Abstract
Salmonella and Shigella species are food- and water-borne pathogens that are responsible for enteric infections in both humans and animals and are still the major cause of morbidity and mortality in the emerging countries. The existence of multiple Salmonella and Shigella serotypes as well as the emergence of strains resistant to antibiotics require the development of broadly protective therapies. Those bacteria utilize a Type III Secretion System (T3SS), necessary for their pathogenicity. The structural proteins composing the T3SS are common to all virulent Salmonella and Shigella spp., particularly the needle-tip proteins SipD (Salmonella) and IpaD (Shigella). We investigated the immunogenicity and protective efficacy of SipD and IpaD administered by intranasal and intragastric routes, in a mouse model of Salmonella enterica serotype Typhimurium (S. Typhimurium) intestinal challenge. Robust IgG (in all immunization routes) and IgA (in intranasal and oral immunization routes) antibody responses were induced against both proteins. Mice immunized with SipD or IpaD were protected against lethal intestinal challenge with S. Typhimurium or Shigella flexneri (100 Lethal Dose 50%). We have shown that SipD and IpaD are able to induce a cross-protection in a murine model of infection by Salmonella and Shigella. We provide the first demonstration that Salmonella and Shigella T3SS SipD and IpaD are promising antigens for the development of a cross-protective Salmonella-Shigella vaccine. These results open the way to the development of cross-protective therapeutic molecules.
Author summary
Salmonella and Shigella are responsible for gastrointestinal diseases and continue to remain a serious health hazard in South and South-East Asia and African countries, even more with the emergence of multi drug resistances. Developed vaccines are either not commercialized (for Shigella) or cover only a limited number of serotypes (for Salmonella). There is thus a crucial need to develop cross-protective therapies. By targeting proteins SipD and IpaD belonging respectively to the injectisome of Salmonella and Shigella and necessary to their virulence, we have shown that these proteins are able to induce immune response and a cross-protection in a murine model of infection by Salmonella and Shigella despite relatively weak identity sequence (38%). Such a candidate vaccine offers promising perspectives to control Salmonella and Shigella diseases.
Introduction
Salmonella and Shigella are GRAM-negative enteropathogenic bacteria belonging to the Enterobacteriaceae family [1,2]. Both are responsible for gastrointestinal diseases ranging from moderate to acute, depending on different factors (e.g pathogen species, ingested dose, or immune status of the host). However, they continue to remain a serious health hazard in South and South-East Asia and African countries [3–7], causing notably severe diarrhea in children under the age of five in sub-Saharan Africa and south Asia [8–10]. Other at-risk populations include military personnel deployed abroad [11–13], travelers and victims of bioterrorist attacks [14,15]. While Salmonella and Shigella consist of only few species (two for Salmonella: S. enterica and S. bongori and four for Shigella: S. flexneri, S. sonnei, S. dysenteriae and S. boydii), there are a multiplicity of subspecies [16–18] making difficult the development of broad range vaccines.
Currently, three types of Salmonella vaccines are licensed: all of them target S. enterica serovar Typhi and do not offer cross-protection against other Salmonella serovars, or against non-typhoidal Salmonella. The situation is even worse for Shigella for which no licensed vaccine is available despite long standing efforts. Hopefully, these efforts will pay off in a next future in regards to the clinical trials currently evaluated worldwide [19–21].
Vaccine strategies can be grouped into two fundamental approaches: live-attenuated vaccines and nonliving vaccines. Live attenuated vaccines are generally more efficient to stimulate the immune response but generally do not induce a broad coverage. Non-living vaccines encompass inactivated whole-organisms or purified recombinant subunits. While offering the safest protection, they suffer from lower immunogenicity and generally require supporting strategies to overcome this hurdle [22–25].
Active immune system stimulation induced by vaccination takes days to weeks to be effective and can only be used to prevent infections. Because T3SS is essential for virulence and is conserved among all pathogenic Salmonella and Shigella strains [26], T3SS proteins appear as ideal candidates for Salmonella-Shigella vaccine and immunotherapy development. Type 3 secretion systems (T3SSs) or injectisomes are bacterial macromolecular organelles that are involved in the pathogenesis of many important human, animal and plant diseases [27] Bacteria that have sustained long-standing close associations with eukaryotic hosts have evolved specific adaptations to survive and replicate in this environment. The study of these systems is leading to unique insights into not only organelle assembly and protein secretion but also mechanisms of symbiosis and pathogenesis [26]. Components of T3SSs are widely distributed in GRAM-negative pathogens and are well conserved with regard to their overall structure, architecture, and function. The T3SS needle of Salmonella and Shigella is built by the helical polymerization of several hundred subunits of a single small protein (PrgI and MxiH respectively). The needle-tip is formed by a pentameric hydrophilic protein complex (SipD and IpaD respectively) connecting the distal end of the needle to the membrane spanning translocon (SipB, SipC for Salmonella and IpaB, IpaC for Shigella) [28–31]. During infection, the bacteria receive an external signal from the host environment and begin to assemble coordinately the constituents of the secretion system [32,33] which ultimately lead to the injection of effectors and/or invasion of the targeted host cell by the bacterium [34–39]. Based on the literature and our results, the needle-tip proteins have proved to be immunogenic in mice and in humans, able to elicit good humoral responses protective against salmonellosis and shigellosis [2,40–44]. Moreover the sequence identity between IpaD and SipD [45], led us to the hypothesis that those needle tip proteins might be suitable targets for the development of a cross Shigella/Salmonella protective immunity. With this aim, we examined the immunogenicity of the Salmonella (SipD) and Shigella (IpaD) proteins, administered alone by comparing intranasal and intragastric immunization routes in a mouse model. We provide the first demonstration that SipD-IpaD are both promising target antigens for a cross-protective Salmonella-Shigella vaccine.
Materials and methods
Ethics statement
Six- to 8-week-old female BALB/c mice were purchased from Janvier Labs, France and maintained in accordance with the French and European regulations on care and protection of laboratory animals (European Community [EC] Directive 86/609, French Law 2001–486, 6 June 2001) and with agreement of the ethical committee (CETEA) no. 15–055 delivered to S. Simon and agreement D-91-272-106 from the Veterinary Inspection Department of Essonne (France). Up to eight mice were kept in each cage and housed in a temperature-regulated-room and had free access to food and water. All animals experiments were performed to ameliorate suffering according to the guideline of the CETEA committee.
Bacterial strains
The Salmonella enterica serovar Typhimurium (CIP 104474, Pasteur Institute collection) and Shigella flexneri 2a (generous gift of Dr A. Phalipon, Pasteur Institute) were used in this study. Bacteria were first grown at 37°C on agar plates (trypticase soy (TCS) containing 0.01% Congo red (Serva) for S. flexneri 2a and LB plates for S. Typhimurium). For infection, a colony (Congo red-positive for S. flexneri 2a) was picked for a 5ml overnight (O/N) culture at 37°C in LB medium, followed by a culture in the same medium with 1:100 of the first culture for 2 h under the same conditions.
