Abstract
The 2019 novel coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has occurred in China and around the world. SARS-CoV-2-infected patients with severe pneumonia rapidly develop acute respiratory distress syndrome (ARDS) and die of multiple organ failure. Despite advances in supportive care approaches, ARDS is still associated with high mortality and morbidity. Mesenchymal stem cell (MSC)-based therapy may be an potential alternative strategy for treating ARDS by targeting the various pathophysiological events of ARDS. By releasing a variety of paracrine factors and extracellular vesicles, MSC can exert anti-inflammatory, anti-apoptotic, anti-microbial, and pro-angiogenic effects, promote bacterial and alveolar fluid clearance, disrupt the pulmonary endothelial and epithelial cell damage, eventually avoiding the lung and distal organ injuries to rescue patients with ARDS. An increasing number of experimental animal studies and early clinical studies verify the safety and efficacy of MSC therapy in ARDS. Since low cell engraftment and survival in lung limit MSC therapeutic potentials, several strategies have been developed to enhance their engraftment in the lung and their intrinsic, therapeutic properties. Here, we provide a comprehensive review of the mechanisms and optimization of MSC therapy in ARDS and highlighted the potentials and possible barriers of MSC therapy for COVID-19 patients with ARDS.
Keywords: mesenchymal stem cells, cell therapy, acute respiratory distress syndrome, SARS-CoV-2, COVID-19, pneumonia
The epidemiology of novel coronavirus disease 2019 (COVID-19) and the management of ARDS
Since December 2019, an increasing number of patients have been infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes pneumonia in China (Chan et al., 2020; Chen et al., 2020a; Chinazzi et al., 2020; Ghinai et al., 2020; Guan et al., 2020; Kucharski et al., 2020; Le et al., 2020). The SARS-CoV-2 pneumonia is defined as a novel coronavirus disease 2019 (COVID-19) by World Health Organization (WHO). Human-to-human transmission has been occurring, and infections have been rapidly spreading in China and around the world (Chen et al., 2020a; Chinazzi et al., 2020; Kucharski et al., 2020). Some patients with severe pneumonia rapidly develop acute respiratory distress syndrome (ARDS) and require intensive care unit (ICU) admission (Guan et al., 2020; Holshue et al., 2020; Sun et al., 2020; Xu et al., 2020a). At this stage, patients worsen in a short period and die of multiple organ failures, such as severe respiratory failure, heart failure, and renal failure. As SARS-CoV-2 is an emerging virus infection, the pathological changes happening in severe patients need to be fully understood, and no effective treatment has been developed to prevent worsening of these severe patients and to treat ARDS due to pneumonia. Current management for SARS-CoV-2-infected patients with severe pneumonia and ARDS remains supportive, including anti-infection, intubated ventilator-assisted breathing therapy, and extracorporeal membrane oxygenation (ECMO) (Chen et al., 2020a; Guan et al., 2020; Kandel et al., 2020; Le et al., 2020; Xu et al., 2020a). Since ARDS is associated with high mortality and morbidity, it is urgent to develop new effective approaches to the control of ARDS.
ARDS is a devastating disorder characterized by acute and refractory hypoxia noncardiogenic pulmonary edema, diffuse alveolar-capillary membrane damage, and reduced compliance (Ranieri et al., 2012). Despite decades of research, there is still no effective pharmacotherapy for ARDS. Although some supportive care approaches are established, ARDS remains devastating and life-threatening (Bellani et al., 2016; Papazian et al., 2019).
A growing body of evidence has shown that cell-based therapy holds therapeutic effects for ARDS. Most studies have focused on the therapeutic effects of mesenchymal stem cells (MSCs), although some studies have also investigated the possible applications of pluripotent stem cells, pulmonary epithelial progenitors, and endothelial progenitor cells (Laffey and Matthay, 2017; Han et al., 2019; Lopes-Pacheco et al., 2019). Mounting studies have examined the efficacy of MSC therapy across a wide array of experimental ARDS models (Table 1). Importantly, some clinical trials have been completed or are in progress to evaluate the safety and efficacy of MSC therapy in patients with ARDS and COVID-19, shown in Table 1 (Zheng et al., 2014; Wilson et al., 2015; Matthay et al., 2019; Bing et al., 2020; Leng et al., 2020).
Table 1.
Preclinical and clinical studies to evaluate therapeutic efficacy of MSCs in ARDS
| MSC sources | Disease | Animal or clinical studies | Key findings | References |
|---|---|---|---|---|
| hUC-MSCs | COVID-19 | Clinical | Transplantion of hUC-MSCs was well tolerated and promoted the recovery in a 65-year-old female critically ill COVID-19 patients. | Bing et al. (2020) |
| Unknown human | COVID-19 | Clinical | Transplantation of MSCs improve the functional outcomes of seven patients with COVID-19 pneumonia, accompanied by the attenuation of inflammation and recovery of the immune system | Leng et al. (2020) |
| hM-MSCs | ARDS induced by the H7N9 virus | Clinical | MSC Transplantation significantly reduced the mortality of the H7N9-induced ARDS | Chen et al. (2020c) |
| hAD-MSCs | ARDS | Clinical (phase I) | Transplantation of MSCs was safe and well-tolerated in the patients. | Zheng et al. (2014) |
| hBM-MSCs | ARDS | Clinical (phase I) | Transplantation of MSCs was tolerated, without adverse effects and differences in the concentrations of IL-6, IL-8, angiopoietin-2, and advanced glycosylation end-product specific receptor (AGER) | Wilson et al. (2015) |
| hBM-MSCs | ARDS | Clinical (phase II) | Transplantation of MSCs improved the oxygenation index and reduced the level of angiopoietin-2 in the plasma. | Matthay et al. (2019) |
| hBM-MSCs | ARDS | Clinical (Compassionate use) | Both patients showed improvement with the resolution of respiratory, hemodynamic, and multiorgan failure. The beneficial effects were associated with a decrease in the biomarkers related to inflammation. | Simonson et al. (2015) |
| rLung-MSC | ARDS induced by LPS | Animal (rat) |
Reduced lung inflammation and pulmonary edema. A decrease in IL-1, IL-1 β, IL-6, and TNF-α levels. Restoration of Treg/Th17 balance. |
Wang et al. (2019) |
| mBM-MSCs | ARDS induced by HCL instillation | Animal (mouse) | Attenuation of fibrosis in the lung. | Islam et al. (2019) |
| mBM-MSCs | ARDS induced by LPS | Animal (mouse) |
Improved the differentiation of MSCs into alveolar epithelial cells. Restoration of the injured structure and function of alveolar epithelial cells. Reduced lung fibrosis. |
Zhang et al. (2019c) |
| rBM-MSCs | ARDS induced by LPS | Animal (rat) |
Improved oxygen saturation. Reduced lung inflammation and pulmonary edema. Reduced IL-6 and TNF-α levels. |
Mokhber Dezfouli et al. (2018) |
|
rBM-, rAD- rlung-MSCs |
ARDS induced LPS | Animal (rat) |
Improved lung function and reduced alveolar collapse. Reduced lung inflammation and lung fibrosis. Reduced TNF-α, IL-1β, KC, and TGF-β levels. Reduced apoptosis in the lung, kidney, and liver. |
Silva et al. (2018) |
| hUC-MSCs | ARDS induced by LPS | Animal (mouse) |
Mitigation of lung injuries. Changing the expression of ARDS-related genes, such as Cyp17a1, Nr1h4, Rps6ka6 Nol3, and Prkg2. |
Huang et al. (2018) |
| hM-MSCs | ARDS induced by LPS | Animal (mouse) |
Reduced lung inflammation and pulmonary edema. Reduced MPO activity and IL-1β level. Increased IL-10 level. |
Xiang et al. (2017) |
| mAD-MSCs | ARDS induced by LPS | Animal (mouse) |
Improved survival. Reduced lung inflammation. Reduced TNF-α and IL-6 levels. |
Pedrazza et al. (2017) |
| hUC-MSCs | ARDS induced by E. coli | Animal (mouse) |
Reduced lung inflammation. Increased bacterial clearance. Reduced alveolar wall thickening. Reduced IL-1α, IL-1β, IL-6, and TNF-α levels. |
Sung et al. (2016) |
| hBM-MSCs | ARDS induced by E. coli | Animal (mouse) |
MSCs transfer their mitochondria to macrophages. Increased phagocytosis activity of macrophages. |
Jackson et al. (2016) |
| hM-MSCs | ARDS induced by the cecal ligation and puncture | Animal (mouse) |
Improved survival. Enhanced bacterial clearance. Reduced inflammation. Reduced TNF-α, MCP1, IL-6, and IL-10 levels. |
Alcayaga-Miranda et al. (2015) |
| hBM-MSCs | ARDS induced by E. coli | Animal (mouse) |
Improved lung recovery. Enhanced bacterial clearance. Increased IL-10 and KGF levels. Reduced IL-16 level. |
Devaney et al. (2015) |
hM, human menstrual blood-derived; hBM, human bone marrow-derived; hAD, human adipose-derived; mBM, mouse bone marrow-derived; hUC, human umbilical cord-derived; mAD, mouse adipose tissue-derived; rlung, rat lung-derived; rBM, rat bone marrow-derived; rAD, rat adipose tissue-derived; E. coli, Escherichia coli; LPS, lipopolysaccharide; IL, interleukin; TNF, tumor necrosis factor; KGF, keratinocyte growth factor; MCP1, monocyte chemoattractant protein 1; TGF, transforming growth factor
In this review, we discussed the mechanisms of MSCs in treating ARDS and the optimization of MSC therapy and highlighted the perspectives related to the therapeutic applications of MSCs in COVID-19 patients with ARDS.
