Abstract
Hsp90 chaperone function requires traversal of a nucleotide-dependent conformational cycle, but the slow and variable rate of Hsp90-mediated ATP hydrolysis is difficult to envision as a determinant of conformational change. A recent study solves this dilemma by showing that Hsp90 samples multiple conformational states in the absence of nucleotides, which serve to influence, but not direct, the cycle. The conformational program of Hsp90 is conserved from bacteria to humans, although the population dynamics are species specific.
The biomedical importance of Hsp90
Although many proteins require the assistance of molecular chaperones to fold properly, the clientele of one such chaperone, heat shock protein 90 (Hsp90), seems to be restricted. Nonetheless, >200 clients of Hsp90 have been identified to date (www.picard.ch/downloads/Hsp90interactors.pdf), and many of these proteins regulate important signaling nodes that integrate diverse environmental inputs and propagate downstream signaling cascades [1-3]. Although not essential in bacteria, Hsp90 is conserved in all eukaryotes in which it seems to have attained a crucial role in maintaining both cellular and organismal viability. Moreover, Hsp90 seems to have been co-opted by cancer cells to enable them to survive both hostile environments within the host (e.g. hypoxia, oxidative stress and chemotherapeutic insult) and a high degree of chronic genetic instability [4,5]. In addition, many viruses seem to require the host Hsp90 chaperone machinery for their successful propagation [6]. It is not surprising, therefore, that Hsp90 has become a popular molecular target for drug development, and several small molecule Hsp90 inhibitors are undergoing various stages of clinical evaluation [7,8].
The Hsp90 chaperone cycle requires conformational flexibility
In light of the burgeoning interest in the possible clinical benefit of Hsp90 inhibitors, many laboratories have been intensively exploring how the protein functions. Most chaperones rely on conformational flexibility for their activity, and Hsp90 is no exception. Although the overall structures of bacterial Hsp90 (HtpG) and eukaryotic Hsp90 proteins are highly similar, only the eukaryotic proteins are known to interact with a large number of co-chaperones that stabilize various Hsp90 conformational states and participate in Hsp90-dependent client protein binding, folding and maturation. Unlike HtpG, eukaryotic Hsp90s also contain an unstructured flexible region of variable length (which is species dependent) that links the nucleotide-binding (and drug-binding) N-domain with the middle (M)-domain, which comprises part of the active site of the ‘split’ ATPase of the chaperone and provides docking sites for client proteins and various co-chaperones. Both bacterial and eukaryotic Hsp90 proteins exist as constitutive dimers through the interaction of the C-domain of each protomer. By contrast, the N-domains transiently dimerize in a highly regulated, ATP-dependent manner involving large movements of both the N- and M- domains of each protomer. Based on available data, the structures of the individual N-, M- and C-domains remain well preserved despite the large conformational rearrangements undergone by the Hsp90 dimer, suggesting that the conformational flexibility of Hsp90 results from displacement of the domains with respect to each other. In support of this hypothesis, studies on small-molecule inhibitor binding to the N-domain nucleotide pocket have shown that a local conformational change in the N-domain is propagated over the length of the protein to both M- and C-domains [9]. Based on data obtained from X-ray crystallography [10], and by analogy to other members of the GHKL (gyrase, Hsp90, histidine kinase, MutL) superfamily of which Hsp90 is a member (also including gyrase, histidine kinases and MutL) [11], nucleotide binding to and hydrolysis by Hsp90 has been thought to drive interconversion between two stable conformational states – an ‘open’ apo state in which N-domains of nucleotide-free Hsp90 are not dimerized and a ‘closed’ ATP-bound state in which N-domains are dimerized [12]. More recently, the use of single particle electron microscopy has provided evidence that HtpG can exist in at least three distinct conformational states – the ‘apo’ or fully open conformation that is nucleotide-free, the ‘closed’ conformation in which both N-domains transiently dimerize in the presence of ATP and a ‘compact’ conformation in the presence of ADP, wherein the N-domains are no longer dimerized but instead make novel intermolecular contacts with their respective M-domain [13]. From this three-state model it follows that, upon ADP release, the open conformation of Hsp90 is re-established and the cycle begins anew. These conformational changes are thought to be critically linked to client protein and co-chaperone binding and release [14].
