Abstract
Congenital heart defects (CHDs) affect approximately 1% of newborns. Epidemiological studies have identified several genetically-mediated maternal phenotypes (e.g., pregestational diabetes, chronic hypertension) that are associated with the risk of CHDs in offspring. However, the role of the maternal genome in determining CHD risk has not been defined. We present findings from gene-level, genome-wide studies that link CHDs to maternal effect genes as well as to maternal genes related to hypertension and proteostasis. Maternal effect genes, which provide the mRNAs and proteins in the oocyte that guide early embryonic development before zygotic gene activation, have not previously been implicated in CHD risk. Our findings support a role for and suggest new pathways by which the maternal genome may contribute to the development of CHDs in offspring.
Introduction
Congenital heart defects (CHDs) are the most common group of birth defects, with a prevalence of approximately 1% in live births [1]. CHDs are also the leading cause of birth defect-related mortality [2] and account for the largest percentage of birth defect-associated hospitalizations and healthcare costs [3]. As for many birth defects, the risk of CHDs is associated with several genetically-mediated, maternal phenotypes, including folate status, obesity, pregestational diabetes, chronic hypertension, and preeclampsia [4, 5]. These associations suggest that the maternal genotype may contribute to the risk of birth defects in offspring, independent of the maternal alleles transmitted to the child. For example, maternal genes involved in folate transport and metabolism may influence the availability of folate to the embryo, which in turn influences the risk of folate-related birth defects.
While there has been some interest in assessing the relationship between birth defects and maternal genotypes (e.g., methylenetetrahydrofolate reductase or MTHFR genotypes) [6–10], studies of the maternal genotype have considered a relatively small number of maternal phenotypes and are limited by gaps in our understanding of the genetic contribution to these phenotypes. Further, studies focused on maternal phenotypes ignore maternal genes that might act through alternate mechanisms to influence the risk of birth defects. For example, studies in model systems indicate that mutations in maternal effect genes (MEGs), which provide the mRNAs and proteins in the oocyte that guide early embryonic development before activation of the embryonic genome, can result in birth defects in offspring [11–13]. While genome-wide association studies (GWAS) provide a comprehensive, agnostic approach for identifying disease associations, only a few GWAS have focused on the maternal genotype [14–17]. Consequently, there is much to be learned about the role of maternal genes in determining the risk of birth defects such as CHDs.
We have previously conducted a single nucleotide polymorphism (SNP)-based GWAS of maternal genetic effects for conotruncal heart defects (CTDs) [14], which affect the cardiac outflow tracts [18] and account for approximately one-third of all CHDs [19]. Although we identified several maternal SNPs with suggestive evidence of association (p ≤ 10−5) with CTDs, no association was genome-wide significant (p < 5 × 10−8). Compared to SNP-based GWAS, gene-based GWAS has the advantage of a less stringent threshold for statistical significance. Furthermore, gene-based analyses can include both common and rare variants [20] and, therefore, capture a greater proportion of the within gene variation than SNP-based analyses, which generally exclude variants with minor allele frequencies (MAFs) less than 5% [21]. Given these advantages, we have undertaken gene-based GWAS and meta-analyses using data from two large CTD datasets to identify maternal genes associated with the risk of CTDs in offspring.
Materials and methods
Study subjects
The Children’s Hospital of Philadelphia (CHOP)
Patients with CTDs and their parents were recruited through the Cardiac Center at CHOP (1992–2010), under a protocol approved by the Institutional Review Board for the Protection of Human Subjects at CHOP [14, 15]. Adult participants provided written consent for themselves and their participating minor children.
Patients with the following diagnoses were eligible to be a CTD case: tetralogy of Fallot, persistent truncus arteriosus, D-transposition of the great arteries, double outlet right ventricle, ventricular septal defects (conoventricular, posterior malalignment, and conoseptal hypoplasia types), aortic-pulmonary window, interrupted aortic arch, and isolated aortic arch anomalies. Medical records, imaging (e.g., echocardiography and cardiac magnetic resonance imaging), and operative reports were used to confirm cardiac diagnoses. Potential cases were tested for the 22q11.2 deletion syndrome using fluorescence in situ hybridization, multiplex ligation-dependent probe amplification, or both, and those with a deletion were excluded [22]. Potential cases were also excluded if they had a clinically diagnosed chromosome abnormality or single-gene mutation.
Pediatric Cardiac Genomics Consortium (PCGC)
Patients with CTDs and their parents were recruited as part of the PCGC Congenital Heart Defect GEnetic NEtwork Study (2010–2012) [23]. Recruitment took place at five main (including CHOP) and four satellite clinical sites. Informed consent was obtained under protocols approved by the Institutional Review Board for each study site. Adult participants provided written consent for themselves and their participating minor children.
Patients recruited through the PCGC included those with the same CTD diagnoses as listed above. Cardiac diagnoses were confirmed through review of medical records and electronic case reports, and potential CTD cases were excluded if they had a clinically diagnosed chromosomal or genetic disorder. Participants recruited at CHOP as part of the PCGC do not overlap with the CHOP participants described above.
Genetic methods
Blood samples were collected from cases, and blood or saliva samples were collected from parents of cases. When blood collection was scheduled in conjunction with a surgical procedure, the sample was collected before any blood transfusion. DNA extraction was performed using standard techniques. Genome-wide microarray genotyping was performed at the CHOP Center for Applied Genomics [14]. The CHOP samples were genotyped using the Illumina HumanOmni-2.5, Illumina HumanHap550 (v2 or v3), or 610 BeadChip platforms, and the PCGC samples were genotyped on the Illumina HumanOmni-1 or 2.5 platforms.
Imputation and Quality Control (QC) procedures
Standard QC procedures were performed for each dataset using Plink version 1.07 [24] and have been previously described [25]. Before imputation, the genotype data were checked for strand and coding errors. Case-parent trios were removed if more than 1% of genotyped SNPs had Mendelian errors. Suspected duplicate samples were identified using pairwise identity-by-descent estimation, and samples with pi-hat greater than 0.6 were removed. Samples with genotyping rates less than 95% were also removed. In addition, SNPs with MAF less than 1%, genotyping rates less than 90%, and all non-autosomal variants were excluded.
Due to differences in microarray genotyping platforms, the CHOP and PCGC case-parent trios data were imputed separately. After the pre-imputation exclusions, the CHOP data from different platforms (HumanOmni-2.5, HumanHap550K v2, 550K v3, and 610K) were combined, and the SNPs present across all platforms (N = 283,977 SNPs) were used for imputation. Similarly, the PCGC data from different platforms (HumanOmni-1 and HumanOmni-2.5) were combined, and the SNPs present on both platforms (N = 624,419 SNPs) were used for imputation.