Reagents
Biotin N-hydroxysuccinimide ester and streptavidin were from Sigma-Aldrich. Goat anti-mouse IgG and IgM polyclonal antibodies were from Jackson ImmunoResearch. Sandwich ELISAs were performed with MaxiSorp 96-well microtiter plates (Nunc, Thermoscientific), and all reagents were diluted in Enzyme Immuno-Assay (EIA) buffer (0.1 M phosphate buffer [pH 7.4] containing 0.15 M NaCl, 0.1% bovine serum albumin [BSA], and 0.01% sodium azide). Plates coated with proteins were saturated in EIA buffer (18 h at 4°C) and washed with washing buffer (0.01 M potassium phosphate [pH 7.4] containing 0.05% Tween 20). AEBSF (serine protease inhibitor) was from Interchim. Spectra/Por dialysis membranes were fromSpectrum Laboratories. Cholera Toxin and Luria Broth were from Sigma. PBS was from Gibco by Life Technologies.
Recombinant SipD and IpaD production and immunizations
The sipd and ipad genes from respectively S. Typhimurium and S. flexneri were synthesized (Genecust) based on the published sequences of Salmonella strain CIP 104474 and of Shigella strain CIP 82.48T and cloned into NdeI/XhoI restriction sites of the IPTG inducible pET22b vector (Novagen), allowing insertion of a poly-histidine tag sequence at the 3′ end of the genes (Table 1).
Table 1. Sequences of the primers used for the cloning of sipd and ipad genes.
| gene | name | sequence | |
|---|---|---|---|
| sipd | sipd_nde1 | 5’-TATACATATGCTTAATATTCAAAATTATTCCGC-3’ | |
| sipd_xho1 | 5’-CAATAGGCCTCGAGTCCTTGCAGGAAGCTTTTGGCGG-3’ | ||
| ipad | ipad_nde1 | 5’-TATACATATGAATATAACAACTCTGACTAATAGTATT-3’ | |
| ipad_xho1 | 5’-CAATAGGCCTCGAGCTTTACCTCTTTTTCAAATAGACA-3’ | ||
Whole proteins SipD and IpaD were expressed and purified by affinity chromatography (Ni-NTA) as described previously [46]. Protein concentrations were determined by measuring absorbance at 280 nm (A280) using the NanoDrop Spectrophotometer and the purity was assessed by SDS PAGE (10–15% gradient Phast Gel, Phast system, GE Healthcare). Purified recombinant proteins were stored at -20°C until use.
Six- to 8-week-old female BALB/c mice were used by groups of 15. For intranasal (IN) immunizations, mice were anesthetized with isoflurane delivered through a vaporizer. Mice were immunized intranasally or intragastrically (IG, with a canula) on days 0, 21 and 42 with 10 μg of SipD or IpaD in 20 μL of PBS (IN) or 300 μg in 200 μL of phosphate-buffered saline (PBS) (IG). The proteins admixed with 1.5 μg (IN) or 10 μg (IG) cholera toxin adjuvant, were incubated for 1 h in a shaker at room temperature before immunization. Mice that received only adjuvant and PBS were included as controls. Animals were monitored daily after immunizations.
LD50 determination and challenge procedures
LD50 determination
5 mL of preculture of S. Typhimurium or S. flexneri 2a was grown in 200 mL of LB at 37°C with agitation (200 rpm) until OD600 nm ~1. Bacteria were centrifuged at 2,000 x g for 15 min at 4°C and pellets were resuspended in PBS. Serial dilutions were performed in sterile PBS and approximately 2 x 102 to 2 x 108 CFU of S. Typhimurium were administered intra-gastrically (200 μL) using a curved gavage needle, or 5 x 105 to 5 x 1010 CFU of S. flexneri 2a were administered intra-nasally (20 μL) to 20–22 week-old female BALB/c mice (5 mice per group). The exact number of CFU of each challenge dose was recalculated by viable counts (plating serial dilutions on LB agar plates). Mice were monitored twice daily for 25 days. The 50% mouse lethal dose (LD 50) for the challenge strains was calculated by the method of Reed and Muench and determined to be ~104 CFU/mL for S. Typhimurium (2X102 CFU/mouse) and ~ 5X108 CFU/mL for S. flexneri 2a (107 CFU/mouse), in agreement with previous publication using this strain [47].
Challenge
On day 84 after primary immunization, mice (N = 15 per group, including control group: mice immunized intranasally with PBS+ adjuvant) were challenged with 100 LD 50 of virulent S. Typhimurium (~ 106 CFU/mL, 200 μL in sterile PBS) via the intragastric route or with 100 LD 50 of virulent S. flexneri 2a (~ 5.1010 CFU/mL, 20 μL in sterile PBS) via intranasal route. Mice were monitored twice daily for 21 days after the challenge and health status, weight and survival were recorded. Any mouse that lost more than 20% of its initial body weight or showed advanced signs of morbidity was euthanized and scored as a death.
Enzyme immunoassays
Labeling with biotin
One hundred μg of MAb or recombinant protein (SipD or IpaD) in 400 μL borate buffer (0.1 M; pH 8.5) was incubated at a 1:20 molar ratio with biotin-N-hydroxysuccinimide ester dissolved in 6 μL of anhydrous dimethylformamide (DMF). The reaction was stopped after 30 min at RT by adding 100 μL of 1 M Tris-HCl (pH 8) for 30 min. Finally, 500 μL of EIA buffer was added and the preparation was stored frozen at -20°C until use.
Evaluation of polyclonal response
Anti-SipD/IpaD antibodies were measured in sera of immunized mice or hybridoma culture supernatants using sandwich ELISA. Briefly, microtiter plates were coated with 100 μL of goat anti-mouse Ig(G+M) antibodies or with rat anti-mouse IgG1, IgG2a, IgG2b antibodies at 10 μg/mL (diluted in 50 mM potassium phosphate buffer) overnight (ON) at RT. Plates were then saturated ON at 4°C with 300 μL/well of EIA buffer. After a washing cycle performed with the washing buffer, 100 μL/well of serial dilutions of mouse sera (from 10−2 to 10−5) were added in duplicate and incubated overnight at 4°C. The plates were then washed 3 times before adding 100 μL/well of biotinylated recombinant SipD or IpaD proteins at 100 ng/mL. Unrelated biotinylated recombinant proteins sharing also an His-tag at their C-terminus were sometimes added as controls (PrgI for SipD immunized mice and MxiH for IpaD immunized mice). After 2 hours of incubation at RT followed by three washing cycles, 100 μL/well of acetylcholinesterase (AChE; EC 3.1.1.7)-labeled streptavidin (1 Ellman unit/mL) were added and incubated for 1 hour at RT. Finally, the plates were washed 3 times and the absorbance was measured at 414 nm after 45 min of reaction with 200 μL/well of Ellman's reagent [48]. Concentrations of Ig(G+M) antibodies were calculated by fitting a calibrated control curve with nonlinear regression and interpolation of absorbance values of test samples by two-phase decay analysis.
Statistical analysis
Graph Pad Prism 5 was used for the graphics generation and statistical analyses. The survival rates were analyzed using a two-tailed Fisher's exact test. Statistical analyses were performed using the non-parametric Mann-Whitney test to compare antibody concentrations between groups. Data are presented as the mean ± standard errors SEM for 10 or 15 samples per group of mice. A P value < 0.05 was considered significant in all determinations.
Results
Immunizations with SipD or IpaD proteins induce Ig(G+M) antibody responses
The SipD and IpaD proteins used to immunize mice were produced in E. coli BL21 (2.3 mg/L and 3 mg/L of culture of SipD and IpaD, respectively). Purity of proteins was assessed by SDS-PAGE electrophoresis and Coomassie blue staining (S1 Fig).
