field work. For all analyses, we deleted singletons by having a look at column variation (< 5 values in a column), hence, several analyses were performed using a subset of the predictors mentioned above.
Categorical variables were hierarchically coded by maximizing the sum of squares between groups. Therefore, the analysis also informs about how levels in categorical predictors are associated with each other. For example, a notation such as site{FAN&STE&GOR&LIE-TAT&TRE} (SI Dataset 1 and 2) contrasts sites Forêt d’Anlier, Steigerwald, Gorczański, and Liesjärvi against Tatrzański and Tre Cime.
Variables revealing the highest statistical significance were added to the model in a stepwise process. As a stopping rule for adding terms we used the minimum Bayesian Information Criterion. Subsequently, the model parameters were estimated using least squares regression.
Results
δ13Cpollen and δ18Opollen of 809 samples of 658 individual trees from nine common tree species have been analysed (Table 3). The samples were taken at seven locations across Europe during three different time periods of flowering (January to March, April to May and May to June).
Table 3. δ13C and δ18O of studied pollen.
| Site name (no.) |
Species | Time of flowering |
Year | Samples (no.) |
δ13C | δ13C | δ13C | δ13C | δ18O | δ18O | δ18O | δ18O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | min. | max. | sd | mean | min. | max. | sd | |||||
| Parc Naturel Forêt d’Anlier (1) | Acer pseudoplatanus | B | 2016 | 6 | -25.2 | -27.1 | -22.5 | 1.7 | 25.7 | 25.0 | 26.7 | 0.6 |
| Alnus glutinosa | A | 2015 | 25 | -28.6 | -30.9 | -25.9 | 1.5 | 22.0 | 21.0 | 23.1 | 0.6 | |
| A | 2016 | 33 | -29.3 | -32.3 | -25.5 | 2.0 | 22.0 | 21.1 | 22.6 | 0.4 | ||
| Betula pendula | B | 2015/1 | 11 | -25.6 | -26.9 | -23.9 | 0.9 | 20.9 | 19.9 | 21.4 | 0.7 | |
| B | 2015/2 | 22 | -25.7 | -28.5 | -23.3 | 1.5 | 24.9 | 22.2 | 26.5 | 1.2 | ||
| B | 2016 | 15 | -24.1 | -25.8 | -21.4 | 1.1 | 24.2 | 22.5 | 25.6 | 0.9 | ||
| Carpinus betulus | B | 2015 | 1 | -25.2 | NA | NA | NA | 29.0 | NA | NA | NA | |
| B | 2016 | 8 | -26.1 | -29.3 | -23.4 | 2.2 | 26.3 | 25.3 | 27.5 | 0.6 | ||
| Corylus avellana | A | 2015 | 27 | -29.0 | -30.7 | -27.6 | 0.8 | 22.8 | 22.1 | 23.4 | 0.4 | |
| A | 2016 | 30 | -28.6 | -32.2 | -26.3 | 1.5 | 22.0 | 20.7 | 23.6 | 0.7 | ||
| Fagus sylvatica | B | 2016 | 23 | -27.3 | -29.3 | -24.7 | 1.6 | 23.2 | 22.4 | 24.3 | 0.5 | |
| Picea abies | C | 2015 | 15 | -26.2 | -29.0 | -23.5 | 1.3 | 24.0 | 23.1 | 25.4 | 0.8 | |
| C | 2016 | 22 | -25.8 | -28.0 | -23.9 | 1.4 | 25.1 | 23.7 | 26.2 | 0.7 | ||
| Pinus sylvestris | C | 2015 | 12 | -27.8 | -29.4 | -26.3 | 1.0 | 25.2 | 21.1 | 28.7 | 2.7 | |
| C | 2016 | 20 | -25.7 | -27.8 | -23.9 | 1.2 | 29.2 | 26.8 | 30.8 | 1.2 | ||
| Quercus robur | B | 2015 | 16 | -26.4 | -29.0 | -25.6 | 0.9 | 26.2 | 24.0 | 29.0 | 1.4 | |
| B | 2016 | 21 | -25.7 | -27.4 | -22.8 | 1.5 | 27.4 | 25.1 | 29.2 | 1.1 | ||
| Parco Naturale Tre Cime (6) | Acer pseudoplatanus | B | 2015 | 3 | -25.5 | -27.3 | -24.6 | 1.6 | 24.7 | 23.9 | 25.1 | 0.7 |
| B | 2016 | 4 | -26.1 | -26.7 | -25.0 | 0.8 | 23.1 | 21.8 | 24.3 | 1.0 | ||
| Betula pendula | B | 2016 | 4 | -24.8 | -26.1 | -23.8 | 1.0 | 23.9 | 22.9 | 25.2 | 1.0 | |
| Fagus sylvatica | B | 2016 | 4 | -27.6 | -28.1 | -27.2 | 0.4 | 24.1 | 23.1 | 26.1 | 1.4 | |
| Picea abies | C | 2015 | 9 | -23.8 | -25.7 | -22.3 | 1.1 | 23.5 | 21.0 | 26.0 | 1.5 | |
| C | 2016 | 31 | -23.2 | -24.6 | -22.0 | 0.9 | 21.6 | 20.0 | 23.2 | 0.9 | ||
| Pinus sylvestris | C | 2015 | 20 | -26.3 | -27.7 | -25.1 | 0.7 | 26.0 | 24.4 | 26.9 | 0.8 | |
| C | 2016 | 14 | -27.0 | -29.1 | -25.3 | 1.1 | 23.6 | 21.2 | 26.0 | 1.9 | ||
| Gorczański Park Narodowy (4) | Acer pseudoplatanus | B | 2015 | 6 | -25.5 | -28.3 | -23.2 | 1.7 | 18.0 | 16.2 | 19.7 | 1.1 |
| B | 2016 | 11 | -24.8 | -27.9 | -23.3 | 1.4 | 18.5 | 16.9 | 20.2 | 1.0 | ||
| Alnus glutinosa | A | 2015 | 26 | -27.4 | -29.6 | -25.2 | 1.4 | 18.1 | 16.9 | 19.2 | 0.6 | |
| A | 2016 | 26 | -26.4 | -29.9 | -22.3 | 1.9 | 19.1 | 17.6 | 21.7 | 1.1 | ||
| Corylus avellana | A | 2015 | 29 | -27.8 | -30.4 | -24.6 | 1.2 | 18.5 | 16.0 | 21.9 | 1.4 | |
| A | 2016 | 27 | -26.5 | -30.4 | -24.4 | 1.6 | 19.2 | 17.5 | 21.2 | 1.3 | ||
| Fagus sylvatica | B | 2015 | 10 | -27.9 | -28.9 | -26.4 | 0.8 | 18.6 | 17.4 | 20.5 | 0.9 | |
| B | 2016 | 13 | -27.4 | -29.5 | -24.9 | 1.8 | 19.5 | 17.4 | 22.1 | 1.3 | ||
| Picea abies | C | 2015 | 17 | -25.6 | -27.8 | -23.2 | 1.23 | 24.8 | 22.5 | 26.5 | 1.0 | |
| C | 2016 | 26 | -25.8 | -26.8 | -24.1 | 0.8 | 21.5 | 20.5 | 22.4 | 0.6 | ||
| Pinus sylvestris | C | 2015/1 | 12 | -26.3 | -27.5 | -25.2 | 0.7 | 25.7 | 24.2 | 26.7 | 0.8 | |
| C | 2015/2 | 52 | -26.3 | -27.9 | -23.9 | 1.3 | 26.9 | 25.6 | 28.4 | 0.8 | ||
| C | 2016 | 13 | -26.9 | -28.9 | -25.6 | 1.1 | 25.0 | 22.0 | 27.4 | 1.4 | ||
| Liesjärvi | Picea abies | C | 2016 | 10 | -25.6 | -27.4 | -24.3 | 0.7 | 22.7 | 21.7 | 24.0 | 0.7 |
| kan. (7) | Pinus sylvestris | C | 2016 | 20 | -27.9 | -29.6 | -26.5 | 0.8 | 23.6 | 21.6 | 24.6 | 0.7 |
| Müritz NP (2) | Alnus glutinosa | A | 2016 | 5 | -31.0 | -32.3 | -30.0 | 1.0 | 22.5 | 22.2 | 23.2 | 0.4 |
| Betula pendula | B | 2016 | 13 | -23.9 | -26.4 | -22.1 | 1.7 | 24.1 | 25.2 | 25.1 | 0.6 | |
| Corylus avellana | A | 2016 | 6 | -25.3 | -27.6 | -23.0 | 1.7 | 23.9 | 23.7 | 24.1 | 0.1 | |
| Pinus sylvestris | C | 2015 | 6 | -26.9 | -28.8 | -26.2 | 0.9 | 28.8 | 28.1 | 29.4 | 0.5 | |
| Steigerwald National Park (3) | Acer pseudoplatanus | B | 2016 | 9 | -23.7 | -24.8 | -23.0 | 1.0 | 24.6 | 24.0 | 25.4 | 0.7 |
| Alnus glutinosa | A | 2016 | 2 | -26.6 | -28.1 | -25.2 | 2.1 | 22.9 | 22.9 | 22.9 | 0.0 | |
| Carpinus betulus | B | 2016 | 10 | -25.6 | -27.1 | -23.5 | 1.4 | 26.1 | 24.7 | 27.3 | 0.9 | |
| Corylus avellana | A | 2016 | 2 | -27.0 | -27.6 | -26.4 | 0.8 | 23.8 | 23.7 | 23.9 | 0.1 | |
| Betula pendula | B | 2016 | 9 | -22.9 | -25.0 | -21.1 | 1.3 | 24.8 | 24.0 | 25.6 | 0.6 | |
| Fagus sylvatica | B | 2016 | 10 | -25.5 | -27.1 | -23.6 | 1.1 | 23.4 | 22.4 | 24.9 | 0.8 | |
| Picea abies | C | 2015 | 7 | -26.1 | -28.8 | -24.9 | 1.3 | 25.2 | 22.6 | 26.9 | 1.6 | |
| C | 2016 | 5 | -25.8 | -27.4 | -23.6 | 1.5 | 21.7 | 19.6 | 22.9 | 1.3 | ||
| Pinus sylvestris | C | 2015 | 5 | -26.0 | -27.3 | -23.9 | 1.4 | 27.6 | 26.3 | 28.7 | 1.0 | |
| Quercus robur | B | 2016 | 3 | -23.3 | -23.6 | -22.8 | 0.4 | 26.3 | 26.2 | 26.5 | 0.1 | |
| Tatrzański | Picea abies | C | 2015 | 18 | -24.3 | -26.4 | -22.1 | 1.1 | 22.6 | 21.1 | 23.9 | 0.8 |
| PN (5) | C | 2016 | 5 | -26.3 | -26.6 | -26.0 | 0.3 | 21.3 | 21.0 | 21.9 | 0.5 |
Stable isotope values of δ13Cpollen and δ18Opollen (min., max., standard deviation) differentiated by sample location (site name and assigned number), species and year. Flowering periods are indicated by letters: (A) January to March, (B) April to May and (C) May to June. The number of samples includes bulk samples of individual trees and sub-samples of different positions within a tree. Missing data is denoted as NA.
δ13Cpollen values of broad-leaved and coniferous tree species
Flowering period January to March: Alnus glutinosa and Corylus avellana
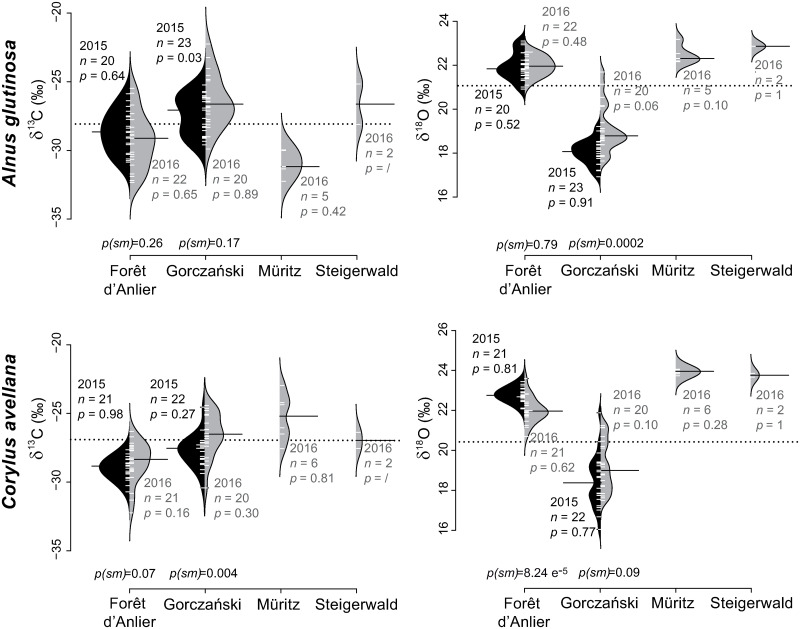
The range of mean δ13Cpollen values from A. glutinosa for all sites and both years is 4.6‰ (-31.0‰ to -26.4‰; Table 3). With the exception of the Gorczański site (2015) the δ13Cpollen values of two consecutive years yield comparable medians at p(sm) = 0.26 and 0.17 (Fig 4). Mean δ13Cpollen values of C. avellana pollen range from -29.1‰ to -25.3‰ (3.8‰; Table 3) and are normally distributed. Similar medians for both years are suggested for Forêt d’Anlier at p(sm) = 0.07, while the null hypothesis of equal medians was rejected at Gorczański at p(sm) = 0.004.
