Abstract
OBJECTIVES:
The aim of this study is to investigate the therapeutic property of hydroalcoholic extract of Fenugreek seeds in nonalcoholic fatty liver disease (NAFLD) in adult patients.
METHODS:
This randomized, placebo-controlled, parallel trial was conducted from November 2014 to June 2017. Patients aged between 18 and 70 years old with confirmed NAFLD were recruited from the Motahhari clinic, affiliated to Shiraz University of Medical Sciences, Iran. Participants either received 1 g hydroalcoholic extract of Fenugreek seeds or placebo daily for 3 months. The primary outcomes were changes in serum alanine transaminase and FibroScan controlled attenuation parameter score. Secondary outcome measures were changes in other laboratory data, liver stiffness measure, liver steatosis percent, and anthropometric variables. Participants were randomly assigned to the groups using blocked randomization method. Participants, investigators, and statistician were blinded to treatments allocation.
RESULTS:
After screening eighty patients, thirty patients met the inclusion criteria and were divided into two groups (1:1). After 3 months, two and four patients did not complete the trial in Fenugreek and placebo group, respectively. The changes in the anthropometrics, laboratories and FibroScan measurements were not statistically significant between the two groups.
CONCLUSION:
The evidence to prove the efficacy of the Fenugreek seeds' hydroalcoholic extract in NAFLD was not strong enough; hence, further experiments are still needed to assess the possible efficacy of Fenugreek on the treatment of NAFLD.
Keywords: Iran, liver diseases, phytotherapy, traditional medicine, trigonella foenum-graecum
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a common metabolic disease with the prevalence of 25.24% (95% confidence interval: 22.10–28.65). The prevalence of NAFLD in the Middle East and South America is the highest in the world (31.79% and 30.45%, respectively).[1] Its prevalence in Iran's urban regions was reported at 40%.[2,3]
From the past, herbal medicines have been used to prevent and treat NAFLD, due to the potential therapeutic effect and low adverse effects.[4]
Fenugreek (Trigonella Foenum-graecum) is used in combination with daily meal in many countries like Iran. Fenugreek is recognized for its numerous pharmacological properties including antidiabetic, anti-hypercholesterolemic, antioxidant, hepatoprotective, and anti-inflammatory effects.[5,6,7,8] The anti-hyperglycemic and insulin-sensitizing effect of Fenugreek along with its antioxidant, anti-inflammatory and hepatoprotective properties suggest that Fenugreek can be used to treat NAFLD.
According to the previously mentioned in vitro and in vivo pharmacological properties of Fenugreek, in this randomized, pilot, placebo-controlled, triple-blind clinical trial we aimed to investigate the therapeutic effect of hydroalcoholic extract of Fenugreek seeds on NAFLD in human.
Methods
Trial design
This study is a 90 days, randomized, placebo-controlled, pilot, triple-blind (participants, investigator, and outcomes assessor), and mono-center clinical trial with parallel design. Patients were randomly assigned with 1:1 allocation ratio to the placebo or fenugreek groups. Patients were enrolled from November 2014 to June 2017, and the last patient finished the study in September 2017.
Study participants
Patients aged 18–70 years old were recruited from the Motahhari clinic, affiliated to Shiraz University of Medical Sciences (SUMS), Iran. Increased serum levels of alanine transaminase (ALT) between 1.5 and 10 times greater than upper normal values and confirmation of fatty liver disease by ultrasonography were used as inclusion criteria. Participants should also have body mass index (BMI) 18.5–40 and be willing to sign the written informed consent form.
Participants were excluded if they had any of these criteria: (1) History of consuming more than one unit of alcohol per day for >10 years (one unit for the spirits: 30–45 cc, wine: 120–150 cc, and beer: 360 cc); (2) consumption of fatty liver control medications, glucose-lowering drugs, cholesterol-lowering drugs, antihypertensive drugs, Vitamin E, coenzyme Q10, corticosteroids and glucocorticoids, and thyroxin within the past 4 months; (3) administration of hepatotoxic drugs, such as methotrexate, tetracycline, amiodarone, tamoxifen, high doses of synthetic estrogens, perhexiline maleate, diethylaminoethoxyhexestrol, carbamazepine, and chloroquine within the past 6 months; (4) Diabetes (type 1 and 2); (5) History of cancer or chemotherapy within the past 2 years; (6) Suffering from other hepatic disease, for example, viral hepatitis B or C, hepatocellular carcinoma, cirrhosis, autoimmune hepatitis, Wilson's disease, primary sclerosing cholangitis, deficiency of alpha-1 antitrypsin, and liver transplantation; (7) Renal failure (creatinine >1.5 × ULN); (8) Chronic pancreatitis; (9) uncontrolled hypertension (above 180 mm Hg systolic blood pressure), heart failure or coronary artery disease; (10) hypothyroidism or hyperthyroidism; (11) Cushing's syndrome; (12) Disorders of the hypothalamic-pituitary axis, and (13) pregnant and lactating women.
