Abstract
Objectives:
To examine associations between mild cognitive impairment (MCI) and falls among primary care patients, and to investigate whether social engagement (SE) modifies these associations.
Design:
Cross sectional analysis using baseline data from an observational cohort study.
Setting:
Primary care.
Participants:
Community-dwelling older adults (N = 430) at risk of mobility decline with a mean age of 76.6 years (range 65-96y).
Main Outcome Measures:
The number of falls in the past year was reported at the baseline interview. MCI was identified using a cutoff of 1.5 SD below the age-adjusted mean on at least 2 of the standardized cognitive performance tests. SE (eg, keeping in touch with friends and family, volunteering, participating social activities…) was assessed with the Late Life Function and Disability Instrument, and required a score above the median value 49.5 out of 100.
Results:
MCI was present among 42% of participants and 42% reported at least 1 fall in the preceding year. Using generalized estimating equations, MCI was associated with a 77% greater rate of falls (P<.05). There was a statistically significant interaction between SE and MCI on the rate of falls (P<.01), such that at a high level of SE, MCI was not statistically associated with falls (P=.83). In participants with lower levels of SE, MCI is associated with 1.3 times greater rate of falls (P<.01).
Conclusions:
While MCI is associated with a greater risk for falls, higher levels of SE may play a protective role.
Keywords: Falls, Mild cognitive impairment, Rehabilitation
Among community dwelling older adults, falls occur with high frequency,1,2 increase the risk for disability, premature nursing-home admission, and mortality.3,4 Cognition is a factor that is linked to mobility skills and specifically fall rates among older adults.5–9 However, for mild cognitive impairment (MCI)—a syndrome at high risk for dementia—the association with falls is suggestive, but lacks clarity.7–10 MCI is a treatment priority for geriatric rehabilitative care because of its high prevalence among community-dwelling older adults (up to 25.2%), and its negative effect on the well-being and functional independence of patients and families,11–13 In contrast to MCI, social engagement (SE) is a factor that has a positive effect on well-being and functional independence and might protect against falls. SE refers to the participation of older adults in different group-related activities such as getting together with friends or family members (other than spouse or partner), volunteering, or attending religious services. This engagement can bring companionship and a sense of belonging, and might also reinforce the practice of healthy behaviors. Previous studies indicate that SE is associated with decreased mortality among older adults.14,15 Low social participation is associated with a sedentary lifestyle and the manifestation of mobility limitations.16–19 Given its favorable effect on functional outcomes negatively affected by MCI, we postulate that SE will be associated with a lower rate of falls in general, and will in addition be a factor that influences the fall rates of patients with MCI.
Although risk factors of falls in older adults are well established, the relationship between SE and falls is not clear. Previous studies suggested that SE can help improve cognition20,21 and protect from physical function decline.22,23 Low levels of SE are associated with increased risk of physical inactivity18 and mobility limitation19 or weak muscle tone and compromised balance,24 which are all factors related to risks of falls. In order to conceptually frame these relationships, we used the World Health Organization’s International Classification of Functioning, Disability and Health (ICF) framework that characterizes the factors that contribute to human functioning.25 The ICF describes the interrelationships between body systems, functional activities (ie, mobility), and an individual’s participation in their life roles. It also accounts for mediating personal and environmental factors. Based on the ICF model, figure 1 shows that MCI corresponds to deficiencies in the body functions and structures affecting mental function. Falls are viewed as a failure in the conduct of mobility activities and SE is considered a combined personal/environmental factor that moderates these associations (figure 1). The ICF model and its associated terminology seem to be suited to the design of this study.
Fig 1.
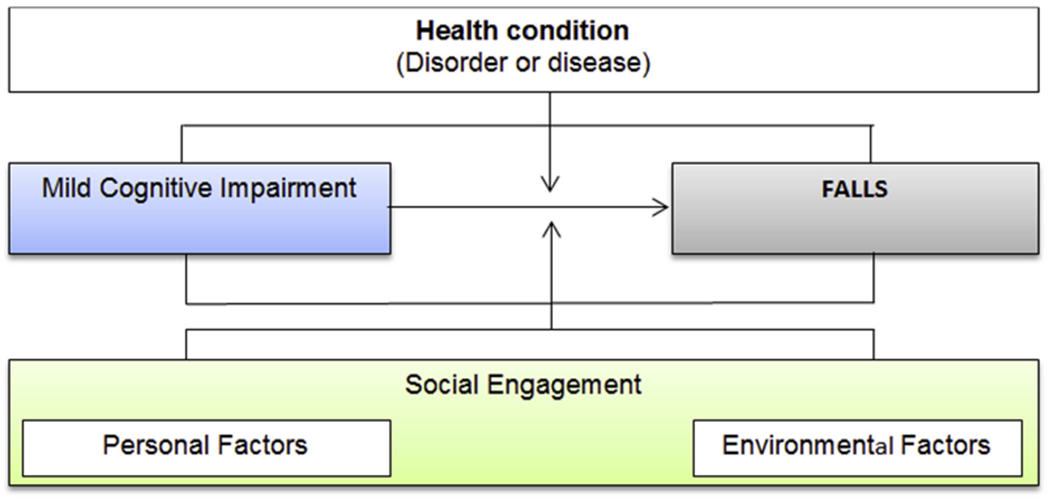
Conceptual framework.