For each dataset, haplotypes were pre-phased using SHAPEIT2 v2.727 [26], and imputation was performed using Impute2 v2.3.0 [27] with pre-phased haplotype data from the 1000 Genomes Project (version: Phase-I integrated v3 variants set) as the reference population. A genotype was imputed, only if the posterior probability value exceeded 0.9, the default calling threshold for Impute2. After imputation, we excluded SNPs with poor imputation quality (Impute2 information metric score less than 0.8), or genotyping rates less than 90%. Samples with genotyping rates less than 95% were removed. Because we were interested in assessing both common and rare SNPs, the post-imputation QC procedures did not include restrictions based on MAFs.
Statistical analysis
Genome-wide gene-based analyses
Maternal genetic effects were evaluated using a case-control approach in which mothers and fathers from the CTD trios were considered as cases and controls, respectively. Genes were defined by their transcription start-stop positions, including untranslated regions (hg19 reference assembly) plus 1kb upstream and downstream. Analyses were conducted separately for the CHOP and PCGC datasets, using the sequence kernel association test for the combined effect of common and rare variants (SKAT-C) [28]. In this approach, separate scores were calculated for the common (MAF ≥ 5%) and rare (MAF < 5%) variants in each gene, and p-values were based on the weighted sum of these scores. We used the SKAT-C default parameters for weighting common and rare SNPs and evaluated all autosomal genes with at least one common and one rare variant in our data.
To control for population stratification bias, only the parents of non-Hispanic Caucasian CTD cases (based on self- or parental-report) were included in the analyses. As race/ethnicity was based on the report rather than genetic data, we adjusted for the first genotypic principal component. Genotypic principal components analyses were conducted using Golden Helix SVS version 8.1 (Golden Helix, Inc., Bozeman, Montana, USA; www.goldenhelix.com), using the default parameter settings (additive genetic model, MAF-based allele classification, and each marker data normalized by its theoretical standard deviation under Hardy Weinberg Equilibrium). A meta-analysis of the gene-based results from the CHOP and PCGC datasets was performed using Fisher’s combination of probability method [29]. For each analysis, the genomic inflation factor (λ) was calculated, and quantile-quantile (Q-Q) plots were constructed to check for deviation of the observed distribution of the test statistic from the expected null distribution.
An association was considered genome-wide significant if the meta-analysis p-value was less than the Bonferroni-corrected p-value, based on the number of genes evaluated. Genes with meta-analysis p < 10−3 were considered to have suggestive evidence of association. For genes with at least suggestive evidence of association in the meta-analysis, we considered those for which the meta-analysis p-value was lower than the p-values in the contributing datasets (i.e., the evidence for an association was stronger in the combined data than in either of the individual datasets) as candidate maternal CTD-related genes. When several contiguous genes met these criteria, which may reflect linkage disequilibrium between variants in genes that are in close proximity rather than independent association signals, we reviewed gene functions [30] to identify the most likely candidate gene in the region.
Gene-set enrichment analyses
Enrichment analyses using MetaCoreTM (Thomson Reuters, Life Science Research; https://portal.genego.com/metacore)), were performed for genes with meta-analysis p < 0.01 to identify enriched gene ontology (GO) processes, diseases (represented by biological markers), pathway maps, and pathway processes. For these analyses, a false-discovery rate (FDR)-corrected p < 0.05 was considered statistically significant.
Post hoc analyses of maternal effect genes (MEGs)
The most significant association in our meta-analysis was with a gene that has been suggested to be a MEG [31]. Given this finding, we elected to conduct an a posteriori, MEG gene-set analysis. For this analysis, we considered a gene to be an established MEG if it was included in at least one of two comprehensive reviews of the MEG literature (Table A of S1 File) [32, 33]. Fisher’s exact test was used to compare the proportion of established MEGs among all genes with meta-analysis p-values below and above a specified p-value cut-point (i.e., p < 0.05 versus p ≥ 0.05). A Fisher’s exact p < 0.05 was considered statistically significant. In addition, we cross-referenced the list of established MEGs with our list of candidate maternal CTD-related genes.
Results
In both the CHOP and PCGC datasets, the most common diagnosis in the offspring was the tetralogy of Fallot (Table 1). After QC exclusions, the CHOP dataset included 423 mothers and 380 fathers, and the PCGC dataset included 216 mothers and 219 fathers.
Table 1. Summary of the conotruncal heart defect phenotypes in the offspring of study subjects.
| Conotruncal Heart Defect Phenotype | CHOP | PCGC | ||
|---|---|---|---|---|
| N = 483 | % | N = 244 | % | |
| Tetralogy of Fallot | 196 | 40.6 | 73 | 29.9 |
| D-transposition of the great arteries | 95 | 19.7 | 52 | 21.3 |
| Ventricular septal defects | 90 | 18.6 | 34 | 13.9 |
| Double outlet right ventricle | 53 | 11.0 | 37 | 15.2 |
| Isolated aortic arch anomalies | 22 | 4.6 | 7 | 2.9 |
| Truncus arteriosus | 15 | 3.1 | 7 | 2.9 |
| Interrupted aortic arch | 6 | 1.2 | 6 | 2.4 |
| Other | 6 | 1.2 | 28 | 11.5 |
Abbreviations: CHOP, The Children’s Hospital of Philadelphia; PCGC, The Pediatric Cardiac Genomics Consortium.
The number of variants and genes included in the CHOP and PCGC datasets are summarized in Table 2. The Q-Q plots (S1 and S2 Figs) and genomic inflation factors (Table 2) for the analyses of the individual datasets provided little evidence for systematic bias (Tables C and D of S1 File). No genome-wide significant associations (p ⪅ 2.3 × 10−6) were detected in either dataset.
Table 2. Summary of the genetic data used in the analyses of the CHOP and PCGC datasets.
| Dataset (# mothers/# fathers) | ||
|---|---|---|
| CHOP (423/380) | PCGC (216/219) | |
| Total variants | 5,605,644 | 6,815,834 |
| Rare variantsa | 3,500,915 | 4,574,369 |
| Number of genes | 21,187 | 22,002 |
| Genomic inflation factor (λ) | 1.06 | 1.05 |
Abbreviations: CHOP, The Children’s Hospital of Philadelphia; PCGC, The Pediatric Cardiac Genomics Consortium.
a Variants with minor allele frequency < 0.05.