Drug and placebo preparation
Hydroalcoholic extraction of Fenugreek seeds was prepared using percolation method for 48 h, and the resulting extract was spray-dried till 2% moisture. The presence of luteolin in the resulting extract was 0.019% according to the below procedure (Barij Essence Pharmaceutical Company procedure) [Figure 1].
Figure 1.
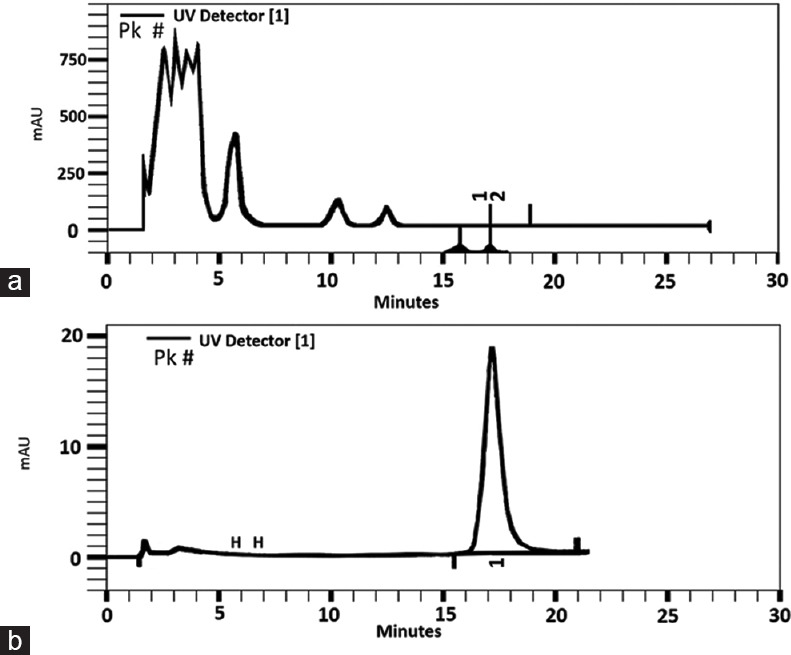
High-performance liquid chromatography profile of fenugreek seed extract, peak 1 shows luteolin content (a). Luteolin (10 μg/mL) (b)
The presence of luteolin in the samples was verified by comparison of the retention time (RT) and ultraviolet (UV) spectral peaks of the sample with those of an authentic sample (Luteolin; Sigma-Aldrich). UV spectra were measured on-line using a Knauer HPLC system (WellChrom, Germany) was equipped with a Spectra System K2600 detector. The optimum HPLC conditions for quantification of luteolin were a gradient system: Methanol/phosphoric acid 4% mixture: 0–12 min.: Methanol 38%–48%; 12–30 min.: Methanol 48%) as mobile phase by a flow rate of 1 ml/min and Hypersi column 100 (150 × 4.6 mm, 5 μm) as the phase of stationary. The detector was set at 350 nm.
Rice flour was filled in placebo capsules. The placebo capsules were the same as the Fenugreek placebo in color, package, shape, and size.
Intervention
Participants either received two oral capsules each containing 500 mg hydroalcoholic extract of Fenugreek seeds (Barij Essence Company, Kashan, Iran) or a placebo daily (one capsule half an hour after lunch and dinner) for 3 months. We recalled the patients on the 45th day after initiating the trial to assess the clinical and paraclinical changes, and ask them about any potential side effects (such as allergic reactions, bloating, transient diarrhea, severe weight loss, dizziness, and cold sweats).
Primary and secondary outcomes
Two primary outcomes were considered as follows: (1) changes in FibroScan controlled attenuation parameter (CAP) score and (2) serum ALT levels. Secondary outcome measures included; complete blood count (CBC), fasting blood sugar (FBS), albumin, aspartate transaminase (AST), alkaline phosphatase (ALK), triglyceride, total cholesterol, uric acid, creatinine, high-density lipoprotein (HDL), low-density lipoprotein (LDL), liver stiffness measure (LSM), steatosis percent, and anthropometric variables.
Follow-up
At baseline, all patients participated in a standard 15-min talk on the standard lifestyle modifications in NAFLD. Lifestyle modifications were high-carbohydrate and high-fat diet restriction and increasing level of physical activity (at least 20-min aerobic exercises daily). They were advised not to take any vitamins, specific pharmacologic treatments for NAFLD, and other supplements during the study. Adherence to the medications assessed by counting the remaining capsules. If the remaining capsule count was >10% of the total amount, the patient was excluded from the study. Each visit included taking a standardized medical history via a standard questionnaire, anthropometric measurements, asking about adverse effects, capsule counts, and blood sampling. The first and the last visits also included the FibroScan assay.
Anthropometric variables measurement
Anthropometric measurements included height, BMI, weight, hip and waist circumference, and waist to hip ratio, which was measured by an expert nurse at baseline, 45 days after, and at the end of the study.