Thus, this study among community dwelling, older primary care patients had 3 objectives (1) to examine whether MCI is associated with self-reported fall rates in the prior year; (2) to investigate the association between SE and self-reported fall rates in the prior year; (3) to understand whether SE can modify the association between MCI and self-reported fall rates in the prior year. We hypothesized that (1) the self-reported fall rates in the prior year will be higher among patients with MCI compared to those without MCI; (2) older adults with higher SE will be less likely to fall than those with lower SE; (3) the relationship between MCI and self-reported fall rates will be modified by SE, such that higher levels of SE will result in a weaker association between MCI and the self-reported fall rates in the prior year.
Methods
Study population
This study is a cross-sectional analysis using baseline data from an observational cohort study among 430 community-dwelling older adults. Details on the methods were previously published.26 The participants were recruited through primary care practices from December 2009 to January 2012, based on patient database screening and telephone interviews. If eligible, they were invited to a screening visit for a face-to-face interview and physical examination that determined final eligibility. Inclusion criteria were: living in the community, aged 65 or older, English proficiency, and at risk of mobility decline by self-reported difficulty walking half a mile (6 blocks) or climbing a flight of stairs (10 steps). Exclusion criteria included the presence of a terminal illness, severe cognitive impairment based on a Mini-Mental State Examination score <18,27 poor physical performance based on a Short Physical Performance Battery (SPPB) score <4, severe visual impairment, amputation of a lower extremity, use of oxygen supplementation, uncontrolled hypertension, heart attack or major surgery in the last 6 months, a planned major surgery, and plan to move away from the Boston area within 2 years.
Primary outcome
Though not a primary outcome of the original study, the number of falls in the previous year was assessed at baseline and used as the dependent variable for this analysis. Falls were determined by the question, “How many times have you fallen to the ground in the past year?” Falls are defined as unintentionally coming to rest on the floor, ground or lower surface. This approach to fall assessment has been found to have good sensitivity and specificity.5,28
Key measures
Cognition
Participants underwent a comprehensive cognitive battery at baseline that evaluated executive function, learning, and memory. The battery consisted of the following tests which are used in clinical settings: Trail Making Test A and B, Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale29 as a non-memory measure in the area of attention and executive function, and the memory measures by Total Recall, Delayed Recall, and Recognition Discrimination indices for Hopkins Verbal Learning Test, Revised.30,31 Details on administration and scoring of these measures are published.6 MCI was defined based on performance on these 3 neuropsychological tests. For each cognitive measure, standardized (z) scores were compared across groups. The raw scores of these tests were converted into age-adjusted standardized scores (z scores) using data from healthy age matched peers.4 Consistent with previous studies,6,32 MCI was operationally defined by using a cutoff of 1.5 SD below the age-adjusted mean of the standardized cognitive performance score of each type of neuropsychological tests. Individuals with impairment on at least 2 of these tests were classified as having MCI.33
Social engagement
SE was assessed with the social role domain of the disability component of the Late Life Function and Disability Instrument (LLFDI).34 It consists of 9 items that reflect performance of social activities and community tasks and is measured by a Likert scale question such as, how often do you… (1) keep in touch with others; (2) visit friends and family in their homes; (3) provide care or assistance to others; (4) work at a volunteer job; (5) take part in active recreation; (6) travel out of town; (7) invite people into your home; (8) go out with others to public places; (9) take part in organized social activities? As part of the LLFDI scoring procedures, these raw scores are transformed to a scaled score between 0-100. The higher score indicates more SE. Using statistical criteria, high SE was defined as a score of at least 49.5, which was the median value of the total score of our participants (range 0-79).
