Abstract
Hepatocyte growth factor/scatter factor (HGF/SF) receptor c-Met is implicated in growth, invasion and metastasis of many tumors. Tumor cells harboring MET gene amplification are initially sensitive to c-Met tyrosine kinase inhibitors (TKI), but escape from long-term treatment has not been investigated. C-Met is a client of heat shock protein 90 (Hsp90) and is destabilized by Hsp90 inhibitors, suggesting that these drugs may inhibit tumors driven by MET amplification, although tumor escape under these conditions also has not been explored. Here, we evaluated the initial inhibitory effects of, and the likelihood of escape from, the Hsp90 inhibitor 17-allylamino-17-demethoxy-geldanamycin (17-AAG) and the c-Met TKI SU11274, using two cell lines harboring MET gene amplification. 17-AAG inhibited cell growth in both cell lines and induced substantial apoptosis, whereas SU11274 was only growth inhibitory in one cell line. In both cell lines, c-Met-dependent Akt, Erk and/or STAT3 signaling, as well as activation of the EGFR family, resumed shortly after treatment with c-Met TKI despite sustained c-Met inhibition. PKC δ upregulation may participate in reactivation of c-Met downstream signaling in both cell lines. In contrast to c-Met TKI, 17-AAG destabilized c-Met protein and durably blocked reactivation of downstream signaling pathways and EGFR family members. Our data demonstrate that downstream signaling in tumor cells overexpressing c-Met is not stably suppressed by c-Met TKI, even though c-Met remains fully inhibited. In contrast, Hsp90 inhibitors provide long-lasting suppression of c-Met-dependent signaling, and these drugs should be further evaluated in tumors driven by MET gene amplification.
Keywords: c-Met, Hsp90, 17-AAG, TKI, oncogene switching
Introduction
The proto-oncogene product c-Met is the receptor for hepatocyte growth factor/scatter factor (HGF/SF). Binding of HGF to c-Met induces receptor dimerization and trans-phosphorylation, providing docking sites for a number of signaling proteins and promoting activation of several signaling networks including phosphoinositide 3-kinase (PI3K)-AKT, Ras-MAPK, phospholipase Cγ (PLCγ) and SRC and signal transducer and activator of transcription (STAT).1 MET gene amplification and activating mutations have been identified in a range of primary human cancers,2-4 and dysregulation of c-Met plays a key role in tumor invasion and metastasis.1 Tumor cells harboring MET gene amplification express high levels of constitutively active c-Met protein. shRNA-mediated knockdown induces significant growth inhibition in such cell lines, but has little or no effect on cell lines lacking MET gene amplification.3 These data suggest that MET gene amplification may identify a subset of tumors addicted to c-Met-driven signaling and therefore sensitive to c-Met inhibitors.5,6
Protein tyrosine kinases such as c-Met have been shown to be novel targets for molecular cancer therapy, and tyrosine kinase inhibitors (TKIs) represent a promising treatment modality. However, no matter the targeted kinase or the TKI used, patients who initially respond to such therapy eventually develop resistance. Recently, multiple studies have been elucidating the mechanisms by which resistance occurs, and several mechanisms have been proposed. These include point mutation of the targeted kinase that interferes with drug binding,7 signaling pathway reactivation secondary to the upregulation of other kinases not targeted by the TKI,8 and escape through utilization of redundant activators of a signaling pathway.9 The latter two mechanisms together comprise a phenomenon known as ‘oncogene switching’, in which the cancer cell is able to re-wire its signaling pathways to compensate for specific inhibition of a targeted kinase. Continuous in vitro incubation with a specific TKI for several days is sufficient to supply the selective pressure necessary to trigger oncogene switching in some cell models.10,11 Whether cells harboring MET gene amplification are capable of evading the effects of c-Met TKI by oncogene switching is not known, but a recent report suggests it to be likely.12
Heat shock protein 90 (Hsp90) is a molecular chaperone that plays an important role in facilitating maturation, stability and activity of its clients.13 Many Hsp90 clients, including ErbB2, Akt, Bcr-Abl, mutated EGFR and mutated p53, play important roles in tumorigenesis, and the anti-tumor activity of Hsp90 inhibitors is currently being evaluated in numerous clinical trials.14 C-Met has been identified as an Hsp90 client, and Hsp90 inhibitors have been reported to destabilize c-Met.15,16 Hsp90 inhibitors also block c-Met-dependent activation of urokinase-type plasminogen activator, as well as HGF/SF-mediated cell scattering and in vitro invasion.17,18 Further, multiple c-Met-activated downstream signaling proteins including PDK1,19 AKT,20,21 RAF22 and STAT3,23 depend to some extent on association with HSP90. Based on these findings, Hsp90 inhibitors are expected to effectively block c-Met downstream signaling at multiple points and to inhibit the proliferation of cancer cells driven by MET gene amplification. However, the in vitro response of MET-dependent cancer cells to long-term exposure to Hsp90 inhibitors has not been rigorously examined, and the likelihood of oncogene switching under such conditions has not been determined.