Height was metered without shoes using stadiometer to the nearest of 0.1 cm. Weight was measured in light dress with a precision of 0.1 kg. Waist circumference was recorded with anthropometric tape in the middle of the 10th rib and the upper border of the iliac crest. BMI was equal to weight (kg) divided by square of the height (m2). Hip circumference was measured as the maximum buttocks' circumference with a precision of 0.5 cm.
Biochemical analysis
Ten milliliter of blood sample was collected from each patient after 8–12 h overnight fasting at the beginning of the trial, 45 days after and at the end of study. The samples were used to measure CBC, FBS, albumin, ALT, AST, ALK, total cholesterol, triglycerides, LDL, HDL, uric acid, and creatinine.
CBC was determined using the KX 21N Sysmex automated hematology analyzer (Sysmex Corporation, Kobe, Japan). The blood samples were centrifuged at 1000 rpm, and serum was separated. FBS was measured using glucose oxidase method (biorexfars, Iran). The measurements of ALT, AST, ALK, albumin, triglycerides, cholesterol, LDL, HDL, uric acid, and creatinine were carried out using biorexfars kits (biorexfars, Iran) and through the spectrophotometry method using Dirui CS-400 chemistry analyzer (Dirui Industrial Co. Ltd, Changchun, China).
FibroScan examination
During the first and last visit, CAP and LSM were measured by FibroScan 502 Touch device (Echosens, Paris, France) as reported previously.[9] Patients fasted about 8–12 h before FibroScan study. FibroScan study was done according to method which was described previously.[10,11] LSM, CAP, and steatosis percent was measured for each patient. Procedure was carried out by an expert hepatologist who was blinded to treatment group.
Sample size
Due to the lack of similar studies, this pilot study was carried out, and by assuming a drop-out rate of 10%–20%, a sample size of 15 patients in each group was considered suitable.
After recruitment of the study participants, statistical power was calculated based on the mean (-35.27) and standard deviation (SD) (22.45) of the difference between CAP score after and before study in fenugreek group. The calculated study power was 96%. Hence, patients' recruitment was stopped.
Randomization
Participants were randomly allocated to the placebo and fenugreek group in the random blocks of 4 subjects. Randomization was done by blocked randomization method. A computer random number generator generated the sequence of permuted blocks. To decrease confounding factors and selection bias, the randomization was done by one appointed researcher.
Blinding
Participants, investigators (the nurse who measures anthropometric variables and hepatologist who perform FibroScan), and the statistician who analyzed the data, were all blinded to treatments allocation until the statistical analysis was complete.
Ethical approval
Before the study, the local Ethics Committee of SUMS reviewed and then approved the patient informed consent form and protocol (CT-P-9362-6352). The study was registered at the NIH clinical trials database (NCT02303314; clinicaltrials. gov) and the Iranian Registry of Clinical Trials (IRCT2013102015083N1; www.irct.ir/). This study was conducted according to the principles of the Declaration of Helsinki (1996) and Good Clinical Practice Guidelines (1996). All patients signed the written informed consent before participation in this research, which was filed at the Gastroenterohepatology Research Center (SUMS, Iran).
Statistical analysis
Shapiro–Wilk test was used to assess normal distribution of data. Comparisons of baseline data between placebo and fenugreek groups was made using Mann–Whitney U test or independent t-test. Changes in anthropometric variables and laboratory data over the course of the study were analyzed using two-way (drug × time) repeated measures ANOVA. The analysis was conducted using SPSS 23 software (SPSS Inc., Chicago, IL, USA). Values of P < 0.05 were considered statistically significant. Study power was calculated using POWER SSC software version 1.0. (Shiraz NIOC Medical Education and Research Center, Shiraz, Iran).
Results
After screening eighty patients, thirty patients were included in the study after they met the inclusion criteria. After 3 months in Fenugreek group two patients did not completed the trial. One of them did not take Fenugreek according to the instructions due to bloating and heartburn, and the other discontinued the study due to personal reasons (migrating to another city). In the placebo group, two patients did not use the drug due to personal reasons, and two other patients did not continue the follow-up. Ultimately, the observation was conducted on 13 patients (all of them were male) who received Fenugreek (Group A), and 11 patients (9 males and 2 females) that received placebo (Group B) [Figure 2].
Figure 2.