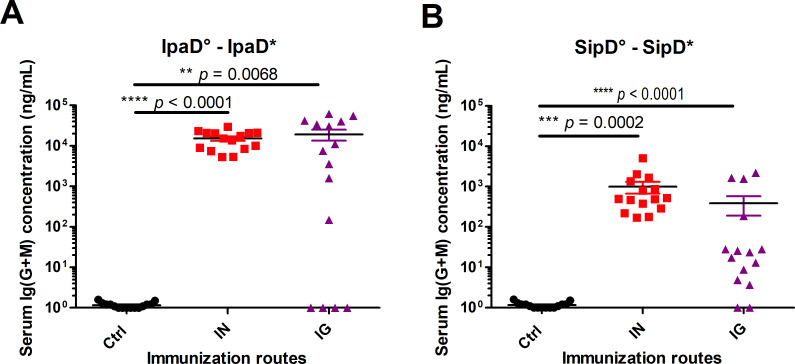
Mice immunized by the intranasal (IN) or the intragastric (IG) route with IpaD (Fig 1A, S2A Fig and S3A Fig) or SipD (Fig 1B, S2B Fig and S3B Fig) developed antigen-specific humoral responses. Total Ig (G+M), were measured using an ELISA test (principle of the ELISA in S4 Fig). Whatever the routes of immunization, the specific antibody titers against IpaD were superior to those obtained with SipD (Figs 1 and S2 and Table 2), probably because of a better immunogenicity of IpaD, compared to SipD. This hypothesis is supported by the results obtained with intragastric immunizations with SipD for which the specific Ig (G+M) responses are more heterogeneous and much lower (two logs, 0.25 μg/mL) than those obtained for IpaD. IpaD-specific Ig(G+M) concentrations reached the highest values by the IG route (23 μg/mL measured at day 84, one month after the third immunization, see Table 2). For both immunization routes, serum Ig (G+M) antibodies to SipD were detected before those to IpaD (after the second immunization) even if the final titer after the third immunization was higher for IpaD (S2 Fig). It should be noted that for the majority of Ig(G+M) measurements (Table 2), the concentrations were below the sum of the concentrations obtained for the different IgG isotypes. This could be due to the antibodies used for the standard curve in the sandwich ELISA: a mixture of specific SipD and IpaD IgG1/IgG2a/IgG2b was used as a standard of Ig(G+M) polyclonal antibodies, which does not exactly reflect the diversity of a polyclonal response (and particularly the IgM production), by comparison with the other tests where each specific isotype was used.
Fig 1. Serum Ig(G+M) concentrations of mice immunized with IpaD or SipD.
Specific serum Ig (G+M) antibodies for IpaD (A) and SipD (B) were quantified by sandwich ELISA 2 weeks after the last immunization as described in experimental procedures. Data represent mean concentrations (ng/mL) and the standard errors (SEM) from 15 individual mice per group (control mice IN immunized with adjuvant + PBS). Asterisks and p values are indicated (**** p < 0.0001, *** 0.0001 < p < 0.001, and ** 0.001 < p < 0.01. Exact p value indicated in the figure) when comparing mice immunized by the IN or IG route versus control mice using a nonparametric Mann-Whitney test.°: indicates injected immunogen; *: indicates biotinylated recombinant protein used for the ELISA analyses.
Table 2. Summary of the homologous (Ig (G+M), IgG1, IgG (2a+2b), IgA) and heterologous (Ig (G+M)) antibody responses after the last immunization with SipD or IpaD by the IN and IG routes.
| Homologous antibody response | Heterologous response | |||||
|---|---|---|---|---|---|---|
| Immunization route | Immunogen | Ig(G+M) | IgG1 | IgG(2a+2b) | IgA titer | Ig (G+M) |
| IN | SipD | 1.7 x 103 | 2.6 x 103 | 4.6 x 102 | 2.4 x 102 | 4.4 x 101 |
| IpaD | 2.9 x 104 | 2.0 x 104 | 6.8 x 103 | 5.2 x 102 | 2.9 x 101 | |
| IG | SipD | 1.2 x 103 | 6.8 x 103 | 9.9 x 102 | 1.7 x 102 | 4.4 x 100 |
| IpaD | 2.6 x 104 | 4.9 x 102 | 8.3 x 103 | 3.8 x 102 | 1.9 x 102 | |
Data represent mean concentrations (ng/mL) for Ig(G+M), IgG1, IgG(2a+2b) responses and IgA titer from each group of mice.
Intranasal and intragastric administrations of SipD elicit serum IgA titers
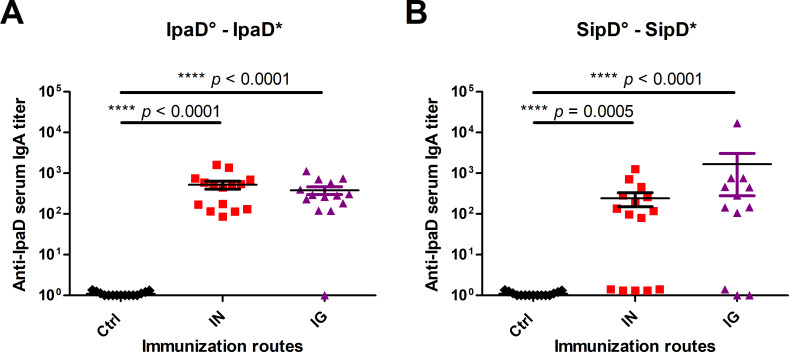
To evaluate the induction of IgA antibodies by the mucosa, the first line of adaptive immune defense against enteric pathogens, IpaD and SipD specific IgA titers in serum from immunized and control mice were measured (Fig 2A and 2B respectively and Table 2). For each protein, the specific IgA titers were equivalent for mice immunized intranasally or intragastrically. It should be noted that for SipD some of the mice did not produce any detectable IgA, contrary to what was noted for IpaD, which supports what we observed for Ig(G+M) responses and the hypothesis of a better immunogenicity of IpaD protein.
Fig 2. IgA titers of mice immunized with IpaD or SipD.
Specific serum IgA antibody titers for IpaD (A) and SipD (B) were quantified by sandwich ELISA 2 weeks after the last immunization as described in experimental procedures. Data represent mean titers and the standard errors (SEM) from 15 individual mice per group (control mice IN immunized with adjuvant + PBS). Asterisks and p values are indicated (**** p < 0.0001 and *** 0.0001 < p < 0.001, exact p value indicated in the figure) when comparing mice immunized by the IN or IG route versus control mice using a nonparametric Mann-Whitney test.°: indicates injected immunogen; *: indicates biotinylated recombinant protein used for the ELISA analyses.