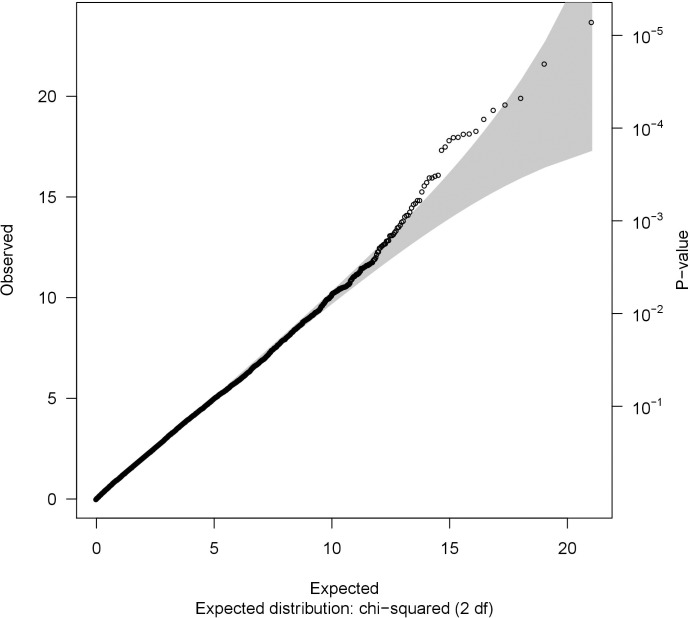
Fisher’s method was used to conduct a meta-analysis of the SKAT-C p-values from the 20,962 genes (Table D of S1 File) that were analyzed in both the CHOP and PCGC datasets. The genomic inflation factor (λ = 1.07) and Q-Q plot provided little evidence of a systematic deviation from the expected distribution (Fig 1). Although no gene achieved genome-wide significance in the meta-analysis (Bonferroni-corrected p < 2.4 × 10−6), the meta-analysis p-value for the germ cell-specific gene, GGN, was of borderline significance (p = 7.1 × 10−6). The meta-analysis also provided suggestive evidence of association for an additional 30 genes (Table 3).
Fig 1. A quantile-quantile plot.
A quantile-quantile plot of meta-analysis p-values obtained by combining SKAT-C test p-values from genome-wide analyses of the CHOP and PCGC datasets.
Table 3. Maternal genes with suggestive evidence of association (p < 10−3) with conotruncal heart defects in the meta-analysis.
| Gene | CHR | CHOP | PCGC | Meta-analysisa | ||
|---|---|---|---|---|---|---|
| # of variants | p-value | # of variants | p-value | p-value | ||
| GGN | 19 | 17 | 6.30 × 10−4 | 18 | 7.23 × 10−4 | 7.10 × 10−6 |
| SPRED3 | 19 | 46 | 5.11 × 10−3 | 44 | 2.71 × 10−4 | 2.01 × 10−5 |
| VARS2 | 6 | 88 | 7.29 × 10−6 | 84 | 4.76 × 10−1 | 4.71 × 10−5 |
| FER1L6-AS1 | 8 | 78 | 5.66 × 10−6 | 153 | 7.30 × 10−1 | 5.54 × 10−5 |
| LOC101927269 | 7 | 18 | 2.80 × 10−1 | 21 | 1.71 × 10−5 | 6.34 × 10−5 |
| LOC151475 | 2 | 25 | 1.49 × 10−5 | 31 | 4.08 × 10−1 | 7.91 × 10−5 |
| SUMO1 | 2 | 84 | 7.48 × 10−1 | 74 | 1.12 × 10−5 | 1.06 × 10−4 |
| PSMD8 | 19 | 40 | 8.51 × 10−4 | 39 | 1.06 × 10−2 | 1.14 × 10−4 |
| SPINT4 | 20 | 43 | 4.38 × 10−1 | 41 | 2.08 × 10−5 | 1.15 × 10−4 |
| SLAIN2 | 4 | 292 | 1.11 × 10−2 | 262 | 8.89 × 10−4 | 1.23 × 10−4 |
| YIF1B | 19 | 52 | 3.20 × 10−2 | 58 | 3.12 × 10−4 | 1.25 × 10−4 |
| CATSPERG | 19 | 162 | 4.72 × 10−3 | 186 | 2.29 × 10−3 | 1.34 × 10−4 |
| FER1L6 | 8 | 785 | 4.51 × 10−5 | 897 | 2.84 × 10−1 | 1.57 × 10−4 |
| LOC101928565 | 1 | 113 | 4.45 × 10−2 | 163 | 3.14 × 10−4 | 1.70 × 10−4 |
| SFTA2 | 6 | 33 | 1.01 × 10−4 | 31 | 2.73 × 10−1 | 3.18 × 10−4 |
| PTPRF | 1 | 342 | 3.56 × 10−1 | 317 | 7.95 × 10−5 | 3.25 × 10−4 |
| PLXND1 | 3 | 224 | 1.26 × 10−3 | 208 | 2.35 × 10−2 | 3.38 × 10−4 |
| TBC1D29 | 17 | 19 | 2.57 × 10−2 | 12 | 1.16 × 10−3 | 3.40 × 10−4 |
| KDM4A | 1 | 161 | 3.21 × 10−1 | 120 | 1.05 × 10−4 | 3.81 × 10−4 |
| TNK2 | 3 | 100 | 9.79 × 10−2 | 99 | 3.77 × 10−4 | 4.14 × 10−4 |
| ZSWIM3 | 20 | 110 | 2.52 × 10−1 | 98 | 1.72 × 10−4 | 4.80 × 10−4 |
| LOC100505978 | 12 | 8 | 1.32 × 10−1 | 10 | 4.15 × 10−4 | 5.92 × 10−4 |
| MYDGF | 19 | 71 | 1.21 × 10−4 | 80 | 4.52 × 10−1 | 5.92 × 10−4 |
| FTH1 | 11 | 14 | 1.93 × 10−4 | 8 | 3.05 × 10−1 | 6.33 × 10−4 |
| WFDC13 | 20 | 27 | 6.72 × 10−1 | 26 | 9.08 × 10−5 | 6.53 × 10−4 |
| WFDC3 | 20 | 89 | 5.44 × 10−1 | 77 | 1.24 × 10−4 | 7.13 × 10−4 |
| H1FOO | 3 | 50 | 2.16 × 10−2 | 46 | 3.50 × 10−3 | 7.92 × 10−4 |
| TBX20 | 7 | 102 | 2.66 × 10−4 | 124 | 3.10 × 10−1 | 8.58 × 10−4 |
| ZNF622 | 5 | 40 | 1.96 × 10−4 | 45 | 4.24 × 10−1 | 8.62 × 10−4 |
| HPS3 | 3 | 193 | 4.54 × 10−4 | 193 | 1.90 × 10−1 | 8.91 × 10−4 |
| STARD7-AS1 | 2 | 37 | 3.53 × 10−1 | 35 | 2.74 × 10−4 | 9.92 × 10−4 |
Abbreviations: CHR, chromosome; CHOP, The Children’s Hospital of Philadelphia; PCGC, The Pediatric Cardiac Genomics Consortium.
a The meta-analysis included 20,992 genes.