In this study, we evaluated the effects of continuous exposure (4–6 days) to either the c-Met TKI SU11274 or the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG) in two cell lines (the non-small cell lung cancer cell line H1993 and the gastric carcinoma cell line MKN45) harboring MET amplification. While c-Met TKI produced durable c-Met inhibition, downstream signaling pathways became reactivated within several days, suggesting utilization of oncogene switching to allow cells to bypass the effects of c-Met inhibition. In contrast, long-term exposure to Hsp90 inhibitor durably blocked not only c-Met activity but also the activity of multiple downstream signaling pathways, while inducing significant apoptosis.
Results
Induction of apoptosis in H1993 cells by 17-AAG or SU11274.
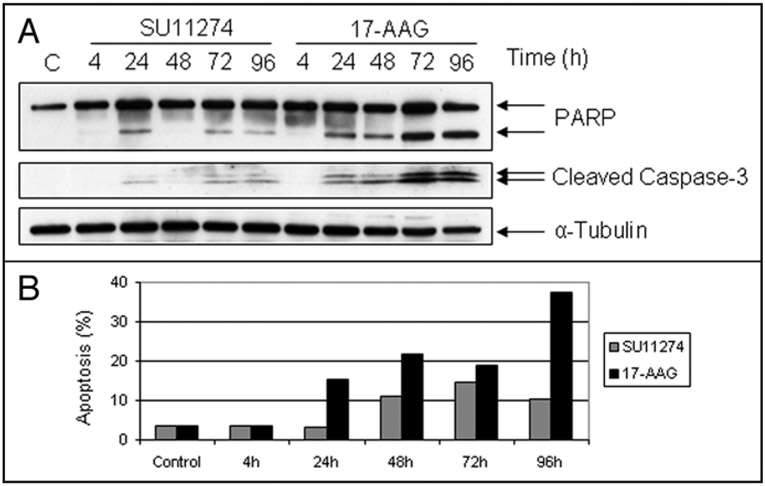
We treated the NSCLC cell line H1993, which harbors MET gene amplification, with the c-Met TKI SU11274 or with the Hsp90 inhibitor 17-Allylamino-17-demethoxy-geldanamycin (17-AAG) for up to 96 h, and we compared effects on PARP and caspase-3 cleavage, and on induction of apoptosis. While SU11274, by all measures examined, was a poor inducer of programmed cell death (Fig. 1), 17-AAG markedly stimulated PARP and caspase-3 cleavage, with a maximal response at 72 h, suggesting that Hsp90 inhibition induced substantial apoptosis in these cells (Fig. 1A). Flow cytometric analysis supported this hypothesis, showing that nearly 40% of H1993 cells underwent apoptosis by 96 h after drug treatment (Fig. 1B).
Figure 1.
Effects of c-Met TKI and Hsp90 inhibitor on induction of apoptosis. (A) H1993 cells were treated with SU11274 (2.5 μM) or 17-AAG (0.5 μM) for the indicated times, and analyzed by immunoblotting using PARP antibody (lower band represents the cleaved PARP) and cleaved Caspase-3 antibody. α-tubulin was used as a loading control. (B) H1993 cells were treated with SU11274 or 17-AAG for the indicated times. Cells were stained with propidium iodide, and the extent of apoptosis was calculated as described in Methods.
Disparate response of signaling pathways downstream of c-Met to long-term exposure of H1993 cells to SU11274 or 17-AAG.