Immune response involved all main IgG isotypes in serum
To investigate further the immune response elicited by both routes of immunization for both proteins, the IpaD and SipD homologous specific IgG1, IgG2a and IgG2b subclasses were measured in serum from immunized IpaD, SipD and control mice after the third immunization for the IN and IG routes (Fig 3 and Table 2). Measurement of the IgG isotype concentrations in sera of immunized mice revealed that all main subclasses contributed to the humoral response whatever the route. Anti-IpaD IgG1 were found in higher concentration after IN route immunization compared with IG route (Fig 3A, left panel), whereas for IgG (2a + 2b), the levels were equivalent (Fig 3A. right panel). For SipD, no difference was found between the two routes either for IgG1 or IgG (2a + 2b) (Fig 3B). It has to be mentioned that whatever the subtypes of anti-SipD immunoglobulins, concentrations were slightly inferior to the ones obtained for IpaD and responses were more heterogeneous, reflecting differences of immunogenicity of the two proteins. IgG1 and IgG(2a+2b) are respectively indicators of the T helper type 2 (humoral) and type 1 (cellular) immune responses. IgG (2a+2b):IgG1 ratios were taken as indicators of the T helper type 1 (Th1, cellular response)/Th2 (humoral response) balance, in order to evaluate the contribution of each pathway to the immune response. As Salmonella and Shigella are facultative intracellular pathogens and multiply in macrophages, one could expect the involvement of the cellular immunity during an infection. IpaD and SipD were able to induce a similar response by the IN route with a ratio close to 1 and slightly in favor of IgG1 production (humoral response) (Fig 4). The balance was more clearly in favor of a cellular response for IpaD by the IG route (ratio around 10), opposite to the result obtained for SipD for which a humoral immunity was favored (ratio IgG (2a+2b):IgG1 close to 0.1). However this result should be taken with caution as it has not been confirmed by measuring directly the T cell specific response.
Fig 3. Serum IgG subtype concentrations of mice immunized with IpaD or SipD.
Serum IgG1 (left panels), IgG2a and IgG2b (right panels) subclasses specific for IpaD (A) and SipD (B) were quantified by sandwich ELISA, 2 weeks after the last immunization. Data represent mean concentrations (ng/mL) and the standard errors (SEM) from 14–16 mice per group. Asterisks and p values are indicated (**** p < 0.0001, exact p value indicated in the figure) when comparing IG or IN immunized mice versus control mice (control mice IN immunized with adjuvant + PBS), as well as IN vs IG routes for IpaD IgG1.°: indicates immunogen injected; *: indicates biotinylated recombinant protein used for the ELISA analyses.
Fig 4.
IgG (2a +2b) / IgG 1 ratio after IpaD (A) and SipD (B) immunizations. Data represent mean titers and the standard errors (SEM) from 15 individual mice per group. Asterisks and p values are indicated (** 0.001 < p < 0.01, ns: non-significant. Exact p value indicated in the figure) when comparing mice immunized by the IN or IG route using a nonparametric Mann-Whitney test.°: indicates injected immunogen; *: indicates biotinylated recombinant protein used for the ELISA analyses.
Heterologous antibody responses
Because the needle-tip proteins SipD and IpaD of the T3SS of Salmonella and Shigella present sequence identities and with the aim of studying the possibility of cross-protection, the humoral responses against SipD in mice immunized with IpaD and against IpaD in mice immunized with SipD were measured. The crossed (heterologous) Ig(G+M) antibody responses were significantly lower (approximately 100-fold) than the specific (homologous) responses (S5 Fig for the kinetics, compare Fig 5 for heterologous response to Fig 1. for the homologous one, and see Table 2). IpaD immunogen seems to induce a higher heterologous antibody response against SipD than the opposite and particularly by the IG route (Table 2), confirming a better immunogenicity/stability of IpaD protein after administration or a better accessibility of the conserved regions between IpaD and SipD when IpaD is used as immunogen. These results are in agreement with the higher production of homologous anti-IpaD antibodies compared to homologous anti-SipD antibodies.
Fig 5. Heterologous serum Ig(G+M) concentrations of mice immunized with IpaD or SipD.
Specific serum Ig (G+M) antibodies against SipD for mice immunized with IpaD (A) and against IpaD for mice immunized with SipD (B) were quantified by sandwich ELISA 2 weeks after the last immunization as described in experimental procedures. Data represent mean concentrations (ng/mL) and the standard errors (SEM) from 15 individual mice per group (control mice IN immunized with adjuvant + PBS). Asterisks and p values are indicated (**** p < 0.0001. Exact p value indicated in the figure. ns: non-significant) when comparing mice immunized by the IN or IG route versus control mice using a nonparametric Mann-Whitney test.°: indicates injected immunogen; *: indicates biotinylated recombinant protein used for the ELISA analyses.
Protective efficacy against lethal S. Typhimurium or S. flexneri 2a challenge
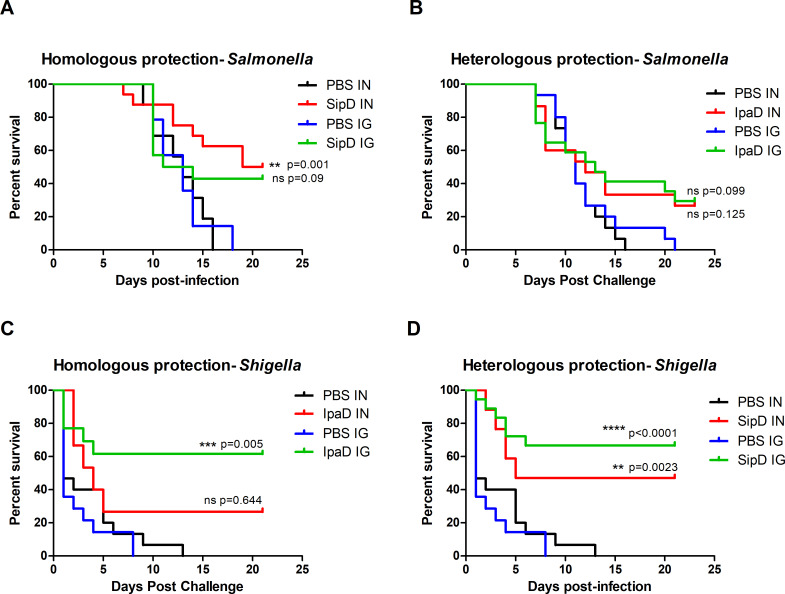
The lethal doses 50% (LD50) of the S. Typhimurium (intragastric infection) and S. flexneri 2a (intranasal infection) strains used in the experiments (see experimental procedures) were determined at 104 CFU/mL for Salmonella (2X102 CFU/mouse) and 5.108 CFU/mL for Shigella (107 CFU/mouse) (S6 Fig) according to the Reed and Muench method [49] which is in agreement other studies ([2,47,50]. To assess first the homologous protective efficacy induced by SipD against S. Typhimurium and IpaD against S. flexneri 2a, immunized and control mice were subjected to intragastric or intranasal challenge, six weeks after the last immunization, with a high dose of bacteria: ~ 100 LD50 of S. Typhimurium (2X104 CFU/mouse) or S. flexneri 2a (109 CFU/mouse) (Fig 6A–6C, respectively, and Table 3). In all challenges, the mortality rate of control animals (mice administered phosphate buffer saline [PBS]/adjuvant) was 100% with death occurring at 16–21 days after challenge by S. Typhimurium and at 8–13 days after challenge by S. flexneri 2a. SipD and IpaD were able to induce efficient homologous protection against challenge by their bacterial counterparts (Fig 6A–6C). The best homologous protective efficacy was induced by IpaD against a S. flexneri 2a challenge (intragastric route, 61% survival rate). In order to evaluate the cross-protective efficacy of each of the proteins, mice immunized intragastrically or intranasally with IpaD or SipD were challenged by S. Typhimurium and S. flexneri 2a, respectively (Fig 6B–6D, and Table 3). Weak cross-protection induced by IpaD was obtained against S. Typhimurium infection by the IN and IG routes (27% and 30%, respectively). Cross-protection induced by SipD against S. flexneri 2a challenge was significant and even superior to the homologous protection induced by IpaD, whatever the route of immunization (47% by IN route, 67% by IG route). We hypothesized that these cross-protections could be due to the production of specific antibodies directed against crucial regions common to both proteins, although their protein sequence identity is relatively weak (38%, S7 Fig).