Of the 31 genes with suggestive evidence for association, ten had meta-analysis p-values lower than the p-values in either individual dataset. These ten genes included one pseudogene (TBC1D29P), one RNA gene (LOC101928565), and eight protein-coding genes. The eight protein-coding genes include two contiguous genes located at 3q22.1 (H1FOO and PLXND1); SLAIN2 at 4p11; and five genes located in an approximately 100,000 base-pair region of 19q13.2 (YIF1B, CATSPERG, PSMD8, GGN, and SPRED3) (Table 4). Based on their known functions (Table 4), the eight protein-coding genes do not appear to be strong candidates for maternal genes that act via a maternal phenotype (e.g., obesity and diabetes). However, the 3q22.1 region includes a known MEG, H1FOO (meta-p = 7.9 × 10−4), and the 19q13.2 region includes a gene that has been proposed to be a MEG, GGN (meta-p = 7.1 ×10−6). Consequently, we propose H1FOO and GGN, as well as SLAIN2 (the single associated gene in the 4p11 region) as the top candidate maternal CTD-related genes identified by our meta-analysis.
Table 4. Maternal protein-coding genes with meta-analysis p-values suggestive of association (p < 10−3) and lower than the p-values from the analysis of individual datasets.
| Chr. | Gene | Positiona | Descriptionb | Maternal Effect Gene | CHOP p-value | PCGC p-value | Meta-analysis p-value |
|---|---|---|---|---|---|---|---|
| 19q13.2 | YIF1B | 38,793,200–38,807,606 | Membrane trafficking protein | 3.20 × 10−2 | 3.12 × 10−2 | 1.25 × 10−4 | |
| CATSPERG | 38,825,443–38,862,589 | Sub-unit of the sperm calcium channel, CATSPER | 4.72 × 10−3 | 2.29 × 10−3 | 1.34 × 10−4 | ||
| PSMD8 | 38,864,190–38,875,464 | Involved in ATP-dependent degradation of ubiquinated proteins | 8.51 × 10−4 | 1.06 × 10−2 | 1.14 × 10−4 | ||
| GGN | 38,873,992–38,879,668 | Germ cell specific gene | Suggested | 6.30 × 10−4 | 7.23 × 10−4 | 7.10 × 10−6 | |
| SPRED3 | 38,879,840–38,891,523 | Negative regulation of MAP kinase signaling | 5.11 × 10−3 | 2.71 × 10−4 | 2.01 × 10−5 | ||
| 3q22.1 | H1FOO | 129,261,057–129,271,310 | Oocyte specific member of the H1 histone family | Established | 2.16 × 10−2 | 3.40 × 10−3 | 7.92 × 10−4 |
| PLXND1 | 129,273,056–129,326,582 | Cell surface receptor for semaphorins | 1.26 × 10−3 | 2.35 × 10−2 | 3.38 × 10−4 | ||
| 4p11 | SLAIN2 | 48,342,613–48,429,215 | Promotes cytoplasmic microtubule nucleation and elongation | 1.11 × 10−2 | 8.89 × 10−4 | 1.23 × 10−4 |
Abbreviations: CHOP, The Children’s Hospital of Philadelphia; PCGC, The Pediatric Cardiac Genomics Consortium.
a Gene transcription start/stop positions (hg19) plus 1 kb upstream and downstream.
b Gene descriptions obtained from GeneCards: https://www.genecards.org/.
Gene-set enrichment analyses
In enrichment analyses of genes with meta-analysis p < 0.01 (N = 204 genes), no pathway map or pathway process was significant. However, there was evidence of enrichment (FDR p < 0.05) for 17 GO processes (Table E of S1 File), including several related to transmembrane transport in general, and calcium ion transport in particular (e.g., GO:1903169, regulation of calcium ion transmembrane transport, p = 2.9 × 10−2). There was also evidence for enrichment of genes for biological markers associated with 24 disease processes including, diseases of proteostasis (e.g., proteostasis deficiencies, p = 8.2 × 10−3) and hypertension (FDR p = 3.0 × 10−2) (Table F of S1 File).
Post hoc analyses of MEGs
Given that the most significant association in our meta-analysis was with GGN (p = 7.1 x 10−6), a gene that has been suggested to be a MEG [31], we elected to conduct an a posteriori, MEG gene-set analysis. Based on two comprehensive reviews of the MEG literature [32, 33], we identified a list of 60 MEGs (Table A of S1 File). In our meta-analysis six of the genes on this list had p < 0.05 (HF1OO, p = 0.0008; KMT2D, p = 0.015; TP73, p = 0.026; BNC1, p = 0.034; ZAR1, p = 0.036; and RNF2, p = 0.0497). Although GGN also had a meta-analysis p < 0.05, this gene was not included in either of the review articles and was therefore omitted from these analyses. The identification of six MEGs with meta-analysis p < 0.05 represents a 2.3-fold enrichment, which is of borderline significance (Fisher’s exact p = 0.057) based on the standard p-value cut-off for a single statistical test (i.e. p < 0.05).
Discussion
Our genome-wide, gene-based analyses of common and rare variants provide suggestive evidence that maternal genes are associated with the risk of CTDs in their offspring. Based on the analyses of individual genes, we identified three candidate CTD-related maternal genes, H1FOO, GGN, and SLAIN2, and propose that these genes are most likely to influence CTD-risk via effects on early embryonic development.
H1FOO (meta-p = 7.1 × 10−6), a known MEG [33], is an oocyte-specific member of the linker histone H1 family [34]. Genes in this family are involved in the determination of higher-order chromatin structure and gene transcription. Knockdown studies of H1foo in mouse one-cell embryos indicate that maternal H1foo influences the progression of DNA replication by reducing the deposition of H3 in the perinuclear region of the male pronucleus, and significantly delays the timing of cleavage into a two-cell embryo [35].
The suspected MEG, GGN, is thought to be involved in DNA repair and is characterized as a germ cell-specific gene. GGN is expressed at high levels in the adult testis [36], and at lower levels in the adult ovary and somatic tissues, as well as in Metaphase-II (MII) oocytes and early embryos [36, 37]. Evidence that GGN may function as a MEG is based on the timing of the loss of viability in mouse Ggn-/- embryos. Specifically, Ggn-/- embryos are present in expected numbers at the two-cell stage but are rarely observed at the morula stage and absent by embryonic day 7.5, consistent with the loss of viability following the depletion of maternal Ggn mRNA stores [31].