Covariates
We also evaluated important demographic and health characteristics that are related to falls among older adults. Age was measured in years as a continuous variable (range 65-96). Sex was characterized by self-report as either men or women. Race was dichotomized into whites and people of color. Education was divided into 3 categories: < 12th grade or general educational development; undergraduate or vocational/technical school; and graduate or professional school. Physical performance was measured by the SPPB35,36 which consisted of 3 subtests—standing balance, walking speed, and the ability to rise from a chair, ranged from 4-12. Pain is a risk factor for falls37 and was measured by the Brief Pain Inventory38 (0 reflecting no pain and 10 reflecting severe or excruciating pain). Depressive symptoms were assessed using the Patient Health Questionnaire.39 Comorbidity was measured using the validated and well-established, Self-administered Comorbidity Questionnaire.40
Statistical analyses
We first inspected the distribution of all variables in table 1. Then, univariate analyses were conducted to compare those with high SE vs low SE, which was defined by Student t-tests or analysis of variance for continuous variables, and chi-squared tests (or Fisher exact test) for categorical variables. To examine for collinearity among potential covariates, a correlation matrix was performed including all of the potential adjustment variables. The covariates with r value <0.5 were utilized. The list of covariates was selected in the adjusted models based on a combination of clinical relevance and statistical criteria (P value <.01). We evaluated for a potential interaction between MCI and SE within the fully adjusted models based on a P value < .05 using a Wald test of the t interaction term. This was done separately evaluating SE as both a continuous variable as well as a categorical variable. Rate ratios (RRs, or the rate of falls in the prior year) and 95% confidence intervals (CI) for the negative binomial regression models were presented. Statistical significance was set at an alpha level of 0.05. All analyses were conducted using SAS, version 9.4.a
Table 1.
Characteristics of study participants by SE status
| Characteristics | Total N=430 | Low SE n=209 | High SE n=221 | P Value |
|---|---|---|---|---|
| Age (y), mean ± SD | 76.56±7.0 | 76.55±7.12 | 76.57±6.91 | .97 |
| Men, n (%) | 139 (32.3) | 70 (33.5) | 69 (31.2) | .61 |
| Living with spouse/partner, n (%) | 148 (34.4) | 62 (29.7) | 86 (38.9) | .04 |
| White and non-Hispanic, n (%) | 355 (82.6) | 171 (81.8) | 184 (83.3) | .69 |
| BMI (kg/m2), n (%) | ||||
| Normal (BMI <25.0) | 102 (23.8) | 50 (23.9) | 52 (23.6) | .70 |
| Overweight (BMI 25.0-29.9) | 162 (37.8) | 75 (35.9) | 87 (39.5) | |
| Obese (BMI ≥30) | 165 (38.5) | 84 (40.2) | 81 (36.8) | |
| Education, n (%) | ||||
| Less than 12th grade or general education | 184 (42.8) | 112 (53.5) | 72 (32.6) | <.0001 |
| Undergraduate or vocational or technical school | 140 (32.6) | 61 (29.2) | 79 (35.7) | |
| Graduate or professional school | 106 (24.7) | 36 (17.2) | 70 (31.7) | |
| Comorbidity score, n (%) | 6.58 (3.7) | 7.02 (3.8) | 6.17 (3.5) | .02 |
| Brief Pain Inventory total, mean ± SD | 2.54±1.9 | 2.71±2.0 | 2.38±1.8 | .07 |
| Depressive symptoms, n (%) | 28 (6.5) | 24 (11.5) | 4 (1.8) | <.0001 |
| Hopkins Verbal Learning Test, mean ± SD | ||||
| Delayed recall | 5.71±3.05 | 5.29±3.13 | 6.11±2.93 | .005 |
| Total recall | 18.73±5.5 | 17.92±5.3 | 19.5±5.6 | .003 |
| Retention | 70.79±30.6 | 66.59±31.90 | 74.76±28.8 | .006 |
| Recognition | 9.48±2.2 | 9.26±2.2 | 9.68±2.1 | .048 |
| DSST, mean ± SD | 36.31±11.1 | 34.2±10.9 | 38.29±10.8 | .0001 |
| Trail A, mean ± SD | 51.1±25.7 | 54.88±28.5 | 47.52±22.2 | .003 |
| Trail B, mean ± SD | 147.3±82.7 | 163.76±87.7 | 131.74±74.5 | <.0001 |
| MCI, n (%) | 180 (42.0) | 107 (51.2) | 74 (33.5) | .0002 |
Abbreviations: BMI, body mass index;DSST, Digit Symbol Substitution Test.