Fig 6. Homologous and heterologous protective efficacies induced by SipD and IpaD immunizations against S. Typhimurium and S. flexneri 2a challenges.
Mice (N = 15) were immunized at days 0, 21 and 42 by the indicated antigens (or by adjuvant + PBS for controls) and routes. Six weeks after the last immunization, at day 84, 100 LD 50 of S. Typhimurium (A, B) or S. flexneri 2a (C, D) was administered (intragastrically and intranasally, respectively) to SipD (A, D) or IpaD (B, C) immunized mice. Survival was monitored for 21 days. Statistical significance was determined using a log-rank (Mantel-Cox) test. Statistically significant differences are indicated by **** p < 0.0001, *** 0.0001 < p < 0.001, and ** 0.001 < p < 0.01. Exact p value indicated in the figure. ns: non-significant) compared to PBS groups.
Table 3. Homologous and cross-protection efficacy induced by SipD and IpaD T3SS protein immunizations by the IN and IG routes from lethal challenge with S. flexneri 2a (intranasal) or S. Typhimurium (intragastric) in mice.
| Immunization route | Immunogen | Challenge | Homologous protection efficacy (%) | Heterologous protection efficacy (%) | P value a |
|---|---|---|---|---|---|
| IN | IpaD | S. flexneri 2a | 27 | 0.644 | |
| IpaD | S. Typhimurium | 27 | 0.125 | ||
| SipD | S. Typhimurium | 50 | 0.001 | ||
| SipD | S. flexneri 2a | 47 | 0.002 | ||
| IG | IpaD | S. flexneri 2a | 62 | 0.0005 | |
| IpaD | S. Typhimurium | 30 | 0.099 | ||
| SipD | S. Typhimurium | 43 | 0.090 | ||
| SipD | S. flexneri 2a | 67 | <0.0001 |
The mice immunized by the intranasal (IN) or intragastric (IG) route with SipD and IpaD were challenged with 109 CFU/mouse of S. flexneri 2a by the IN route (LD 50 = 107 CFU/mouse) or with 2X104 CFU/mouse of S. Typhimurium by the IG route (LD 50 = 2X102 CFU/mouse). The mortality rate of the immunized group was compared with that of the PBS-immunized control animals using the log-rank (Mantel-Cox) test.
Heterologous protection induced by SipD was equivalent to the homologous protection induced by IpaD against S. flexneri 2a infection (40% vs 27% by IN route, 60% vs 61.5% by IG route) while the Ig(G+M) antibody concentration able to cross-react with SipD in IpaD-immunized mice seemed to be higher than the one produced against IpaD in SipD-immunized mice. It has to be noted that cross-reactive IgA in SipD-immunized mice were not measured and could bring substantial protection against S. flexneri 2a infection.
Discussion
Infections caused by Shigella and Salmonella (typhoidal as well as nontyphoidal, and particularly invasive nontyphoidal Salmonella (iNTS)) are associated with a high burden in terms of mortality and morbidity especially in low income countries and in children under 5 years of age [51]. For this reason, long-standing efforts have been made to understand the immunological mechanisms underlying these infections and to develop effective therapies against them. Vaccines targeting typhoidal Salmonella are already marketed, but none protect against non-typhoidal Salmonella. No licensed vaccine exists for Shigella, though some developments have been the subject of clinical studies with varying degrees of success. The existence of multiple Shigella and Salmonella serotypes and the increase of multiresistant iNTS as well as Shigella clones highlight the need for development of a broad-spectrum protective vaccine [52,53].
Different studies show the importance of the humoral response in the fight against Salmonella and Shigella infections [54–60]. For Shigella, numerous data provide evidence of the immunogenicity/protective role of T3SS proteins and particularly IpaB/IpaD [42,59,61–64], which have been evaluated as parts of a bi-component recombinant vaccine [43]. Although it is recognized that the mouse model of Shigella pulmonary infection is not ideal to mimick an intestinal infection, it is currently the one used by scientific community for the evaluation of vaccines in development. For Salmonella, studies on the importance of a protective humoral response are scarcer and sometimes controversial ([65] and for review see [66]). We have shown in a preliminary study that SipD induced a good humoral response and was protective against a S. Typhimurium challenge [46], and more recently Martinez-Becerra and coll. have shown that two fusion proteins mixed together and composed of SipB/SipD and SseB/SseC have the potential to provide a cross-protective effect against two serovars of Salmonella enterica [67]. However, unlike for IpaB/IpaD of Shigella, the SipB/SipD fusion protein alone was unable to elicit protection.
Based on these data and because of the sequence identity, the strong similarity in the three-dimensional structures and the mechanism of action between SipD and IpaD, as well as the role of the humoral response against these proteins and their importance in protecting against Shigella and Salmonella infections, we have hypothesized that broad-spectrum cross-protection against Salmonella and Shigella infections can be induced by using SipD or IpaD as immunogen. The results of this study show that by using indifferently SipD or IpaD, good protection (60%) against Shigella flexneri 2a infection is obtained despite very high challenging doses (100 LD50). In a comparative study, using the same model of Shigella pulmonary infection, immunizations with IpaD yielded 70 to 90% cross-protection against 5 and 11 LD50 of S. sonnei and S. flexneri, respectively, which decreased dramatically to around 20% with 9 and 24 LD50 of S. sonnei and S. flexneri, respectively [68]. Interestingly, we found in this study that protection against S. flexneri was equivalent using SipD or IpaD, whereas the immune responses induced by SipD were lower than those induced by IpaD. This difference might be due to better immunogenicity of IpaD compared to SipD. This hypothesis is supported by the results obtained with IG immunizations with SipD for which the specific Ig (G+M) responses are more heterogeneous and much lower (two logs) than those obtained for IpaD, which could be partly explained by the heterogeneity in the degradation of the proteins by the gastric acid of the stomach. In addition, while Shigella infections are carried out intranasally, protection is better when immunizations are performed intragastrically. This may be related, among other things, to a slightly higher IgA titer by the IG route than by the IN route, induced by immunizations with SipD. All antibody subclasses are involved in the humoral response for both SipD and IpaD, regardless of route of immunization, and this highlights the importance of humoral (and particularly mucosal) immunity in protecting against Salmonella and Shigella infection. It has to be noted that protection against Shigella infection is better when mice are immunized by the IG route compared to the IN route. This might be correlated to the IgG subtype measurement balance in favor of a cellular response for IpaD by the IG route suggesting a significant contribution of the Th1 response for protection after IN challenge. It has to be noted that this hypothesis has not been verified by a direct measurement of the T cell specific response.
Although IpaD is also able to induce protection against Salmonella infection (100 LD50), it is nevertheless lower than that obtained for Shigella with SipD. This might be due to different factors that could be linked altogether: i) Salmonella has two type three secretion systems involved in the pathogenicity [69], ii) pathogenicity mechanisms are different between Salmonella and Shigella and particularly in regard to the involvement of the innate and adaptative immune response, and iii) a more important systemic dissemination of Salmonella in the murine model [70,71]. Nevertheless, the protective effect obtained using SipD/IpaD as immunogen underscores the importance of the extracellular life cycles of Salmonella and Shigella for their pathogenicity and dissemination and highlight the role of conserved regions of needle-tip proteins SipD/IpaD in this protection.