Although SLAIN2 has not previously been implicated as a MEG, SLAIN2 mRNA is abundant in both MII oocytes and one-cell embryos and declines thereafter [37]. Further, SLAIN2 is involved in microtubule dynamics and organization [38], which are essential for several post-fertilization processes, including meiotic spindle assembly, separation of the parental genomes, and pronuclei migration [39–42]. Hence, both the expression pattern and known functions of SLAIN2 are compatible with a potential role as a MEG.
Additional evidence that MEGs may be associated with CTDs in offspring is provided by the observed 2.3-fold enrichment of established MEGs among genes with p < 0.05 in our meta-analysis. The established MEGs with meta-analysis p < 0.05 include: H1FOO (discussed above); the transcriptional regulators, BNC1 and KMT2D; RNF2, which is involved in chromatin remodeling; and, TP73 and ZAR1, which are involved in cell cycle regulation [32]. Although MEGs have not previously been implicated as potential maternal risk factors for CTDs, studies in model systems demonstrate that mutations in MEGs can have a range of consequences for offspring, including embryonic lethality, developmental delay, and congenital malformations [11–13]. Similarly, women carrying a MEG mutation (e.g., NLRP5, NLRP7, and PADI6) experience a range of reproductive outcomes, including hydatidiform moles, periods of infertility, reproductive loss, offspring with multi-locus imprinting disorders, and unaffected children [43–46]. Although somewhat anecdotal, it is of interest that one (of five) woman with an NLRP5 mutation, ascertained following the birth of a child with a multi-locus imprinting disorder, also had a child with an isolated (i.e., apparently non-syndromic) CHD (atrial septal and ventricular defects) [43].
The observed enrichment of genes mapping to GO processes related to ion transmembrane transport, and specifically to calcium ion transport, could also be driven by MEGs. Although a detailed understanding of the genetic regulation of these oscillations is lacking, the known MEG, NLRP5 (also known as MATER), is required for calcium homeostasis. Specifically, oocytes from mouse Mater hypomorphs exhibit lower first peak amplitudes and higher frequencies of calcium oscillations (as compared to wild-type oocytes), likely due to a reduction in calcium stores in the endoplasmic reticulum [47].
Our analyses also identified the enrichment of genes related to hypertension. Maternal pregestational hypertension is associated with an increased risk of several birth defects, including, but not limited, to CHDs [48–51]. These associations appear to be independent of medications taken for the treatment of hypertension [48, 49], suggesting either that maternal hypertension, per se, has a negative impact on development (e.g., via an effect on blood flow to the uterus) or that hypertension and birth defects share common risk factors (e.g., genes with pleiotropic effects).
Our analyses also identified enrichment of genes related to proteostasis deficiency and diseases associated with protein misfolding and aggregation (e.g., amyotrophic lateral sclerosis). During pregnancy, the accumulation of misfolded proteins in body fluids and the placenta is associated with preeclampsia, a maternal condition that is also associated with an increased risk of birth defects, including CHDs [50–54].
The results of our study must be viewed in light of both its strengths and limitations. We used a case-control study design, in which we compared the mothers (cases) and fathers (controls) of individuals with CTDs, to identify maternal CTD-related genes. Compared to a case-control design using unrelated, female controls, our design has the advantage of controlling for the potentially confounding effects of the genotype inherited by the child but is subject to bias arising from differences in allele frequencies between males and females. However, sex differences in allele frequencies appear to be uncommon (< 1% of variants) in autosomal genes [55]. In addition, while our analyses assess whether there are gene-level differences between mothers and fathers, they do not indicate which group might carry more (or less) disease-related alleles. Our observed associations could, therefore, be driven by paternal rather than maternal effects. However, since embryonic development prior to zygotic gene activation is primarily driven by maternal gene products, and the maternal genome has a direct effect on the in utero environment, any true associations detected in our study are most likely due to maternal genes. Nonetheless, additional studies (e.g., in model systems) will be required to confirm and establish the mechanisms underlying these observed associations.
Our analyses were based on two large CTD datasets that were ascertained in the United States using similar recruitment, and systematic case confirmation (phenotyping) approaches. Furthermore, our gene-based analyses had a lower multiple-testing burden than SNP-based GWAS. However, our sample sizes were relatively small for our genome-wide approach, and the criterion for achieving statistical significance (corrected-p ~ 2.5 × 10−6) remained quite high. Consequently, associations with maternal CTD-related genes may have been missed in our analyses due to low power. Genes with suggestive evidence of association and genes associated with enriched terms, therefore, appear to be strong targets for further investigations of the maternal genetic contribution to CTDs.
In our analyses, we combined data across different CTD phenotypes, which could have obscured associations if the maternal contribution to individual phenotypes is distinct. However, the predominance of evidence suggests that maternally mediated risk factors tend to be related to a broad spectrum of malformations. For example, maternal hypertension, preeclampsia, diabetes, and obesity are all associated with a spectrum of cardiac and non-cardiac malformations. Hence, for studies of maternal risk factors, the potential for improved power resulting from analyses of similar birth defects (e.g., the various CTD phenotypes) outweighs concerns regarding the potential impact of phenotypic heterogeneity.
This study is the first gene-based GWAS of maternal genotypes and CTDs. We have, however, previously conducted SNP-based, common-variant GWAS and meta-analysis using the same datasets as in the current gene-based analyses [14]. In our SNP-based meta-analysis, we identified several variants with suggestive evidence of association (p ≤ 10−5); however, none were located in, or within 1kb of the genes with suggestive evidence of association in the current, gene-based analyses. Based on these same two datasets, we have also reported that the risk of CTDs is associated with a maternal genetic risk score for hypertension [10]. However, only one variant included in the genetic risk score falls within a gene that was included in our enrichment analyses (i.e., rs11862778 in MTHFR). Hence, these two analyses appear to provide largely independent evidence that genes related to maternal hypertension are associated with the risk of CTDs.
In conclusion, our analyses provide provocative new insights into the potential influence of the maternal genome on embryonic development. While our results are specific to CTDs, both maternal conditions (e.g., hypertension) and MEGs are associated with a range of adverse reproductive outcomes, suggesting that our findings may have much broader implications for the understanding of birth-defect etiology. Further, our findings suggest a link between birth defects and other adverse pregnancy outcomes (e.g., reproductive loss and infertility). Confirmation of such a link would have broad implications for reproductive counseling and planning. Given these initial, compelling findings, additional studies of the relationship between the maternal genome and birth defects are warranted.