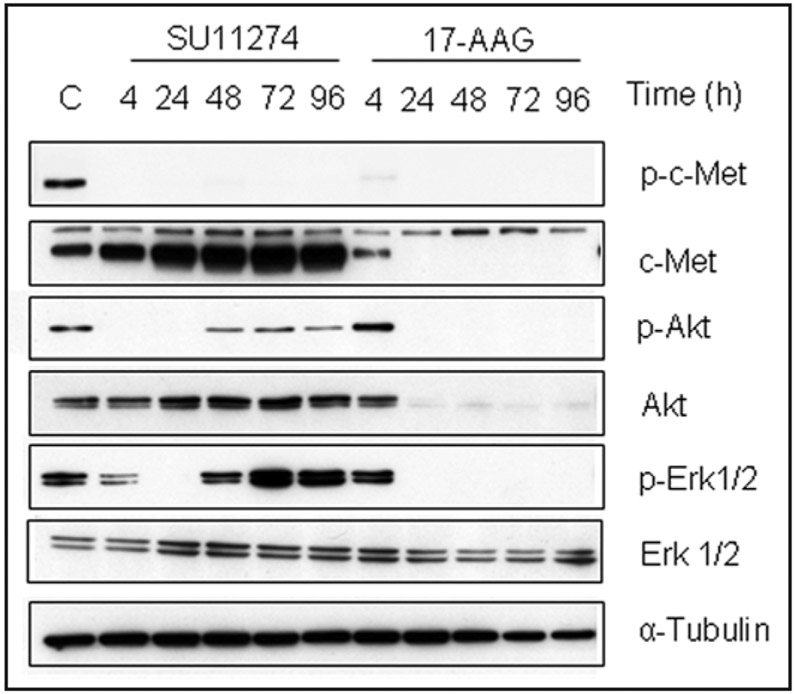
Auto-phosphorylation of c-Met on tyrosine Y1349 in its C-terminal tail provides a docking site for recruitment of signal transducers. Thus, phosphorylation of this site is necessary for activation of downstream signaling pathways including PI3K-AKT and Ras-MAPK/ERK. We therefore examined the effects of chronic exposure of H1993 cells to either SU11274 or 17-AAG on the activation status of these downstream signaling pathways (Fig. 2). Although SU11274 caused complete inhibition of c-Met auto-phosphorylation for the duration of the experiment, inhibition of Akt and Erk signaling was not durable. Akt and Erk phosphorylation, initially inhibited, rebounded by 48 h despite continuous drug exposure. In contrast, 17-AAG efficiently and durably downregulated c-Met and Akt protein expression, and blocked the activation of Erk1/2 presumably by destabilizing c-Raf, an intermediate in this signaling pathway.22
Figure 2.
Effects of c-Met TKI and Hsp90 inhibitor on Akt and Erk signaling. H1993 cells were treated with SU11274 or 17-AAG for the indicated times and lysates were immunoblotted as shown. ‘C’ = untreated cells.
Activation of EGFR family members is initially inhibited but rapidly recovers in the face of prolonged c-Met inhibition.
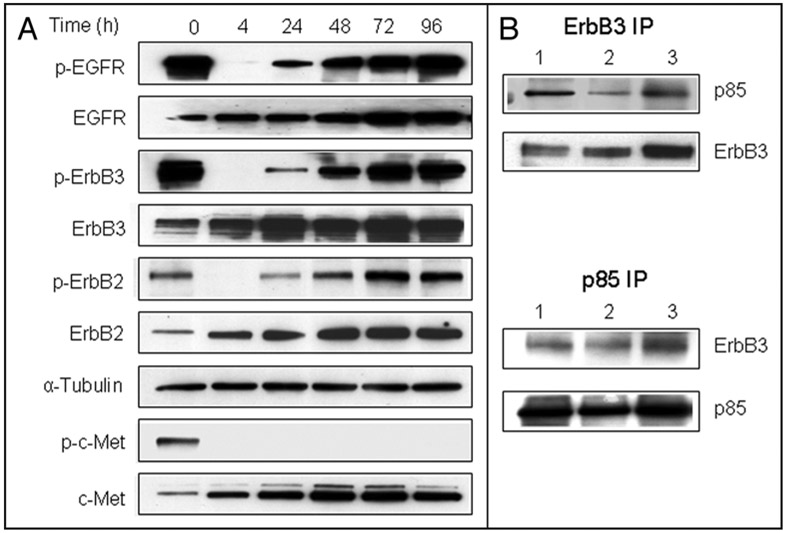
c-Met was reported to co-precipitate with EGFR family member ErbB3 and to activate ErbB3/PI3K signaling in tumor cell lines with MET amplification.8 Recent work showed that c-Met interacts with EGFR and overexpressed c-Met can directly activate EGFR.24 Therefore, we examined the activation status of the EGFR family in H1993 cells chronically exposed to SU11274 (Fig. 3A). Western blot analysis demonstrated that three EGFR family members—EGFR, ErbB2 and ErbB3—were constitutively activated in H1993 cells. Within 4 h of exposure to SU11274, phosphorylation of these three proteins was markedly inhibited. However, this inhibition was transient and activation status was restored within 48–72 h. Phosphorylated ErbB3 is the principal EGFR family member that recruits PI3K to activate the PI3K-Akt pathway.25 Reciprocal immunoprecipitation analysis confirmed the steady-state association between ErbB3 and the p85 subunit of PI3K in H1993 cells. In parallel with the transient inhibition of ErbB3 phosphorylation, ErbB3/PI3K association decreased after 4 h exposure to SU11274, but robustly reappeared by 48 h (Fig. 3B).