Mechanisms of MSC adoptive transfer in treating ARDS
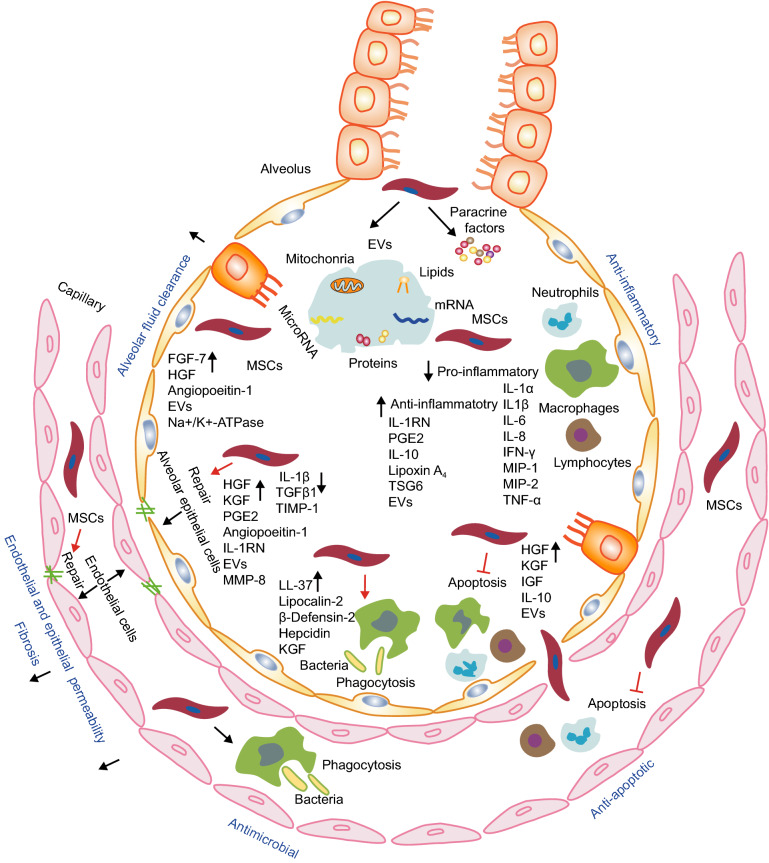
MSCs are harvested from a variety of sources, including the bone marrow, adipose tissue, placenta, and umbilical cord blood (Le Blanc and Mougiakakos, 2012). MSCs possess specific properties that make them attractive candidates for therapeutic use in kinds of diseases, including inflammatory autoimmune diseases, tissue damage repair, etc. They are non-immunogenic, have low tumorigenicity and short lifespan in vivo, possess immunomodulatory and anti-inflammatory effects, and also can detect injured microenvironments and direct their responses for triggering the regeneration process (Stappenbeck and Miyoshi, 2009; Le Blanc and Mougiakakos, 2012). Preclinical and clinical studies have suggested that in vivo administration of MSCs exerts anti-inflammatory and anti-apoptotic effects, enhances epithelial and endothelial cell recovery, promotes microbial and alveolar fluid clearance, and reduces lung and distal organ injuries in the treatment of ARDS (Fig. 1).
Figure 1.
The mechanisms of MSC therapy in ARDS. The therapeutic effects of MSCs in ARDS involve multiple mechanisms via their secretion of soluble paracrine protein factors and extracellular vesicles (EVs). MSCs can exert anti-inflammatory, anti-apoptotic, and anti-microbial effects, protect the pulmonary endothelial and alveolar epithelial cells, enhance alveolar fluid clearance, and inhibit lung fibrosis
Induction of anti-inflammatory response
In vivo transfer of MSCs can mitigate inflammation by reducing levels of inflammatory mediators and increasing levels of anti-inflammatory and pro-resolution factors in experimental ARDS models (Fig. 1). The anti-inflammatory effects of MSCs have been mostly attributed to their release of paracrine factors, because of low engraftment of donor-derived MSCs into at the host lung tissue after MSC therapy (Qin et al., 2012; Millar et al., 2019). In support of this, MSC-conditioned media have been suggested to reduce pro-inflammatory mediator levels and inflammatory cell counts in ARDS models (Ionescu et al., 2012; Goolaerts et al., 2014; Chen et al., 2015; Su et al., 2019; Xu et al., 2019). It was recently shown that certain therapeutic effects of MSCs depend on their ability to secrete extracellular vesicles. Extracellular vesicles can transfer messenger RNA (mRNA), microRNA, proteins, lipids, and even organelles such as mitochondria to the target cells and tissues, altering gene expression and modulating the behavior of target cells to attenuate the inflammatory response, consequently mediating and exerting MSC therapeutic effects (Hao et al., 2019; Lee et al., 2019; Abraham and Krasnodembskaya, 2020).
Accumulating evidence demonstrates that patients with severe COVID-19 suffer from the hyperinflammatory syndrome or cytokine storm syndrome, characterized by hypercytokinaemia, such as an increase in the levels of interleukin (IL)-1β, IL-2, Interferon γ-induced protein 10 (IP10), granulocyte colony-stimulating factor (GCSF), IL-7, interferon γ (IFNγ), monocyte chemotactic protein 1 (MCP1), macrophage Inflammatory Protein 1 α (MIP1α), and Tumor necrosis factor α (TNFα) (Chen et al., 2020a; Haberman et al., 2020; Koff and Williams, 2020; Mehta et al., 2020; Schett et al., 2020). A retrospective, multicentre study of 150 COVID-19 patients, showed that the patients that died had higher levels of serum ferritin, C-reactive proteins, and IL-6 as compared to the survivors, indicating that hyperinflammation contributed to death (Schett et al., 2020; Stebbing et al., 2020; Vaninov, 2020). Similarly, a retrospective, multicentre cohort study containing 191 COVID-19 patients also found increased ferritin and IL-6 (Mehta et al., 2020; Vaninov, 2020). Yang et al., conducted a multiplex screen for 48 cytokines in 53 moderate and severe COVID-19 patients and revealed a significant increase in the levels of 14 cytokines in COVID-19 patients as compared with healthy controls. Among them, the increased IP-10, MCP3, hepatocyte growth factor (HGF), monokine induced gamma interferon (MIG), and MIP1α were closely linked to the disease severity (Yang et al., 2020). Moreover, highly proinflammatory monocyte-derived FCN1+ macrophages were present in bronchoalveolar lavage fluid of the severe COVID-19 patients but not the mild patients (Liao et al., 2020). A markedly higher number of CD14+CD16+ inflammatory monocytes were observed in the peripheral blood of severe COVID-19 patients compared to mild cases (Zhou et al., 2020b). These data suggest that anti-inflammatory effects conferred by MSCs might suppress the hyperinflammatory syndrome in severe COVID-19 patients.
Reduction of cell apoptosis
Apoptosis of both resident and immune cells contribute to ARDS progression, as apoptotic cells recruit inflammatory cells and result in tissue remodeling. In vivo administration of MSCs into ARDS models can reduce the apoptotic cell counts in the lung tissues and distal organs in ARDS (Silva et al., 2018). MSCs have also been reported to reduce apoptosis of the alveolar epithelial cells and endothelial cell by secretion of keratinocyte growth factor (KGF), angiopoietin-1, and HGF (Bernard et al., 2018; Chen et al., 2019a; Meng et al., 2019). Alveolar macrophage apoptosis induced by lipopolysaccharide (LPS) was also attenuated after MSC treatment, which may be partially mediated by inhibition of the Wnt/β-catenin pathway (Li et al., 2015). A reduction of tumor necrosis factor-α (TNF-α) that induces cell death may also contribute to the anti-apoptotic effects of MSC therapy (Kim et al., 2011; Güldner et al., 2015).
Lymphocytopenia is common in COVID-19 patients, which mostly impairs the CD4+ T cell subsets (Chen et al., 2020b; Goyal et al., 2020; Yang et al., 2020). The apoptosis of lymphocytes induced by SARS-CoV-2 infection leads to lymphocytopenia in critically ill patients with COVID-19 pneumonia (Chen et al., 2020b; Zheng et al., 2020). Mild patients have only mild lymphocytopenia, whereas severe patients have severe lymphocytopenia, suggesting apoptosis of lymphocytes reflects the severity of COVID-19 pneumonia to some extent. Moreover, SARS-CoV-2 infection results in apoptosis of alveolar epithelial cells and endothelial cells (Monteil et al., 2020; Varga et al., 2020; Walls et al., 2020). For this reason, MSC-based therapy may bring about beneficial effects for COVID-19 patients possibly by inhibiting apoptosis of resident cells and immune cells.