Fig 4. Pollen-isotopes of broad-leaved species flowering January to March: Alnus glutinosa and Corylus avellana.
The bean plots show the values of δ13Cpollen and δ18Opollen of the two species, Alnus glutinosa and Corylus avellana, from four different locations (Fig 1) sampled in 2015 (black) and 2016 (grey). Localities Steigerwald and Müritz were only sampled in 2016. n indicates the number of trees sampled on each occasion (i.e. year). p-values indicate whether the pollen-isotope values of one year are normally distributed, whereas p(sm) represents the probability for equal medians in samples of two consecutive years. The dotted line represents the mean over all localities and both years. The means of each sampling are indicated by a black bar.
Flowering period April to May: Acer pseudoplatanus, Betula pendula, Carpinus betulus, Fagus sylvatica and Quercus robur
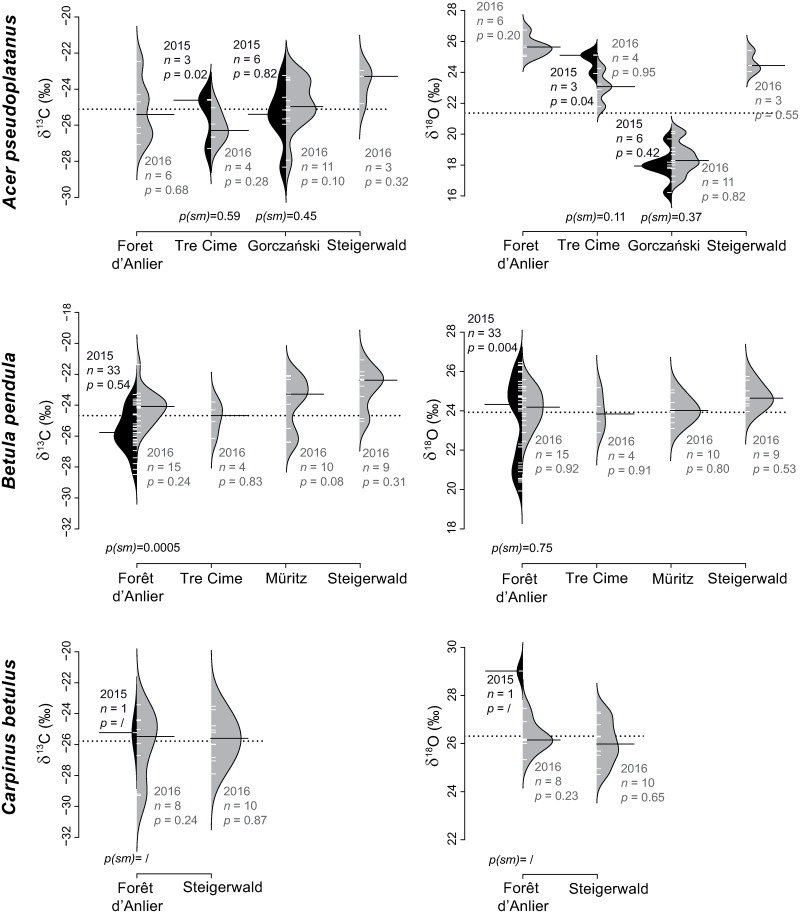
Mean δ13Cpollen values of A. pseudoplatanus range from -26.1‰ to -23.7‰ (2.4‰; Table 3, Fig 5). With one exception at Tre Cime (2015, p = 0.02), the δ13Cpollen values of all sites are normally distributed and they yield similar medians for both vegetation periods. δ13Cpollen values of B. pendula are also normally distributed. Their mean ranges from -25.7‰ to -22.9‰ (2.8‰; Table 3, Fig 5), but the distributions yield unequal medians for both years at the site Forêt d’Anlier (p(sm) = 0.0005). Mean δ13Cpollen of C. betulus range between -26.1‰ and -25.6‰ (0.5‰) and the values reveal a normal distribution at each site (Table 3, Fig 5).
Fig 5. Pollen-isotopes of broad-leaved species flowering April to May: Acer pseudoplatanus, Betula pendula and Carpinus betulus.
The broad-leaved species Acer pseudoplatanus, Betula pendula and Carpinus betulus were sampled at two to four locations. The bean plots show δ13Cpollen values (left) and δ18Opollen values (right) of 2015 (black) and 2016 (grey). n indicates the number of individuals. p-values indicate whether pollen-isotope values of a single year are normally distributed (sign. level = 0.05), whereas p(sm) represents the probability for equal medians in samples of two consecutive years. The dotted line represents the mean over all localities and both years. The means of each sampling are indicated by a black bar.
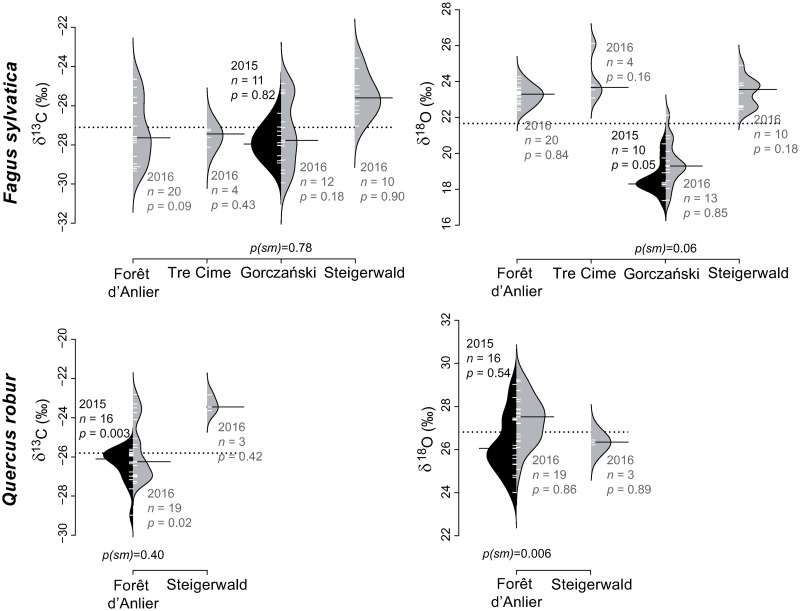
The range of F. sylvatica mean δ13Cpollen values is -27.9‰ to -25.5‰ (2.4‰) and the values are normally distributed with similar medians for both years at the site Gorczański (Table 3, Fig 6). Mean δ13Cpollen values of Q. robur range from -26.4‰ to -23.3‰ (3.1‰). The δ13Cpollen values are not normally distributed at Forêt d’Anlier (2015: p = 0.003; 2016: p = 0.02), but the carbon isotope values of both years are similar at this site (p(sm) = 0.40).
Fig 6. Pollen-isotopes of broad-leaved species flowering April to May: Fagus sylvatica and Quercus robur.
The broad-leaved species Fagus sylvatica and Quercus robur were sampled at two to four locations. The bean plots show δ13Cpollen values (left) and δ18Opollen values (right) of 2015 (black) and 2016 (grey). n indicates the number of individuals. p-values indicate whether the pollen-isotope values of a single year are normally distributed (sign. level = 0.05), whereas p(sm) represents the probability for equal medians in samples of two consecutive years. The dotted line represents the mean over all localities and both years. The means of each sampling are indicated by a black bar.
Flowering period May to June: Pinus sylvestris and Picea abies
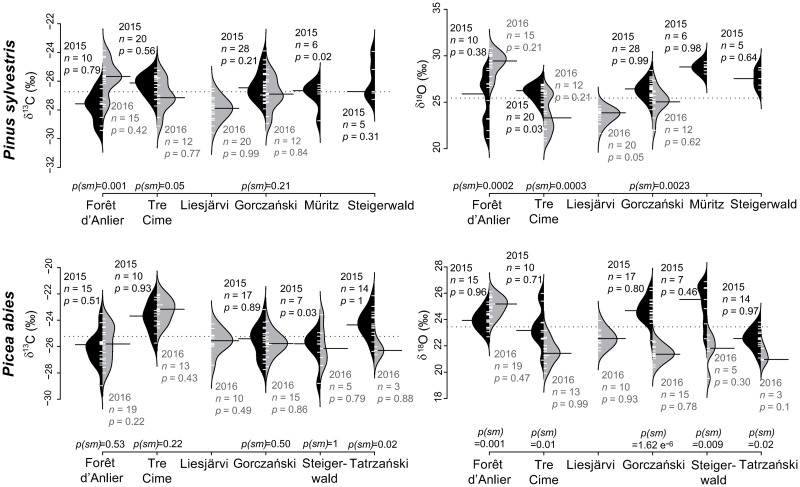
Pinus sylvestris mean δ13Cpollen values range between -27.9‰ and -26.0‰ (1.9‰; Table 3, Fig 7) and the values are normally distributed with one exception at Müritz (2015; p = 0.02). Medians of both years are mostly comparable, only Forêt d’Anlier yields statistically distinct medians at p(sm) = 0.001. The mean δ13Cpollen values of P. abies range from -26.3‰ to -23.2‰ (3.1‰; Table 3) and only the samples from Steigerwald (2015; p = 0.03) are not normally distributed. Between-year equal medians are present for all locations except Tatrzański (p(sm) = 0.02).
Fig 7. Pollen-isotopes of coniferous species flowering May to June: Pinus sylvestris and Picea abies.
Pinus sylvestris and Picea abies were sampled at six locations. The bean plots show δ13Cpollen values (left) and δ18Opollen values (right) of 2015 (black) and 2016 (grey). n indicates the number of individuals, p represents the probability of normally distributed pollen-isotopes within one year and p(sm) indicates the probability for similar medians in samples of two consecutive years. The dotted line represents the mean over all localities and both years. The means of each sampling are indicated by a black bar.
Intra-tree variability, intra-annual variability and variability with elevation of δ13Cpollen
Intra-tree variability of δ13Cpollen
64% of δ13Cpollen values of samples taken at lower branches of the broad-leaved species A. pseudoplatanus A. glutinosa and C. avellana are more negative in comparison to the branches higher up in the same individuals (Fig 3, Table 4). The isotopic values of the samples taken at different positions within the canopy are mostly ranging within one or two standard deviations from the mean isotope value of the tree. δ13Cpollen values from pollen growing at the east side of a tree are likely to be higher, whereas values of the west tend to show lower δ13Cpollen values in comparison to the mean isotope value of the trees. Deviations from the mean at northern and southern positions appear to be species-specific. The δ13Cpollen value of A. pseudoplatanus is lower in the North (-0.7‰ from the mean value) and A. glutinosa yields lower values in the North in two out of three samples (Table 4). The carbon isotope depletion in the North averages at -0.4‰ for A. glutinosa. In contrast, C. avellana exhibits higher pollen-isotope values in the North (+0.5‰) compared to the intra-tree average of this species. Twenty samples taken at different positions within the canopy of a single P. abies tree show higher δ13Cpollen values at eastern and western positions and lower values at the southern exposition (Table 5). The analysis of 34 individual inflorescences from low and high positions demonstrates the intra-branch variability in pollen-isotopes of six neighbouring P. sylvestris trees (Table 6). δ13Cpollen values from the same branch of one individual tree differ in a range of 0‰ to 1.2‰ (Table 6). The average δ13Cpollen difference between inflorescences from the same branch is 0.3‰.
Table 4. Isotopic deviation of δ13Cpollen values and δ18Opollen values between different sampling heights and cardinal directions.