But is the Hsp90 chaperone cycle driven by ATP binding and hydrolysis?
Although ATP binding and hydrolysis was thought to determine conformational flexibility of Hsp90, as seen for other GHKL superfamily members, Hsp90 ATPase activity is low, and it has been difficult to envisage ATP hydrolysis as the driving force behind the chaperone cycle. Recently, several studies have highlighted the intrinsic flexibility of the Hsp90 protein in the absence of nucleotide [13,15-17]. Southworth and Agard [18] now provide compelling evidence that nucleotide binding per se does not, in fact, drive conformational change in Hsp90. Instead, their data show that multiple Hsp90 conformations, representing the three conformational states described before for HtpG, co-exist in a dynamic steady-state equilibrium in the absence of nucleotide and that this equilibrium is only moderately perturbed by nucleotide binding. Thus, in considering HtpG dynamics, the addition of either AMPPNP (a non-hydrolyzable ATP analog) or ADP slightly skew the equilibrium to favor the closed or compact state, respectively. However, unlike HtpG, under similar conditions yeast Hsp90 adopted only two distinct conformations (open and closed), whereas human Hsp90 showed no obvious conformational changes in the presence of either nucleotide (i.e. remaining in an open conformation).
Conservation of the three-stage Hsp90 chaperone cycle from bacteria to humans
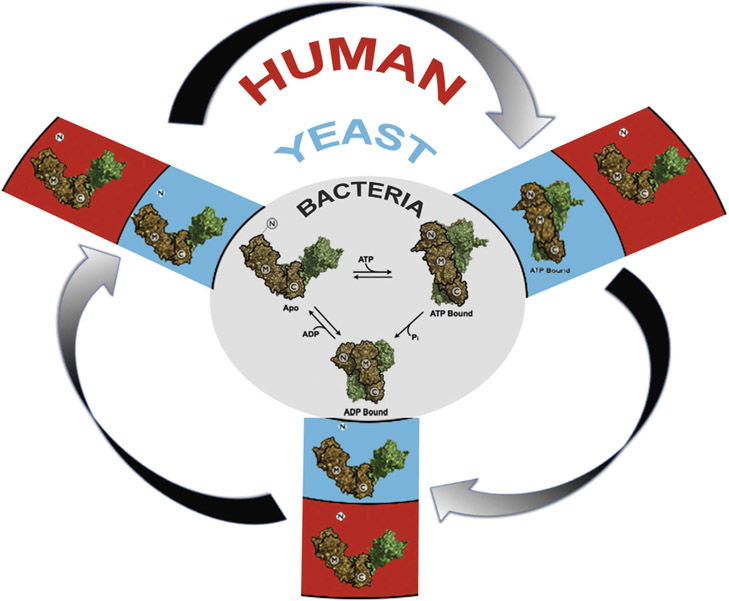
Although the data from HtpG fit the three-state conformational model described earlier, the data collected for both yeast and human Hsp90 seem not to support the generality of the model. Moreover, yeast and human Hsp90 seem to display distinctly different conformational responses to nucleotide. However, by using a cross-linking technique to trap rarely populated conformational states, Southworth and Agard [18] show that, indeed, both yeast and human Hsp90 proteins conform to the three-state model that is more easily observable in HtpG. Their data support and help to explain recent kinetic studies that suggest a conserved ATPase cycle among Hsp90 proteins from different species and provide evidence for transient N-domain dimerization in human Hsp90 [19,20]. Perhaps unexpectedly, an important conclusion stemming from these observations is that, whereas the three-state conformational model describing the Hsp90 chaperone cycle is indeed conserved from bacteria to humans, the population occupancy of each conformation at equilibrium is unique for different species (Figure 1). This, in turn, suggests that evolutionary processes have optimized the kinetics of the Hsp90 cycle to meet the distinct chaperoning requirements of each species [18].
Figure 1.