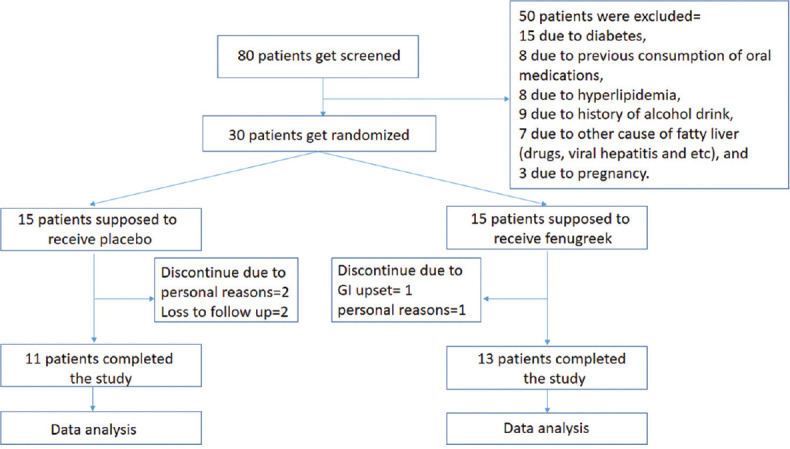
CONSORT diagram of the effect of fenugreek on the treatment of nonalcoholic fatty liver disease
The mean (SD) age of Group A and B were 39.08 (11.00) and 39.55 (8.70), respectively (P = 0.910). Baseline characteristics and paraclinical data of patients are shown in Table 1.
Table 1.
Baseline data of patients
| Parameters | Mean (SD) | P | |
|---|---|---|---|
| Fenugreek | Placebo | ||
| Sex | |||
| Male (N) | 13 | 9 | |
| Female (N) | 0 | 2 | |
| Age | 39.08 (11.00) | 39.55 (8.70) | 0.910* |
| BMI | 29.16 (3.92) | 28.38 (4.59) | 0.661* |
| Weight | 88.54 (11.90) | 80.41 (16.61) | 0.177* |
| Waist to hip ratio | 1.05 (0.21) | 0.96 (0.06) | 0.179* |
| Alanine aminotransferase | 57.00 (17.18) | 60.64 (24.90) | 0.677* |
| AST | 34.38 (7.37) | 37.00 (11.44) | 0.506* |
| ALK | 221.08 (42.14) | 248.64 (75.30) | 0.297* |
| Albumin | 4.72 (0.40) | 4.67 (0.34) | 0.783* |
| FBS | 90.31 (12.95) | 90.00 (14.04) | 0.839# |
| Triglyceride | 167.85 (71.36) | 173.82 (88.13) | 0.856* |
| Total cholesterol | 188.92 (26.30) | 174.73 (43.90) | 0.338* |
| LDL | 113.23 (24.93) | 100.27 (33.93) | 0.293* |
| HDL | 42.85 (8.91) | 46.27 (11.28) | 0.414* |
| Uric acid | 5.45 (1.49) | 4.49 (1.21) | 0.100* |
| Creatinine | 0.96 (0.16) | 0.92 (0.13) | 0.450# |
| Hemoglobin | 15.20 (1.32) | 15.51 (1.36) | 0.578* |
| White blood cells | 6.48 (1.41) | 6.86 (1.60) | 0.543* |
| Platelet | 216.85 (64.16) | 241.27 (35.29) | 0.192# |
| Liver stiffness measure | 5.57 (1.16) | 5.32 (1.89) | 0.708* |
| CAP score | 314.62 (36.11) | 286.56 (54.04) | 0.158* |
| Steatosis percent | 71.08 (20.63) | 50.67 (32.69) | 0.122* |
*Independent samples t-test, #Mann-Whitney U-test. AST=Aspartate transaminase, ALK=Alkaline phosphatase, FBS=Fasting blood sugar, LDL=Low-density lipoprotein, HDL=High-density lipoprotein, CAP=Controlled attenuation parameter, BMI=Body mass index, SD=Standard deviation
Mean creatinine level was raised statistically significant in Group A in comparison with Group B (F = (2, 44) = 3.776, P = 0.031). Although the observed change was statistically significant, it was not clinically significant.
While CAP score and steatosis percentage of Fenugreek group reduced more than placebo group, this was not statistically significant.
The changes in BMI, weight, waist-to-hip ratio, ALT, AST, ALK, albumin, FBS, triglycerides, cholesterol, LDL, HDL, uric acid, CBC, and LSM were not statistically significant by consideration of treatment group.
Table 2 summarizes anthropometric, laboratory, and FibroScan data of patients in the study.
Table 2.