| ID | direct. | pos. | δ13C | δ18O | var. δ13C | var. δ18O | av. δ13C | av. δ18O | de. δ13C | de. δ18O | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (‰) | (‰) | (L-H) | (L-H) | (‰) | (‰) | (‰) | (‰) | |||||||
| Acer_1 (Steigerwald) | North | L | -25.7 | 25.8 | - | - | -25.7 | 25.8 | ||||||
| H | - | - | ||||||||||||
| South | L | -24.8 | 25.1 | -0.4 | 0.2 | -24.6 | 25.0 | N- 0.7 | N +0.4 | |||||
| H | -24.3 | 24.8 | W +0.0 | -24.8 (av.tree) | E +0.3 | W -0.1 | 25.4 (av.tree) | E +0.3 | ||||||
| East | L | -25.2 | 25.3 | -1.1 | -1.0 | -24.6 | 25.7 | |||||||
| H | -24 | 26.2 | S +0.4 | S -0.5 | ||||||||||
| West | L | -25.4 | 25.0 | -1 | -0.8 | -24.9 | 25.3 | |||||||
| H | -24.4 | 25.7 | ||||||||||||
| Alnus_1 (F. d’Anlier) | North | L | - | - | - | - | -26.4 | 21.4 | ||||||
| H | -26.4 | 21.4 | ||||||||||||
| South | L | - | - | - | - | -25.8 | 21.9 | N +0.3 | N -0.2 | |||||
| H | -25.8 | 21.9 | W -1.4 | -26.7 (av.tree) | E +0.2 | W +0.1 | 21.6 (av.tree) | E -0.2 | ||||||
| East | L | - | - | - | - | -26.5 | 21.4 | |||||||
| H | -26.5 | 21.4 | S +1.1 | S +0.3 | ||||||||||
| West | L | -28.1 | 21.6 | - | - | -28.1 | 21.6 | |||||||
| H | - | - | ||||||||||||
| Alnus_9 (F. d’Anlier) | North | L | -29.9 | 21.6 | -2.3 | -1.3 | -28.8 | 22.3 | ||||||
| H | -27.6 | 22.9 | ||||||||||||
| South | L | -27.6 | 21.7 | - | - | -27.6 | 21.7 | N -0.5 | N +0.4 | |||||
| H | - | - | W +0.4 | -28.3 (av.tree) | E +0.6 | W -0.7 | 21.8 (av.tree) | E +0.4 | ||||||
| East | L | - | - | - | - | -29.0 | 22.2 | |||||||
| H | -29.0 | 22.2 | S +0.7 | S -0.2 | ||||||||||
| West | L | -27.5 | 21.1 | 0.9 | -0.1 | -27.9 | 21.1 | |||||||
| H | -28.4 | 21.2 | ||||||||||||
| Alnus_11 (Gorczański) | North | L | -28.7 | 19.0 | -0.9 | -0.7 | -28.3 | 19.4 | ||||||
| H | -27.8 | 19.8 | ||||||||||||
| South | L | -26.5 | 18.5 | -0.6 | -0.2 | -26.2 | 18.6 | N -0.9 | N +0.3 | |||||
| H | -25.9 | 18.7 | W -0.1 | -27.3 (av.tree) | E -0.2 | W +0.3 | 19.1 (av.tree) | E -0.1 | ||||||
| East | L | -27.6 | 19.1 | - | - | -27.6 | 19.1 | |||||||
| H | - | - | S +1.2 | S -0.5 | ||||||||||
| West | L | -27.6 | 19.5 | -0.3 | 0.2 | -27.5 | 19.4 | |||||||
| H | -27.3 | 19.3 | ||||||||||||
| Corylus_11 (Gorczański) | North | L | -24.6 | 19.1 | 0.8 | -0.3 | -25.0 | 19.3 | ||||||
| H | -25.3 | 19.4 | ||||||||||||
| South | L | -25.4 | 18.9 | 0.9 | -0.3 | -27.8 | 19 | N +0.7 | N -0.0 | |||||
| H | -26.2 | 19.1 | W +0.1 | -25.1 (av.tree) | E +1.4 | W +0.1 | 19.3 (av.tree) | E +0.3 | ||||||
| East | L | -24.2 | 20.1 | 0.1 | 1.0 | -24.3 | 19.6 | |||||||
| H | -24.3 | 19.1 | S -2.2 | S -0.3 | ||||||||||
| West | L | -25.1 | 19.3 | 0.8 | -0.1 | -25.5 | 19.4 | |||||||
| H | -25.9 | 19.4 | ||||||||||||
| Corylus_19 (Gorczański) | North | L | -27.0 | 20.3 | -2.5 | -0.5 | -25.7 | 20.5 | ||||||
| H | -24.5 | 20.8 | ||||||||||||
| South | L | -27.5 | 18.9 | 0.3 | -0.1 | -27.6 | 18.9 | N +1.4 | N +1.1 | |||||
| H | -27.8 | 19.0 | W -1.0 | -27.2 (av.tree) | E -0.0 | W -0.4 | 19.4 (av.tree) | E -0.2 | ||||||
| East | L | -27.7 | 19.6 | -1.1 | 0.7 | -27.2 | 19.2 | |||||||
| H | -26.6 | 18.9 | S -0.5 | S -0.5 | ||||||||||
| West | L | -28.6 | 18.4 | -0.9 | -1.2 | -28.1 | 19.0 | |||||||
| H | -27.7 | 19.6 | ||||||||||||
| Corylus_7 (F. d’Anlier) | North | L | -29.6 | 23.4 | -0.9 | -0.1 | -29.2 | 23.5 | ||||||
| H | -28.7 | 23.5 | ||||||||||||
| South | L | -30.4 | 22.2 | -1.1 | -0.1 | -29.8 | 22.3 | N +0.2 | N +1.0 | |||||
| H | -29.3 | 22.3 | W -0.5 | -29.2 (av.tree) | E +0.9 | W -1.2 | 22.7 (av.tree) | E +0.4 | ||||||
| East | L | -29.9 | 23.0 | -2.9 | 0.0 | -28.4 | 23.0 | |||||||
| H | -27.0 | 23.0 | S -0.5 | S -0.3 | ||||||||||
| West | L | -29.8 | 21.4 | - | - | -29.8 | 21.4 | |||||||
| H | - | - | ||||||||||||
| Corylus_21 (F. d’Anlier) | North | L | -29.0 | 23.1 | -0.7 | 0.1 | -28.6 | 23.1 | ||||||
| H | -28.3 | 23.0 | ||||||||||||
| South | L | -27.0 | 22.3 | 1.5 | -0.5 | -27.7 | 22.5 | N -0.4 | N +0.5 | |||||
| H | -28.5 | 22.7 | W -1.3 | -28.4 (av.tree) | E +1.3 | W -0.3 | 22.6 (av.tree) | E -0.2 | ||||||
| East | L | - | - | - | - | -26.9 | 22.4 | |||||||
| H | -26.9 | 22.4 | S +0.4 | S -0.1 | ||||||||||
| West | L | -29.2 | 22.5 | 0.5 | 0.4 | -29.5 | 22.3 | |||||||
| H | -29.7 | 22.1 |
Analysis of samples from eight individual trees of three species (Acer pseudoplatanus, Alnus glutinosa and Corylus avellana) taken at each cardinal direction and from two different positions on each tree. ID = individual identification (including species, number and site); direct. = cardinal direction; pos. = position on the tree (L = low; H = high); var. = variance; av. = average; de. = deviation from the mean value. The mean isotope values of the tree are noted in the black box (av.tree).
Table 5. Intra-tree analysis of a single Pices abies tree from Tre Cime, Italy.
| ID | direct. | pos. | inf._ID | δ13C | δ18O | av. δ13C | av. δ18O | var. δ13C | var. δ18O | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (‰) | (‰) | (‰) | (‰) | (L-H) | (L-H) | |||||||
| Picea_12 (Tre Cime) | South | L | - | -22.4 | 23.4 | -22.5 | 23.1 | -0.1 | 1.9 | |||
| S_low_1 | -22.5 | 23.4 | de. δ13C | |||||||||
| S_low_2 | -22.8 | 21.6 | (‰) | |||||||||
| S_low_3 | -22.2 | 23.9 | N | |||||||||
| H | - | -22.5 | 21.1 | -22.3 | 21.3 | W +0.1 | -22.2 (av.tree) | E +0.2 | ||||
| S_high_1 | -22.3 | 20.6 | ||||||||||
| S_high_2 | -22.8 | 21.9 | S -0.4 | |||||||||
| S_high_3 | -21.7 | 21.5 | ||||||||||
| East | L | - | -22.6 | 22.9 | -22.5 | 23.2 | -1.0 | -0.4 | ||||
| E_low_1 | -21.3 | 22.6 | ||||||||||
| E_low_2 | -23.6 | 24.2 | ||||||||||
| H | - | -21.2 | 23.5 | -21.5 | 23.7 | de. δ18O | ||||||
| E_high_1 | -21.8 | 23.7 | (‰) | |||||||||
| E_high_2 | -21.4 | 23.8 | N | |||||||||
| West | L | - | -22.3 | 23 | -21.7 | 23.1 | 0.6 | -0.5 | W +0.3 | 22.9 (av.tree) | E +0.5 | |
| W_low_1 | -21.8 | 22.6 | ||||||||||
| W_low_2 | -21.1 | 23.6 | S -0.8 | |||||||||
| H | - | -21.8 | 23.2 | -22.3 | 23.5 | |||||||
| W_high_1 | -22.4 | 24.3 | ||||||||||
| W_high_2 | -22.8 | 23 |
Analysis of samples from three cardinal directions (south, east and west; the northern side was not flowering), different sampling heights (low/high) and the comparison of individual inflorescences from each sampling position. ID = individual identification (including species, number and site); direct. = cardinal direction; pos. = position on the tree (L = low; H = high); inf._ID = inflorescence identification per branch; var. = variance; av. = average; de. = deviation from the mean value. The mean isotope values of the tree are noted in the black box (av.tree).
Table 6. Intra-tree variability of δ13Cpollen and δ18Opollen values from single inflorescences of six Pinus sylvestris trees.
| Tree ID |
Position | Branch ID |
Inf. ID |
δ13C | δ18O | de. δ13C | de. δ18O |
|---|---|---|---|---|---|---|---|
| (‰) | (‰) | (‰) | (‰) | ||||
| Pinus 1 | L | low_1 | 1 | -25.3 | 27.0 | 0.7 | 0.8 |
| L | 2 | -26.0 | 27.8 | ||||
| L | low_2 | 1 | -24.8 | 28.0 | 0.4 | 0.4 | |
| L | 2 | -25.2 | 28.4 | ||||
| H | high_1 | 1 | -25.7 | 27.2 | 0.1 | 1.4 | |
| H | 2 | -25.6 | 28.6 | ||||
| H | high_2 | 1 | -25.4 | 27.3 | 0.2 | 0.1 | |
| H | 2 | -25.2 | 27.2 | ||||
| Pinus 2 | L | low_1 | 1 | -23.7 | 26.4 | 0.0 | 0.2 |
| L | 2 | -23.7 | 26.6 | ||||
| L | low_2 | 1 | -24.0 | 26.5 | 0.3 | 0.7 | |
| L | 2 | -23.7 | 27.2 | ||||
| H | high_1 | 1 | -24.5 | 26.1 | 0.1 | 0.2 | |
| H | 2 | -24.4 | 26.3 | ||||
| H | high_2 | 1 | -23.8 | 26.4 | 0.0 | 0.7 | |
| H | 2 | -23.8 | 27.1 | ||||
| Pinus 3 | L | low_1 | 1 | -23.3 | 27.4 | 1.2 | 0.1 |
| L | 2 | -24.5 | 27.5 | ||||
| L | low_2 | 1 | -24.7 | 26.7 | 0.2 | 0.2 | |
| L | 2 | -24.9 | 26.9 | ||||
| H | high_1 | 1 | -24.8 | 26.9 | 0.0 | 0.8 | |
| H | 2 | -24.8 | 27.7 | ||||
| H | high_2 | 1 | -25.0 | 26.6 | 0.2 | 0.4 | |
| H | 2 | -25.2 | 26.2 | ||||
| Pinus 4 | L | low_1 | 1 | -25.4 | 27.6 | 0.8 | 0.5 |
| L | 2 | -24.6 | 27.1 | ||||
| H | high_1 | 1 | -24.6 | 27.5 | 0.0 | 0.3 | |
| H | 2 | -24.6 | 27.8 | ||||
| Pinus 5 | L | low_1 | 1 | -26.4 | 26.3 | 0.2 | 0.7 |
| L | 2 | -26.6 | 27.0 | ||||
| Pinus 6 | L | low_1 | 1 | -27.8 | 27.2 | 0.6 | 0.3 |
| L | 2 | -27.2 | 26.9 | ||||
| H | high_1 | 1 | -27.0 | 27.4 | 0.9 | 0.1 | |
| H | 2 | -27.9 | 27.3 | ||||
| Average: | 0.3 | 0.5 |
The samples of six individual Pinus sylvestris trees from the same location represent single inflorescences from different positions (low/high) on the tree. The isotopic difference between the inflorescences shows the variability of δ13Cpollen and δ18Opollen values on branches and within trees at a high resolution. Tree ID = individual identification, (including species, number and site); Position = position on the tree (L = low; H = high); Branch ID = branch identification at each tree; Inf. ID = inflorescence identification of each branch; de. = deviation from the mean value.
δ13Cpollen values at different stages of pollen maturation
The δ13Cpollen values of B. pendula collected in March (-25.6‰) and May (-25.7‰) 2015 and of P. sylvestris collected in May (-26.3‰) and June (-26.3‰) 2015 are normally distributed and the statistical test reveals similar medians of the isotope values for both samplings of B. pendula and P. sylvestris (Fig 8).
Fig 8. Intra-annual comparison of δ13Cpollen and δ18Opollen values of Betula pendula (Forêt d’Anlier) and Pinus sylvestris (Gorczański).
Both species were sampled twice in 2015. The bean plots show δ13Cpollen values (left) and δ18Opollen values (right). Colours indicate the sampling date (orange = 10 March 2015; red = 5 May 2015; light blue = 20 May 2015; dark blue = 1 June 2015). n is the number of individuals sampled. The p-values indicate whether the pollen-isotope values of one year are normally distributed (sign. level = 0.05), whereas p(sm) represents the probability for equal medians in samples of the same year. The dotted line represents the mean over all localities and both years. The means of each sampling are indicated by a black bar.
Isotope variability of δ13Cpollen with elevation
The δ13Cpollen values of P. abies at Tatrzański Park Narodowy increase with altitude from -26.6‰ (1053 m a.s.l) up to -22.1‰ (1344 m a.s.l.) (Fig 9). The coefficient R2 of 0.52 reveals a weak linear correlation with elevation.
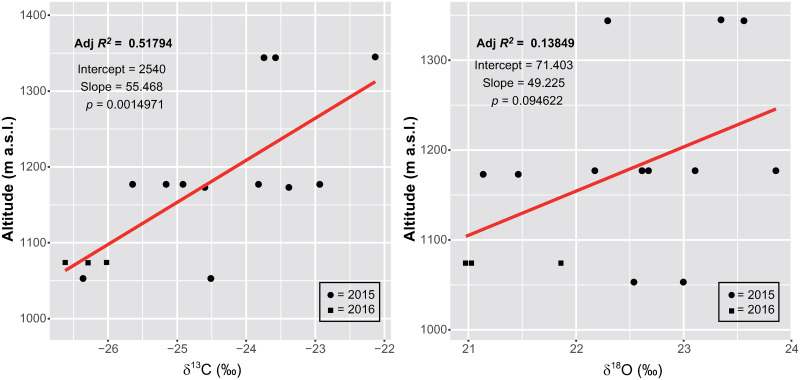
Fig 9. 300 m altitudinal transect of Picea abies at Tatrzański Park Narodowy.