Initiation of the anti-microbial innate response
MSCs therapy has been proven to promote bacterial clearance in the lung in animal models of ARDS. Such effects of MSCs are associated with the secretion of anti-microbial peptides and proteins, including LL-37, lipocalin-2, and β-defensin-2, hepcidin, and KGF (Krasnodembskaya et al., 2010; Gupta et al., 2012; Alcayaga-Miranda et al., 2015; Sung et al., 2016). Alternative mechanisms underlying the bacterial clearance by MSCs include, at least partially, the enhancement of the phagocytic activity of macrophages and monocytes via promoting mitochondrial transfer via tunneling nanotubes in the model of ARDS (Krasnodembskaya et al., 2012; Lee et al., 2013; Jackson et al., 2016). Therefore, these studies together show that MSCs can enhance the innate immune responses against bacterial infection via direct and indirect mechanisms.
Some critically ill patients with COVID-19 had co-infections of bacteria and fungi. A retrospective study of 113 deceased patients showed many of them developed secondary bacterial infections, which were highly associated with death (Chen et al., 2020b). Another study of 339 elderly patients with COVID-19 showed that about 42% of the patients developed bacterial infections, and bacterial infection was increased significantly in dead patients (Wang et al., 2020a). Empirical antibacterial therapy could promote recovery (Chen et al., 2020b). It is speculated that MSC transplantation may mitigate bacterial infections in severe patients with COVID-19.
Protection of pulmonary endothelial cells and alveolar epithelial cell damage
Breakdown of alveolar-capillary membrane integrity is a hallmark of ARDS, which contributes to edema formation and tissue remodeling. MSC therapy has been shown to preserve or restore the alveolar epithelial and pulmonary endothelial lining by secreting HGF via extracellular vesicles, reducing inflammatory damage, and increasing autophagy (Yang et al., 2015b; Zhou and You, 2016; Hu et al., 2018; Meng et al., 2019). MSC therapy has also been demonstrated to differentiate directly into type II alveolar epithelial cells for the regeneration by activation of wnt/β-catenin signaling (Liu et al., 2013; Cai et al., 2015; Li et al., 2017; Zhang et al., 2019b). In addition to differentiation into alveolar epithelial cells, MSCs can protect the alveolar epithelial cells against the inflammation-induced damage and oxidative injuries by secretion of angiopoietin-1, interleukin 1 (IL-1) receptor antagonist (IL-1RN), prostaglandin E2 (PGE2), HGF, and KGF or scavenging oxidants and radicals (Fang et al., 2010; Goolaerts et al., 2014; Bernard et al., 2018; Yan et al., 2019). Moreover, MSCs can regulate tissue remodeling processes and attenuate the lung fibrosis by increasing metalloproteinase (MMP)-8 and decreasing the levels of tissue inhibitor of metalloproteinase (TIMP)-1, IL-1 β, and transforming growth factor-β1 (TGF-β1) in the animal model of ARDS (Maron-Gutierrez et al., 2013; Silva et al., 2018). Taken together, MSCs can protect pulmonary endothelial and epithelial cell damage and restore their impaired barrier functions.
The SARS-CoV-2 infects the host using the angiotensin-converting enzyme II (ACE2) receptor (Lan et al., 2020; Shang et al., 2020; Wang et al., 2020b; Yan et al., 2020; Yuan et al., 2020; Zhao et al., 2020; Zhou et al., 2020a), which is widely expressed on the alveolar epithelial type II cells and the endothelial cells in the lung and many other organs, including the heart, liver, kidney, and intestines. In patients with COVID-19, post-mortem analysis demonstrated that the SARS-CoV-2 infected the endothelial cells in the lungs, kidneys, intestines, hearts, and livers and caused endotheliitis and endothelial apoptosis (Varga et al., 2020). Moreover, the SARS-CoV-2 binds to endothelial cells in the lungs and subsequently activates bradykinin 1 receptor (B1R) and B2R on endothelial cells, which result in lung angioedema (van de Veerdonk et al., 2020). In vitro, SARS-CoV-2 can infect the human-engineered blood vessel organoids (Monteil et al., 2020). Alveolar epithelial type II cells produce surfactants that can reduce surface tension, which is essential for the gas exchange function. SARS-CoV-2 viruses infect alveolar epithelial type II cells and result in severe injuries to them in COVID-19 patients (Walls et al., 2020; Zhao et al., 2020; Zhou et al., 2020a). The observation of severe injuries to endothelial cells and alveolar epithelial type II cells by SARS-CoV-2 infection may provide a rationale for MSC transplantation therapy to protect the endothelial cells and alveolar epithelial cells in COVID-19 patients.
Improvement of alveolar fluid clearance
Removal of excessive alveolar and interstitial fluid facilitates lung function recovery in ARDS, as fluid impairs surfactant concentration and gas exchange. MSCs can improve alveolar fluid clearance by the secretion of paracrine factors and regulating the function of membrane channels and transporters. In vivo administration of MSCs has been shown to increase the alveolar fluid clearance in ARDS models and in ex vivo perfused human lung that is injured by endotoxin or Escherichia coli (Lee et al., 2013; Hao et al., 2015; Park et al., 2019; Zhang et al., 2019b). These effects may be associated with the secretion of fibroblast growth factor-7 (FGF-7) and extracellular microvesicles (Park et al., 2019). In a lung injury model induced by influenza infection, treatment with MSCs promotes the alveolar fluid clearance by secreting angiopoietin-1 and KGF and suppressing the downregulation of Na+/K+-ATPase (Chan et al., 2016). In the bronchoalveolar lavage fluid collected from COVID-19 patients, a large number of SARS-CoV-2 viruses are detected (Lu et al., 2020; Zhou et al., 2020a), and highly inflammatory monocyte-derived FCN1+ macrophages are present and responsible for producing enormous cytokines (Liao et al., 2020), indicating inflammatory microenvironment in the alveolar fluid. Accordingly, the promotion of alveolar fluid clearance by MSC transplantation may possibly contribute to the recovery of lung function in severe patients with COVID-19.
Attenuation of lung and distal organ injuries
Multiple organ failure can occur as ARDS aggravates, which ultimately results in increased morbidity and mortality. MSC administration can reduce the histopathological impairment of lung tissues and promote the functional recovery in the ARDS models (Mokhber Dezfouli et al., 2018; Silva et al., 2018; Zhang et al., 2019b). Administration of MSCs can also improve the repair and function recovery in other distal organs, including heart (Lee et al., 2009; Golpanian et al., 2016), liver (Lee et al., 2018; Silva et al., 2018), kidney (Perico et al., 2017; Silva et al., 2018), and gut (Garcia-Olmo and Schwartz, 2015; Molendijk et al., 2015). Therefore, MSC administration may not only improve the lung function recovery but also delay or suppress the progression of ARDS into multiple organ injuries. Since ACE2 is expressed in other many organs in addition to lungs, SARS-CoV-2 infection leads to injuries to multiple organs, including heart, kidney, liver, and nervous systems (Li et al., 2020; Ronco and Reis, 2020; Wang et al., 2020a; Xu et al., 2020b; Zhang et al., 2020). Given the confirmed beneficial effects for multiple organs after MSC transplantation, MSCs may be used to prevent and mitigate multiple organ failure in COVID-19 patients.
Strategies to optimize MSC therapy in ARDS
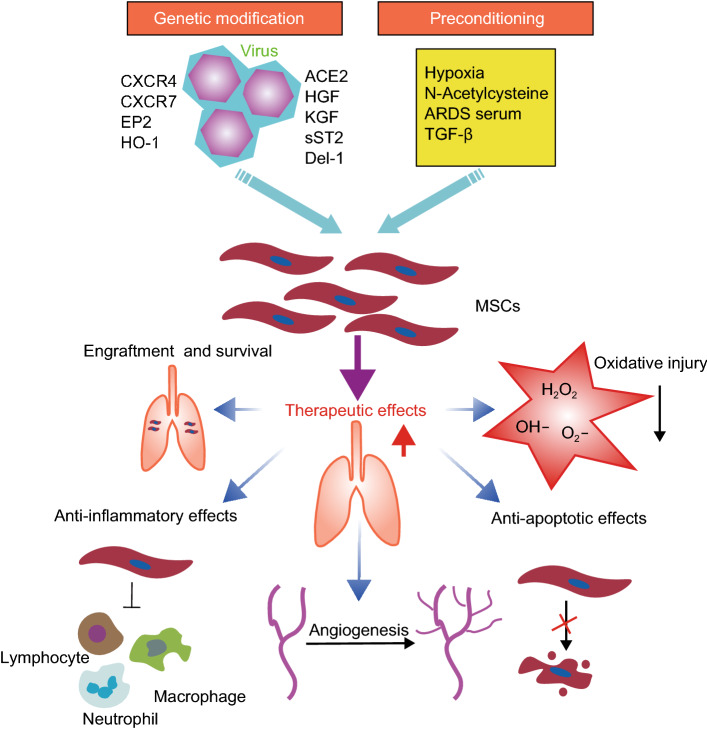
Low engraftment and poor survival of transplanted MSCs are obstacles for clinical translation of this therapy. Several strategies have been developed to improve MSC engraftment and survival in the lung in the experimental models of ARDS (Fig. 2).