The Boston RISE study was approved by the Institutional Review Board at Partners and Spaulding Rehabilitation Network/ Massachusetts General Hospital. All participants provided written informed consent.
Results
Characteristics of our sample based upon SE status are presented in table 1. Overall, the mean age of participants is 76.6±7.0 years (range 65-96y); 32% of participants were men, and 82% were white. Table 1 indicates that 42% of participants exhibited MCI and 42% reported at least 1 fall in the preceding year. The median score of SE is 49.5 (range 0-79).
There were no statistical differences in terms of age, sex, race, marital status, body mass index, and pain between participants with limited SE vs participants with high SE. Compared to the participants with low SE, those with high SE were more likely to live with a spouse or partner (38.9% vs 29.8, P value =.04), had higher education levels, had a lower number of comorbidities (6.2 vs 7.0, P value =.02), and had a lower prevalence of depressive symptoms (1.8% vs 12.0%, P value <.0001). Participants with high SE also had better cognitive performance across all cognitive measures.
Table 2 indicates that MCI was strongly associated with the number of falls after adjusting for age, race, sex, education, pain, and comorbidity (RR=1.90, 95% CI [1.34-2.70]). Similarly, table 2 also shows the association between SE and the number of falls after adjusting for the covariates (Model 3: RR=0.64, 95% CI [0.46-0.90]).
Table 2.
Separate models evaluating the association between either MCI or SE and the rate of falls in the prior year
| Association Between MCI and the Rate of Falls |
Association Between SE and the Rate of Falls |
|||||||
|---|---|---|---|---|---|---|---|---|
| Different Models | RR | 95% CI | P Value | RR | 95% CI | P Value | ||
| Model 1: unadjusted model | 1.46 | 1.05 | 2.04 | .02 | 0.67 | 0.48 | 0.93 | .02 |
| Model 2: adjusted for age, race, sex, and education | 1.79 | 1.26 | 2.53 | <.0001 | 0.60 | 0.43 | 0.84 | .003 |
| Model 3: adjusted for age, race, sex, education, pain, and comorbidity | 1.90 | 1.34 | 2.70 | <.0001 | 0.64 | 0.46 | 0.90 | .01 |
There was a statistically significant interaction between SE and MCI in both the unadjusted and adjusted models (P<.05) (fig 2). Among participants with low SE, MCI was associated with a higher fall rate in the prior year (RR=1.97, 95% CI [1.17-3.31]). However, there was no statistically significant association between MCI and falls among participants with a high SE (RR=1.03, 95% CI [0.60-1.74]) (table 3).
Fig 2.

Numbers of falls in the previous year per 100 person-years stratified by MCI and SE status. High engagement was defined as a score of at least 49.5.
Table 3.
The association between MCI and the rate of falls in the prior year stratified by SE status
| Low SE |
High SE |
|||||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | |||
| MCI | 1.97 | 1.17 | 3.31 | 1.03 | 0.60 | 1.74 |
| Age | 0.98 | 0.95 | 1.02 | 0.97 | 0.94 | 1.01 |
| Men | 0.94 | 0.58 | 1.54 | 0.74 | 0.44 | 1.24 |
| Education | ||||||
| - Education: < 12th grade | ||||||
| - 12th grade or general education | 1.53 | 0.74 | 3.16 | 1.67 | 0.57 | 4.89 |
| - Undergraduate or vocational/technical school | 2.01 | 0.92 | 4.40 | 2.14 | 0.76 | 6.03 |
| - Graduate or professional school | 2.82 | 1.20 | 6.66 | 2.74 | 0.94 | 8.02 |
| White and non-Hispanic | 1.71 | 0.91 | 3.21 | 2.77 | 1.32 | 5.81 |
| Pain | 1.04 | 0.91 | 1.19 | 0.99 | 0.86 | 1.14 |
| Comorbidity | 1.05 | 0.98 | 1.12 | 1.01 | 0.94 | 1.08 |
As part of post hoc sensitivity analyses, we reanalyzed our final models in order to ascertain whether the inclusion of self-reported depression (derived from the comorbidity questionnaire) and mobility performance (SPPB) altered the findings. The results from the sensitivity analyses were similar to the primary analyses.
Discussion
The main findings of this study are (1) that MCI is associated with falls in the prior year; (2) that SE is inversely associated with falls in the prior year; (3) that SE moderates the association between MCI and falls. This is the first study to investigate the association between SE and falls among older adult primary care patients. Our sample consisted of primary care patients with a heightened risk for mobility problems and falls, thus representing the type of patients that would be prioritized in fall prevention and treatment programs.