Supporting information
(PDF)
(PDF)
Table A. Human homologs of mammalian maternal effect genes identified in reviews by Condic (2016) and Zhang and Smith (2015). Table B. A gene-based analysis of the CHOP cohort using the SKAT-C test. The mothers and fathers of patients with CTDs were considered as 'cases' and 'controls,' respectively, for this analysis. Table C. A gene-based analysis of the PCGC cohort using the SKAT-C test. The mothers and fathers of patients with CTDs were considered as 'cases' and 'controls,' respectively, for this analysis. Table D. A gene-based meta-analysis of SKAT-C test results from the CHOP and PCGC cohort analyses. Table E. Gene Ontology (GO) processes from MetaCore enrichment analysis of top genes (meta-analysis p < 0.01) from the SKAT-C test, and color-coded GO process clusters identified through REVIGO. Table F. Enriched diseases (by biological markers) in the MetaCore enrichment analysis of top genes (meta-analysis p < 0.01) from the SKAT-C test.
(XLSX)
Acknowledgments
Members of the PCGC include (listed alphabetically, by institution): Boston Children’s Hospital, Boston, Massachusetts (Jane Newburger and Amy Roberts), Children’s Hospital of Los Angeles, Los Angeles, California (Richard Kim), Children’s Hospital of Philadelphia (Elizabeth Goldmuntz)*, Cincinnati Children’s Hospital, Cincinnati, Ohio (Eileen C. King), Columbia University Medical School, New York, New York (Wendy Chung), Harvard Medical School, Boston, Massachusetts (Christine Seidman and Jonathan Seidman), Icahn School of Medicine at Mount Sinai, New York, New York (Bruce Gelb), J. David Gladstone Institutes, San Francisco, California (Deepak Srivastava and Daniel Bernstein), New England Research Institutes, Watertown, Massachusetts (Sharon Tennstedt, Kimberly Dandreo, and Julie Miller), Stanford University, Stanford, California (Daniel Bernstein), Steve and Alexandra Cohen Children’s Medical Center of New York, New Hyde Park, New York (Angela Romano-Adesman), University of Rochester School of Medicine and Dentistry, Rochester, New York (George Porter), University of Utah, Salt Lake City, Utah (Martin Tristani-Firouzi and H. Joseph Yost), Yale School of Medicine, New Haven, Connecticut (Martina Brueckner and Richard Lifton).
*Lead PCGC author: goldmuntz@email. chop.edu
Data Availability
Data underlying the figures in this manuscript are provided in the Supporting Information as follows: Fig 1 (Table D of S1 File); S1 Fig (Table B of S1 File); S2 Fig (Table C of S1 File). The genotype data used in these studies are available at: Pediatric Cardiac Genomics Consortium: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs001194.v2.p2 CHOP pediatric controls: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000490.v1.p1 CHOP CTD trios: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000881.v1.p1
Funding Statement
This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01HD070454, AJA, EG, BM, LEM, FM, AS, DT; R21HD097347, AJA, LEM; R03HD098552, LEM) (https://www.nichd.nih.gov/), the National Heart, Lung, and Blood Institute (P50-HL74731) (https://www.nhlbi.nih.gov/), including the PCGC (U01-HL098188, U01HL131003, U01-HL098147, U01-HL098153, U01-HL098163, U01-HL098123, U01-HL098162) (https://benchtobassinet.com) and the Cardiovascular Development Consortium (U01-HL098166) (https://benchtobassinet.com/?page_id=1644), as well as the National Human Genome Research Institute (U54HG006504) (https://www.genome.gov), and the National Center for Research Resources (M01-RR-000240, RR024134, which is now the National Center for Advancing Translational Sciences (UL1TR000003) (https://ncats.nih.gov). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources. Array genotyping of the CHOP cohorts was funded by an Institutional Development Fund to The Center for Applied Genomics from The Children’s Hospital of Philadelphia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002;39(12):1890–900. Epub 2002/06/27. 10.1016/s0735-1097(02)01886-7 . [DOI] [PubMed] [Google Scholar]
- 2.Yang Q, Chen H, Correa A, Devine O, Mathews TJ, Honein MA. Racial differences in infant mortality attributable to birth defects in the United States, 1989–2002. Birth defects research Part A, Clinical and molecular teratology. 2006;76(10):706–13. Epub 2006/10/06. 10.1002/bdra.20308 . [DOI] [PubMed] [Google Scholar]
- 3.Arth AC, Tinker SC, Simeone RM, Ailes EC, Cragan JD, Grosse SD. Inpatient Hospitalization Costs Associated with Birth Defects Among Persons of All Ages—United States, 2013. MMWR Morbidity and mortality weekly report. 2017;66(2):41–6. Epub 2017/01/20. 10.15585/mmwr.mm6602a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115(23):2995–3014. Epub 2007/05/24. 10.1161/CIRCULATIONAHA.106.183216 . [DOI] [PubMed] [Google Scholar]
- 5.Patel SS, Burns TL. Nongenetic risk factors and congenital heart defects. Pediatric cardiology. 2013;34(7):1535–55. Epub 2013/08/22. 10.1007/s00246-013-0775-4 . [DOI] [PubMed] [Google Scholar]
- 6.Brody LC, Conley M, Cox C, Kirke PN, McKeever MP, Mills JL, et al. A polymorphism, R653Q, in the trifunctional enzyme methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase is a maternal genetic risk factor for neural tube defects: report of the Birth Defects Research Group. American journal of human genetics. 2002;71(5):1207–15. Epub 2002/10/18. 10.1086/344213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hobbs CA, MacLeod SL, Jill James S, Cleves MA. Congenital heart defects and maternal genetic, metabolic, and lifestyle factors. Birth defects research Part A, Clinical and molecular teratology. 2011;91(4):195–203. Epub 2011/03/09. 10.1002/bdra.20784 . [DOI] [PubMed] [Google Scholar]