Figure 2.
The strategies to optimize MSC therapy in ARDS. MSCs can be genetically modified to overexpress beneficial genes or pre-treated with a series of preconditioning strategies, which can promote their therapeutic effects. The improvement of therapeutic effects may depend on an increase in the engraftment and survival of MSCs in the lung, a decrease in the oxidative injury, and enhanced effects of anti-inflammation, anti-apoptosis, and angiogenesis
The strategies using genetic modification to overexpress beneficial genes on MSCs have enhanced the migration, survival, and therapeutic potential of MSCs transfer in the models of ARDS (Han et al., 2019). Stromal-derived factor-1 (SDF-1) and the chemokine receptor 4 (CXCR4) signaling axis directs the migration of MSCs. However, low expression of CXCR4 on MSCs impairs their homing to the damaged tissues. Overexpression of CXCR4 via viral vector has increased the homing and engraftment of MSCs into the injured lung of LPS-induced lung injury (ALI) or radiation-induced ALI models (Yang et al., 2015a; Zhang et al., 2019a). Similarly, overexpression of another SDF-1 receptor Cxcr7 in MSCs also enhances the therapeutic effects on LPS-ALI model (Shao et al., 2019). PGE2, released in the inflammatory site, can promote the migration of MSCs via activation of the E-prostanoid 2 (EP2) receptor (Goolaerts et al., 2014; Zhu et al., 2017; Xu et al., 2019). MSCs overexpressing EP2 increase their migration to the sites of lung injury in the mouse model of LPS-ALI (Han et al., 2019). In addition to improving the migration of MSCs, genetic modification enhances the intrinsic capacity of MSCs to reduce inflammation and apoptosis. Heme oxygenase-1 (HO-1) is a stress-response protein that has anti-inflammatory, anti-apoptotic, and anti-oxidative effects. Compared to the unmodified MSCs, MSCs transfected with HO-1 gene show higher potential to ameliorate cytokine levels in serum and the neutrophil counts and total protein concentration in the bronchoalveolar lavage fluid, and improve the histological structure of the injured lung in the LPS-induced ALI rat model (Chen et al., 2018; Chen et al., 2019b). Angiotensin-converting enzyme 2 (ACE2) can protect against lung injury by degrading the profibrotic peptide angiotensin II. Overexpression of the ACE2 gene in bone marrow-derived MSCs has been reported to increase the potential of MSCs to migrate to lung tissue, mitigate the inflammation, and endothelial injury in the LPS-induced ALI mouse model (He et al., 2015; Xu et al., 2018). Growth factors, such as HGF (Hu et al., 2016; Chen et al., 2017a; Meng et al., 2019), KGF (Chen et al., 2013), and angiopoietin-1 (Mei et al., 2007; Xu et al., 2008; Shao et al., 2018), have shown pro-angiogenic, anti-inflammatory, anti-oxidative, and pro-proliferation effects. MSCs modified with these growth factor genes have significantly improved their therapeutic effects on lung injury. Overexpression of anti-inflammatory and anti-oxidative molecules, including soluble IL-1 receptor-like-1 (sST2) (Martínez-González et al., 2013), developmental endothelial locus-1 (Del-1) (Zhao et al., 2014), and manganese superoxide dismutase (Chen et al., 2017b), in MSCs also have shown an enhanced ability to treat lung injury.
Nevertheless, viral transfection is associated with the risk of triggering oncogenes and tumorigenesis. Moreover, the establishment of genetically modified MSCs is time-consuming, and it may be hard to administrate genetically modified MSCss immediately following the onset of ARDS. An alternative approach, such as preparation and modification of allogenic MSCs to set up a cell bank for the application needs to be established.
A series of preconditioning strategies have been developed to enhance the therapeutic capacity of MSCs in animal ARDS models. Hypoxic culture of MSCs can stimulate the secretion of bioactive molecules, including vascular endothelial growth factor (VEGF), angiopoietin 1, HGF, insulin-like growth factor 1 (IGF-1), and bal-2, which are associated with pro-angiogenic, anti-apoptotic, and anti-oxidative effects (Chacko et al., 2010; Zhang et al., 2014). Treatment with hypoxia-conditioned MSCs also extends the survival of engrafted cells, improves pulmonary respiratory functions, and reduced inflammatory and pro-fibrotic factor production (Lan et al., 2015). Pre-treatment with N-Acetylcysteine, a precursor of glutathione with anti-oxidative effect, has been demonstrated to strengthen the therapeutic potential of MSCs in ALI mouse models by increasing MSC engraftment and survival rate in the lung tissue (Wang et al., 2013). Pre-activation with serum obtained from ARDS patients enhances the anti-inflammatory capacity of MSCs, evidenced by an increase in the secretion of IL-10 and IL-1-RN (Bustos et al., 2013). Moreover, the serum-preactivated MSCs are more effective in reducing lung injury scores, inflammatory cell counts, and pulmonary edema after transplantation into a mouse model of ARDS (Bustos et al., 2013). Moreover, pretreatment with low levels of TGF-β1 has been shown to increase the MSC survival in the lung following transplantation in a rat model of LPS-induced ALI (Li et al., 2016). These preconditioning strategies might be promising for increasing MSC potency in the management of ARDS.
Clinical trials of MSC therapy in patients with ARDS
To date, 13 clinical trials assessing the safety and efficacy of MSC therapy in ARDS patients have been registered in the US National Institutes of Health (https://clinicaltrials.gov). Three clinical trials have been completed. The first study to evaluate the safety of MSCs in treating ARDS patients was conducted in China (NCT01902082). Zheng et al., conducted a phase I, single-center, randomized, double-blind, placebo-controlled clinical trial, in which ARDS patients received an intravenous injection of allogenic adipose-derived MSCs (1 × 106 cells/kg) (Zheng et al., 2014). The in vivo administered MSCs seemed to be safe and well-tolerated in the patients. The two groups showed no significant difference in the length of hospital day, ventilator-free days, and ICU-free days within 4 weeks after the treatment (Zheng et al., 2014). Wilson et al., conducted another multicenter, open-label, dose-escalation, phase I clinical trials in the USA (NCT01775774) (Wilson et al., 2015). Patients with moderate-severe ARDS received a single intravenous injection of allogeneic bone marrow-derived MSCs at 1, 5, 10 × 106 cells/kg. All dose levels were tolerated, without administration-related adverse effects. No significant differences were observed in the concentrations of related biomarkers such as IL-6, IL-8, angiopoietin-2, and advanced glycosylation end-product specific receptor (AGER) (Wilson et al., 2015). The same group continued to conduct phase IIa clinical trials, in which patients received a high dose level of allogeneic bone marrow MSCs (10 × 106 cells/kg) (NCT02097641) (Matthay et al., 2019). No MSC-related hemodynamic and respiratory adverse effects were observed in all patients. Patients receiving MSC treatment showed an improvement in the oxygenation index. A reduced level of angiopoietin-2 in the plasma was found, indicating that MSC administration attenuated endothelial injury. In a Swedish case report, two patients with severe, refractory ARDS received an intravenous infusion of allogeneic bone marrow-derived MSCs (2 × 106 cells/kg) at a compassionate use (Simonson et al., 2015). Both patients showed improvement with the resolution of respiratory, hemodynamic, and multiorgan failure. The beneficial effects were associated with a decrease in the biomarkers related to inflammation (Simonson et al., 2015). In addition, transplantation of menstrual blood-derived MSCs could reduce the mortality in patients with H7N9 virus-induced ARDS without adverse effects after the five-year follow-up period in China (Chen et al., 2020c). Because H7N9 and COVID-19 share similar complications, MSC transplantation may be useful for treating COVID-19.