Our findings confirmed the results of other studies that observed an association between MCI and falls.7,41 This association is consistent in unadjusted and adjusted models. In a systematic review of 26 prospective studies, Muir et al reported that cognitive impairment diagnosed through clinical assessment increased the future risk for falls among a sample of both community- and institution-dwelling older adults by 1.78 times.41 Our estimate for falls in the prior year is consistent with their prospective data (1.9 times increased rate of self-reported falls in the prior year).
An important finding of our study is confirming the association between SE and the rate of falls in the prior year. Previous literature on the relationship between SE and falls or physical functions is somewhat limited and has shown inconsistent findings.15,42 This could be, in part, due to inconsistent measuring of SE across studies, ranging from number of intimate friends to frequency of participation in a variety of social activities. In addition, some studies focused on all falls, whereas some focused on fall-related injury or fractures.15,42
Our study indicates that SE has a meaningful influence on the association between MCI and falls. Among older adults with limited SE, MCI was associated with a 97% higher fall rate in the prior year. In contrast, MCI was not associated with falls in the prior year among older adults with high SE. This relationship was independent of personal factors including physical activity and performance-based measures of mobility. To our knowledge, this is the first study to identify the moderating effect of SE in the relationship between MCI and falls. One possible explanation, as proposed by social behavior models (eg, social cognition theory), is that patients are influenced by the external and internal social environments when interacting with other people (family members, friends, and others). Their influence may help patients to more frequently exercise and recognize environmental hazards that may lead to falls.23,43 These assumptions of social behavior models should be tested in larger scale population based studies to confirm our findings.
We fully acknowledge that our study is cross-sectional and thus causality cannot be ascertained. However, SE is rarely prioritized in fall care and our findings do suggest the possibility that SE may have a protective role with regard to falls, especially among older adults with MCI. Others have reported that SE has a favorable effect on physical function.17,44 There are a number of mechanisms by which SE might have this favorable effect. SE may stimulate neuron connections, heighten awareness of the risk of falls, enhance self-esteem, and provide a sense of mastery, control, and self-efficacy in MCI patients.22,43 Moreover, those that are more socially engaged may be more physically active. We did not, however, observe any influence of patient reported physical activity within our multivariable models.
From a treatment perspective, our findings are thought provoking. It may suggest that by optimizing SE among patients that falls might be ameliorated, especially patients with MCI. Enhancing SE is not typically considered within advocated fall prevention and rehabilitation paradigms.45–47 SE is modifiable, whereas many other fall risk factors are not. It is intriguing to consider this possibility as other well founded interventions such as exercise or rehabilitation, may be challenging to implement among those with MCI. However, we cannot speculate deeply regarding causality as this is a cross-sectional study. This hypothesis needs stronger support from prospective studies.
Study limitations
In addition to the cross-sectional design, our study has other limitations. The measure of falls was based on self-report of falls in the previous year. This approach may be susceptible to recall bias but it has been found to be a valid alternative to time intensive fall diaries.28 Also, the study is not a population-based study and thus our findings may not generalize to older adults outside the primary care setting and to other regions outside the greater area. Further, reverse causation could be an issue for the interpretation of our results. To address this, future study should examine these relationships through an analysis of longitudinal data, so SE can be examined prior to falls occurred. Further, confounding by indication could be an issue since all participants were older primary-care patients with mobility limitations. This limits generalizability to all populations. Last but not least, Boston RISE did not have information on the physical environmental factors, such as home and neighborhood environment data, so we were not able to adjust for the physical environment.
Our study does have certain strengths. It was grounded in a model of clinical care, mirroring Centers for Disease Control— advocated screening practices for older adult primary care patients.45 We employed a well-validated measure of SE that more broadly ascertains settings and levels of engagement than prior studies. The variety of questions measuring social participation within the LLFDI is an important strength in which LLFDI included various questions assessing engagement in both informal context (eg, visiting with friends or family) and formal context (eg, taking part in organized social activities). However, future work is needed to assess whether different types of participation have differing effects on the relationship between cognition and falls. The LLFDI also quantifies frequency of participation in social activities, which may be a more sensitive measure of SE than the quality of it.
Conclusions
In our study of vulnerable older primary care patients, we observed lower rates of falls among those with high SE. Interestingly, this association was maintained even among those with MCI. This highlights the possibility that SE is a modifiable risk factor for falls that could enhance future treatment strategies. We need to confirm these relationships within prospective studies before advancing this hypothesis further.