The plots show the linear regression analysis of δ13Cpollen values and altitude (left) and δ18Opollen values and altitude (right). Samples were taken in 2015 (black dots) and 2016 (black squares). The red line indicates the slope of the linear regression model. Adjusted R2 indicates the proportion of variance explained by the linear association of isotopes and elevation.
Stepwise regression analysis for δ13Cpollen
By species
The most relevant factor influencing δ13Cpollen values for several species is site with very high probability values of Prob > F = < 0001* (Table 7; S1 Dataset). The year of sampling influenced δ13Cpollen of B. pendula, C. avellana and Q. robur. The factor soil and the maturity of the pollen are important for A. glutinosa and P. sylvestris. The factors proximity to water and water classification occur only once in the analytical outcome.
Table 7. Environmental factors affecting stable pollen-isotope composition for each species.
| Plant species | Influencing factor on δ13Cpollen | Prob > F | Influencing factor on δ18Opollen | Prob > F |
|---|---|---|---|---|
| A. pseudoplatanus | proximity to water | 0.0266* | site {GOR-FAN&DOL&STE} | <.0001* |
| site {DOL&STE-FAN} | 0.0043* | |||
| A. glutinosa | soil | 0,0097 | year | 0.0034* |
| maturity | 0.0012* | site {GOR-FAN&MUR&STE} | <.0001* | |
| site {FAN-MUR&STE} | 0.0162* | |||
| site {MUR-STE} | 0.0009* | |||
| maturity | <.0001* | |||
| B. pendula | year | <.0001* | year | 0.0165* |
| month {mar-apr&may} | <.0001* | |||
| C. betulus | water classification | 0.0186* | proximity to water | 0.0048* |
| C. avellana | year | 0.0047* | site {GOR-FAN&STE&MUR} | <.0001* |
| site {FAN—GOR&STE&MUR} | <.0001* | site {FAN-STE&MUR} | 0.0003* | |
| F. sylvatica | site {GOR&TRE&FAN—STE} | 0.0001* | site {GOR-FAN&STE&TRE} | <.0001* |
| maturity | <.0001* | |||
| P. abies | site {FAN&STE&GOR&LIE -TAT&TRE} | <.0001* | year | <.0001* |
| site {TAT-TRE} | 0.0022* | site {TRE&TAT&LIE&GOR-STE&FAN} | 0.0055* | |
| site {TRE&TAT-LIE} | 0.0068* | |||
| water_classification | <.0001* | |||
| P. sylvestris | maturity | 0.0009* | site {LIE&TRE&GOR—STE&FAN&MUR} | <.0001* |
| soil | 0.0018* | month | 0.0124* | |
| maturity | <.0001* | |||
| Q. robur | year | 0.0240* | year | 0.0146* |
Relevant environmental impact factors on δ13Cpollen and δ18Opollen values for each species. Prob > F gives the probability value after Levene´s test [46]. The factor site groups locations by similarity. Abbreviations for locations: FAN (Forêt d’Anlier); GOR (Gorczański); MUR (Müritz); STE (Steigerwald); TAT (Tatrzański); TRE (Tre Cime); LIE (Liesjärvi).
By site
The site-specific statistical evaluation of the deviation of δ13Cpollen values from the means reveals the factor species as the most important one (Table 8; S2 Dataset). Species are often hierarchically grouped by statistical similarity. The year of sampling influences the pollen variability at two sites (Forêt d’Anlier and Gorczański) and also maturity occurs twice (Tre Cime and Tatrański). Soil (Gorczański) and altitude (Tatrzański) are once listed as influencing factors.
Table 8. Environmental factors affecting stable pollen-isotope composition at each site.
| Site | Influencing factor on δ13Cpollen | Prob > F | Influencing factor on δ18Opollen | Prob > F |
|---|---|---|---|---|
| Parc Naturel Forêt d’Anlier | species {A.glutinosa & C.avellana–F.sylvatica & P.sylvestris & Q. robur & P.abies & C.betulus & A.pseudoplatanus & B. pendula} | <.0001* | species {A.glutinosa & C.avellana & F.sylvatica & B.pendula & P.abies–A.pseudoplatanus & C.betulus & Q.robur & P.sylvestris } | <.0001* |
| species {F.sylvatica & P.sylvestris—Q.robur & P.abies & C.betulus & A.pseudoplatanus & B.pendula} | <.0001* | species {A.glutinosa & C.avellana—F.sylvatica & B.pendula & P.abies } | 0.0189* | |
| species {Q.robur & P.abies & C.betulus—A.pseudoplatanus & B.pendula} | 0.0004* | species {F.sylvatica & B.pendula–P.abies} | 0.0002* | |
| year | 0.0060* | species {F.sylvatica—B.pendula} | <.0001* | |
| species {A.pseudoplatanus & C.betulus & Q.robur—P.sylvestris} | <.0001* | |||
| species {A.pseudoplatanus–C.betulus & Q.robur} | 0.0042* | |||
| year | 0.0004* | |||
| month | <.0001* | |||
| maturity | 0.0017* | |||
| water classification | 0.0013* | |||
| PN Tre Cime | species {F.sylvatica & P.sylvestris & A.pseudoplatanus—B.pendula & P.abies} | <.0001* | species {P.abies—A.pseudoplatanus & B.pendula & F.sylvatica & P.sylvestris} | <.0001* |
| species {B.pendula- P.abies} | 0.0010* | year | <.0001* | |
| maturity | 0.0085* | |||
| Liesjärvi k. | species | <.0001* | species | 0.0028* |
| Gorczański Park Narodowy | species {F.sylvatica & C.avellana & A.glutinosa & P.sylvestris—P.abies & A.pseudoplatanus} | <.0001* | species {A.pseudoplatanus & A.glutinosa & C.avellana & F.Sylvatica—P.abies & P.sylvestris} | <.0001* |
| year | 0.0096* | species {P.abies—P.sylvestris} | <.0001* | |
| soil | <0.0006* | altitude | 0.0204* | |
| month | 0.0021* | |||
| Müritz NP | species {A.glutinosa—P.sylvestris & C.avellana & B.pendula} | <.0001* | species {A.glutinosa & C.avellana & B.pendula—P.sylvestris} | <.0001* |
| species {A.glutinosa—C.avellana & B.pendula} | <.0001* | |||
| Steigerwald National Park | species {C.avellana & A.glutinosa & P.sylvestris & P.abies & C.betulus & F.sylvatica–A.pseudoplatanus & Q.robur & B.pendula} | <.0001* | species {A.glutinosa & F.sylvatica & C.avellana & P.abies & A.pseudoplatanus & B.pendula—C.betulus & Q.robur & P.sylvestris} | 0.0017* |
| species {A.glutinosa & F.sylvatica & C.avellana & P.abies–A.pseudoplatanus & B.pendula} | <.0001* | |||
| year | <.0001* | |||
| altitude | 0.0028* | |||
| Tatrzański PN | altitude | 0.0026* | year | 0.0127* |
| maturity | 0.0196* |
Relevant environmental factors on δ13Cpollen values and δ18Opollen for each site. Prob > F gives the probability value after Levene´s test [46]. Hierarchical clusters follow statistical similarity of the δ13Cpollen and δ18Opollen values within the factor species. The significance level lies at 0.05.
δ18Opollen values of broad-leaved and coniferous tree species
Flowering period January to March: Alnus glutinosa and Corylus avellana
Mean δ18Opollen values of A. glutinosa range from 18.1‰ to 22.9‰ (4.8‰; Table 3). Values of each year are normally distributed (Fig 4). However, their medians are statistically distinct for site Gorczański at p(sm) = 0.0002. Mean C. avellana δ18Opollen values range from 18.5‰ to 23.9‰ (5.4‰, Table 3). The pollen-isotope values are normally distributed but years 2015 and 2016 yield statistically distinct medians for Forêt d’Anlier.
Flowering period April to May: Acer pseudoplatanus, Betula pendula, Carpinus betulus, Fagus sylvatica and Quercus robur
Mean δ18Opollen values of A. pseudoplatanus range between 18.0‰ and 25.7‰ (7.7‰; Table 3, Fig 5). Values of each year are normally distributed except for site Tre Cime (2015, at p = 0.04). The statistical test reveals similar medians between years. Mean B. pendula δ18Opollen values range from 23.5‰ to 24.8‰ (1.3‰) and are normally distributed with one exception (Forêt d’Anlier 2015, p = 0.004; Fig 5). The δ18Opollen values of 2015 and 2016 yield similar medians (p(sm) = 0.75). Mean δ18Opollen values of C. betulus range from 26.1‰ to 26.3‰ (0.2‰; Table 3) and are also normally distributed (Fig 5). The comparatively low mean δ18Opollen values of F. sylvatica range from 18.6‰ to 24.1‰ (5.5‰; Table 3) and the values are normally distributed with similar medians for both years from Gorczański (Fig 6). Mean Q. robur δ18Opollen values range between 26.2‰ and 27.4‰ (1.2‰; Table 3) and are normally distributed but statistically distinct between both years at p(sm) = 0.006 (Fig 6).
Flowering period May to June: Pinus sylvestris and Picea abies
Mean δ18Opollen values of P. sylvestris range from 23.6‰ to 29.2‰ (5.6‰; Table 3) and the values are normally distributed except for the samples at Tre Cime collected in 2015 (p = 0.03). All comparisons between the two vegetation periods are statistically distinct. Mean P. abies δ18Opollen values range between 21.3‰ and 25.2‰ (3.9‰; Table 3). The values are normally distributed within each sampling (Fig 7) but comparisons between 2015 and 2016 are statistically distinct for all sites.
Intra-tree variability, intra-annual variability and variability with elevation of δ18Opollen
Intra-tree variability of δ18Opollen
68% of the δ18Opollen values of samples from A. pseudoplatanus, A. glutinosa and C. avellana taken at the lower branches are more negative in comparison to the values of the higher branches from the same individual (Fig 3, Table 4). The δ18Opollen values were often lower at southern and western positions, whereas values from the northern position seem to be generally higher for all species (Table 4). Twenty samples taken at different positions within the canopy of a P. abies tree show higher δ18Opollen at eastern and western positions, and lower values at the southern exposition (Table 5). Similar to the broad-leaved species, δ18Opollen values from lower branches of P. abies tend to be lower than those from higher branches (Table 5). The analysis of individual inflorescences demonstrates an intra-branch variability ranging between 0‰ and 1.4‰ (Table 6). The average deviation for inflorescences from the same branch is 0.5‰ for δ18Opollen.
δ18Opollen values at different stages of pollen maturation
The δ18Opollen values of B. pendula are normally distributed (Fig 8), but the medians are statistically distinct. The mean δ18Opollen values increase by 4‰ within 11 weeks (from 20.9‰ on 10 March to 24.9‰ on 5 May). δ18Opollen values of P. sylvestris are also normally distributed for each sampling (Fig 8). However, they increase by 1.2‰ within 12 days: δ18Opollen were at 25.7‰ on 20 May and at 26.9‰ on 1 June and the medians are statistically distinct.
Isotope variability of δ18Opollen with elevation
δ18Opollen values of P. abies at Tatrzański Park Narodowy (Fig 9) are scattered throughout the elevation transect and do not correlate with elevation (R2 = 0.14). The values range from 21.0‰ (1074 m a.s.l.) to 23.9‰ (1177 m a.s.l.).
Stepwise regression analysis of δ18Opollen
By species
δ18Opollen values were found to be affected by variable factors. The most important factor was site, which impacts the δ18Opollen variability in six species (Table 7). The year of sampling influences the δ18Opollen variability in four species, whereas the maturity of the pollen determines the δ18Opolle variability in three species and the factor month of sampling occurs twice. The factors proximity to water and water classification are rarely influential and, if significant, they were species-specific.
By site
δ18Opollen value variability within sites is mostly determined by the factor species (Table 8). The year of sampling is important in four cases and the month of sampling and altitude occur twice in the analytical outcome. The factors maturity of the pollen and water classification are each listed once.
Discussion
δ13Cpollen values of broad-leaved species flowering January to March
Alnus glutinosa and Corylus avellana
Like other winter-deciduous tree species, A. glutinosa and C. avellana (both family Betulaceae) have a similar timing of leaf unfolding and senescence, i.e. a similar duration of the photosynthetically active period in one year [47–52]. Nonetheless, their δ13Cpollen values are generally low compared to tree species flowering April to June (A. glutinosa: -28.2‰, C. avellana: -27.4‰; Table 3). Catkins of both species occur before the leaf buds emerge (Fig 2), thus the pollen can only be polymerized from stored fatty and amino acids, phenols and other precursors of sporopollenin predominately accumulated during previous vegetation periods. Alnus glutinosa and C. avellana pollen are present and fully developed in shape before winter dormancy, but the size of maturing pollen grains increases in early spring until a few days before pollen shedding [53]. This indicates that some biopolymers are still added to the pollen grains and possibly exchanged right before pollination.