To our knowledge, a cross-protective effect of a T3SS-1 component against Shigella and Salmonella infections has never been described before this study. The novelty of the results obtained here should highlight the major role of SipD and IpaD in Salmonella and Shigella virulence and although give first evidence of the interest of these proteins as potential targets to protect broadly against Salmonella and Shigella infection in development of new vaccines. The role of SipD/IpaD effectors in systemic dissemination of these bacteria strengthens the protective effect obtained using these proteins as immunogens and underscores the importance of their extracellular life cycle for their pathogenicity and dissemination. The common molecular mechanisms governing the cross-protection induced by SipD or IpaD remain now to be deciphered. Because of the key role of SipD and IpaD in the virulence of the bacteria, they are well conserved among the different Salmonella and Shigella strains and species and thus appears as good targets for broad-spectrum coverage against different Salmonella and Shigella species and serotypes. However, further investigations are needed to evaluate this possibility.
Supporting information
SDS-PAGE / Coomassie blue staining (reducing conditions) of purified recombinant proteins. PolyHis-IpaD (37.1 kDa, lane 2) and polyHis-SipD (38.2 kDa, lane 3) are shown with molecular mass markers in kilodaltons (kDa) (lane 1).
(TIF)
Mice were immunized three times (time indicated with arrows) with IpaD (A) or SipD (B) by the IN route (left panels) or IG route (right panels) as described in Materials and Methods. Homologous responses of Ig(G+M) antibodies specific for IpaD or SipD were quantified by sandwich ELISA. Data represent mean concentrations (ng/mL) and the standard errors (SEM) from 14–16 individual mice per group. (**** p < 0.0001, *** 0.0001 < p < 0.001, ** 0.001 < p < 0.01 and * 0.01 < p < 0.1. ns: non significant) comparing the antibody responses on days post-immunization versus those on day 0 (nonparametric Mann-Whitney test).°: indicates injected immunogen; *: indicates biotinylated recombinant protein.
(TIF)
Mice were immunized three times intranasally (IN) or intragastrically (IG) with IpaD (A) or SipD (B) as described in Materials and Methods. Example of specificity of Ig(G+M) responses is shown for one mouse per route of immunization, and was assessed by using biotinylated unrelated recombinant proteins, sharing the same His-tag as IpaD and SipD at their C-terminus. Control (ctl) His-tagged MxiH (needle protein of Shigella injectisome) or His-tagged PrgI (needle protein of Salmonella injectisome) were used for mice immunized with IpaD and SipD respectively and quantified by sandwich ELISA. Data represent absorbance units obtained with sera of mice diluted 1000 fold.
(TIF)
A sandwich ELISA test was performed to measure the concentrations of circulating antibodies (immune response after immunizations (Ig(G+M), IgG1, IgG2a, IgG2b and IgA, see experimental procedures)
(TIF)
Mice were immunized three times (time indicated with arrows) with IpaD (A) or SipD (B) by the IN route (left panels) or IG route (right panels) as described in Materials and Methods. Heterologous responses of Ig(G+M) antibodies specific for SipD (from mice immunized with IpaD) or SipD (from mice immunized with IpaD) were quantified by sandwich ELISA. Data represent mean concentrations (ng/mL) and the standard errors (SEM) from 14–16 individual mice per group. (**** p < 0.0001, *** 0.0001 < p < 0.001, ** 0.001 < p < 0.01 and * 0.01 < p < 0.1. ns: non significant) comparing the antibody responses on days post-immunization versus those on day 0 (nonparametric Mann-Whitney test).°: indicates injected immunogen; *: indicates biotinylated recombinant protein.
(TIF)
Serial dilutions of S. Typhimurium (from 2.102 to 2.108 CFU) and S. flexneri 2a (5.105 to 5.1010 CFU) were administered intragastrically (S. Typhimurium) or intranasally (S. flexneri 2a) to 20- to 22-week-old female BALB/c mice (5 mice per group). The 50% mouse lethal dose (LD 50) was calculated by the method of Reed and Muench.
(TIF)
Alignment sequences of IpaD from S. flexneri 2a (accession number SVF87366.1) and SipD from S. Typhimurium (accession number AAA86617.1) were performed using BLAST (Basic local alignment search tool) from NCBI (https://blast.ncbi.nlm.nih.gov/). SipD sequence is represented in blue and IpaD sequence in red. Identical aminoacids are represented in black and similar aminoacids by a “+”. Sequence identity is 38.17%.
(TIF)
Acknowledgments
We thank Dr Armelle Phalipon for the generous gift of the Shigella flexneri 2a strain.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Bakhos Jneid obtained a grant from the PhD program of the Commissariat à l’Energie Atomique et aux Energies Alternatives. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Velge P, Wiedemann A, Rosselin M, Abed N, Boumart Z, Chaussé AM, et al. Multiplicity of Salmonella entry mechanisms, a new paradigm for Salmonella pathogenesis. MicrobiologyOpen. 2012;1: 243–258. 10.1002/mbo3.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Becerra FJ, Kissmann JM, Diaz-McNair J, Choudhari SP, Quick AM, Mellado-Sanchez G, et al. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect Immun. 2012;80: 1222–1231. 10.1128/IAI.06174-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet Lond Engl. 2013;382: 209–222. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 4.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal Salmonella disease, 2010(1). Emerg Infect Dis. 2015;21 10.3201/eid2106.140999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tennant SM, MacLennan CA, Simon R, Martin LB, Khan MI. Nontyphoidal salmonella disease: Current status of vaccine research and development. Vaccine. 2016;34: 2907–2910. 10.1016/j.vaccine.2016.03.072 [DOI] [PubMed] [Google Scholar]
- 6.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5: 540–553. 10.1038/nrmicro1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine MM, Kotloff KL, Nataro JP, Muhsen K. The Global Enteric Multicenter Study (GEMS): Impetus, Rationale, and Genesis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;55: S215–S224. 10.1093/cid/cis761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farag TH, Nasrin D, Wu Y, Muhsen K, Blackwelder WC, Sommerfelt H, et al. Some Epidemiologic, Clinical, Microbiologic, and Organizational Assumptions That Influenced the Design and Performance of the Global Enteric Multicenter Study (GEMS). Clin Infect Dis. 2012;55: S225–S231. 10.1093/cid/cis787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker CLF, Aryee MJ, Boschi-Pinto C, Black RE. Estimating diarrhea mortality among young children in low and middle income countries. PloS One. 2012;7: e29151 10.1371/journal.pone.0029151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scallan E, Mahon BE, Hoekstra RM, Griffin PM. Estimates of illnesses, hospitalizations and deaths caused by major bacterial enteric pathogens in young children in the United States. Pediatr Infect Dis J. 2013;32: 217–221. 10.1097/INF.0b013e31827ca763 [DOI] [PubMed] [Google Scholar]