Species-dependent conformational equilibrium model for the Hsp90 molecular chaperone cycle. Whereas E. coli Hsp90 (gray), yeast Hsp90 (blue) and human Hsp90 (red) all can be found to occupy an ‘open’ (apo), ‘closed’ (ATP) and ‘compact’ (ADP) conformation; steady-state occupancy of each conformation is a unique characteristic of each species. Nonetheless, multiple conformational states are sampled to one degree or another by the apo-Hsp90s in each species. N-, M- and C-domains are labeled as shown; individual Hsp90 protomers are colored brown or green, respectively.
Implications and future directions
It has been difficult to reconcile the slow and species-dependent rate of ATP hydrolysis catalyzed by Hsp90 with the concept that the conformational changes of the chaperone are driven by nucleotide binding and hydrolysis. It has also been unpalatable to consider that the mechanics of the Hsp90 cycle might not be universally shared among species. The data presented by Southworth and Agard [18] now provide an elegant solution to both of these difficulties, and they might help to explain the disparate rates of ATP hydrolysis by Hsp90 proteins of different organisms [14]. Moreover, it also has not been clear why eukaryotic Hsp90s, unlike HtpG, require additional ‘co-chaperones’ for function, nor why, again unlike the bacterial chaperone, the N- and M-domains of the eukaryotic chaperone have become separated by a long, unstructured linker region. A frequent speculation proposes that the greater structural diversity and number of client proteins in eukaryotes has required both increased flexibility of Hsp90 and a need to more finely regulate its ATPase activity. In considering the species dependence of the conformational equilibrium of Hsp90, it would be intriguing to determine whether the length and composition of the linker region has any role in fixing the steady-state distribution of conformational states. Likewise, the appearance of co-chaperones in eukaryotes might be related to the disparate energetics of the bacterial and eukaryote Hsp90 cycles. According to Southworth and Agard [18], HtpG–ATP binding is essentially isoenergetic with N-domain dimerization, which is probably the rate-limiting step of the overall chaperone cycle. By contrast, yeast Hsp90 undergoes a shift to the closed state (N-domains dimerized) in the presence of ATP, whereas a conformational change in human Hsp90 remains unfavorable under these conditions. Various co-chaperones are known to accelerate (e.g. Ahal [activator of heat shock protein 90 ATPase]) or inhibit (e.g. Hop [Hsp organizing protein], Cdc37 [cell-division cycle 37 homolog] and p23) the ATPase activity of Hsp90, and it is certainly possible that the unique energetics of the yeast and human Hsp90 cycle requires their participation to properly regulate the process (in a species-dependent manner) [12]. The involvement of a panoply of co-chaperones to assist in, stabilize and promote the dissolution of Hsp90 N-terminal dimerization probably provides a much greater degree of control of, and ability to fine tune, Hsp90 function. Post-translational modification of eukaryotic Hsp90 (e.g. phosphorylation and acetylation) can also impact both nucleotide and co-chaperone binding [21,22], thus providing a further layer of regulation of the Hsp90 cycle not found in bacteria, a requirement no doubt made necessary by the increasingly important utilization of Hsp90 in maintaining cellular and organismal homeostasis in the face of complex environmental fluctuations.
Several additional questions arise from these new observations (Box 1), but translating these exciting structural insights to a cellular context ultimately remains the next, daunting task for those investigators intrigued by the complex dance of Hsp90 crucial to its role in maintaining cellular homeostasis.
Box 1. Some future directions for further exploration.
Paralogs of eukaryotic Hsp90 exist in the endoplasmic reticulum (e.g. Grp94 [glucose regulated protein 94]) and mitochondria (e.g. Trap1 [TNF receptor-associated protein 1]) and they possess detectable ATPase activity, yet no co-chaperones have yet been identified in either subcellular compartment [19,23]. Does the three-state conformational model hold for Grp94 and Trap1? If it does hold, what are the respective conformational equilibria? Most Hsp90 inhibitors currently in development bind to the nucleotide pocket in the N-domain. Recently, two such inhibitors, radicicol and 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin), were reported to induce a compact conformation of Hsp90 [9]. Is this similar to the compact conformation induced by ADP and sampled by apo-Hsp90? Unlike nucleotide binding, does drug binding to yeast or human Hsp90 noticeably shift the conformational equilibrium? Can one explain the apparently increased affinity of cancer cell Hsp90 for these drugs by invoking a unique conformational equilibrium in which sampling of the ‘drug-favorable’ conformation is increased relative to normal cells (perhaps determined by Hsp90 usage, distinct co-chaperone interactions and/or post-translational modifications)?