Follow-up data of patients
| Parameter | Drug | Mean (SD) | P* | ||
|---|---|---|---|---|---|
| First visit | Second visit | Third visit | |||
| BMI | Fenugreek | 29.16 (3.92) | 28.67 (3.96) | 28.54 (4.01) | F=(2, 44)=2.053, |
| Placebo | 28.38 (4.59) | 28.31 (4.90) | 28.16 (4.89) | P=0.155 | |
| Weight | Fenugreek | 88.54 (11.90) | 87.04 (11.92) | 86.65 (12.18) | F=(2, 44)=1.784, |
| Placebo | 80.41 (16.61) | 80.05 (16.99) | 79.64 (16.83) | P=0.190 | |
| Waist to hip ratio | Fenugreek | 1.05 (0.21) | 1.03 (0.20) | 1.04 (0.20) | F=(2, 44)=1.402, |
| Placebo | 0.96 (0.06) | 0.97 (0.06) | 0.97 (0.08) | P=0.257 | |
| Alanine aminotransferase | Fenugreek | 57.00 (17.18) | 56.00 (35.90) | 58.00 (37.06) | F=(2, 44)=1.006, |
| Placebo | 60.64 (24.90) | 58.09 (42.21) | 47.55 (36.80) | P=0.374 | |
| AST | Fenugreek | 34.38 (7.37) | 31.08 (12.00) | 32.77 (12.22) | F=(2, 44)=2.843, |
| Placebo | 37.00 (11.44) | 39.67 (21.31) | 29.82 (16.47) | P=0.081 | |
| ALK | Fenugreek | 221.08 (42.14) | 213.62 (33.81) | 218.77 (37.87) | F=(2, 44)=2.189, |
| Placebo | 248.64 (75.30) | 250.82 (84.92) | 228.18 (80.10) | P=0.137 | |
| Albumin | Fenugreek | 4.72 (0.40) | 4.62 (0.40) | 4.56 (0.23) | F=(2, 44)=0.806, |
| Placebo | 4.67 (0.34) | 4.70 (0.35) | 4.71 (0.28) | P=0.453 | |
| FBS | Fenugreek | 90.31 (12.95) | 93.85 (9.08) | 88.77 (10.23) | F=(2, 44)=1.191, |
| Placebo | 90.00 (14.04) | 84.64 (11.51) | 84.00 (13.02) | P=0.314 | |
| Triglyceride | Fenugreek | 167.85 (71.36) | 198.00 (94.80) | 166.77 (87.11) | F=(2, 44)=2.876, |
| Placebo | 173.82 (88.13) | 198.36 (119.14) | 242.18 (143.09) | P=0.067 | |
| Cholesterol | Fenugreek | 188.92 (26.30) | 189.38 (29.47) | 192.46 (19.53) | F=(2, 44)=1.534, |
| Placebo | 174.73 (43.90) | 187.55 (48.38) | 198.64 (53.19) | P=0.231 | |
| LDL | Fenugreek | 113.23 (24.93) | 118.08 (23.86) | 116.54 (25.23) | F=(2, 44)=1.766, |
| Placebo | 100.27 (33.93) | 109.64 (36.10) | 121.27 (40.50) | P=0.195 | |
| HDL | Fenugreek | 42.85 (8.91) | 46.23 (5.63) | 44.08 (8.36) | F=(2, 44)=1.193, |
| Placebo | 46.27 (11.28) | 45.36 (10.96) | 41.82 (7.31) | P=0.313 | |
| Uric acid | Fenugreek | 5.45 (1.49) | 5.56 (1.39) | 6.10 (1.38) | F=(2, 44)=0.896, |
| Placebo | 4.49 (1.21) | 4.60 (1.34) | 4.66 (1.39) | P=0.416 | |
| Creatinine | Fenugreek | 0.96 (0.16) | 0.91 (0.13) | 1.01 (0.10) | F=(2, 44)=3.776, |
| Placebo | 0.92 (0.13) | 0.91 (0.13) | 0.87 (0.16) | P=0.031 | |
| Hemoglobin | Fenugreek | 15.20 (1.32) | 15.16 (1.60) | 15.34 (1.55) | F=(2, 44)=0.356, |
| Placebo | 15.51 (1.36) | 15.33 (1.04) | 15.37 (1.00) | P=0.702 | |
| White blood cells | Fenugreek | 6.48 (1.41) | 6.21 (1.17) | 6.08 (0.83) | F=(2, 44)=2.215, |
| Placebo | 6.86 (1.60) | 7.21 (2.52) | 7.59 (2.44) | P=0.121 | |
| Platelet | Fenugreek | 216.85 (64.16) | 221.15 (63.96) | 214.69 (62.94) | F=(2, 44)=0.638, |
| Placebo | 241.27 (35.29) | 257.18 (50.71) | 248.45 (54.35) | P=0.533 | |
| Liver stiffness measure | Fenugreek | 5.30 (0.78) | NP | 5.88 (1.45) | F=(1, 17)=1.072, |
| Placebo | 5.32 (1.89) | NP | 5.20 (1.62) | P=0.315 | |
| CAP score | Fenugreek | 309.10 (39.06) | NP | 273.90 (47.75) | F=(1, 17)=3.023, |
| Placebo | 286.56 (54.04) | NP | 281.44 (48.18) | P=0.100 | |
| Steatosis percent | Fenugreek | 68.30 (22.90) | NP | 46.70 (28.16) | F=(1, 17)=4.014, |
| Placebo | 50.67 (32.69) | NP | 52.67 (26.55) | P=0.061 | |
*Two-way repeated measures ANOVA. NP=Not performed, AST=Aspartate transaminase, ALK=Alkaline phosphatase, FBS=Fasting blood sugar, LDL=Low-density lipoprotein, HDL=High-density lipoprotein, CAP=Controlled attenuation parameter, BMI=Body mass index, SD=Standard deviation
Discussion
In this randomized controlled clinical trial, we investigated the effect of oral Fenugreek seeds hydroalcoholic extract in the treatment of NAFLD. Although Fenugreek reduced CAP score and steatosis percentage in comparison with placebo group, this reduction was not statistically significant.