- 11.Sanders JW, Isenbarger DW, Walz SE, Pang LW, Scott DA, Tamminga C, et al. An observational clinic-based study of diarrheal illness in deployed United States military personnel in Thailand: presentation and outcome of Campylobacter infection. Am J Trop Med Hyg. 2002;67: 533–538. 10.4269/ajtmh.2002.67.533 [DOI] [PubMed] [Google Scholar]
- 12.Kasper MR, Blair PJ, Touch S, Sokhal B, Yasuda CY, Williams M, et al. Infectious etiologies of acute febrile illness among patients seeking health care in south-central Cambodia. Am J Trop Med Hyg. 2012;86: 246–253. 10.4269/ajtmh.2012.11-0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasper MR, Lescano AG, Lucas C, Gilles D, Biese BJ, Stolovitz G, et al. Diarrhea outbreak during U.S. military training in El Salvador. PloS One. 2012;7: e40404 10.1371/journal.pone.0040404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma T, Heywood A, MacIntyre CR. Chinese travellers visiting friends and relatives—A review of infectious risks. Travel Med Infect Dis. 2015;13: 285–294. 10.1016/j.tmaid.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Török TJ, Tauxe R V., Wise RP, Livengood JR, Sokolow R, Mauvais S, et al. A large community outbreak of salmonellosis caused by intentional contamination of restaurant salad bars. JAMA. 1997;278: 389–395. 10.1001/jama.1997.03550050051033 [DOI] [PubMed] [Google Scholar]
- 16.Ansong C, Tolić N, Purvine SO, Porwollik S, Jones M, Yoon H, et al. Experimental annotation of post-translational features and translated coding regions in the pathogen Salmonella Typhimurium. BMC Genomics. 2011;12: 433 10.1186/1471-2164-12-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bäumler AJ, Tsolis RM, Ficht TA, Adams LG. Evolution of host adaptation in Salmonella enterica. Infect Immun. 1998;66: 4579–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phalipon A, Sansonetti PJ. Shigella’s ways of manipulating the host intestinal innate and adaptive immune system: a tool box for survival? Immunol Cell Biol. 2007;85: 119–129. 10.1038/sj.icb7100025 [DOI] [PubMed] [Google Scholar]
- 19.Chen WH, Kotloff KL. Shigella Vaccine Development: Finding the Path of Least Resistance. Clin Vaccine Immunol CVI. 2016;23: 904–907. 10.1128/CVI.00444-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Launay O, Lewis DJM, Anemona A, Loulergue P, Leahy J, Sciré AS, et al. Safety Profile and Immunologic Responses of a Novel Vaccine Against Shigella sonnei Administered Intramuscularly, Intradermally and Intranasally: Results From Two Parallel Randomized Phase 1 Clinical Studies in Healthy Adult Volunteers in Europe. EBioMedicine. 2017;22: 164–172. 10.1016/j.ebiom.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AKM. Shigellosis. Lancet Lond Engl. 2017. 10.1016/S0140-6736(17)33296-8 [DOI] [PubMed] [Google Scholar]
- 22.MacLennan CA, Martin LB, Micoli F. Vaccines against invasive Salmonella disease: current status and future directions. Hum Vaccines Immunother. 2014;10: 1478–1493. 10.4161/hv.29054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaminski RW, Oaks EV. Inactivated and subunit vaccines to prevent shigellosis. Expert Rev Vaccines. 2009;8: 1693–1704. 10.1586/erv.09.127 [DOI] [PubMed] [Google Scholar]
- 24.Tennant SM, Levine MM. Live attenuated vaccines for invasive Salmonella infections. Vaccine. 2015;33 Suppl 3: C36–41. 10.1016/j.vaccine.2015.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkatesan MM, Ranallo RT. Live-attenuated Shigella vaccines. Expert Rev Vaccines. 2006;5: 669–686. 10.1586/14760584.5.5.669 [DOI] [PubMed] [Google Scholar]
- 26.Demers J-P, Sgourakis NG, Gupta R, Loquet A, Giller K, Riedel D, et al. The common structural architecture of Shigella flexneri and Salmonella typhimurium type three secretion needles. PLoS Pathog. 2013;9: e1003245 10.1371/journal.ppat.1003245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galán JE. Salmonella interactions with host cells: type III secretion at work. Annu Rev Cell Dev Biol. 2001;17: 53–86. 10.1146/annurev.cellbio.17.1.53 [DOI] [PubMed] [Google Scholar]
- 28.Deng W, Marshall NC, Rowland JL, McCoy JM, Worrall LJ, Santos AS, et al. Assembly, structure, function and regulation of type III secretion systems. Nat Rev Microbiol. 2017;15: 323–337. 10.1038/nrmicro.2017.20 [DOI] [PubMed] [Google Scholar]
- 29.Francis CL, Starnbach MN, Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low-oxygen conditions. Mol Microbiol. 1992;6: 3077–3087. 10.1111/j.1365-2958.1992.tb01765.x [DOI] [PubMed] [Google Scholar]
- 30.Goosney DL, Knoechel DG, Finlay BB. Enteropathogenic E. coli, Salmonella, and Shigella: masters of host cell cytoskeletal exploitation. Emerg Infect Dis. 1999;5: 216–223. 10.3201/eid0502.990205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsot C. Shigella type III secretion effectors: how, where, when, for what purposes? Curr Opin Microbiol. 2009;12: 110–116. 10.1016/j.mib.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 32.Burkinshaw BJ, Strynadka NCJ. Assembly and structure of the T3SS. Biochim Biophys Acta. 2014;1843: 1649–1663. 10.1016/j.bbamcr.2014.01.035 [DOI] [PubMed] [Google Scholar]
- 33.Deng W, Marshall NC, Rowland JL, McCoy JM, Worrall LJ, Santos AS, et al. Assembly, structure, function and regulation of type III secretion systems. Nat Rev Microbiol. 2017;15: 323–337. 10.1038/nrmicro.2017.20 [DOI] [PubMed] [Google Scholar]
- 34.Francis CL, Starnbach MN, Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low-oxygen conditions. Mol Microbiol. 1992;6: 3077–3087. 10.1111/j.1365-2958.1992.tb01765.x [DOI] [PubMed] [Google Scholar]
- 35.Goosney DL, Knoechel DG, Finlay BB. Enteropathogenic E. coli, Salmonella, and Shigella: masters of host cell cytoskeletal exploitation. Emerg Infect Dis. 1999;5: 216–223. 10.3201/eid0502.990205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsot C. Shigella type III secretion effectors: how, where, when, for what purposes? Curr Opin Microbiol. 2009;12: 110–116. 10.1016/j.mib.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 37.Campbell-Valois F-X, Pontier SM. Implications of Spatiotemporal Regulation of Shigella flexneri Type Three Secretion Activity on Effector Functions: Think Globally, Act Locally. Front Cell Infect Microbiol. 2016;6: 28 10.3389/fcimb.2016.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashida H, Mimuro H, Sasakawa C. Shigella manipulates host immune responses by delivering effector proteins with specific roles. Front Immunol. 2015;6: 219 10.3389/fimmu.2015.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashida H, Ogawa M, Mimuro H, Sasakawa C. Shigella infection of intestinal epithelium and circumvention of the host innate defense system. Curr Top Microbiol Immunol. 2009;337: 231–255. 10.1007/978-3-642-01846-6_8 [DOI] [PubMed] [Google Scholar]
- 40.Markham AP, Barrett BS, Esfandiary R, Picking WL, Picking WD, Joshi SB, et al. Formulation and immunogenicity of a potential multivalent type III secretion system-based protein vaccine. J Pharm Sci. 2010;99: 4497–4509. 10.1002/jps.22195 [DOI] [PubMed] [Google Scholar]