Ambient air temperatures shortly before the onset of flowering affect the catkin development of both species and the onset date for trees flowering in early spring is highly variable, even between consecutive years [54]. However, the responsiveness of the plant to favorable weather conditions does not seem to have any marked effect on its δ13Cpollen values. The measurements indicate similar medians for consecutive years for A. glutinosa (p(sm) = 0.26 and 0.17; Fig 4), although flowering started roughly two weeks earlier at all sites sampled in 2016 (personal observation). Corylus avellana δ13Cpollen values deviate more strongly between the two years (p(sm) = 0.07 and 0.004; Fig 4). But even though both species react differently over two vegetation periods, they show similar patterns between particular sites (Fig 4): The signals of the western sampling location of Forêt d’Anlier are similar to the signals of the eastern sampling location of Gorczański (Fig 1).
Especially noticeable are the low values of δ13Cpollen for A. glutinosa from Müritz (2016) compared to those of other sites. The mean δ13Cpollen value is -31.0‰ and thus 2.8‰ lower than the average A. glutinosa pollen-isotope value of -28.2‰ from all sampling sites. However, all values lie within the range of a normal distribution (p = 0.81; Fig 4), so we can exclude measurement errors or tree-individual outliers to have caused the observed low mean δ13Cpollen value. Additionally, C. avellana from the same site and year (Müritz 2016) does not show exceptionally low values in comparison to data of that species measured at other sites. At the moment, we do not have a valid explanation for the unusual deviation of δ13Cpollen values of A. glutinosa sampled at Müritz (2016).
δ13Cpollen values of broad-leaved species flowering April to May
Acer pseudoplatanus
Mean δ13Cpollen values of A. pseudoplatanus (Sapindaceae; -25.1‰) are intermediate compared to other broad-leaved species in this study. Depending on location and year of sampling, the δ13Cpollen values of A. pseudoplatanus are closest to Q. robur (-25.7‰, Forêt d’Anlier 2016) and B. pendula (-24.8‰, Tre Cime 2016), but they show no consistent offset or similarity to any other species examined. In general, Acer ssp. are not dependent on prevailing spring temperatures to start seasonal development but instead require a specific amount of daylight [55]. Their leaves emerge early in the season and immediately exhibit a high rate of photosynthesis [56]. However, Acer ssp. are easily affected by short-term weather events and under unfavourable conditions, their carbon fixation during photosynthesis is rather ineffective, up to 50% less compared to the genus Quercus under the same conditions [57]. Additionally, A. pseudoplatanus is very sensitive to cold air inversions in spring and autumn [58]. Inter-annual variations in leaf senescence are highly correlated with precipitation: in dry years, high respiration rates cause early senescence and even premature leaf fall [59]. Therefore, photosynthetically active periods of Acer ssp. are highly variable even between consecutive years. Hence, their pollen-isotope values may be challenging to interpret.
Fagus sylvatica and Quercus robur
δ13Cpollen values of Q. robur show a mean of -25.1‰, whereas the mean value of F. sylvatica is 2‰ lower (-27.1‰). Deviations between the species of the family Fagaceae can be explained by plant physiology and individual phenology. Both were examined at two locations in 2016: Forêt d’Anlier and Steigerwald (Fig 6 and Table 3). Catkins of the genera Quercus and Fagus emerge during the same period in late April or early May, simultaneously with their first leaves and twigs. This happens roughly two weeks before anthesis is complete and the pollen starts to shed [60]. They both carry winter-dormant leaf and flower buds [61], but the spring ontogeny is genus-specific [50].
Quercus robur shows a high sensitivity to favourable spring temperatures regarding bud burst, whereas F. sylvatica needs twelve hours of daylight to start developing [58]. Trees of the genus Quercus are slow in leaf unfolding and it takes about two months until full performance of carbon fixation in the leaves [62]. Thus, the length of the vegetation period in which carbon can be fixated and stored out of a positive net productivity from photosynthesis is shorter for Quercus in comparison to Fagus. In general, warmer spring temperatures tend to increase carbon uptake, whereas warmer summer and autumn temperatures decrease the uptake due to larger respiration rates [63]. The impact of prevailing temperature on pollen-isotope values during different stages of a vegetation period has yet to be examined in detail.
Betula pendula and Carpinus betulus
Mean δ13Cpollen values of B. pendula (-24.3‰) are on average 1.3‰ less negative than the mean values of C. betulus (-25.6‰), even though they belong to the same family (Betulaceae), flower during the same period from mid-April to mid-May and prefer similar habitats (Figs 2 and 5; [60]). The total phase of pollen development spans early September to early April, and the size of B. pendula pollen grains still increases two to three weeks before pollen release [53], which implies a continuous addition and exchange of biomolecules during pollen maturation. Stach et al. [64] reported a positive correlation of aerial pollen counts of B. pendula with temperature and rainfall of the year before pollination. Thus, plant physiological reactions to prevailing environmental conditions can be assumed for B. pendula. Carpinus betulus is known to be particularly sensitive to frost damage and tends to prolong catkin proliferation when temperatures in spring are low. That affects the positive net productivity of photosynthesis in early spring [65] and leads to a shift of pollen development and maturation, which may have an effect on the δ13Cpollen values.
Differences in pollen-isotopes within families and subfamilies
δ13Cpollen deviate much less within one plant family than between families [14]. However, our findings show that this result does not apply to European taxa of the Betulaceae family. Four tree taxa of this family have been examined in this study (A. glutinosa, C. avellana, B. pendula and C. betulus). Taxonomically and genetically, the family is divided into two subfamilies: Coryloideae (including Corylus and Carpinus) and Betuloideae (including Betula and Alnus). Pollen grains of the members within the subfamilies are almost similar in shape and size [66]. However, due to their different flowering periods from January to March (A. glutinosa and C. avellana) and April to May (B. pendula and C. betulus), they are isotopically very different. We found that isotopic differences within these subfamilies were higher than between-family differences (Table 9; differences within the Betuloideae δ13Cpollen 3.9‰ and within the Coryloideae δ13Cpollen 1.8‰). Analysing all Betulaceae pollen together would imply a loss of information in the isotope signal.
Table 9. Mean isotope values of the examined plant families in Europe.
| Plant Family | Subfamily | Species | δ13Cpollen | δ18Opollen |
|---|---|---|---|---|
| (‰) | (‰) | |||
| Betulaceae | -26.4 ± 2.3 | 22.8 ± 2.7 | ||
| Betuloideae | A. glutinosa | -28.2 ± 1.9 | 21.1 ± 2.1 | |
| B. pendula | -24.3 ± 1.6 | 24.1 ± 1.6 | ||
| Coryloideae | C. avellana | -27.4 ± 1.7 | 21.7 ± 2.2 | |
| C. betulus | -25.6 ± 1.7 | 24.5 ± 1.0 | ||
| Fagaceae | -26.2 ± 1.6 | 23.5 ± 3.2 | ||
| Pinaceae | -26.0 ± 1.5 | 24.5 ± 2.3 | ||
| Sapindaceae | -25.1 ± 1.5 | 22.4 ± 2.5 |
Mean δ13Cpollen and δ18Opollen pollen-isotope values and standard deviations of the plant families and of the species within the two subfamilies of the Betulaceae. Mean values for each species include all sites and samplings of 2015 and 2016.
δ13Cpollen values of coniferous trees flowering May to June
Picea abies and Pinus sylvestris
Even though both species of the family Pinaceae prefer similar habitats [67], the mean carbon isotope offset between the two species is 1.5‰. The mean δ13Cpollen value of P. abies is -25.3‰ (2015: -25.2‰; 2016: -25.4‰), that of P. sylvestris lies at -26.8‰ (2015: -26.6‰; 2016: -26.9‰). Thus, analysing bulk coniferous pollen would reduce the environmental information incorporated in the plant material. However, comparison of the mean δ13Cpollen values of P. sylvestris and P. abies within the sampling sites reveals almost equal signals for both species between 2015 and 2016. Between-year mean δ13Cpollen values of P. abies deviate by 0.2‰ and that of P. sylvestris by 0.3‰. Schwarz [13] reported low δ13Cpollen ranges between four consecutive years for Pinus retinosa (0.53‰) and between three years for Pinus strobus (1.03‰). Carbon molecules (mostly soluble sugars from the previous vegetation periods) can be allocated in the individuals [68] and are remobilized directly after the resumption of growth in spring [13, 69]. Thus, the usage of stored carbon as basic modules for the pollen might compensate seasonal variations in the δ13Cpollen. Hence, between-year median δ13Cpollen values (p(sm); Fig 7) of most sites cannot be statistically distinguished for both species. The stored carbon differs in age between 0.7 years [70] and up to ten years [68].
Tree growth and photosynthetic activity of P. sylvestris starts approximately 40 days prior to budburst [71]. Pinus sylvestris δ13Cpollen values are found to correlate with temperature four to six weeks prior to pollen release [25]. Hence, it can be assumed that the plant uses newly fixated carbon in substantial portions to finish pollen maturation. There is no support for a correlation between North American Pinus-species δ13Cpollen and temperature [13]. Thus, this correlation might be species-specific to P. sylvestris [22].
Intra-site pollen-isotope variability of the δ13Cpollen values of P. abies range between 0.6‰ and 5.5‰ and that of P. sylvestris range between 2.5‰ and 4.0‰. Factors determining the intra-site isotope variability of C. atlantica and herbaceous species are microclimate, physiological differences between the individuals, water and nutrient availability and the number of trees in the direct vicinity [15, 18]. These factors may also account for the isotope variability of P. abies and P. sylvestris. Compared to P. abies, P. sylvestris has a broader physiological tolerance range to a variety of environmental conditions [67, 72]. Hence, with larger plasticity, physiological reactions turn out smaller and their pollen-isotope values fluctuate less within one location. In general, the pollen-isotope ranges of both species are broader in pollen sampled in 2015 than in samples of 2016.
Intra-tree variability of δ13Cpollen
δ13Cpollen values vary between 1.1‰ and 3.5‰ within an individual tree (Table 4). In most cases the values are higher at the eastern exposed side than at the southern and western sides. With less insolation, open stomata do not discriminate as much against the heavy aerial δ13C isotopes [73]. That phenomenon is also expressed by circumferential variations of 1–3‰ in leaf-tissue δ13C [73] and Leavitt [74] mentioned an average of 0.5–1.5‰ deviation from the mean within a circumferential tree ring cellulose analysis. In addition, the difference between mean δ13Cpollen values can be up to 3‰ within a height difference of 6 m (Table 4). For 64% of the individuals, δ13Cpollen values of the lower samples of a tree are lower compared to the upper ones (Table 4). These results agree with Schleser [73] who described an enrichment of 13C in leaf-tissue of 1–4‰ from bottom to top within one tree. The intra-tree variability between several inflorescences within one branch can be as high as 1.2‰ for δ13Cpollen (Table 6). However, average differences of pollen-isotope values in neighbouring inflorescences of P. sylvestris are as little as 0.3‰ (δ13Cpollen, Table 6).
Due to different carbon and oxygen sources the δ13Cpollen shows a higher intra-tree variability than δ18Opollen (Table 6). Carbon is fixated in the leaves as the product of photosynthesis. The rate of photosynthesis varies in relation to the position of the leaf on the tree, which leads to isotopic differences between the cardinal directions. Stable isotopes of tree ring cellulose and other plant material vary in one individual [75, 76] and it is generally suggested to pool several cores/samples in order to get the average representative isotope weight for the tree/year relation [2].
δ13Cpollen values at different stages of pollen maturation
Betula pendula
Mean δ13Cpollen values of B. pendula do not vary much over the flowering period and both samplings (11 weeks apart) cannot be statistically distinguished (-25.6‰ and -25.7‰; Fig 8, black bar). Pollen primordia are already built by the end of the previous year and the pollen maturation continues after a winter dormancy. In spring, the catkins emerge at the same time as the first leaf buds. Photosynthesis is not yet profitable this early in the year, thus the trees use stored and pooled carbon molecules to build new plant tissue in the beginning of the vegetation period [77]. This explains why the carbon composition of the pollen remained constant.
Pinus sylvestris
Mean δ13Cpollen values of P. sylvestris did not change within 12 days (-26.3‰ and -26.3‰; Fig 8). Comparatively little is known about timing, exact molecular processes and chemical compositions during pollen development [78, 79]. Plants may use only storage molecules to build plant tissue in spring and early summer [13, 77]. However, Loader and Hemming [22] reported a correlation between δ13Cpollen and temperature approximately six weeks prior to pollen release. Thus, at least in parts, P. sylvestris uses newly accumulated carbon molecules to build pollen. Perhaps we detected no change in the δ13Cpollen of P. sylvestris because the sporopollenin of the grain wall had already been synthesised during the previous weeks.