In China, about 10 clinical trials have been registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn/) to investigate the safety and efficacy of transplantation therapy of bone marrow or umbilical cord mesenchymal stem cells for COVID-19 patients with severe pneumonia or ARDS. Liang and colleagues reported that transplantation of human umbilical cord-derived MSCs could modulate the immune response and promote the functional recovery in a 65-year-old female patient with critically ill COVID-19 and severe complications such as respiratory failure and multiple organ failure (Bing et al., 2020). The patient received three doses of 5 × 107 million allogeneic umbilical cord-MSCs every three days. Following the second dose, the vital signs were improved, and she did not require the ventilator. Two days after the third dose, she was transferred out of the ICU. Recently, one published study involved 7 COVID-19 patients (1 critically serious ill, 4 serious ill, and 2 commons) in the MSC-treated group and 3 patients in the control group, with a 14-day follow-up (Leng et al., 2020). In the MSC-treated group, patients received one dose of MSC injection at 1 × 106 cells per kilogram of weight. No serious adverse side effects were observed in the MSC-treated groups. All the 7 patients in the MSC-treated group recovered. A few days after MSC transplantation, oxygen saturation and biomarkers for inflammation were decreased, and peripheral lymphocytes were increased. Moreover, transplanted MSCs were negative for ACE2, indicating MSCs are free from SARS-CoV-2 infection. This study suggests that MSCs can suppress hyperinflammation and improve the immune system. Some other study groups have also reported beneficial effects of MSC therapy for COVID-19 before the publication of their data. Accordingly, Atluri and colleagues consider MSCs as a potential alternative therapy for treating critically ill COVID-19 patients (Atluri et al., 2020).
Challenges for clinical use of MSC therapy in ARDS
Despite remarkable advances in the control of ARDS with MSC therapy, further research is needed to elucidate several issues, including the optimal MSC source and dose, the time window of MSC administration, administration routes, and frequency (single vs. multiple-dose regimen). Although bone marrow is the most common source for isolating MSCs, the harvesting procedure is invasive, and the cell numbers are limited. Moreover, ARDS affects the immunomodulatory effects of bone marrow MSCs and impairs their potential use for autologous transplantation (Antebi et al., 2018). Although several studies have evaluated the therapeutic effects of MSCs from other sources in ARDS (Zheng et al., 2014; Ren et al., 2018; Silva et al., 2018), it remains unclear about which one may provide superior therapeutic effects. Cell doses are critical for the clinical use of MSC therapy. In experimental models, the number of MSCs administered at a single dose range from 5 × 104 to 3.6 × 107 cells (McIntyre et al., 2016). In the clinical setting, this range in a 25-g mouse would correspond to 2 × 106 to 1.44 × 109 cells/kg in humans. Such quantities are faced with technical and operational challenges. Of note, some safety concerns may be associated with the use of high doses of MSCs. To date, 1 × 107 cells/kg is the highest dose ever used in clinical studies. Accordingly, the optimal number of MSCs administered should be clearly defined for treating ARDS to find a balance between therapeutic effects and undesired safety events. Although a single dose of MSCs has been shown to provide therapeutic benefits, more than one dose may be required to induce a more efficient tissue repair or even to maintain benefits.
The therapeutic window and index for MSC therapies should be further characterized in experimental studies of ARDS. MSC administration has been performed by either local or systemic routes in different experimental models. Local administration via intratracheal infusion delivers cells directly to the site of injury, whereas systemic administration via intravenous infusion allows wide distribution throughout the body. MSCs administered intravenously would encounter the first-class pulmonary effect (Fischer et al., 2009), which results in significant retention of cells in the lung, thereby providing advantages for lung tissue repair. Therefore, ongoing clinical trials and most experimental studies have used the intravenous route for MSC administration. ECMO has been a common therapeutic strategy for patients with severe ARDS. However, MSCs administered intravenously were found to adhere to membrane oxygenator fibers during ECMO in an ex vivo model of ARDS, resulting in a significant reduction of flow through the circuit (Millar et al., 2019). Thus, MSC transfer should be performed before ECMO or during a pause in the flow or by intratracheal infusion. Especially for patients with severe ARDS requiring continuous high-flow ECMO, intratracheal administration may be optional for this clinical situation.
Conclusions and perspectives
Tremendous progress has been made in investigating MSC-based therapy in experimental ARDS models and patients with ARDS. The safety of MSC therapy has also been demonstrated in early-stage clinical studies with a relatively small number of patients.
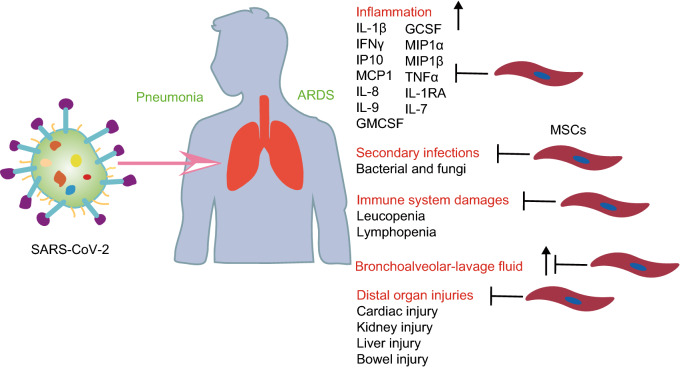
SARS-CoV-2 infections caused severe pneumonia and ARDS, with significant pathophysiological changes (Fig. 3). Pulmonary inflammation and extensive lung injury are detected, evidenced by high levels of proinflammatory cytokines in serum (eg., IL-1β, IFNγ, IP10, MCP1, GCSF, MIP1α, MIP1β, and TNFα) (Cao et al., 2020; Chen et al., 2020a; Haberman et al., 2020; Mehta et al., 2020; Schett et al., 2020). Moreover, patients can have secondary infections. In view of the high levels of cytokines induced by SARS-CoV-2 infections, MSC therapy is likely to reduce inflammation-induced lung injury in SARS-CoV-2-infected patients with ARDS. In addition, the blood counts of patients showed leukopenia and lymphopenia, indicating that SARS-CoV-2 consumes immune cells (especially, lymphocytes) and impairs the body’s immune system functions (Cao et al., 2020; Chen et al., 2020a). Administration of MSCs may promote the recovery of the mounts of white blood cells and lymphocytes because MSCs have shown immunomodulatory and anti-apoptotic effects that protect macrophages, neutrophils, and monocytes against apoptosis. Patients with SARS-CoV-2–infected pneumonia may become worse to develop distal organ injuries such as acute cardiac injury, acute kidney injury, liver injury, and bowel injury (Chan et al., 2020; Chen et al., 2020a; Zhu et al., 2020). Given that the systemic administration of MSC has shown protective effects on these distal organs, MSC treatment may decrease the progression of patients with ARDS into multiple organ failure. Apart from the above benefits, MSC can enhance epithelial and endothelial recovery and promote microbial and alveolar fluid clearance by their paracrine secretion, transfer of extracellular vesicles, or cell-cell contact. Therefore, MSC-based therapy may be a potential alternative strategy for treating COVID-19 patients with ARDS.
Figure 3.