- 41.Riddle MS, Kaminski RW, Williams C, Porter C, Baqar S, Kordis A, et al. Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine. Vaccine. 2011;29: 7009–7019. 10.1016/j.vaccine.2011.07.033 [DOI] [PubMed] [Google Scholar]
- 42.Ndungo E, Randall A, Hazen TH, Kania DA, Trappl-Kimmons K, Liang X, et al. A Novel Shigella Proteome Microarray Discriminates Targets of Human Antibody Reactivity following Oral Vaccination and Experimental Challenge. mSphere. 2018;3 10.1128/mSphere.00260-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heine SJ, Diaz-McNair J, Martinez-Becerra FJ, Choudhari SP, Clements JD, Picking WL, et al. Evaluation of immunogenicity and protective efficacy of orally delivered Shigella type III secretion system proteins IpaB and IpaD. Vaccine. 2013;31: 2919–2929. 10.1016/j.vaccine.2013.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jneid B, Moreau K, Plaisance M, Rouaix A, Dano J, Simon S. Role of T3SS-1 SipD Protein in Protecting Mice against Non-typhoidal Salmonella Typhimurium. PLoS Negl Trop Dis. 2016;10: e0005207 10.1371/journal.pntd.0005207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato H, Frank DW. Multi-Functional Characteristics of the Pseudomonas aeruginosa Type III Needle-Tip Protein, PcrV; Comparison to Orthologs in other Gram-negative Bacteria. Front Microbiol. 2011;2: 142 10.3389/fmicb.2011.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jneid B, Moreau K, Plaisance M, Rouaix A, Dano J, Simon S. Role of T3SS-1 SipD Protein in Protecting Mice against Non-typhoidal Salmonella Typhimurium. PLoS Negl Trop Dis. 2016;10: e0005207 10.1371/journal.pntd.0005207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phalipon A, Kaufmann M, Michetti P, Cavaillon JM, Huerre M, Sansonetti P, et al. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J Exp Med. 1995;182: 769–778. 10.1084/jem.182.3.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7: 88–95. 10.1016/0006-2952(61)90145-9 [DOI] [PubMed] [Google Scholar]
- 49.Reed LJ, Muench H. A SIMPLE METHOD OF ESTIMATING FIFTY PER CENT ENDPOINTS. Am J Epidemiol. 1938;27: 493–497. 10.1093/oxfordjournals.aje.a118408 [DOI] [Google Scholar]
- 50.van de Verg LL, Mallett CP, Collins HH, Larsen T, Hammack C, Hale TL. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect Immun. 1995;63: 1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17: 909–948. 10.1016/S1473-3099(17)30276-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AKM. Shigellosis. Lancet Lond Engl. 2018;391: 801–812. 10.1016/S0140-6736(17)33296-8 [DOI] [PubMed] [Google Scholar]
- 53.Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;59: 933–941. 10.1093/cid/ciu468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michetti P, Mahan MJ, Slauch JM, Mekalanos JJ, Neutra MR. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60: 1786–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infect Immun. 2000;68: 3344–3348. 10.1128/iai.68.6.3344-3348.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacLennan CA, Gondwe EN, Msefula CL, Kingsley RA, Thomson NR, White SA, et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest. 2008;118: 1553–1562. 10.1172/JCI33998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dougan G, John V, Palmer S, Mastroeni P. Immunity to salmonellosis. Immunol Rev. 2011;240: 196–210. 10.1111/j.1600-065X.2010.00999.x [DOI] [PubMed] [Google Scholar]
- 58.Simon JK, Maciel M, Weld ED, Wahid R, Pasetti MF, Picking WL, et al. Antigen-specific IgA B memory cell responses to Shigella antigens elicited in volunteers immunized with live attenuated Shigella flexneri 2a oral vaccine candidates. Clin Immunol Orlando Fla. 2011;139: 185–192. 10.1016/j.clim.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cam PD, Pál T, Lindberg AA. Immune response against lipopolysaccharide and invasion plasmid-coded antigens of shigellae in Vietnamese and Swedish dysenteric patients. J Clin Microbiol. 1993;31: 454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimanovich AA, Buskirk AD, Heine SJ, Blackwelder WC, Wahid R, Kotloff KL, et al. Functional and Antigen-Specific Serum Antibody Levels as Correlates of Protection against Shigellosis in a Controlled Human Challenge Study. aClin Vaccine Immunol CVI. 2017;24 10.1128/CVI.00412-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oberhelman RA, Kopecko DJ, Salazar-Lindo E, Gotuzzo E, Buysse JM, Venkatesan MM, et al. Prospective study of systemic and mucosal immune responses in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect Immun. 1991;59: 2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van de Verg LL, Herrington DA, Boslego J, Lindberg AA, Levine MM. Age-specific prevalence of serum antibodies to the invasion plasmid and lipopolysaccharide antigens of Shigella species in Chilean and North American populations. J Infect Dis. 1992;166: 158–161. 10.1093/infdis/166.1.158 [DOI] [PubMed] [Google Scholar]
- 63.Kotloff KL, Simon JK, Pasetti MF, Sztein MB, Wooden SL, Livio S, et al. Safety and immunogenicity of CVD 1208S, a live, oral DeltaguaBA Deltasen Deltaset Shigella flexneri 2a vaccine grown on animal-free media. Hum Vaccin. 2007;3: 268–275. 10.4161/hv.4746 [DOI] [PubMed] [Google Scholar]
- 64.Simon JK, Wahid R, Maciel M, Picking WL, Kotloff KL, Levine MM, et al. Antigen-specific B memory cell responses to lipopolysaccharide (LPS) and invasion plasmid antigen (Ipa) B elicited in volunteers vaccinated with live-attenuated Shigella flexneri 2a vaccine candidates. Vaccine. 2009;27: 565–572. 10.1016/j.vaccine.2008.10.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forbes SJ, Eschmann M, Mantis NJ. Inhibition of Salmonella enterica serovar typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin A antibody. Infect Immun. 2008;76: 4137–4144. 10.1128/IAI.00416-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kurtz JR, Goggins JA, McLachlan JB. Salmonella infection: Interplay between the bacteria and host immune system. Immunol Lett. 2017;190: 42–50. 10.1016/j.imlet.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez-Becerra FJ, Kumar P, Vishwakarma V, Kim JH, Arizmendi O, Middaugh CR, et al. Characterization and Protective Efficacy of Type III Secretion Proteins as a Broadly Protective Subunit Vaccine against Salmonella enterica Serotypes. Infect Immun. 2018;86 10.1128/IAI.00473-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Becerra FJ, Kissmann JM, Diaz-McNair J, Choudhari SP, Quick AM, Mellado-Sanchez G, et al. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect Immun. 2012;80: 1222–1231. 10.1128/IAI.06174-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silva-Herzog E, Detweiler CS. Salmonella enterica replication in hemophagocytic macrophages requires two type three secretion systems. Infect Immun. 2010;78: 3369–3377. 10.1128/IAI.00292-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vazquez-Torres A, Fang FC. Cellular routes of invasion by enteropathogens. Curr Opin Microbiol. 2000;3: 54–59. 10.1016/s1369-5274(99)00051-x [DOI] [PubMed] [Google Scholar]
- 71.Lawley TD, Chan K, Thompson LJ, Kim CC, Govoni GR, Monack DM. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2006;2: e11 10.1371/journal.ppat.0020011 [DOI] [PMC free article] [PubMed] [Google Scholar]