δ13Cpollen values of the elevation transect
δ13Cpollen values of P. abies increase by 3.4‰ (mean value at 1053 m a.s.l: -26.6‰; mean value at 1344 m a.s.l.: -23.2‰) within the elevation transect covering roughly 300 m at the Tatrzański Mountains. The values correlate with elevation (Adj R2 = 0.52; Fig 9). Although the increase of 3.4‰ is statistically distinct, it cannot be exclusively attributed to an altitudinal effect. In general, the discrimination of δ13C is linearly related to the ratio of intercellular to ambient CO2 partial pressures, and thus δ13C values increase with increasing altitude [80]. Hultine and Marshall [81] found a linear relationship between δ13C from Picea ssp. needle tissue and elevation where the values of the δ13Cneedle increase by approximately 0.5‰ per 300 m in altitude, whereas Warren et al. [82] reported an increase of 2.5‰ over 1000 m of δ13Cwood from 14 different coniferous species. The P. abies individuals chosen for the elevation transect were all located on the same slope facing north, thus ensuring comparable environmental conditions of e.g. wind, insolation and precipitation. However, the inflorescences from higher elevations were still immature, whereas they were already flowering at lower altitude. Flowering of P. abies starts with a delay of three days with every additional 100 m of elevation [83]. Bell et al. [15] found that local environmental constraints mask the effect of altitude. They concluded that the source of air masses and moisture input seem to determine the pollen-isotopic weight more than the actual altitude in the mountains. In addition, Treydte et al. [84] pointed out, that an existing temperature signal of P. abies in δ13Ctree ring was independent of elevation. Influencing factors on pollen-isotope values are very variable in mountainous areas. Which combination of factors caused the high 3.4‰ isotope offset over 300 m remains uninvestigated for now.
Stepwise regression analysis
Factors influencing δ13Cpollen of each species
δ13Cpollen values of all species are determined by several variable factors, and the outcome of the statistical analysis is sometimes inconclusive (Table 7). The factor site strongly influences C. avellana (Prob > F = <0.0001), where the maritime site (Forêt d’Anlier) contrasts the continental sites (Gorczański, Steigerwald, Müritz). Picea abies δ13Cpollen values seem to be grouped by altitude, where the mountainous sites of Tre Cime and Tatrzański are contrasting the lower-altitude sites (Forêt d’Anlier, Steigerwald, Gorczański and Liesjärvi). However, these groupings could also be caused by genetic predispositions affecting phenological responses [65, 85, 86]. Pinus sylvestris is known to have different haplotypes in Europe, one of which is restricted to the Southern Alps. Another haplotype of P. sylvestris spreads throughout central Europe [87] and can be found at all other sites of this study. Pinus sylvestris has distinct δ13Cpollen values at northern and southern European sites, which seems to be caused by a different reaction to local temperatures due to their genetic background [22].
Factors influencing δ13Cpollen at each site
δ13Cpollen values are mostly determined by the factor species which largely overprints other local non-climatic factors (e.g. the proximity of the tree to the next water body, type of soil, slope angle; Table 8). Species groupings of the factor species at each site are variable and do not seem to follow plant family affiliation or flowering period. In addition, the factor maturity is important for the mountainous sites Tre Cime and Tatrański.
General considerations for the usage of δ13Cpollen in palaeoclimate studies
Species-specific isotope values and patterns display susceptibility to different environmental factors due to their phenology and physiology (Table 3; Figs 4–7). Therefore, the pollen must be separated by species prior to isotope analysis. Without separation, the individual isotope signal within the pollen will be lost and the method cannot be applied in palaeoclimate studies [14, 38]. To date, the separation process is done manually which makes it very time-consuming to pick a sufficient amount of pollen for stable-isotope analysis [38, 88]. The amount of pollen needed to obtain representative average isotopic values is variable. Bell et al. [38] suggest to use a sample size of at least 30 μg carbon when using standard stable-isotope measuring techniques. New technology makes it feasible to decrease this amount. Nelson et al. [35] developed an isotope measuring technique using single pollen grains in order to estimate their photosynthetic pathway and Roij et al. [88] even measured as little as 0.042 μg carbon with a precision better than 0.5‰. However, we identified significantly different pollen-isotope values within one site and even within one tree and therefore suggest using a higher amount of carbon/pollen depending on the pollen type to obtain significant mean pollen-isotope values.
Furthermore, mostly sporopollenin of the pollen wall is preserved in fossil pollen [25]. The analysis of raw pollen material, as reported by this study, shows the general variability between species and sites, but patterns and ranges cannot directly be compared to fossil pollen-isotope values [14]. To accomplish that, extant pollen will have to be treated chemically prior to analysis. The method of sporopollenin-extraction with sulfuric acid has been tested for several species and a consistent offset between raw and the chemically treated pollen material was found [15, 25]. The same accounts for the species of this study: treatment with sulfuric acid resulted in a stable but species-specific offset when compared to raw pollen material (unpublished data).
δ18Opollen values of broad-leaved species flowering January to March
Alnus glutinosa and Corylus avellana
Mean δ18Opollen values of A. glutinosa (21.1‰) and C. avellana (21.5‰) of the family Betulaceae are exceptionally low (Table 3). The trees sampled for this study grew on saturated soils with ample water supply out of consistent precipitation throughout the winter months and snowmelt in February and March. Therefore, both species did not face water stress during times of sampling, which may explain the observed low δ18Opollen values. Alnus glutinosa and C. avellana generally prefer areas with aggravated or missing drainage close to a streamside [89]. In case of insufficient water supply, riparian trees are able to switch sources and especially A. glutinosa is drought resistant [47]. In general, there is no fractionation of oxygen isotopes during water uptake by the roots [90]. The xylem water remains unaltered during transport until it reaches evaporative tissue or is used to build plant tissue, such as flower primordia and pollen grains [91]. Since A. glutinosa and C. avellana flower before leaf proliferation, the water is not passing transpiring leaf tissue, where the isotopes would be altered by evaporative 18O enrichment. Mean δ18Opollen values of A. glutinosa and C. avellana from Gorczański are exceptionally low, but display broader ranges than the δ18Opollen values from Forêt d’Anlier (Fig 4). We expect variable interactions of non-climate environmental influences, e.g. elevation, slope and density of the surrounding vegetation, to have caused the wider distribution of δ18Opollen values at Gorczański.
δ18Opollen of broad-leaved species flowering April to May
Acer pseudoplatanus
Mean δ18Opollen values of A. pseudoplatanus (Sapindaceae) are highly variable between the sites (range of 18.0‰ to 25.7‰). Mean isotope values are comparable to B. pendula and F. sylvatica, depending on the location and year of sampling (Table 3). Noteworthy are the pollen-isotope values at Gorczański, where A. pseudoplatanus exhibits the lowest δ18Opollen values of all samplings in this study (2015: 18.0‰, 2016: 18.5‰). Although A. pseudoplatanus is flowering in May after leaf proliferation, the values are even lower than δ18Opollen of A. glutinosa and C. avellana.
Fagus sylvatica and Quercus robur
δ18Opollen of Q. robur are on average 3‰ higher than the values of F. sylvatica at those sites where both species from the family Fagaceae occur (Forêt d’Anlier and Steigerwald). This likely results from the developmental delay in spring. Quercus robur is slower in leaf unfolding, water uptake and in establishing a positive net productivity out of photosynthesis [58, 62]. Oxygen isotope values of precipitation are enriched by evaporation before seeping into the soil, especially during summer months. An increased activity of Q. robur in summer and the uptake of water enriched in 18O might explain the differences in δ18Opollen between the two species. The δ18Opollen values of F. sylvatica from Gorczański are exceptionally low (2015: 18.6‰; 2016: 19.5‰). This was also recognized in the May-flowering species A. pseudoplatanus (Table 3). However, we do not have a valid explanation for this site-specific phenomenon at the moment.
Betula pendula and Carpinus betulus
Mean δ18Opollen values of species of the Betuloideae deviate by more than 3‰ where C. betulus (27.1‰) yields higher values than B. pendula (24.1‰). In general, the mean δ18Opollen values within the species are very similar between the investigated sites and do not deviate much from the overall mean (Fig 5, dotted line). In particular the 2016 oxygen isotope values of B. pendula are very similar (Fig 5, black bar), implying that the δ18Opollen values of these two species are less affected by local environmental events but rather by trans-regional trends. Isotope values of other species deviate more between sites per year (Figs 4, 6 and 7).
δ18Opollen values of coniferous trees flowering May to June
Picea abies and Pinus sylvestris
Mean δ18Opollen values of P. abies and P. sylvestris (both Pinaceae) are significantly different, even within the same site (p(sm); Fig 7). Schwarz [13] pointed out that oxygen pollen-isotopes are highly sensitive to environmental conditions. We expect the varying interaction of all local conditions (e.g. micro-climate at the sampling site and water availability) to have caused the different δ18Opollen values between the vegetation periods 2015 and 2016. However, all European sites compared, both species show similar pollen-isotope patterns. The year-to-year differences between mean δ18Opollen values of P. abies from all sites is 1.7‰ (2015: 24.0‰; 2016: 22.3‰), whereas the differences between P. sylvestris pollen-isotopes is 1.4‰ (2015: 26.8‰; 2016: 25.4‰). With an offset of only 0.3‰ between both, the species seem to react to regional environmental conditions in a similar fashion. Nevertheless, their species-specific isotope values are still very distinct. Picea abies (overall average: 23.2‰) and P. sylvestris (overall average: 26.1‰) yield an average offset of 2.9‰
The δ18Opollen values at Forêt d’Anlier are higher for 2016 compared to 2015, whereas the δ18Opollen values at all other locations in this study are higher for 2015 (Fig 7). Both species display the same patterns, therefore weather conditions, which were not investigated further in this study, are expected to have caused the δ18Opollen inversions. The North Atlantic Oscillation (NAO) is known to influence phenology and temporal variability of seasonal tree development in Europe [92]. The site of Forêt d’Anlier lies closest to the Atlantic Ocean and is therefore more strongly influenced by the NAO than more inland-located sites.
In general, δ18O values of precipitation become more negative with increasing altitude and latitude. The δ18Ocellulose of coniferous trees shows a clear latitudinal pattern at a transect in North America covering the area between 65° N and 33° N [93]. Our study covers an area from 60° N to 46° N, but the pollen-isotopes do not show any pattern resembling this latitudinal effect. The northernmost site (Liesjärvi) yields low δ18Opollen values for P. sylvestris (23.6‰), but average values of δ18Opollen for P. abies (22.7‰). The southernmost site (Tre Cime, 1067m a.s.l.) should yield the highest δ18Opollen values. However, compared to other central European sites the δ18Opollen values at Tre Cime are overprinted by an additional altitudinal effect leading to comparatively low δ18Opollen values for both species (P. abies: 22.6‰, P. sylvestris: 24.8‰; Fig 7). All sites of this study are influenced by a variety of location-dependent factors, such as longitude, proximity to the ocean and altitude that overprint the latitudinal effect.
Intra-tree variability of δ18Opollen
δ18Opollen values differ between 0.6‰ and 2.1‰ between different cardinal directions in a single tree. Increasing tree height from 1 m to 7 m accounts for a statistically distinct increase in δ18Opollen of 1.3‰ (Table 4). In general, lower samples yield lower isotope values than upper samples. In addition, the southern and western samples yield lower isotope values than the northern samples (Table 4). The δ18Opollen intra-branch variability can be as high as 1.4‰ (Table 6), but the average difference between neighbouring inflorescences is 0.5‰, which can be considered to lie within a natural tolerance range.
δ18Opollen values at different stages of pollen maturation
Betula pendula
δ18Opollen values (20.9‰ and 24.9‰; Fig 8) are dependent on floral development. Oxygen molecules within the pollen tissue are exchanged until pollination. Rowley et al. [94] reported pollen alteration on the exact day of pollen shedding, where last cells from the pollen developmental tissue are removed and a nutritional transfer takes place. The coating of the outer grain wall contains mostly lipids and proteins and the inside holds cell organelles which develop only late in the pollen maturation process. Therefore, we assume that the variability of the δ18Opollen values is caused by alteration and exchange of volatile components of the outer and inner pollen grains. Another reason for the highly variable δ18Opollen of both samplings might be oxidation of the grain wall. The catkins of B. pendula on 10 March were very young and tightly closed. The ones sampled on 5 May were mostly open, dry and some catkins already exceeded the time of pollination. Therefore, pollen in open catkins may have been oxidized subsequent to air exposure changing the δ18Opollen values.
Pinus sylvestris
Mean δ18Opollen noticeably changes (25.7‰ and 26.9‰; Fig 8, black arrow) over a time period of 12 days. On 20 May the P. sylvestris inflorescences were still closed, whereas on 1 June the flowers were open and pollinating. Thus, as with B. pendula, the difference of δ18Opollen might result from changes of attached nutrients and volatile grain wall components. In addition, the immature sacculae of coniferous pollen contain a liquid that is only reabsorbed a few days before pollination [94]. The isotope composition of that liquid remains unknown.
δ18Opollen values of the elevation transect
δ18Opollen values do not correlate with altitude (Adj. R2 = 0.14; Fig 9). The mean δ18Opollen value increases by 1.2‰ over 300 m elevation. However, considering the different maturity of the inflorescences at the time of sampling, the isotopic weight of immature pollen grains on the mountaintop was most likely affected by further maturation.
Stepwise regression analysis
Factors influencing δ18Opollen of each species
δ18Opollen values are especially dependent on the geographic location, hence the factor site (a combination of latitude, longitude and altitude) particularly important for all species. The broad-leaved trees A. glutinosa and C. avellana as well as F. sylvatica mostly differ between the western sites (Forêt d’Anlier, Steigerwald and Müritz) in relation to the eastern site of Gorczański. The sampling sites for the coniferous pollen are separated in a similar way compared to the broad-leaved species, apart from Gorczański, which is also grouped with the high-latitude site of Liesjärvi and the mountainous site of Tre Cime (Table 7). These groupings indicate differences between maritime and continental (and high-altitude) sampling sites. The factor maturity makes a noticeable difference for A. glutinosa, F. sylvatica and P. sylvestris (Table 7), because volatile components and cell organelles increase in size and volume until the end of the pollen maturation process.