Figure 3.
Reactivation of EGFR family members in the presence of c-Met TKI. (A) H1993 cells were treated with SU11274 for the indicated times and lysates were immunoblotted with the indicated antibodies. (B) H1993 cells were treated with DMSO (lane 1), SU11274 for 4 h (lane 2) or 48 h (lane 3). Extracts were immunoprecipitated (IP) with anti-ErbB3 or anti-p85 antibodies. Immunoprecipitates were subjected to SDS-PAGE and immunoblotted with indicated antibodies.
PKC δ participates in reactivation of ErbB3 and Akt.
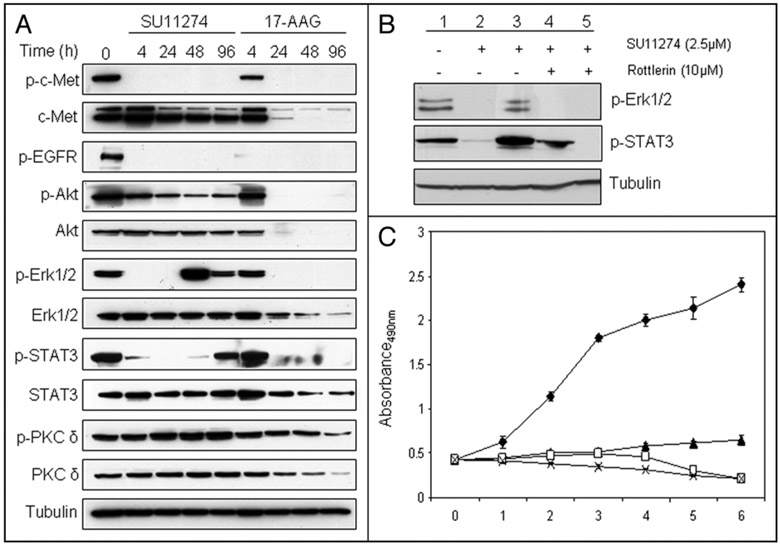
Restoration of ErbB3 activity can explain the reactivation of Akt in the face of continued c-Met inhibition. Because EGFR has been reported to be activated via a PKC δ-mediated pathway in some cell models,26,27 we next examined whether PKC δ activation was involved in the restoration of ErbB3 signaling and Akt activation in the presence of SU11274. First, western blot analysis showed that SU11274 significantly and time-dependently induced the expression of PKC δ protein. Phosphorylation of PKC δ at Ser664, important for the release of PKC δ from a detergent-insoluble fraction into the cytosol,28 also increased significantly over time in the presence of SU11274. In contrast, the Hsp90 inhibitor 17-AAG caused a decrease in both total PKC δ protein and phospho-Ser664 PKC δ, suggesting that PKC δ is an Hsp90 client (Fig. 4A). Next, we pretreated the cells with SU11274 for 48 h before adding, for the final 4 h, the PKC δ inhibitor rottlerin, GO6976 (an inhibitor blocking the activities of classical PKCs only), or CI-1033 (an irreversible ErbB TKI). Inclusion of rottlerin for the final 4 h effectively inhibited the re-activation of Akt, ErbB2 and ErbB3 (Fig. 4B). GO6976 was ineffective, while CI-1033 had an intermediate impact on the restoration of ErbB/AKT signaling. Taken together, these data suggest that PKC δ may participate in ErbB3 and Akt reactivation following chronic inhibition of c-Met.
Figure 4.
Involvement of PKC δ in the reactivation of EGFR family members and downstream Akt and Erk signaling following prolonged c-Met TKI treatment. (A) H1993 cells were treated with SU11274 or 17-AAG for the indicated times and analyzed by immunoblotting using anti-PKC δ or anti-PKC δ (pS664) antibodies. Tubulin was used as a loading control. (B) H1993 cells were treated with SU1274 for 4 h (lane 2) or 48 h (lane 3–6). CI-1033 (1.5 μM, lane 4), rottlerin (10 μM, lane 5), or GO6976 (10 μM, lane 6) were added 4 hours before the cells were harvested. Untreated (lane 1) or treated cells were analyzed by immunobloting with the indicated antibodies. (C) H1993 cells were incubated continuously with DMSO (◆), CI-1033 (1.5 μM, ∎), SU11274 (2.5 μM, ▴), 17-AAG (0.5 μM, *), SU11274 and CI-1033 (◻), or SU11274 and rottlerin (1 μM, ▵). Cell density was monitored for 6 consecutive days by MTS assay. Each point represents the mean of 4 determinations; error bars, SD.