Insulin resistance has a central role in the pathophysiology of NAFLD. Obesity, hyperlipidemia, diabetes mellites type 2, metabolic syndrome, and hypertension are common metabolic comorbidities associated with NAFLD.[1] Recommendation of healthy diet and physical activity is the main treatment of patients without non-alcoholic steatohepatitis (NASH) or fibrosis, and there is no need to recommend any medication. Decreasing 7%–10% of body weight is the goal of lifestyle modification in obese patients, can improve liver enzymes and histology.[12]
Pharmacotherapy must be done for individuals with NASH, especially for patients with stage F2 and higher of fibrosis and high-risk individuals for disease progression (i.e., persistently increased ALT, diabetes). Pioglitazone and vitamin E could be used for patients with NASH.[12,13]
Fenugreek has many therapeutic properties. It can decrease serum glucose and cholesterol level. Furthermore, it has anti-inflammatory, antioxidant and hepatoprotective properties. 4-hydroxyisoleucine, diosgenin, and fiber from Fenugreek have been shown to have positive effects on level of blood sugar, inflammation, insulin resistance, liver function, and lipids.[14]
4-hydroxyisoleucine has many beneficial effects on type 2 diabetes mellitus. 4-Hydroxyisoleucine can rise secretion of insulin and inhibit the molecular pathways that contribute to insulin resistance. It has been shown to improve glucose to insulin ratios, level of blood glucose, and gluconeogenese of liver. Furthermore, it can increase HDL level and decreased total cholesterol and triglycerides. Furthermore, AST and ALT decrease after administration of 4-Hydroxyisoleucine.[15]
Another study reviewed the effect of 4-Hydroxyisoleucine on insulin resistance related to obesity. It revealed that 4-hydroxyisoleucine can reduce blood glucose, total cholesterol, and triglycerides, and improved liver function.[16]
Previous studies also showed hepatoprotective effect of Fenugreek on hepatocellular injury in animal models.
A study by Raju and Bird evaluated the effect of Fenugreek on rat liver. They reported that Fenugreek reduced the storage of triglyceride in the liver without changing the glucose or plasma insulin levels. They suggested that tumor necrosis factor alpha might have played an important role in this process.[17]
Kaviarasan et al. showed polyphenol extract of Fenugreek seeds has favorable effect on lipid profile and decrease peroxidation, crosslinking, and collagen and aldehyde content in liver of ethanol-induced rats.[18] In another study, they showed the administration of polyphenol extract of Fenugreek seeds in ethanol-induced liver injury restored the levels of liver injury markers, increased hepatocyte viability and reduced apoptotic nuclei.[19]
Reddy and Srinivasan evaluated the hepatoprotective and antioxidant effect of Fenugreek seeds powder in mice with high-cholesterol diet. Activities of hepatic antioxidant enzymes like glutathione peroxidase and glutathione reductase were higher in Fenugreek treated mice. Therefore, they suggested that Fenugreek seeds have antioxidant and hepatoprotective effect.[20]
Mohamed et al. investigated the efficacy of aqueous extract of Termis, Fenugreek, and Nigella seeds in the NAFLD in obese diabetic albino rats. Diabetes was induced by subcutaneous injection of alloxan solution. Fenugreek alone and with the mixture of other plants depressed glucose level, increased insulin level, and improved fatty changes in rats' liver.[21]
Mbarki et al. studied the protective effect of powdered Fenugreek seeds on CCl4 induced toxicity in the liver and kidneys of the male rat. The results disclosed that Fenugreek seeds significantly protected the liver and kidneys from CCl4 toxicity.[22]
Furthermore, several investigations on human showed the beneficial properties of Fenugreek on hyperglycemia, hyperlipidemia, and insulin resistance.