The potential of MSC-based therapy in COVID-19 patients with severe pneumonia and ARDS by targeting pathophysiological changes. SARS-CoV-2 infections caused severe pneumonia and ARDS, with significant pathophysiological changes, including inflammation, immune system damages (leukopenia and lymphopenia), secondary infections, and distal organ injuries. However, MSCs have the potential to target these pathophysiological events, acting as a alternative strategy for treating COVID-19 patients with ARDS
Acknowledgments
ABBREVIATIONS
ACE2, angiotensin-converting enzyme II; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; B1R, bradykinin 1 receptor; COVID-19, 2019 novel coronavirus disease; CXCR4, chemokine receptor 4; ECMO, extracorporeal membrane oxygenation; EP2, E-prostanoid 2; FGF-7, fibroblast growth factor-7; GCSF, granulocyte colony-stimulating factor; HGF, hepatocyte growth factor; HO-1, Heme oxygenase-1; ICU, intensive care unit; IFN γ, interferon γ; IL, interleukin; IL-1RN, interleukin 1 receptor antagonist; IP10. Interferon γ-induced protein 10; KGF, keratinocyte growth factor; LPS, lipopolysaccharide; MCP1, monocyte chemotactic protein 1; MIG, monokine induced gamma interferon; MIP1 α, macrophage Inflammatory Protein 1 α; mRNA, messenger RNA; MSC, Mesenchymal stem cell; MMP, metalloproteinase; PGE2, prostaglandin E2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SDF-1, Stromal-derived factor-1; TGF-β1, transforming growth factor-β1; TIMP, tissue inhibitor of metalloproteinase; TNFα, Tumor necrosis factor α
COMPLIANCE WITH ETHICS GUIDELINES
Hua Qin and Andong Zhao declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Abraham A, Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9:28–38. doi: 10.1002/sctm.19-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcayaga-Miranda F, Cuenca J, Martin A, Contreras L, Figueroa FE, Khoury M. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther. 2015;6:199. doi: 10.1186/s13287-015-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antebi B, Mohammadipoor A, Batchinsky AI, Cancio LC. The promise of mesenchymal stem cell therapy for acute respiratory distress syndrome. J Trauma Acute Care Surg. 2018;84:183–191. doi: 10.1097/TA.0000000000001713. [DOI] [PubMed] [Google Scholar]
- Atluri S, Manchikanti L, Hirsch JA. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23:E71–E83. [PubMed] [Google Scholar]
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- Bernard O, Jeny F, Uzunhan Y, Dondi E, Terfous R, Label R, Sutton A, Larghero J, Vanneaux V, Nunes H, Boncoeur E, Planès C, Dard N. Mesenchymal stem cells reduce hypoxia-induced apoptosis in alveolar epithelial cells by modulating HIF and ROS hypoxic signaling. Am J Physiol Lung Cell Mol Physiol. 2018;314:L360–L371. doi: 10.1152/ajplung.00153.2017. [DOI] [PubMed] [Google Scholar]
- Bing L, Jun-hui C, Tao L, Hai-ying W, Wen-jie Y, Yan-jiao L, Jian-chun L, Cong-tao Y, Fang-ang N, Zhao-xia M et al (2020) Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. ChinaXiv 02.00084v1 [DOI] [PMC free article] [PubMed]
- Bustos ML, Huleihel L, Meyer EM, Donnenberg AD, Donnenberg VS, Sciurba JD, Mroz L, McVerry BJ, Ellis BM, Kaminski N, Rojas M. Activation of human mesenchymal stem cells impacts their therapeutic abilities in lung injury by increasing interleukin (IL)-10 and IL-1RN levels. Stem Cells Transl Med. 2013;2:884–895. doi: 10.5966/sctm.2013-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S-x, Liu A-r, Chen S, He H-l, Chen Q-h, Xu J-Y, Pan C, Yang Y, Guo F-M, Huang Y-z, Liu L, Qiu H-B. Activation of Wnt/β-catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res Ther. 2015;6:65. doi: 10.1186/s13287-015-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299:C1562–C1570. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MCW, Kuok DIT, Leung CYH, Hui KPY, Valkenburg SA, Lau EHY, Nicholls JM, Fang X, Guan Y, Lee JW, Chan RWY, Webster RG, Matthay MA, Peiris JSM. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci USA. 2016;113:3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, Xing F, Liu J, Yip CC-Y, Poon RW-S, Tsoi H-W, Lo SK-F, Chan K-H, Poon VK-M, Chan W-M, Ip JD, Cai J-P, Cheng VC-C, Chen H, Hui CK-M, Yuen K-Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li C, Gao X, Li C, Liang Z, Yu L, Li Y, Xiao X, Chen L. Keratinocyte growth factor gene delivery via mesenchymal stem cells protects against lipopolysaccharide-induced acute lung injury in mice. PLoS ONE. 2013;8:e83303. doi: 10.1371/journal.pone.0083303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Hao H, Li C, Du Y, Hu Y, Li J, Liang Z, Li C, Liu J, Chen L. Mesenchymal stem cell conditioned medium promotes proliferation and migration of alveolar epithelial cells under septic conditions in vitro via the JNK-P38 signaling pathway. Cell Physiol Biochem. 2015;37:1830–1846. doi: 10.1159/000438545. [DOI] [PubMed] [Google Scholar]
- Chen S, Chen X, Wu X, Wei S, Han W, Lin J, Kang M, Chen L. Hepatocyte growth factor-modified mesenchymal stem cells improve ischemia/reperfusion-induced acute lung injury in rats. Gene Ther. 2017;24:3–11. doi: 10.1038/gt.2016.64. [DOI] [PubMed] [Google Scholar]
- Chen H-X, Xiang H, Xu W-H, Li M, Yuan J, Liu J, Sun W-J, Zhang R, Li J, Ren Z-Q, Zhang X-M, Du B, Wan J, Wu B-Y, Zeng Q, He K-L, Yang C. Manganese superoxide dismutase gene-modified mesenchymal stem cells attenuate acute radiation-induced lung injury. Hum Gene Ther. 2017;28:523–532. doi: 10.1089/hum.2016.106. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang Y, Wang W, Liu Z, Meng J, Han Z. Mesenchymal stem cells modified with heme oxygenase-1 have enhanced paracrine function and attenuate lipopolysaccharide-induced inflammatory and oxidative damage in pulmonary microvascular endothelial cells. Cell Physiol Biochem. 2018;49:101–122. doi: 10.1159/000492847. [DOI] [PubMed] [Google Scholar]
- Chen X-X, Tang L, Han Z-H, Wang W-J, Meng J-G. Coculture with bone marrow-derived mesenchymal stem cells attenuates inflammation and apoptosis in lipopolysaccharide-stimulated alveolar epithelial cells via enhanced secretion of keratinocyte growth factor and angiopoietin-1 modulating the Toll-like receptor-4 signal pathway. Mol Med Rep. 2019;19:1891–1902. doi: 10.3892/mmr.2019.9836. [DOI] [PubMed] [Google Scholar]
- Chen X, Wu S, Tang L, Ma L, Wang F, Feng H, Meng J, Han Z. Mesenchymal stem cells overexpressing heme oxygenase-1 ameliorate lipopolysaccharide-induced acute lung injury in rats. J Cell Physiol. 2019;234:7301–7319. doi: 10.1002/jcp.27488. [DOI] [PubMed] [Google Scholar]
- Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Di Wu, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, Chen L, Gao H, Lu X, Yu L, Dai X, Xiang C, Li L. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, Pastore Y, Piontti A, Mu K, Rossi L, Sun K, Viboud C, Xiong X, Yu H, Halloran ME, Longini IM, Vespignani A. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020 doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaney J, Horie S, Masterson C, Elliman S, Barry F, O’Brien T, Curley GF, O’Toole D, Laffey JG. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax. 2015;70:625–635. doi: 10.1136/thoraxjnl-2015-206813. [DOI] [PubMed] [Google Scholar]
- Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Olmo D, Schwartz DA. Cumulative evidence that mesenchymal stem cells promote healing of perianal fistulas of patients with Crohn’s disease-going from bench to bedside. Gastroenterology. 2015;149:853–857. doi: 10.1053/j.gastro.2015.08.038. [DOI] [PubMed] [Google Scholar]
- Ghinai I, McPherson TD, Hunter JC, Kirking HL, Christiansen D, Joshi K, Rubin R, Morales-Estrada S, Black SR, Pacilli M, Fricchione MJ, Chugh RK, Walblay KA, Ahmed NS, Stoecker WC, Hasan NF, Burdsall DP, Reese HE, Wallace M, Wang C, Moeller D, Korpics J, Novosad SA, Benowitz I, Jacobs MW, Dasari VS, Patel MT, Kauerauf J, Charles EM, Ezike NO, Chu V, Midgley CM, Rolfes MA, Gerber SI, Lu X, Lindstrom S, Verani JR, Layden JE. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the damaged heart: mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiol Rev. 2016;96:1127–1168. doi: 10.1152/physrev.00019.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goolaerts A, Pellan-Randrianarison N, Larghero J, Vanneaux V, Uzunhan Y, Gille T, Dard N, Planès C, Matthay MA, Clerici C. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol. 2014;306:L975–L985. doi: 10.1152/ajplung.00242.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020 doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, Du B, Li L-J, Zeng G, Yuen K-Y, Chen R-C, Tang C-L, Wang T, Chen P-Y, Xiang J, Li S-Y, Wang J-L, Liang Z-J, Peng Y-X, Wei L, Liu Y, Hu Y-H, Peng P, Wang J-M, Liu J-Y, Chen Z, Li G, Zheng Z-J, Qiu S-Q, Luo J, Ye C-J, Zhu S-Y, Zhong N-S. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güldner A, Maron-Gutierrez T, Abreu SC, Xisto DG, Senegaglia AC, Barcelos PRdS, Silva JD, Brofman P, de Abreu MG, Rocco PRM. Expanded endothelial progenitor cells mitigate lung injury in septic mice. Stem Cell Res Ther. 2015;6:230. doi: 10.1186/s13287-015-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman R, Axelrad J, Chen A, Castillo R, Yan Di, Izmirly P, Neimann A, Adhikari S, Hudesman D, Scher JU. Covid-19 in immune-mediated inflammatory diseases—case series from New York. N Engl J Med. 2020 doi: 10.1056/NEJMc2009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Li Y, Li Y. Strategies to enhance mesenchymal stem cell-based therapies for acute respiratory distress syndrome. Stem Cells Int. 2019;2019:5432134. doi: 10.1155/2019/5432134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q, Zhu Y-g, Monsel A, Gennai S, Lee T, Xu F, Lee J-W. Study of bone marrow and embryonic stem cell-derived human mesenchymal stem cells for treatment of escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells Transl Med. 2015;4:832–840. doi: 10.5966/sctm.2015-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q, Gudapati V, Monsel A, Park JH, Hu S, Kato H, Lee JH, Zhou L, He H, Lee JW. Mesenchymal stem cell-derived extracellular vesicles decrease lung injury in mice. J Immunol. 2019;203:1961–1972. doi: 10.4049/jimmunol.1801534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H-l, Liu L, Chen Q-h, Cai S-x, Han J-B, Hu S-L, Chun P, Yang Y, Guo F-M, Huang Y-z, Qiu H-B. MSCs modified with ACE2 restore endothelial function following LPS challenge by inhibiting the activation of RAS. J Cell Physiol. 2015;230:691–701. doi: 10.1002/jcp.24794. [DOI] [PubMed] [Google Scholar]