Factors influencing δ18Opollen at each site
δ18Opollen values are also often determined by species. However, the statistical groupings are even more variable than those of δ13Cpollen. At most of the sites where pollen of the same species were sampled in two consecutive years (Forêt d’Anlier, Tre Cime, Steigerwald and Tatrańzki), the factor year significantly affects the pollen-isotope values, reflecting comparative results of Figs 4 to 7 (Table 8).
Conclusions
Most of the δ13Cpollen and δ18Opollen are normally distributed and all examined species yield specific pollen-isotope patterns, even if the isotope ranges of the species partly overlap.
δ13Cpollen and δ18Opollen ranges show gradients between maritime and continental study sites (W–E transect) as well as between those which differ in day length (N–S transect).
Mean species-specific pollen-isotope values vary inter-annually, on average 1.0‰ for δ13Cpollen and 1.6‰ for δ18Opollen. Due to storage of carbon molecules for building new plant tissue, the variation within δ13Cpollen is lower than the variation within δ18Opollen.
Broad-leaved tree species flowering before leaf proliferation (January to March; Alnus glutinosa and Corylus avellana) yield significantly lower δ13Cpollen and δ18Opollen values than broad-leaved species flowering later in spring (April to May).
The coniferous species Pinus sylvestris and Picea abies show similar reactions to local environmental conditions, but their specific δ13Cpollen and δ18Opollen values are significantly different.
Intra-annual pollen-isotope analysis reveals that δ13Cpollen values do not significantly change during the last stages of the pollen maturation process, whereas δ18Opollen can be altered during that time.
δ13Cpollen and δ18Opollen differ between sampling positions on trees. Samples from lower positions yield lower isotopic weights than samples from upper positions. Isotope values of single inflorescences can vary within branches up to 1.2‰ for δ13Cpollen and 1.4‰ for δ18Opollen. There is also a circumferential variability of pollen-isotopes at different cardinal directions of up to 3.5‰ for δ13Cpollen and 2.1‰ for δ18Opollen. Because of that variability, an appropriate amount of pollen is needed for stable isotope analysis to enhance the precision of the measured mean δ13Cpollen and δ18Opollen values.
δ13Cpollen values increase with elevation by 3.4‰ over 300 m. Even though the two variates are significantly correlated, the difference is too high to be explained by altitude alone. Thus, additional unknown factors must have influenced the isotopic trend. δ18Opollen values do not change linearly with elevation.
Stepwise regression analysis reveals that the most important factor determining pollen-isotope values of any species in this study is their geographic location (factor site). In addition, the statistical analysis shows that the pollen-isotopes within one site are mostly determined by the factor species.
Stable carbon and oxygen isotope analysis of fossil pollen can improve the precision of palaeoenvironmental investigations. The applicability of different pollen types in palaeoclimate research relies on the specific plant physiological traits as well as on the pollen abundance in fossil archives. However, due to species-specific pollen-isotope ranges and patterns, fossil pollen should be separated by species as thoroughly as possible. After separation, varying vegetation periods and ecological preferences of the host-plant will allow climate reconstructions on an intra-seasonal scale.
Supporting information
Eight variables influencing the stable isotope composition in pollen (δ13C, δ18O) for each species were explored by means of stepwise regression analysis. They include the categorical variables year (of sampling), month (of sampling), maturity (of the pollen at the time of sampling), slope, water (proximity to water body), water classification (type of water body), soil and site.
(DOCX)
Nine variables influencing the stable isotope composition in pollen (δ13C, δ18O) at each site were explored by means of stepwise regression analysis. They include the continuous variable altitude and the categorical variables year (of sampling), month (of sampling), maturity (of the pollen at the time of sampling), slope, water (proximity to water body), water classification (type of water body), soil and species.
(DOCX)
Acknowledgments
We are thankful to the students who helped during fieldwork and sample preparation in the laboratory: Julian Hennig, Camilla Brunello, Franziska Pritzke, Alena Zippel, Hendrik Schultz and Vanessa Skiba. Also, we thank Maike Glos (Freie Universität Berlin) for discussion, support and consultations concerning laboratory methods. We also acknowledge Raffella Dibona and all colleagues from the Centro Studi Ambiente Alpino in San Vito Di Cadore for accommodation during fieldwork in Italy and discussions about the biogeography in the adjoining Dolomite Mountains. Additionally, we are very grateful to Vincent Duponcheel (Forêt d’Anlier), Nils Altvater, Ralf Pauli (both Müritz NP) and Pawel Czarnota (Gorczański NP) for regional information about the national parks, local phenology and seasonality as well as pollinator behaviour. We also thank two anonymous reviewers for many positive comments and suggestions to improve an earlier version of the manuscript.
Data Availability
The pollen-isotope dataset is publicly availiable at PANGEA Database under https://doi.org/10.1594/PANGAEA.910977.
Funding Statement
This work was supported by the Deutsche Forschungsgesellschaft (DFG) [grant number RI 809/33-1]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. Stable isotopes in plant ecology. Annu. Rev. Ecol. Evol. S. 2002; 33: 507–559. [Google Scholar]
- 2.McCarroll D, Loader NJ. Stable isotopes in tree rings. Quat. Sci. Rev. 2004; 23: 771–801. [Google Scholar]
- 3.Helle G, Schleser GH. Beyond CO2-fixation by Rubisco–an interpretation of 13C/12C variations in tree rings from novel intra-seasonal studies on broad-leaf trees. Plant Cell Environ. 2004; 27: 367–380. [Google Scholar]
- 4.Jones PD, Briffa KR, Osborn TJ, Lough JM, Van Ommen TD, Vinther BM, et al. High-resolution palaeoclimatology of the last millennium: a review of current status and future prospects. Holoc. 2009; 19: 3–49. [Google Scholar]
- 5.Gessler A, Ferrio JP, Hommel R, Treydte K, Werner RA, Monson RK. Stable isotopes in tree rings: towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol. 2014; 348: 796–818. 10.1093/treephys/tpu040 [DOI] [PubMed] [Google Scholar]
- 6.van der Sleen P, Zuidema PA, Pons TL. Stable isotopes in tropical tree rings: theory, methods and applications. Funct. Ecol. 2017; 31: 1674–1689. [Google Scholar]
- 7.Lockheart MJ, Van Bergen PF, Evershed R. Variations in the stable carbon isotope compositions of individual lipids from the leave of modern angiosperms: implications for the study of higher land plant-derived sedimentary organic matter. Org. Geochem. 1997; 26: 137–153. [Google Scholar]
- 8.Graham HV, Patzkowsky ME, Wing SL, Parker GG, Fogel ML, et al. Isotopic characteristics of canopies in simulated leaf assemblages. Geochim. Cosmochim. Acta 2014; 144: 82–95. [Google Scholar]
- 9.O’Leary MH. Carbon isotopes in photosynthesis. Bioscience 1988; 38: 328–336. [Google Scholar]
- 10.Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu. Rev. of Plant Physiol. Plant Molecul. Biol. 1989; 40: 503–537. [Google Scholar]
- 11.Ehleringer JR, Dawson TE. Water uptake by plants: perspectives from stable isotope composition. Plant Cell Environ. 1992; 15: 1073–1082. [Google Scholar]
- 12.Flanagan LB, Farquhar GD. Variation in the carbon and oxygen isotope composition of plant biomass and its relationship to water-use efficiency at the leaf-and ecosystem-scales in a northern Great Plains grassland. Plant Cell Environ. 2014; 37: 425–438. 10.1111/pce.12165 [DOI] [PubMed] [Google Scholar]
- 13.Schwarz DM. A Stable Isotope Investigation Of Pollen From Pinery Provincial Park, Southwestern Ontario, Canada. The University of Western Ontario. Electronic Thesis and Dissertation Repository. 2016; 4254.
- 14.Jahren AH. The carbon stable isotope composition of pollen. Rev. Palaeobot. Palynol. 2004; 132: 291–313. [Google Scholar]
- 15.Bell BA, Fletcher WJ, Ryan P, Grant H, Ilmen R. Stable carbon isotope analysis of Cedrus atlantica pollen as an indicator of moisture availability. Rev. Palaeobot. Palynol. 2017; 244: 128–139. [Google Scholar]
- 16.Amundson R, Evett RR, Jahren AH, Bartolome J. Stable carbon isotope composition of Poaceae pollen and its potential in paleovegetational reconstructions. Rev. Palaeobot. Palynol. 1997; 99: 17–24. [Google Scholar]
- 17.Nelson DM. Carbon isotopic composition of Ambrosia and Artemisia pollen: assessment of a C3-plant paleophysiological indicator. New Phytol. 2012; 195: 787–793. 10.1111/j.1469-8137.2012.04219.x [DOI] [PubMed] [Google Scholar]
- 18.King DC, Schubert BA, Jahren AH. Practical considerations for the use of pollen δ13C value as a paleoclimate indicator. Rapid Commun. Mass Spectrom. 2012; 26: 2165–2172. 10.1002/rcm.6333 [DOI] [PubMed] [Google Scholar]
- 19.Nelson DM, Hu FS, Michener RH. Stable-carbon isotope composition of Poaceae pollen: an assessment for reconstructing C3 and C4 grass abundance. Holoc. 2006; 16: 819–825. [Google Scholar]
- 20.Descolas-Gros C, Schölzel C. Stable isotope ratios of carbon and nitrogen in pollen grains in order to characterize plant functional groups and photosynthetic pathway types. New Phytol. 2007; 176: 390–401. 10.1111/j.1469-8137.2007.02176.x [DOI] [PubMed] [Google Scholar]
- 21.Urban MA, Nelson DM, Kelly R, Ibrahim T, Dietze M, Pearson A, et al. A hierarchical Bayesian approach to the classification of C3 and C4 grass pollen based on SPIRAL δ13C data. Geochim. Cosmochim. Acta. 2013; 121: 168–176. [Google Scholar]
- 22.Loader NJ, Hemming DL. Spatial variation in pollen δ13C correlates with temperature and seasonal development timing. Holoc. 2001; 11: 587–592. [Google Scholar]
- 23.Chesson LA, Tipple BJ, Erkkila BR, Cerling TE, Ehleringer JR. B-HIVE: Beeswax hydrogen isotopes as validation of environment. Part I: Bulk honey and honeycomb stable isotope analysis. Food Chem. 2011; 125: 576–581. [Google Scholar]
- 24.Chesson LA, Tipple BJ, Erkkila BR, Ehleringer JR. Hydrogen and oxygen stable isotope analysis of pollen collected from honey. Grana 2013; 52: 305–315. [Google Scholar]
- 25.Loader NJ, Hemming DL. The stable isotope analysis of pollen as an indicator of terrestrial palaeoenvironmental change: a review of progress and recent developments. Quat. Sci. Rev. 2004; 23: 893–900. [Google Scholar]
- 26.Saurer M, Siegenthaler U. 13C/12C isotope ratios in trees are sensitive to relative humidity. Dendrochro. 1989; 7: 9–13. [Google Scholar]
- 27.Saurer M, Borella S, Leuenberger M. δ18O of tree rings of beech (Fagus silvatica) as a record of δ18O of the growing season precipitation. Tellus B. 1997; 49: 80–92. [Google Scholar]
- 28.Traverse A. Paleopalynology Topics in Geobiology. 2nd ed Dordrecht: Springer Netherlands; 2007. [Google Scholar]
- 29.Speer J. Fundamentals of Tree Ring Research. University of Arizona Press; 2010. [Google Scholar]
- 30.Birks HJB, Birks HH. Quaternary palaeoecology. London: E. Arnold; 1980. [Google Scholar]
- 31.Moore PD, Webb JA, Collinson ME. Pollen Analysis. Second ed Oxford, UK: Blackwell Scientific Publishing; 1991. [Google Scholar]
- 32.Bezrukova EV, Tarasov PE, Solovieva N, Krivonogov SK, Riedel F. Last glacial–interglacial vegetation and environmental dynamics in southern Siberia: Chronology, forcing and feedbacks. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010; 296: 185–198. [Google Scholar]
- 33.Demske D, Tarasov PE, Nakagawa T. Atlas of pollen, spores and further non-pollen palynomorphs recorded in the glacial-interglacial late Quaternary sediments of Lake Suigetsu, central Japan. Quat. Int. 2013; 290: 164–238. [Google Scholar]
- 34.Müller C. Bi-decadal climate reconstruction derived from a 1200-year long pollen record from the NE Qinghai-Tibet Plateau and its implications for discussion of the late Holocene environmental dynamics. Quat. Int. 2017; 444: 1–10. [Google Scholar]
- 35.Nelson DM, Hu FS, Scholes DR, Joshi N, Pearson A. Using SPIRAL (Single Pollen Isotope Ratio AnaLysis) to estimate C3- and C4-grass abundance in the paleorecord. Earth Planet Sci. Lett. 2008; 269: 11–16. [Google Scholar]
- 36.Kamenik C, Van der Knaap WO, Van Leeuwen JF, Goslar T. Pollen/climate calibration based on a near‐annual peat sequence from the Swiss Alps. J. Quat. Sci. 2009; 24: 529–546. [Google Scholar]
- 37.Urban MA, Nelson DM, Jiménez-Moreno G, Hu FS. Carbon isotope analyses reveal relatively high abundance of C4 grasses during early–middle Miocene in southwestern Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016; 443: 10–17. [Google Scholar]
- 38.Bell BA, Fletcher WJ, Cornelissen HL, Campbell JF, Ryan P, Grant H et al. Stable carbon isotope analysis on fossil Cedrus pollen shows summer aridification in Morocco during the last 5000 years. J. Quat. Sci. 2019; 34: 323–332. [Google Scholar]
- 39.Griener KW, Nelson DM, Warny S. Declining moisture availability on the Antarctic Peninsula during the Late Eocene. Palaeogeor. Palaeoclimatol. Palaeoecol. 2013; 383: 72–78. [Google Scholar]
- 40.Ahas R, Aasa A, Menzel A, Fedotova VG, Scheifinger H. Changes in European spring phenology. Int. J. Climatol. 2002; 22: 1727–1738. [Google Scholar]
- 41.Panagos P, Van Liedekerke M, Jones A, Montanarella L. European Soil Data Centre: Response to European policy support and public data requirements. Land Use Policy 2012; 29: 329–338. [Google Scholar]
- 42.Paul C, Skrzypek G, Forizs I. Normalization of Measured Stable Isotopic Compositions to Isotope Reference Scales–a Review. Rapid Commun. Mass Spectrom. 2007; 21: 3000–3014. [DOI] [PubMed] [Google Scholar]
- 43.R Core Team (2017) R: A Language and Environment for Statistical Computing. https://www.R-project.org/
- 44.RStudio Team. RStudio: Integrated Development for R. RStudio, Inc., Boston 2015; MA URL http://www.rstudio.com/.