A meta-analysis of clinical trials by Neelakantan et al. supported the positive effects of Fenugreek seeds on controlling blood sugar in diabetic patients.[23]
Another meta-analysis studied the effects of Fenugreek on lipid profile and blood glucose profile and showed potential glucose and total cholesterol lowering property.[6]
A study investigated level of insulin resistance in women with the polycystic ovarian syndrome after consuming hydroalcoholic extract of Fenugreek seeds. It concluded that adjuvant therapy (Fenugreek plus metformin) did not improved the insulin resistance in comparison with metformin alone.[24]
Another study on polycystic ovarian syndrome patients showed Fenugreek seeds extract had beneficial effects on the cyst size and menstrual cycle, but no statistically significant changes were reported in serum levels of ALT, AST, sugar, triglycerides and HDL.[25]
Rafraf et al. compared the effect of powdered whole Fenugreek seeds with placebo on type 2 diabetic patients. Fenugreek seeds significantly decreased FBS, hemoglobin A1c, total cholesterol, insulin resistance, and triglycerides level. Level of adiponectin in serum was increased.[26]
The study on the efficacy and safety of a polyherbal formulation including Fenugreek showed it was effective in decreasing the levels of lipid profile and blood sugar in type 2 diabetic individuals.[27]
Robert et al. studied the effect of Fenugreek seeds mixed in bread on postprandial blood sugar in healthy humans. They revealed Fenugreek seeds powder plus 10% of wheat flour can reduce the glycemic index of bread and postprandial blood glucose.[28]
A clinical trial by Tavakoly et al. reported that Fenugreek seeds powder can reduce inflammation and oxidative stress. It decreased high-sensitivity C-reactive protein and increased superoxide dismutase in type 2 diabetes mellitus patients.[29]
In our study, a possible reason for lack of efficacy of fenugreek was potential difference between level of lifestyle modification, physical activity, and restriction of diet with high amount of fat and carbohydrate in patients of two groups. Further studies should consider this issue and record level of lifestyle changes by quantitative questionnaire for each patient.
In this study, we excluded patients who suffered from diabetes mellitus and hypertriglyceridemia, so the glucose and lipid-lowering effect could not be compared with other studies. While, FibroScan assay can be considered as the best noninvasive method for follow up of NAFLD, histologic findings are presently the gold standard method and it can be accounted as another limitation.
Conclusion
Even though previous in vitro and animal studies showed Fenugreek has insulin-sensitizing, hepatoprotective, and antioxidant effects, the findings of this study was not strong enough to confirm in vivo effect on the liver enzymes and FibroScan assay in patients with NAFLD. Further investigations with large sample size and higher dose and longer administration time of Fenugreek are still needed to be conducted to evaluate the possible efficacy of Fenugreek in the treatment of NAFLD.
Financial support and sponsorship
This article was supported financially by the Vice Chancellor for Research of SUMS (grant number: 92-01-21-6352).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This article was extracted from the MD thesis written by Amir Hossein Babaei and supported by the Vice Chancellor for Research of SUMS (grant number: 92-01-21-6352). The authors would like to thank Mr. H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Amirkalali B, Poustchi H, Keyvani H, Khansari MR, Ajdarkosh H, Maadi M, et al. Prevalence of non-alcoholic fatty liver disease and its predictors in North of Iran. Iran J Public Health. 2014;43:1275–83. [PMC free article] [PubMed] [Google Scholar]
- 3.Lankarani KB, Ghaffarpasand F, Mahmoodi M, Lotfi M, Zamiri N, Heydari ST, et al. Non alcoholic fatty liver disease in southern Iran: A population based study. Hepat Mon. 2013;13:e9248. doi: 10.5812/hepatmon.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao H, Qiao YJ, Zhao YL, Tao XF, Xu LN, Yin LH, et al. Herbal medicines and nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22:6890–905. doi: 10.3748/wjg.v22.i30.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babaei A, Motamedifar M, Khalifat S, Mohammadi A, Zamani K, Motamedifar A. In vitro study of antibacterial property and cytotoxic effects of aqueous, ethanolic, methanolic, and hydroalcoholic extracts of fenugreek seed. Pak J Med Health Sci. 2018;12:906–10. [Google Scholar]
- 6.Gong J, Fang K, Dong H, Wang D, Hu M, Lu F. Effect of fenugreek on hyperglycaemia and hyperlipidemia in diabetes and prediabetes: A meta-analysis. J Ethnopharmacol. 2016;194:260–8. doi: 10.1016/j.jep.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Nagulapalli Venkata KC, Swaroop A, Bagchi D, Bishayee A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol Nutr Food Res. 2017;61:1600950. doi: 10.1002/mnfr.201600950. [DOI] [PubMed] [Google Scholar]
- 8.Sepaskhah M, Mohammadi A, Nabavizadeh S, Faridi P, Babaei A. Comparison of the efficacy of oral fenugreek seed extract and azithromycin in the treatment of acne vulgaris: A randomized, triple-blind controlled pilot clinical trial. Iran J Dermatol. 2019;22:58–64. [Google Scholar]