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Li J, Xu X, Liu A, He H, Xu J, Chen Q, Liu S, Liu L, Qiu H, Yang Y. The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res Ther. 2016;7:66. doi: 10.1186/s13287-016-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Park J, Liu A, Lee J, Zhang X, Hao Q, Lee J-W. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl Med. 2018;7:615–624. doi: 10.1002/sctm.17-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Liu H, Zhang X, Wen G, Zhu C, Zhao Y, Niu W, Qin Y, Chen H, Bai C, Liu G. Transcriptomic analysis of lung tissues after hUC-MSCs and FTY720 treatment of lipopolysaccharide-induced acute lung injury in mouse models. Int Immunopharmacol. 2018;63:26–34. doi: 10.1016/j.intimp.2018.06.036. [DOI] [PubMed] [Google Scholar]
- Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thébaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967–L977. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam D, Huang Y, Fanelli V, Delsedime L, Wu S, Khang J, Han B, Grassi A, Li M, Xu Y, Luo A, Wu J, Liu X, McKillop M, Medin J, Qiu H, Zhong N, Liu M, Laffey J, Li Y, Zhang H. Identification and modulation of microenvironment is crucial for effective mesenchymal stromal cell therapy in acute lung injury. Am J Respir Crit Care Med. 2019;199:1214–1224. doi: 10.1164/rccm.201802-0356OC. [DOI] [PubMed] [Google Scholar]
- Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, O’Kane CM, Krasnodembskaya AD. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel N, Chungong S, Omaar A, Xing J. Health security capacities in the context of COVID-19 outbreak: an analysis of International Health Regulations annual report data from 182 countries. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Chang YS, Choi SJ, Kim JK, Yoo HS, Ahn SY, Sung DK, Kim SY, Park YR, Park WS. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir Res. 2011;12:108. doi: 10.1186/1465-9921-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff WC, Williams MA. Covid-19 and immunity in aging populations—a new research agenda. N Engl J Med. 2020 doi: 10.1056/NEJMp2006761. [DOI] [PubMed] [Google Scholar]
- Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee J-W, Matthay MA. antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee J-W, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1003–L1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharski AJ, Russell TW, Diamond C, Liu Y, Edmunds J, Funk S, Eggo RM, Sun F, Jit M, Munday JD, Davies N, Gimma A, van Zandvoort K, Gibbs H, Hellewell J, Jarvis CI, Clifford S, Quilty BJ, Bosse NI, Abbott S, Klepac P, Flasche S. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. biology and potential therapeutic value. Am J Respir Crit Care Med. 2017;196:266–273. doi: 10.1164/rccm.201701-0107CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y-W, Choo K-B, Chen C-M, Hung T-H, Chen Y-B, Hsieh C-H, Kuo H-P, Chong K-Y. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther. 2015;6:97. doi: 10.1186/s13287-015-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020 doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Le HT, Nguyen LV, Tran DM, Do HT, Tran HT, Le YT, Phan PH. The first infant case of COVID-19 acquired from a secondary transmission in Vietnam. Lancet Child Adolesc Health. 2020 doi: 10.1016/S2352-4642(20)30091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- Lee C-W, Chen Y-F, Wu H-H, Lee OK. Historical perspectives and advances in mesenchymal stem cell research for the treatment of liver diseases. Gastroenterology. 2018;154:46–56. doi: 10.1053/j.gastro.2017.09.049. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park J, Lee J-W. Therapeutic use of mesenchymal stem cell-derived extracellular vesicles in acute lung injury. Transfusion. 2019;59:876–883. doi: 10.1111/trf.14838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187:751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min K-J, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Zhang H, Zeng M, He W, Li M, Huang X, Deng DYB, Wu J. Bone marrow mesenchymal stem cells protect alveolar macrophages from lipopolysaccharide-induced apoptosis partially by inhibiting the Wnt/β-catenin pathway. Cell Biol Int. 2015;39:192–200. doi: 10.1002/cbin.10359. [DOI] [PubMed] [Google Scholar]
- Li D, Liu Q, Qi L, Dai X, Liu H, Wang Y. Low levels of TGF-β1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol Med Rep. 2016;14:1681–1692. doi: 10.3892/mmr.2016.5416. [DOI] [PubMed] [Google Scholar]
- Li Y, Shi X, Yang L, Mou Y, Li Y, Dang R, Li C. Hypoxia promotes the skewed differentiation of umbilical cord mesenchymal stem cells toward type II alveolar epithelial cells by regulating microRNA-145. Gene. 2017;630:68–75. doi: 10.1016/j.gene.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Chen L, Li J, Wang X, Wang F et al (2020) The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing
- Liu A-r, Liu Le, Chen S, Yang Y, Zhao H-J, Liu L, Guo F-M, Lu X-M, Qiu H-B. Activation of canonical wnt pathway promotes differentiation of mouse bone marrow-derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J Cell Physiol. 2013;228:1270–1283. doi: 10.1002/jcp.24282. [DOI] [PubMed] [Google Scholar]
- Lopes-Pacheco M, Robba C, Rocco PRM, Pelosi P. Current understanding of the therapeutic benefits of mesenchymal stem cells in acute respiratory distress syndrome. Cell Biol Toxicol. 2019 doi: 10.1007/s10565-019-09493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron-Gutierrez T, Silva JD, Asensi KD, Bakker-Abreu I, Shan Y, Diaz BL, Goldenberg RCS, Mei SHJ, Stewart DJ, Morales MM, Rocco PRM, Dos Santos CC. Effects of mesenchymal stem cell therapy on the time course of pulmonary remodeling depend on the etiology of lung injury in mice. Crit Care Med. 2013;41:e319–e333. doi: 10.1097/CCM.0b013e31828a663e. [DOI] [PubMed] [Google Scholar]
- Martínez-González I, Roca O, Masclans JR, Moreno R, Salcedo MT, Baekelandt V, Cruz MJ, Rello J, Aran JM. Human mesenchymal stem cells overexpressing the IL-33 antagonist soluble IL-1 receptor-like-1 attenuate endotoxin-induced acute lung injury. Am J Respir Cell Mol Biol. 2013;49:552–562. doi: 10.1165/rcmb.2012-0406OC. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre LA, Moher D, Fergusson DA, Sullivan KJ, Mei SHJ, Lalu M, Marshall J, Mcleod M, Griffin G, Grimshaw J, Turgeon A, Avey MT, Rudnicki MA, Jazi M, Fishman J, Stewart DJ. Efficacy of mesenchymal stromal cell therapy for acute lung injury in preclinical animal models: a systematic review. PLoS ONE. 2016;11:e0147170. doi: 10.1371/journal.pone.0147170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei SHJ, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S-S, Guo F-M, Zhang X-W, Chang W, Peng F, Qiu H-B, Yang Y. mTOR/STAT-3 pathway mediates mesenchymal stem cell-secreted hepatocyte growth factor protective effects against lipopolysaccharide-induced vascular endothelial barrier dysfunction and apoptosis. J Cell Biochem. 2019;120:3637–3650. doi: 10.1002/jcb.27642. [DOI] [PubMed] [Google Scholar]
- Millar JE, von Bahr V, Malfertheiner MV, Ki KK, Redd MA, Bartnikowski N, Suen JY, McAuley DF, Fraser JF. Administration of mesenchymal stem cells during ECMO results in a rapid decline in oxygenator performance. Thorax. 2019;74:194–196. doi: 10.1136/thoraxjnl-2017-211439. [DOI] [PubMed] [Google Scholar]
- Mokhber Dezfouli MR, Jabbari Fakhr M, Sadeghian Chaleshtori S, Dehghan MM, Vajhi A, Mokhtari R. Intrapulmonary autologous transplant of bone marrow-derived mesenchymal stromal cells improves lipopolysaccharide-induced acute respiratory distress syndrome in rabbit. Crit Care. 2018;22:353. doi: 10.1186/s13054-018-2272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk I, Bonsing BA, Roelofs H, Peeters KCMJ, Wasser MNJM, Dijkstra G, van der Woude CJ, Duijvestein M, Veenendaal RA, Zwaginga J-J, Verspaget HW, Fibbe WE, van der Meulen-de Jong AE, Hommes DW. Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2015;149:918-27.e6. doi: 10.1053/j.gastro.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian L, Aubron C, Brochard L, Chiche J-D, Combes A, Dreyfuss D, Forel J-M, Guérin C, Jaber S, Mekontso-Dessap A, Mercat A, Richard J-C, Roux D, Vieillard-Baron A, Faure H. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensiv Care. 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim S, Lim H, Liu A, Hu S, Lee J, Zhuo H, Hao Q, Matthay MA, Lee J-W. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019;74:43–50. doi: 10.1136/thoraxjnl-2018-211576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazza L, Cunha AA, Luft C, Nunes NK, Schimitz F, Gassen RB, Breda RV, Donadio MVF, Souza Wyse AT, Pitrez PMC, Rosa JL, de Oliveira JR. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. J Cell Physiol. 2017;232:3552–3564. doi: 10.1002/jcp.25816. [DOI] [PubMed] [Google Scholar]