- 8.Boyles AL, Wilcox AJ, Taylor JA, Shi M, Weinberg CR, Meyer K, et al. Oral facial clefts and gene polymorphisms in metabolism of folate/one-carbon and vitamin A: a pathway-wide association study. Genetic epidemiology. 2009;33(3):247–55. Epub 2008/12/03. 10.1002/gepi.20376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lupo PJ, Canfield MA, Chapa C, Lu W, Agopian AJ, Mitchell LE, et al. Diabetes and obesity-related genes and the risk of neural tube defects in the national birth defects prevention study. American journal of epidemiology. 2012;176(12):1101–9. Epub 2012/11/08. 10.1093/aje/kws190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplinski M, Taylor D, Mitchell LE, Hammond DA, Goldmuntz E, Agopian AJ, et al. The association of elevated maternal genetic risk scores for hypertension, type 2 diabetes and obesity and having a child with a congenital heart defect. PloS one. 2019;14(5):e0216477 Epub 2019/05/30. 10.1371/journal.pone.0216477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim KH, Lee KA. Maternal effect genes: Findings and effects on mouse embryo development. Clinical and experimental reproductive medicine. 2014;41(2):47–61. 10.5653/cerm.2014.41.2.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahadevan S, Sathappan V, Utama B, Lorenzo I, Kaskar K, Van den Veyver IB. Maternally expressed NLRP2 links the subcortical maternal complex (SCMC) to fertility, embryogenesis and epigenetic reprogramming. Sci Rep. 2017;7:44667 10.1038/srep44667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juan T, Geminard C, Coutelis JB, Cerezo D, Poles S, Noselli S, et al. Myosin1D is an evolutionarily conserved regulator of animal left-right asymmetry. Nat Commun. 2018;9(1):1942 Epub 2018/05/18. 10.1038/s41467-018-04284-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agopian AJ, Goldmuntz E, Hakonarson H, Sewda A, Taylor D, Mitchell LE, et al. Genome-Wide Association Studies and Meta-Analyses for Congenital Heart Defects. Circulation Cardiovascular genetics. 2017;10(3):e001449 Epub 2017/05/05. 10.1161/CIRCGENETICS.116.001449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agopian AJ, Mitchell LE, Glessner J, Bhalla AD, Sewda A, Hakonarson H, et al. Genome-wide association study of maternal and inherited loci for conotruncal heart defects. PloS one. 2014;9(5):e96057 Epub 2014/05/08. 10.1371/journal.pone.0096057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell LE, Agopian AJ, Bhalla A, Glessner JT, Kim CE, Swartz MD, et al. Genome-wide association study of maternal and inherited effects on left-sided cardiac malformations. Hum Mol Genet. 2015;24(1):265–73. Epub 2014/08/21. 10.1093/hmg/ddu420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi M, Murray JC, Marazita ML, Munger RG, Ruczinski I, Hetmanski JB, et al. Genome wide study of maternal and parent-of-origin effects on the etiology of orofacial clefts. American journal of medical genetics Part A. 2012;158A(4):784–94. Epub 2012/03/16. 10.1002/ajmg.a.35257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson TR. Conotruncal cardiac defects: a clinical imaging perspective. Pediatric cardiology. 2010;31(3):430–7. Epub 2010/02/19. 10.1007/s00246-010-9668-y . [DOI] [PubMed] [Google Scholar]
- 19.Kloesel B, DiNardo JA, Body SC. Cardiac Embryology and Molecular Mechanisms of Congenital Heart Disease: A Primer for Anesthesiologists. Anesth Analg. 2016;123(3):551–69. Epub 2016/08/20. 10.1213/ANE.0000000000001451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santorico SA, Hendricks AE. Progress in methods for rare variant association. BMC Genet. 2016;17 Suppl 2:6 Epub 2016/02/13. 10.1186/s12863-015-0316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorlov IP, Gorlova OY, Sunyaev SR, Spitz MR, Amos CI. Shifting paradigm of association studies: value of rare single-nucleotide polymorphisms. American journal of human genetics. 2008;82(1):100–12. Epub 2008/01/09. 10.1016/j.ajhg.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldmuntz E, Clark BJ, Mitchell LE, Jawad AF, Cuneo BF, Reed L, et al. Frequency of 22q11 deletions in patients with conotruncal defects. Journal of the American College of Cardiology. 1998;32(2):492–8. Epub 1998/08/26. 10.1016/s0735-1097(98)00259-9 . [DOI] [PubMed] [Google Scholar]
- 23.Hoang TT, Goldmuntz E, Roberts AE, Chung WK, Kline JK, Deanfield JE, et al. The Congenital Heart Disease Genetic Network Study: Cohort description. PloS one. 2018;13(1):e0191319 Epub 2018/01/20. 10.1371/journal.pone.0191319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81(3):559–75. Epub 2007/08/19. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sewda A, Agopian AJ, Goldmuntz E, Hakonarson H, Morrow BE, Taylor D, et al. Gene-based genome-wide association studies and meta-analyses of conotruncal heart defects. PloS one. 2019;14(7):e0219926 Epub 2019/07/18. 10.1371/journal.pone.0219926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nature genetics. 2012;44(8):955–9. Epub 2012/07/24. 10.1038/ng.2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529 Epub 2009/06/23. 10.1371/journal.pgen.1000529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ionita-Laza I, Lee S, Makarov V, Buxbaum JD, Lin X. Sequence kernel association tests for the combined effect of rare and common variants. American journal of human genetics. 2013;92(6):841–53. Epub 2013/05/21. 10.1016/j.ajhg.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher RAS. Statistical methods for research workers. Fourth ed.—revised and enlarged ed: Edinburgh Oliver & Boyd; 1932. [Google Scholar]
- 30.Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr Protoc Bioinformatics. 2016;54:1 30 1–1 3. Epub 2016/06/21. 10.1002/cpbi.5 . [DOI] [PubMed] [Google Scholar]
- 31.Jamsai D, O'Connor AE, Deboer KD, Clark BJ, Smith SJ, Browne CM, et al. Loss of GGN leads to pre-implantation embryonic lethality and compromised male meiotic DNA double strand break repair in the mouse. PloS one. 2013;8(2):e56955 Epub 2013/03/02. 10.1371/journal.pone.0056955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Condic ML. The Role of Maternal-Effect Genes in Mammalian Development: Are Mammalian Embryos Really an Exception? Stem Cell Rev. 2016;12(3):276–84. Epub 2016/02/20. 10.1007/s12015-016-9648-6 . [DOI] [PubMed] [Google Scholar]
- 33.Zhang K, Smith GW. Maternal control of early embryogenesis in mammals. Reprod Fertil Dev. 2015;27(6):880–96. Epub 2015/02/20. 10.1071/RD14441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker M, Becker A, Miyara F, Han Z, Kihara M, Brown DT, et al. Differential in vivo binding dynamics of somatic and oocyte-specific linker histones in oocytes and during ES cell nuclear transfer. Mol Biol Cell. 2005;16(8):3887–95. Epub 2005/06/10. 10.1091/mbc.e05-04-0350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funaya S, Ooga M, Suzuki MG, Aoki F. Linker histone H1FOO regulates the chromatin structure in mouse zygotes. FEBS Lett. 2018;592(14):2414–24. Epub 2018/07/03. 10.1002/1873-3468.13175 . [DOI] [PubMed] [Google Scholar]