- 45.Kampstra P. Beanplot: A Boxplot Alternative for Visual Comparison of Distributions. J. Stat. Softw. 2008; 28: 1–9. 10.18637/jss.v028.i07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schönwiese C. Klimaänderungen: Daten, Analysen, Prognosen. Springer-Verlag, 2013. [Google Scholar]
- 47.McVean DN. Alnus glutinosa (L.) Gaertn. J Ecol. 1953; 41: 447–466. [Google Scholar]
- 48.Kikuzawa K. Leaf phenology as an optimal strategy for carbon gain in plants. Can. J. Bot. 1995; 73: 158–163. [Google Scholar]
- 49.Kramer K. Phenology and growth of European trees in relation to climate change. University of Wageningen Press, Netherlands; 1996. [Google Scholar]
- 50.Vitasse Y, Porté AJ, Kremer A, Michalet R, Delzon S. Responses of canopy duration to temperature changes in four temperate tree species: relative contributions of spring and autumn leaf phenology. Oecol. 2009; 161: 187–198. [DOI] [PubMed] [Google Scholar]
- 51.Črepinšek Z, Štampar F, Kajfež-Bogataj L, Solar A. The response of Corylus avellana L. phenology to rising temperature in north-eastern Slovenia. Int. J. Biomet. 2012; 56: 681–694. [DOI] [PubMed] [Google Scholar]
- 52.Gallinat AS, Primack RB, Wagner DL. Autumn, the neglected season in climate change research. Trends Ecol. Evol. 2015; 30: 169–176. 10.1016/j.tree.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 53.Krizo M, Slobodnik B. Changes in the size of pollen grains and structure of the tapetal layer in Betula pendula (Roth) during the maturation of pollen. Bull. Polish Acad. Sci. Biol. Sci. 1997; 45: 2–4. [Google Scholar]
- 54.Myszkowska D, Jenner B, Puc M, Stach A, Nowak M, Malkiewicz M, et al. Spatial variations in the dynamics of the Alnus and Corylus pollen seasons in Poland. Aerobiol. 2010; 26: 209–221. [Google Scholar]
- 55.Vitasse Y, Delzon S, Dufrêne E, Pontailler JY, Louvet JM, Kremer A, et al. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric. For. Meteorol. 2009; 149: 735–744. [Google Scholar]
- 56.Sparks TH, Carey PD. The responses of species to climate over two centuries: an analysis of the Marsham phenological record, 1736–1947. J. Ecol. 1995; 83: 321–329. [Google Scholar]
- 57.Morecroft MD, Roberts JM. Photosynthesis and stomatal conductance of mature canopy oak (Quercus robur) and sycamore (Acer pseudoplatanus) trees throughout the growing season. Funct. Ecol. 1999; 13: 332–342. [Google Scholar]
- 58.Schuster C, Kirchner M, Jakobi G, Menzel A. Frequency of inversions affects senescence phenology of Acer pseudoplatanus and Fagus sylvatica. Int. J. Biometeorol. 2014; 58: 485–498. 10.1007/s00484-013-0709-0 [DOI] [PubMed] [Google Scholar]
- 59.Archetti M, Richardson AD, O’Keefe J, Delpierre N. Predicting climate change impacts on the amount and duration of autumn colors in a New England forest. PLoS One 2013; 8: e57373 10.1371/journal.pone.0057373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lejoly-Gabriel M, Leuschner RM. Comparison of air-borne pollen at Louvain-la-Neuve (Belgium) and Basel (Switzerland) during 1979 and 1980. Grana 1983; 22: 59–64. [Google Scholar]
- 61.Brett DW. The inflorescence of Fagus and Castanea, and the evolution of the cupules of the Fagaceae. New Phytol. 1964; 63: 96–118. [Google Scholar]
- 62.Morecroft MD, Stokes VJ, Morison JIL. Seasonal changes in the photosynthetic capacity of canopy oak (Quercus robur) leaves: the impact of slow development on annual carbon uptake. Int. J. Biometeorol. 2003; 47: 221–226. 10.1007/s00484-003-0173-3 [DOI] [PubMed] [Google Scholar]
- 63.Piao SL, Ciais P, Friedlingstein P, Peylin P, Reichstein M, Luyssaert S, et al. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 2008; 451: 49–52. 10.1038/nature06444 [DOI] [PubMed] [Google Scholar]
- 64.Stach A, Emberlin J, Smith M, Adams-Groom B, Myszkowska D. Factors that determine the severity of Betula spp. pollen seasons in Poland (Poznań and Krakow) and the United Kingdom (Worcester and London). Int. J. Biometeorol. 2008; 52: 311–321. 10.1007/s00484-007-0127-2 [DOI] [PubMed] [Google Scholar]
- 65.Chuine I, Belmonte J, Mignot A. A modelling analysis of the genetic variation of phenology between tree populations. J. Ecol. 2000; 88: 561–570. [Google Scholar]
- 66.Beug HJ. Leitfaden der Pollenbestimmung für Mitteleuropa und angrenzende Gebiete. Stuttgart: Gustav Fischer; 2004. [Google Scholar]
- 67.George E, Seith B, Schaeffer C, Marschner H. Responses of Picea, Pinus and Pseudotsuga roots to heterogeneous nutrient distribution in soil. Tree Physiol. 1997; 17: 39–45. 10.1093/treephys/17.1.39 [DOI] [PubMed] [Google Scholar]
- 68.Richardson AD, Carbone MS, Keenan TF, Czimczik CI, Hollinger DY, Murakami P, et al. Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol. 2013; 197: 850–861. 10.1111/nph.12042 [DOI] [PubMed] [Google Scholar]
- 69.Loescher WH, McCamant T, Keller JD. Carbohydrate reserves, translocation, and storage in woody plant roots. Hortic. Sci. 1990; 25: 274–281. [Google Scholar]
- 70.Gaudinski JB, Torn MS, Riley WJ, Swanston C, Trumbore SE, Joslin JD, et al. Use of stored carbon reserves in growth of temperate tree roots and leaf buds: analyses using radiocarbon measurements and modeling. Glob. Chang. Bio. 2009; 15: 992–1014. [Google Scholar]
- 71.Michelot A, Simard S, Rathgeber C, Dufrêne E, Damesin C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012; 32: 1033–1045. 10.1093/treephys/tps052 [DOI] [PubMed] [Google Scholar]
- 72.Ingestad T. Mineral nutrient requirements of Pinus silvestris and Picea abies seedlings. Physiol. Plant. 1979; 45: 373–380. [Google Scholar]
- 73.Schleser GH. 13C/12C in growth rings and leaves: carbon distribution in trees In: Fossil plants and spores: modern techniques. London: Geo. Soc; 1999; 306–9. [Google Scholar]
- 74.Leavitt SW. Tree-ring C–H–O isotope variability and sampling. Sci. Total Environ. 2010; 408: 5244–5253. 10.1016/j.scitotenv.2010.07.057 [DOI] [PubMed] [Google Scholar]
- 75.Leavitt SW, Long A. Sampling strategy for stable carbon isotope analysis of tree rings in pine. Nature 1984; 311: 145–147. [Google Scholar]
- 76.Leavitt SW, Long A. Stable-carbon isotope variability in tree foliage and wood. Ecol. Soc. Am. 1986; 67: 1002–1010. [Google Scholar]
- 77.Luomajoki A. Timing of microsporogenesis in trees with reference to climatic adaptation: a review. Acta For. Fenn. 1986; 196: Article id: 7642. [Google Scholar]
- 78.Datta R, Chamusco KC, Chourey PS. Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiol. 2002; 130: 1645–1656. 10.1104/pp.006908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blackmore S, Wortley AH, Skvarla JJ, Rowley JR. Pollen wall development in flowering plants. New Phytol. 2007; 174: 483–498. 10.1111/j.1469-8137.2007.02060.x [DOI] [PubMed] [Google Scholar]
- 80.Körner C, Farquhar GD, Wong SC. Carbon isotope discrimination by plants follows latitudinal and altitudinal trends. Oecol. 1991; 88: 30–40. [DOI] [PubMed] [Google Scholar]
- 81.Hultine KR, Marshall JD. Altitude trends in conifer leaf morphology and stable carbon isotope composition. Oecol. 2000; 123: 32–40. [DOI] [PubMed] [Google Scholar]
- 82.Warren CR, McGrath JF, Adams MA. Water availability and carbon isotope discrimination in conifers. Oecol. 2001; 127: 476–486. [DOI] [PubMed] [Google Scholar]
- 83.Rötzer T, Chmielewski FM. Phenological maps of Europe. Clim. Res. 2001; 18: 249–257. [Google Scholar]
- 84.Treydte K, Schleser GH, Schweingruber FH, Winiger M. The climatic significance of δ13C in subalpine spruces (Lötschental, Swiss Alps) a case study with respect to altitude, exposure and soil moisture. Tellus B. 2001; 53: 593–611. [Google Scholar]
- 85.Willis CG, Ruhfel B, Primack R B, Miller-Rushing AJ, Davis CC. Phylogenetic patterns of species loss in Thoreau’s woods are driven by climate change. PNAS 2008; 105: 17029–17033. 10.1073/pnas.0806446105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilczek AM, Burghardt LT, Cobb AR, Cooper MD, Welch SM, Schmitt J. Genetic and physiological bases for phenological responses to current and predicted climates. Philos. Trans. R. Soc. Lond. Biol. Sci. 2010; 365: 3129–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheddadi R, Vendramin GG, Litt T, François L, Kageyama M, Lorentz S, et al. Imprints of glacial refugia in the modern genetic diversity of Pinus sylvestris. Glob. Ecol. Biogeogr. 2006; 15: 271–282. [Google Scholar]
- 88.van Roij L, Sluijs A, Laks JJ, Reichart GJ. Stable carbon isotope analyses of nanogram quantities of particulate organic carbon (pollen) with laser ablation nano combustion gas chromatography/isotope ratio mass spectrometry. Rapid Commun. Mass Spectrom. 2017; 31: 47–58. 10.1002/rcm.7769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bennett KD, Birks HJB. Postglacial history of alder (Alnus glutinosa (L.) Gaertn.) in the British Isles. J. Quat. Sci. 1990; 5: 123–133. [Google Scholar]
- 90.Dongmann G, Nürnberg HW, Förstel H, Wagener K. On the enrichment of H 2 18 O in the leaves of transpiring plants. Radiat. Environ. Biophys. 1974; 11: 41–52. 10.1007/BF01323099 [DOI] [PubMed] [Google Scholar]
- 91.Ehleringer JR, Dawson TE. Water uptake by plants: perspectives from stable isotope composition. Plant Cell Environ. 1992; 15: 1073–1082. [Google Scholar]
- 92.Scheifinger H, Menzel A, Koch E, Peter C, Ahas R. Atmospheric mechanisms governing the spatial and temporal variability of phenological phases in central Europe. Int. J. Climatol.: J. R. Meteorl. Soc. 2002; 22: 1739–1755. [Google Scholar]
- 93.Burk RL, Stuiver M. Oxygen isotope ratios in trees reflect mean annual temperature and humidity. Science 1981; 211: 1417–1419. 10.1126/science.211.4489.1417 [DOI] [PubMed] [Google Scholar]
- 94.Rowley JR, Skvarla JJ, Walles B. Microsporogenesis in Pinus sylvestris L. VIII. Tapetal and late pollen grain development. Plant Sys. Evol. 2000; 225: 201–224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Eight variables influencing the stable isotope composition in pollen (δ13C, δ18O) for each species were explored by means of stepwise regression analysis. They include the categorical variables year (of sampling), month (of sampling), maturity (of the pollen at the time of sampling), slope, water (proximity to water body), water classification (type of water body), soil and site.
(DOCX)
Nine variables influencing the stable isotope composition in pollen (δ13C, δ18O) at each site were explored by means of stepwise regression analysis. They include the continuous variable altitude and the categorical variables year (of sampling), month (of sampling), maturity (of the pollen at the time of sampling), slope, water (proximity to water body), water classification (type of water body), soil and species.
(DOCX)
Data Availability Statement
The pollen-isotope dataset is publicly availiable at PANGEA Database under https://doi.org/10.1594/PANGAEA.910977.