References
- 1.Neckers L (2002) Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol. Med 8, S55–S61 [DOI] [PubMed] [Google Scholar]
- 2.Millson SH, et al. (2005) A two-hybrid screen of the yeast proteome for Hsp90 interactors uncovers a novel Hsp90 chaperone requirement in the activity of a stress-activated mitogen-activated protein kinase, Slt2p (Mpk1p). Eukaryot. Cell 4, 849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao R et al. (2005) Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120, 715–727 [DOI] [PubMed] [Google Scholar]
- 4.Isaacs JS et al. (2003) Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell 3, 213–217 [DOI] [PubMed] [Google Scholar]
- 5.Whitesell L and Lindquist SL (2005) HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 5, 761–772 [DOI] [PubMed] [Google Scholar]
- 6.Geller R et al. (2007) Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes Dev. 21, 195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp S and Workman P (2006) Inhibitors of the HSP90 molecular chaperone: current status. Adv. Cancer Res 95, 323–348 [DOI] [PubMed] [Google Scholar]
- 8.Barginear MF et al. (2008) The heat shock protein 90 chaperone complex: an evolving therapeutic target. Curr. Cancer Drug Targets 8, 522–532 [DOI] [PubMed] [Google Scholar]
- 9.Phillips JJ et al. (2007) Conformational dynamics of the molecular chaperone Hsp90 in complexes with a co-chaperone and anticancer drugs. J. Mol. Biol 372, 1189–1203 [DOI] [PubMed] [Google Scholar]
- 10.Ali MM et al. (2006) Crystal structure of an Hsp90-nucleotide-p23/ Sbal closed chaperone complex. Nature 440, 1013–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta R and Inouye M (2000) GHKL, an emergent ATPase/kinase superfamily. Trends Biochem. Sci 25, 24–28 [DOI] [PubMed] [Google Scholar]
- 12.Pearl LH and Prodromou C (2006) Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem 75, 271–294 [DOI] [PubMed] [Google Scholar]
- 13.Shiau AK et al. (2006) Structural Analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell 127, 329–340 [DOI] [PubMed] [Google Scholar]
- 14.Wandinger SK et al. (2008) The Hsp90 chaperone machinery. J. Biol. Chem 283, 18473–18477 [DOI] [PubMed] [Google Scholar]
- 15.Bron P et al. (2008) Apo-Hsp90 coexists in two open conformational states in solution. Biol. Cell 100, 413–425 [DOI] [PubMed] [Google Scholar]
- 16.Krukenberg KA et al. (2008) Multiple conformations of E. coli Hsp90 in solution: insights into the conformational dynamics of Hsp90. Structure 16, 755–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W et al. (2004) Biochemical and structural studies of the interaction of Cdc37 with Hsp90. J. Mol Biol 340, 891–907 [DOI] [PubMed] [Google Scholar]
- 18.Southworth DR and Agard D (2008) Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol. Cell 32, 631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frey S et al. (2007) The ATPase cycle of the endoplasmic chaperone Grp94. J. Biol. Chem 282, 35612–35620 [DOI] [PubMed] [Google Scholar]
- 20.Richter K et al. (2008) Conserved conformational changes in the ATPase cycle of human Hsp90. J. Biol. Chem 283, 17757–17765 [DOI] [PubMed] [Google Scholar]
- 21.Scroggins BT and Neckers L (2007) Post-translational modification of heat-shock protein 90: impact on chaperone function. Expert Opinion on Drug Discovery 2, 1403–1414 [DOI] [PubMed] [Google Scholar]
- 22.Scroggins BT et al. (2007) An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol. Cell 25, 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leskovar A et al. (2008) The ATPase cycle of the mitochondrial Hsp90 analog Trap1. J. Biol. Chem 283, 11677–11688 [DOI] [PubMed] [Google Scholar]