- 9.Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–62. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 10.Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–71. doi: 10.1038/ajg.2012.331. [DOI] [PubMed] [Google Scholar]
- 11.Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: A prospective cohort study. Gut. 2016;65:1359–68. doi: 10.1136/gutjnl-2015-309265. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts. 2016;9:65–90. doi: 10.1159/000443344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Dayyat HM, Rayyan YM, Tayyem RF. Non-alcoholic fatty liver disease and associated dietary and lifestyle risk factors. Diabetes Metab Syndr. 2018;12:569–75. doi: 10.1016/j.dsx.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Fuller S, Stephens JM. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: Mechanisms of actions and potential effects on metabolic syndrome. Adv Nutr. 2015;6:189–97. doi: 10.3945/an.114.007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zafar MI, Gao F. 4-hydroxyisoleucine: A potential new treatment for type 2 diabetes Mellitus. BioDrugs. 2016;30:255–62. doi: 10.1007/s40259-016-0177-2. [DOI] [PubMed] [Google Scholar]
- 16.Avalos-Soriano A, De la Cruz-Cordero R, Rosado JL, Garcia-Gasca T. 4-Hydroxyisoleucine from fenugreek (Trigonella foenum-graecum): Effects on insulin resistance associated with obesity. Molecules. 2016;21:1596. doi: 10.3390/molecules21111596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raju J, Bird RP. Alleviation of hepatic steatosis accompanied by modulation of plasma and liver TNF-alpha levels by Trigonella foenum graecum (fenugreek) seeds in Zucker obese (fa/fa) rats. Int J Obes (Lond) 2006;30:1298–307. doi: 10.1038/sj.ijo.0803254. [DOI] [PubMed] [Google Scholar]
- 18.Kaviarasan S, Viswanathan P, Anuradha CV. Fenugreek seed (Trigonella foenum graecum) polyphenols inhibit ethanol-induced collagen and lipid accumulation in rat liver. Cell Biol Toxicol. 2007;23:373–83. doi: 10.1007/s10565-007-9000-7. [DOI] [PubMed] [Google Scholar]
- 19.Kaviarasan S, Anuradha CV. Fenugreek (Trigonella foenum graecum) seed polyphenols protect liver from alcohol toxicity: A role on hepatic detoxification system and apoptosis. Pharmazie. 2007;62:299–304. [PubMed] [Google Scholar]
- 20.Reddy RR, Srinivasan K. Hepatoprotective and antioxidant effect of fenugreek (Trigonella foenum-graecum) seeds in mice under lithogenic condition. Journal of Food Biochemistry. 2011;35(6):1619–26. [Google Scholar]
- 21.Mohamed WS, Mostafa AM, Mohamed KM, Serwah AH. Effects of fenugreek, Nigella, and termis seeds in nonalcoholic fatty liver in obese diabetic albino rats. Arab J Gastroenterol. 2015;16:1–9. doi: 10.1016/j.ajg.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Mbarki S, Alimi H, Bouzenna H, Elfeki A, Hfaiedh N. Phytochemical study and protective effect of Trigonella foenum graecum (Fenugreek seeds) against carbon tetrachloride-induced toxicity in liver and kidney of male rat. Biomed Pharmacother. 2017;88:19–26. doi: 10.1016/j.biopha.2016.12.078. [DOI] [PubMed] [Google Scholar]
- 23.Neelakantan N, Narayanan M, de Souza RJ, van Dam RM. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: A meta-analysis of clinical trials. Nutr J. 2014;13:7. doi: 10.1186/1475-2891-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassanzadeh Bashtian M, Emami SA, Mousavifar N, Esmaily HA, Mahmoudi M, Mohammad Poor AH. Evaluation of fenugreek (Trigonella foenum-graceum L.), effects seeds extract on insulin resistance in women with polycystic ovarian syndrome. Iran J Pharm Res. 2013;12:475–81. [PMC free article] [PubMed] [Google Scholar]
- 25.Swaroop A, Jaipuriar AS, Gupta SK, Bagchi M, Kumar P, Preuss HG, et al. Efficacy of a novel fenugreek seed extract (Trigonella foenum-graecum, furocyst) in polycystic ovary syndrome (PCOS) Int J Med Sci. 2015;12:825–31. doi: 10.7150/ijms.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rafraf M, Malekiyan M, Asghari-Jafarabadi M, Aliasgarzadeh A. Effect of fenugreek seeds on serum metabolic factors and adiponectin levels in type 2 diabetic patients. Int J Vitam Nutr Res. 2014;84:196–205. doi: 10.1024/0300-9831/a000206. [DOI] [PubMed] [Google Scholar]
- 27.Zarvandi M, Rakhshandeh H, Abazari M, Shafiee-Nick R, Ghorbani A. Safety and efficacy of a polyherbal formulation for the management of dyslipidemia and hyperglycemia in patients with advanced-stage of type-2 diabetes. Biomed Pharmacother. 2017;89:69–75. doi: 10.1016/j.biopha.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Robert SD, Ismail AA, Rosli WI. Reduction of postprandial blood glucose in healthy subjects by buns and flatbreads incorporated with fenugreek seed powder. Eur J Nutr. 2016;55:2275–80. doi: 10.1007/s00394-015-1037-4. [DOI] [PubMed] [Google Scholar]
- 29.Tavakoly R, Maracy MR, Karimifar M, Entezari MH. Does fenugreek (Trigonella foenum-graecum) seed improve inflammation, and oxidative stress in patients with type 2 diabetes mellitus? A parallel group randomized clinical trial. Eur J Integr MED. 2018;18:13–7. [Google Scholar]