We next tested the long-term effect of these inhibitors on proliferation of H1993 cells (Fig. 4C). After up to 6 days of incubation, SU11274 alone only moderately inhibited H1993 cell growth, while the combination of SU121274 and CI-1033 was much more effective. Surprisingly, treatment with CI-1033 alone had no impact on cell growth. Combination of SU11274 and rottlerin also was quite effective in inhibiting cell growth, while 17-AAG was most able to reduce cell number by 6 days, consistent with our earlier finding that 17-AAG induced significant apoptosis in H1993 cells.
17-AAG, but not SU11274, durably inhibits c-Met signaling and cell growth in MKN45 cells.
To verify the efficacy of 17-AAG in preventing escape from c-Met inhibition in an additional MET-amplified cell model, we examined the gastric cancer cell line MKN45. In this cell line, unlike the NSCLC cell line H1993, SU11274 exposure resulted in sustained Akt and EGFR inhibition, although a residual pool of activated Akt appeared to be unaffected by continuous exposure to c-Met TKI (Fig. 5A). Phosphorylation of ERK1/2 and STAT3, while transiently inhibited, reappeared within 48–96 h of continuous exposure to SU11274 (Fig. 5A). As was the case with H1993 cells, PKC δ phosphorylation was moderately increased over time in MKN45 cells after exposure to SU11274. As in H1993 cells, 17-AAG treatment resulted in robust and durable inhibition of each of these parameters.
Figure 5.
Effects of c-Met TKI and Hsp90 inhibitor on cell signaling and proliferation of MKN45 cells. (A) MKN45 cells were treated with SU11274 (2.5 μM) or 17-AAG (0.5 μM) for the indicated times, and analyzed by immunoblotting using specific antibodies. (B) MKN45 cells were treated with SU11274 for 4 h (lane 2) or 96 h (lane 3–5). Rottlerin (10 μM) was added for 4 h (lane 4) or 96 h (lane 5). Cell lysates were analyzed by immunobloting with the indicated antibodies. (C) MKN45 cells were incubated continuously with DMSO (◆), SU11274 (▴), 17-AAG (◻) or SU11274 and rottlerin (*), and cell proliferation was monitored by MTS assay. Each point represents the mean of 4 determinations; error bars, SD.
To investigate the role of PKC δ in the reactivation of ERK and STAT3 in this cell model following c-Met TKI treatment, MKN45 cells were co-treated with SU11274 together with the PKC δ-specific inhibitor rottlerin for either 4 h or 96 h. At both short and long incubation times, combination of rottlerin and SU11274 significantly inhibited the activation of both ERK and STAT3 (Fig. 5B). Unlike H1993 cells, SU11274-treated MKN45 cells were strongly growth inhibited for up to 6 days, although the combination of SU11274 and rottlerin proved more cytotoxic, as did single agent treatment with 17-AAG (Fig. 5C).