- Perico L, Morigi M, Rota C, Breno M, Mele C, Noris M, Introna M, Capelli C, Longaretti L, Rottoli D, Conti S, Corna D, Remuzzi G, Benigni A. Human mesenchymal stromal cells transplanted into mice stimulate renal tubular cells and enhance mitochondrial function. Nat Commun. 2017;8:983. doi: 10.1038/s41467-017-00937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z-h, Xu J-f, Qu J-M, Zhang J, Sai Y, Chen C-m, Wu L, Yu L. Intrapleural delivery of MSCs attenuates acute lung injury by paracrine/endocrine mechanism. J Cell Mol Med. 2012;16:2745–2753. doi: 10.1111/j.1582-4934.2012.01597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Ren H, Zhang Q, Wang J, Pan R. Comparative effects of umbilical cord- and menstrual blood-derived MSCs in repairing acute lung injury. Stem Cells Int. 2018;2018:7873625. doi: 10.1155/2018/7873625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020 doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G, Sticherling M, Neurath MF. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020;20:271–272. doi: 10.1038/s41577-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Shen J, Zhou F, He D. Mesenchymal stem cells overexpressing Ang1 attenuates phosgene-induced acute lung injury in rats. Inhal Toxicol. 2018;30:313–320. doi: 10.1080/08958378.2018.1521483. [DOI] [PubMed] [Google Scholar]
- Shao Y, Zhou F, He D, Zhang L, Shen J. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed Pharmacother. 2019;109:1233–1239. doi: 10.1016/j.biopha.2018.10.108. [DOI] [PubMed] [Google Scholar]
- Silva JD, Lopes-Pacheco M, Paz AHR, Cruz FF, Melo EB, de Oliveira MV, Xisto DG, Capelozzi VL, Morales MM, Pelosi P, Cirne-Lima E, Rocco PRM. Mesenchymal stem cells from bone marrow, adipose tissue, and lung tissue differentially mitigate lung and distal organ damage in experimental acute respiratory distress syndrome. Crit Care Med. 2018;46:e132–e140. doi: 10.1097/CCM.0000000000002833. [DOI] [PubMed] [Google Scholar]
- Simonson OE, Mougiakakos D, Heldring N, Bassi G, Johansson HJ, Dalén M, Jitschin R, Rodin S, Corbascio M, El Andaloussi S, Wiklander OPB, Nordin JZ, Skog J, Romain C, Koestler T, Hellgren-Johansson L, Schiller P, Joachimsson P-O, Hägglund H, Mattsson M, Lehtiö J, Faridani OR, Sandberg R, Korsgren O, Krampera M, Weiss DJ, Grinnemo K-H, Le Blanc K. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl Med. 2015;4:1199–1213. doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su VY-F, Lin C-S, Hung S-C, Yang K-Y. Mesenchymal stem cell-conditioned medium induces neutrophil apoptosis associated with inhibition of the NF-κB pathway in endotoxin-induced acute lung injury. Int J Mol Sci. 2019 doi: 10.3390/ijms20092208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Chen J, Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study. Lancet Digit Health. 2020;2:e201–e208. doi: 10.1016/S2589-7500(20)30026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung DK, Chang YS, Sung S, Yoo HS, Ahn SY, Park WS. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta- defensin- 2 via toll- like receptor 4 signalling. Cell Microbiol. 2016;18:424–436. doi: 10.1111/cmi.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Netea MG, van Deuren M, van der Meer JW, de Mast Q, Brüggemann RJ, van der Hoeven H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. Elife. 2020 doi: 10.7554/eLife.57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol. 2020;20:277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-Y, Li X-Z, Wang L-B. Therapeutic implications of mesenchymal stem cells in acute lung injury/acute respiratory distress syndrome. Stem Cell Res Ther. 2013;4:45. doi: 10.1186/scrt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shi M, Tong L, Wang J, Ji S, Bi J, Chen C, Jiang J, Bai C, Zhou J, Song Y. Lung-resident mesenchymal stem cells promote repair of LPS-induced acute lung injury via regulating the balance of regulatory T cells and Th17 cells. Inflammation. 2019;42:199–210. doi: 10.1007/s10753-018-0884-6. [DOI] [PubMed] [Google Scholar]
- Wang L, He W, Yu X, Hu D, Bao M, Liu H, Zhou J, Jiang H. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen K-Y, Wang Q, Zhou H, Yan J, Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee J-W, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang B, Chen L, Wang X, Zhao Y, Wang Y, Xiang C. Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of LPS-induced acute lung injury. Int J Mol Sci. 2017 doi: 10.3390/ijms18040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Qu J, Cao L, Sai Y, Chen C, He L, Yu L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214:472–481. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- Xu X-P, Huang L-L, Hu S-L, Han J-B, He H-l, Xu J-Y, Xie J-F, Liu A-r, Liu S-Q, Liu L, Huang Y-z, Guo F-M, Yang Y, Qiu H-B. Genetic modification of mesenchymal stem cells overexpressing angiotensin II Type 2 receptor increases cell migration to injured lung in LPS-induced acute lung injury mice. Stem Cells Transl Med. 2018;7:721–730. doi: 10.1002/sctm.17-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Shao Y, Ye K, Qu Y, Memet O, He D, Shen J. Mesenchymal stem cell-derived exosomes attenuate phosgene-induced acute lung injury in rats. Inhal Toxicol. 2019;31:52–60. doi: 10.1080/08958378.2019.1597220. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;395:470. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang F-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Fu X, Jia Y, Ma X, Tao J, Yang T, Ma H, Liang X, Liu X, Yang J, Wei J. Nrf2/Keap1/ARE signaling mediated an antioxidative protection of human placental mesenchymal stem cells of fetal origin in alveolar epithelial cells. Oxid Med Cell Longev. 2019;2019:2654910. doi: 10.1155/2019/2654910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020 doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-X, Zhang N, Wang H-W, Gao P, Yang Q-P, Wen Q-P. CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J Biol Chem. 2015;290:1994–2006. doi: 10.1074/jbc.M114.605063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Chen Q-h, Liu A-r, Xu X-P, Han J-B, Qiu H-B. Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res Ther. 2015;6:250. doi: 10.1186/s13287-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shen C, Li J, Yuan J, Wei J, Huang F, Wang F, Li G, Li Y, Xing L, Peng L, Yang M, Cao M, Zheng H, Wu W, Zou R, Li D, Xu Z, Wang H, Zhang M, Zhang Z, Gao GF, Jiang C, Liu L, Liu Y. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Wu NC, Zhu X, Lee C-CD, So RTY, Lv H, Mok CKP, Wilson IA. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020 doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu L, Huo Y, Yang Y, Wang Y. Hypoxia-pretreated human MSCs attenuate acute kidney injury through enhanced angiogenic and antioxidative capacities. Biomed Res Int. 2014;2014:462472. doi: 10.1155/2014/462472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhu Y, Wang J, Hou L, Li W, An H. CXCR4-overexpressing umbilical cord mesenchymal stem cells enhance protection against radiation-induced lung injury. Stem Cells Int. 2019;2019:2457082. doi: 10.1155/2019/2457082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li Q, Liu W, Liu Z, Shen H, Zhao M. Mesenchymal stem cells alleviate acute lung injury and inflammatory responses induced by paraquat poisoning. Med Sci Monit. 2019;25:2623–2632. doi: 10.12659/MSM.915804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen J, Xue M, Tang Y, Xu J, Liu L, Huang Y, Yang Y, Qiu H, Guo F. Overexpressing p130/E2F4 in mesenchymal stem cells facilitates the repair of injured alveolar epithelial cells in LPS-induced ARDS mice. Stem Cell Res Ther. 2019;10:74. doi: 10.1186/s13287-019-1169-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensiv Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y-F, Xiong W, Wu X-L. Mesenchymal stem cell-based developmental endothelial locus-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. Mol Med Rep. 2014;9:1583–1589. doi: 10.3892/mmr.2014.1988. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W (2020) Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2 [DOI] [PMC free article] [PubMed]
- Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, Xu J. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, Xu Y, Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, You Z. Mesenchymal stem cells alleviate LPS-induced acute lung injury in mice by MiR-142a-5p-controlled pulmonary endothelial cell autophagy. Cell Physiol Biochem. 2016;38:258–266. doi: 10.1159/000438627. [DOI] [PubMed] [Google Scholar]
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020 doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Xiong Y, Xia Y, Zhang R, Tian D, Wang T, Dai J, Wang L, Yao H, Jiang H, Yang K, Liu E, Shi Y, Fu Z, Gao L, Zou L. Therapeutic effects of human umbilical cord-derived mesenchymal stem cells in acute lung injury mice. Sci Rep. 2017;7:39889. doi: 10.1038/srep39889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]