- 36.Jamsai D, Sarraj MA, Merriner DJ, Drummond AE, Jones KT, McLachlan RI, et al. GGN1 in the testis and ovary and its variance within the Australian fertile and infertile male population. Int J Androl. 2011;34(6 Pt 1):624–32. Epub 2010/12/01. 10.1111/j.1365-2605.2010.01127.x . [DOI] [PubMed] [Google Scholar]
- 37.Park SJ, Shirahige K, Ohsugi M, Nakai K. DBTMEE: a database of transcriptome in mouse early embryos. Nucleic Acids Res. 2015;43(Database issue):D771–6. Epub 2014/10/23. 10.1093/nar/gku1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Vaart B, Manatschal C, Grigoriev I, Olieric V, Gouveia SM, Bjelic S, et al. SLAIN2 links microtubule plus end-tracking proteins and controls microtubule growth in interphase. J Cell Biol. 2011;193(6):1083–99. Epub 2011/06/08. 10.1083/jcb.201012179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marlow FL. Recent advances in understanding oogenesis: interactions with the cytoskeleton, microtubule organization, and meiotic spindle assembly in oocytes. F1000Res. 2018;7 Epub 2018/05/15. 10.12688/f1000research.13350.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuentes R, Mullins MC, Fernandez J. Formation and dynamics of cytoplasmic domains and their genetic regulation during the zebrafish oocyte-to-embryo transition. Mech Dev. 2018;154:259–69. Epub 2018/08/06. 10.1016/j.mod.2018.08.001 . [DOI] [PubMed] [Google Scholar]
- 41.Reichmann J, Nijmeijer B, Hossain MJ, Eguren M, Schneider I, Politi AZ, et al. Dual-spindle formation in zygotes keeps parental genomes apart in early mammalian embryos. Science. 2018;361(6398):189–93. Epub 2018/07/14. 10.1126/science.aar7462 . [DOI] [PubMed] [Google Scholar]
- 42.Inoue D, Wittbrodt J, Gruss OJ. Loss and Rebirth of the Animal Microtubule Organizing Center: How Maternal Expression of Centrosomal Proteins Cooperates with the Sperm Centriole in Zygotic Centrosome Reformation. Bioessays. 2018;40(4):e1700135 Epub 2018/03/10. 10.1002/bies.201700135 . [DOI] [PubMed] [Google Scholar]
- 43.Docherty LE, Rezwan FI, Poole RL, Turner CL, Kivuva E, Maher ER, et al. Mutations in NLRP5 are associated with reproductive wastage and multilocus imprinting disorders in humans. Nat Commun. 2015;6:8086 Epub 2015/09/02. 10.1038/ncomms9086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Begemann M, Rezwan FI, Beygo J, Docherty LE, Kolarova J, Schroeder C, et al. Maternal variants in NLRP and other maternal effect proteins are associated with multilocus imprinting disturbance in offspring. J Med Genet. 2018;55(7):497–504. Epub 2018/03/27. 10.1136/jmedgenet-2017-105190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soellner L, Kopp KM, Mutze S, Meyer R, Begemann M, Rudnik S, et al. NLRP genes and their role in preeclampsia and multi-locus imprinting disorders. J Perinat Med. 2018;46(2):169–73. Epub 2017/07/29. 10.1515/jpm-2016-0405 . [DOI] [PubMed] [Google Scholar]
- 46.Qian J, Nguyen NMP, Rezaei M, Huang B, Tao Y, Zhang X, et al. Biallelic PADI6 variants linking infertility, miscarriages, and hydatidiform moles. European journal of human genetics: EJHG. 2018;26(7):1007–13. Epub 2018/04/26. 10.1038/s41431-018-0141-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim B, Zhang X, Kan R, Cohen R, Mukai C, Travis AJ, et al. The role of MATER in endoplasmic reticulum distribution and calcium homeostasis in mouse oocytes. Dev Biol. 2014;386(2):331–9. Epub 2014/01/01. 10.1016/j.ydbio.2013.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li DK, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. BMJ. 2011;343:d5931 Epub 2011/10/20. 10.1136/bmj.d5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramakrishnan A, Lee LJ, Mitchell LE, Agopian AJ. Maternal Hypertension During Pregnancy and the Risk of Congenital Heart Defects in Offspring: A Systematic Review and Meta-analysis. Pediatric cardiology. 2015;36(7):1442–51. Epub 2015/05/09. 10.1007/s00246-015-1182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellizzi S, Ali MM, Abalos E, Betran AP, Kapila J, Pileggi-Castro C, et al. Are hypertensive disorders in pregnancy associated with congenital malformations in offspring? Evidence from the WHO Multicountry cross sectional survey on maternal and newborn health. BMC Pregnancy Childbirth. 2016;16(1):198 Epub 2016/07/31. 10.1186/s12884-016-0987-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber KA, Mayo JA, Carmichael SL, Stevenson DK, Winn VD, Shaw GM. Occurrence of Selected Structural Birth Defects Among Women With Preeclampsia and Other Hypertensive Disorders. American journal of epidemiology. 2018;187(4):668–76. Epub 2017/10/12. 10.1093/aje/kwx269 . [DOI] [PubMed] [Google Scholar]
- 52.Boyd HA, Basit S, Behrens I, Leirgul E, Bundgaard H, Wohlfahrt J, et al. Association Between Fetal Congenital Heart Defects and Maternal Risk of Hypertensive Disorders of Pregnancy in the Same Pregnancy and Across Pregnancies. Circulation. 2017;136(1):39–48. Epub 2017/04/21. 10.1161/CIRCULATIONAHA.116.024600 . [DOI] [PubMed] [Google Scholar]
- 53.Sheriff FR, Lopez A, Lupo PJ, Seth A, Jorgez C, Agopian AJ. Maternal hypertension and hypospadias in offspring: A systematic review and meta-analysis. Birth Defects Res. 2019;111(1):9–15. Epub 2018/11/10. 10.1002/bdr2.1415 . [DOI] [PubMed] [Google Scholar]
- 54.Nelson DB, Chalak LF, McIntire DD, Leveno KJ. Is preeclampsia associated with fetal malformation? A review and report of original research. J Matern Fetal Neonatal Med. 2015;28(18):2135–40. Epub 2014/10/30. 10.3109/14767058.2014.980808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lucotte EA, Laurent R, Heyer E, Segurel L, Toupance B. Detection of Allelic Frequency Differences between the Sexes in Humans: A Signature of Sexually Antagonistic Selection. Genome Biol Evol. 2016;8(5):1489–500. Epub 2016/05/18. 10.1093/gbe/evw090 [DOI] [PMC free article] [PubMed] [Google Scholar]