Discussion
Based on the currently accepted paradigm of ‘oncogene addiction’, oncogenic tyrosine kinases have been considered to be promising targets for anticancer drugs. TKIs have been developed to treat cancers driven by BCR-ABL,29 VEGFR,30 EGFR family members31,32 and MET.33,34 Uniformly, however, prolonged therapeutic inactivation of tyrosine kinases has created selective pressure for the development of cellular resistance mechanisms. In some cases, these mechanisms may involve a growth advantage for previously existing low-abundance mutations of the targeted kinase that abrogate TKI binding.7,35 Other mechanisms allow cancer cells to utilize the inherent redundancy of cellular signaling networks to bypass the inhibited kinase while maintaining activation of downstream pathways.9,11,35 Indeed, in NSCLC, MET amplification and c-Met activation have been suggested as one means of escape from, or inherent insensitivity to, EGFR TKI.8,36-38
Amplified c-Met has been reported to drive the activity of EGFR family members and, conversely, mutated and amplified EGFR can drive c-Met activity.24 Very recently, ligand-induced EGFR family kinase activation has been shown to confer initial resistance to c-Met TKI in MET-amplified gastric carcinoma cell lines, suggesting that, as is the case with other targeted kinases, escape from prolonged c-Met TKI exposure can readily occur.12
Our data indicate that critical downstream signaling pathways driven by c-Met, including Akt, Erk and STAT3 are spontaneously reactivated in MET-amplified NSCLC and gastric carcinoma cells chronically exposed to c-Met TKI. In H1993 cells, EGFR family members (EGFR, ErbB2 and ErbB3), after initially being inhibited in response to c-Met TKI as previously reported for a gastric carcinoma cell model,12 became reactivated over a period of many hours even in the absence of added EGFR family ligands. Not surprisingly, combined inhibition of ErbB proteins and c-Met resulted in significantly greater growth inhibition than did inhibition of either pathway alone. Since prolonged exposure (≥24 h) to c-Met TKI resulted in markedly enhanced expression of c-Met protein, residual (but undetectable in our assays) c-Met kinase activity might play a role in reactivating EGFR family members. However, other possibilities should not be excluded, and our data demonstrate that c-Met inhibitor-dependent activation of PKC δ at least partially mediates the reactivation process in both cell lines. This observation is not entirely without precedent, as PKC δ has previously been reported to activate EGFR and Erk in glioblastoma cells and in bronchial epithelial cells, respectively.26,27
In contrast to c-Met TKI, the Hsp90 inhibitor 17-AAG induced complete and durable inhibition, not only of c-Met itself, but also of the several downstream signaling pathways examined in this study. Reactivation of these signaling molecules remained undetectable for at least 96 hours without evidence of rebound, suggesting that 17-AAG is a potentially robust inhibitor of c-Met-driven cancers. Similar observations regarding ErbB-driven cancers have recently been reported.11 In the case of both ErbB- and c-Met-driven cancers, prolonged Hsp90 inhibition, in contrast to specific TKI, results in significant apoptosis, likely because the impact of Hsp90 inhibition is long-lasting and multifactorial. Importantly, although Hsp90 inhibitors are cleared rapidly from blood and normal tissues, pharmacologically active concentrations have been measured in tumor xenografts for at least 48 hours after a single bolus administration.39,40
Although TKIs are an emerging class of anticancer therapies that have shown promising clinical activity, initial or delayed resistance to single TKIs is a common occurrence, in part due to the plasticity of regulation of downstream signaling pathways. Given the fact that key components of these pathways, as well as many receptor tyrosine kinases that feed into them, depend to one degree or another on Hsp90 for their stability and activity, Hsp90 inhibitors might be expected to significantly reduce the likelihood of escape of tyrosine kinase-addicted cancers from targeted therapy.
Materials and Methods
Reagents.
SU11274, rottlerin and GO6976 were purchased from Calbiochem (San Diego, CA). 17-AAG was obtained from the National Cancer Institute (Bethesda, MD). CI-1033 was purchased from Selleck Chemicals Co., Ltd. (Riverside, CA).
Cell culture.
The non-small cell lung cancer (NSCLC) cell line H1993 and the gastric cancer cell line MKN45 were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, and incubated at 37°C in a humidified 5% CO2 atmosphere.
Western blot analysis.
Cells were treated with 17-AAG or SU11274 for the indicated times. Medium containing inhibitors was changed daily. Cells were washed with cold PBS and lysed in cell lysis buffer [20 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 10% glycerol, 50 mM NaF, 5 mM Na3VO4, 10 mM β-glycerophosphate, 2.5 mM sodium pyrophosphate] supplemented with complete protease inhibitor cocktail (Roche Applied Science, Basel, Switzerland). Protein concentration was measured using the BCA protein assay reagent (Pierce Biotechnology, Rockford, IL). 10–30 μg protein from each lysate was separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with antibodies specific for α-tubulin (as loading control; Calbiochem, San Diego, CA), ErbB3 (Santa Cruz Biotechnology, Santa Cruz, CA), ErbB2 and ErbB2 pTyr1248 (Lab Vision, Freemont, CA), PKCδ pS664 and EGFR pTyr1086 (Invitrogen, Carlsbad, CA), phosphotyrosine and PI3 kinase p85 subunit (Millipore, Billerica, MA), PKC δ and EGFR (BD Biosciences, San Jose, CA), PARP, cleaved Caspase-3, c-Met, c-Met pTyr1349, Akt, Akt pS473, Erk1/2, Erk1/2 pT202/204, STAT3 and STAT3 pTyr705 (Cell Signaling Technology, Danvers, MA).
Protein immunoprecipitation.
Cells were treated as above. An equivalent amount of total cell protein (800 μg) was immunoprecipitated by adding either 1.5 μg anti-ErbB3 antibody (Santa Cruz Biotechnology) or 3 μg anti-p85 antibody (Millipore) and incubating overnight at 4°C. Protein G-agarose beads (Invitrogen) were then added to each sample and tubes were rotated at 4°C for 1 h. Beads were pelleted and SDS-containing sample loading buffer was added. After heating to 100°C for 5 min, samples were subjected to SDS-PAGE, proteins were transferred to nitrocellulose membranes, and immunoblotted as described above.
Cell proliferation assay.
Cell proliferation was monitored using CellTiter 96 Aqueous MTS reagent (Promega, Madison, WI) according to the manufacturer’s instructions. Briefly, 3,000–5,000 cells were seeded into 96-well plates and grown in RPMI-1640 medium with 10% FBS. After 24 h, the medium was replaced with 100 μl of complete medium containing various inhibitors and cells were incubated for up to six days. At the end of the experiment, medium was discarded, 100 μl fresh medium containing 20 μl MTS solution was added to each well, and the plate was incubated at 37°C for 1 h. Cell number is proportional to absorbance, which was read on an automated spectrophotometric plate reader at a wavelength of 490 nm.
Analysis of cell cycle and apoptosis by fluorescence-activated cell sorting (FACS).
Cells were cultured in 10 cm plates and collected by centrifugation at 1,000 rpm for 5 min. Pellets were fixed with 70% ethanol for 4 h at −20°C and re-centrifuged. Pellets were resuspended in propidium iodide (PI) staining solution (50 μg/ml PI, 0.1 mg/ml RNase A, 0.05% Triton X-100 in PBS) and incubated for 40 min at 37°C. Tubes were centrifuged for 5 min at 1,500 rpm, supernatant was removed, and the cell pellets were resuspended in PBS. Samples were analyzed using a FACSCalibur instrument (BD Biosciences, San Jose, CA) calibrated to measure only specific PI-emitted fluorescence. Signal threshold was established based on the fluorescence of untreated control cells. Apoptotic cells appeared as a hypodiploid peak (SubG1) due to nuclear fragmentation and loss of DNA.
References
- 1.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003; 4:915–25. [DOI] [PubMed] [Google Scholar]
- 2.Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer 2006; 6:637–45· [DOI] [PubMed] [Google Scholar]
- 3.Lutterbach B, Zeng Q, Davis LJ, Hatch H, Hang G, Kohl NE, et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res 2007; 67:2081–8. [DOI] [PubMed] [Google Scholar]
- 4.Danilkovitch-Miagkova A, Zbar B. Dysregulation of Met receptor tyrosine kinase activity in invasive tumors. J Clin Invest 2002; 109:863–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolen GA, Sordella R, Muir B, Mohapatra G, Barmettler A, Archibald H, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci USA 2006; 103:2316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puri N, Ahmed S, Janamanchi V, Tretiakova M, Zumba O, Krausz T, et al. c-Met is a potentially new therapeutic target for treatment of human melanoma. Clin Cancer Res 2007; 13:2246–53. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005; 352:786–92. [DOI] [PubMed] [Google Scholar]
- 8.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007; 316:1039–43. [DOI] [PubMed] [Google Scholar]
- 9.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 2007; 318:287–90. [DOI] [PubMed] [Google Scholar]
- 10.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokar KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 2007; 445:437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pashtan I, Tsutsumi S, Wang S, Xu W, Neckers L. Targeting Hsp90 prevents escape of breast cancer cells from tyrosine kinase inhibition. Cell Cycle 2008; 7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachleitner-Hofmann T, Sun MY, Chen CT, Tang L, Song L, Zeng Z, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther 2008; 7:3499–508. [DOI] [PubMed] [Google Scholar]
- 13.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med 2002; 8:55–61. [DOI] [PubMed] [Google Scholar]
- 14.Taldone T, Gozman A, Maharaj R, Chiosis G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol 2008; 8:370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koga F, Tsutsumi S, Neckers LM. Low dose geldanamycin inhibits hepatocyte growth factor and hypoxia-stimulated invasion of cancer cells. Cell Cycle 2007; 6:1393–402. [DOI] [PubMed] [Google Scholar]
- 16.Maulik G, Kijima T, Ma PC, Ghosh SK, Lin J, Shapiro GI, et al. Modulation of the c-Met/heatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res 2002; 8:620–7. [PubMed] [Google Scholar]
- 17.Xie Q, Gao CF, Shinomiya N, Sausville E, Hay R, Gustafson M, et al. Geldanamycins exquisitely inhibit HGF/SF-mediated tumor cell invasion. Oncogene 2005; 24:3697–707. [DOI] [PubMed] [Google Scholar]
- 18.Webb CP, Hose CD, Koochekpour S, Jeffers M, Oskarsson M, Sausville E, et al. The geldanamycins are potent inhibitors of the hepatocyte growth factor/scatter factor-met-urokinase plasminogen activator-plasmin proteolytic network. Cancer Res 2000; 60:342–9. [PubMed] [Google Scholar]
- 19.Fujita N, Sato S, Ishida A, Tsuruo T. Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-depedent kinase-1. J Biol Chem 2002; 277:10346–53. [DOI] [PubMed] [Google Scholar]
- 20.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA 2000; 97:10832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 is destabilized by inhibitors of Hsp90 function. J Biol Chem 2002; 277:39858–66. [DOI] [PubMed] [Google Scholar]
- 22.Schulte TW, Blagosklonny MV, Romanova L, Mushinski JF, Monia BP, Johnston JF, et al. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol Cell Biol 1996; 16:5839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang SA, Moser C, Gaumann A, Klein D, Glockzin G, Popp FC, et al. Targeting heat shock protein 90 in pancreatic cancer impairs insulin-like growth factor-1 receptor signaling, disrupts an interleukin-6/signal-transducer and activator of transcription 3/hypoxia-inducible factor-1 alpha autocrine loop, and reduces orthotopic tumor growth. Clin Cancer Res 2007; 13:6459–68. [DOI] [PubMed] [Google Scholar]
- 24.Guo A, Villen J, Kornhauser J, Lee KA, Stokes MP, Rikova K, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci USA 2008; 105:692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellyer NJ, Kim MS, Koland JG. Heregulin-dependent activation of phosphoinositide 3-kinase ang Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem 2001; 276:42153–61. [DOI] [PubMed] [Google Scholar]
- 26.Amos S, Martin PM, Polar GA, Parsons SJ, Hussaini IM. Phorbol 12-myristate 13-acetate induces epidermal growth factor receptor transactivation via protein kinase Cdelta/c-Src pathways in glioblastoma cells. J Biol Chem 2005; 280:7729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, He D, Saatian B, Watkins T, Spannhake EW, Pyne NJ, et al. Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase C delta, Lyn kinase, and matrix metalloproteinases. J Biol Chem 2006; 281:19501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keranen LM, Dutil EM, Newton AC. Protein Kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol 1995; 5:1394–403. [DOI] [PubMed] [Google Scholar]
- 29.Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 2002; 99:1928–37. [DOI] [PubMed] [Google Scholar]
- 30.Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 2005; 65:4389–400. [DOI] [PubMed] [Google Scholar]
- 31.Baselga J, Albanell J, Ruiz A, Lluch A, Gascón P, Guillém V, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol 2005; 23:5323–33. [DOI] [PubMed] [Google Scholar]
- 32.Spector NL, Xia W, Burris H 3rd, Hurwitz H, Dees EC, Dowlati A, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol 2005; 23:2502–12. [DOI] [PubMed] [Google Scholar]
- 33.Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res 2003; 63:7345–55. [PubMed] [Google Scholar]
- 34.Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 2007; 67:4408–17. [DOI] [PubMed] [Google Scholar]
- 35.Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res 2008; 14:2895–9. [DOI] [PubMed] [Google Scholar]
- 36.Cappuzzo F, Jänne PA, Skokan M, Finocchiaro G, Rossi E, Ligorio C, et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol 2009; 20:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zucali PA, Ruiz MG, Giovannetti E, Destro A, Varella-Garcia M, Floor K, et al. Role of c-MET expression in non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitors. Ann Oncol 2008; 19:1605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 2007; 104:20932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L, Eiseman JL, Egorin MJ, D’Argenio DZ. Physiologically-based pharmacokinetics and molecular pharmacodynamics of 17-(allylamino)-17-demethoxygeldanamycin and its active metabolite in tumor-bearing mice. J Pharmacokinet Pharmacodyn 2003; 30:185–219. [DOI] [PubMed] [Google Scholar]
- 40.Eiseman JL, Lan J, Lagattuta TF, Hamburger DR, Joseph E, Covey JM, et al. Pharmacokinetics and pharmacodynamics of 17-demethoxy 17-[[(2-dimethylamino) ethyl]amino]geldanamycin (17DMAG, NSC 707545) in C.B-17 SCID mice bearing MDA-MB-231 human breast cancer xenografts. Cancer Chemother Pharmacol 2005; 55:21–32. [DOI] [PubMed] [Google Scholar]