Acknowledgments
Supported by the National Institute on Aging (grant no. R01AG032052-03 PI-J.F.B.), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant no. 1K24HD070966-01 PI-J.F.B.), and the National Center for Research. Resources in a grant to the Harvard Clinical and Translational Science Center (grant no. 1UL1 RR025758-01). Dr L.Q. was also supported in part by the National Institutes of Health (grant no. P2CHD065702), the National Institute for Neurological Disorders and Stroke, the National Institute of Biomedical Imaging and Bioengineering, and the Boston Claude D. Pepper Older Americans Independence Center, National Institutes on Aging (grant no. P30-AG031679).
List of abbreviations:
- CI
confidence interval
- ICF
International Classification of Functioning, Disability and Health
- LLFDI
Late Life Function and Disability Instrument
- MCI
mild cognitive impairment
- RR
rate ratio
- SE
social engagement
- SPPB
Short Physical Performance Battery
Footnotes
Presented to the American Congress of Rehabilitation Medicine, October 23-28, 2017, Atlanta, GA.
Disclosures: none.
Supplier
SAS, version 9.4; SAS Institute, Inc.
References
- 1.Quach LT, Burr JA. Arthritis, depression, and falls among communitydwelling older adults: evidence from the health and retirement study. J Appl Gerontol 2018;37:1133–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreland BL, Durbin LL, Kasper JD, Mielenz TJ. Rehabilitation utilization for falls among community-dwelling older adults in the United States in the National Health and Aging Trends Study. Arch Phys Med Rehabil 2018;99:1568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman F, Chaudhury H. Falls and the physical environment: a review and a new multifactorial falls-risk conceptual framework. Can J Occup Ther 2008;75:82–95. [DOI] [PubMed] [Google Scholar]
- 4.Geusens P, Milisen K, Dejaeger E, Boonen S. Falls and fractures in postmenopausal women: a review. J Br Menopause Soc 2003;9:101–6. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, Speechley M, GinterSF. Riskfactors for falls among elderly persons living in the community. N Engl J Med 1988;319:1701–7. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen MM, Holt NE, Grande L, et al. Mild cognitive impairment status and mobility performance: an analysis from the Boston RISE study. J Gerontol A Biol Sci Med Sci 2014;69:1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyrovolas S, Koyanagi A, Lara E, Santini ZI, Haro JM. Mild cognitive impairment is associated with falls among older adults: findings from the Irish Longitudinal Study on Ageing (TILDA). Exp Gerontol 2016;75:42–7. [DOI] [PubMed] [Google Scholar]
- 8.Welmer AK, Rizzuto D, Laukka EJ, Johnell K, Fratiglioni L. Cognitive and physical function in relation to the risk of injurious falls in older adults: a population-based study. J Gerontol A Biol Sci Med Sci 2017;72:669–75. [DOI] [PubMed] [Google Scholar]
- 9.van der Wardt V, Logan P, Hood V, Booth V, Masud T, Harwood R. The association of specific executive functions and falls risk in people with mild cognitive impairment and early-stage dementia. Dement Geriatr Cogn Disord 2015;40:178–85. [DOI] [PubMed] [Google Scholar]
- 10.Lipardo DS, Aseron AMC, Kwan MM, Tsang WW. Effect of exercise and cognitive training on falls and fall-related factors in older adults with mild cognitive impairment: a systematic review. Arch Phys Med Rehabil 2017;98:2079–96. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet 2006;367:1262–70. [DOI] [PubMed] [Google Scholar]
- 12.Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement 2012;8:14–21. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 2018;90:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Soc Sci Med 2000;51: 843–57. [DOI] [PubMed] [Google Scholar]
- 15.Corbett DB, Rejeski WJ, Tudor-Locke C, et al. Social participation modifies the effect of a structured physical activity program on major mobility disability among older adults: results from the LIFE Study. J Gerontol B Psychol Sci Soc Sci 2018;73:1501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heseltine R, Skelton DA, Kendrick D, et al. “Keeping Moving”: factors associated with sedentary behaviour among older people recruited to an exercise promotion trial in general practice. BMC Fam Pract 2015;16:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren M, Ganley KJ, Pohl PS. The association between social participation and lower extremity muscle strength, balance, and gait speed in US adults. Prev Med Rep 2016;4:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D, Subramanian SV, Gortmaker SL, Kawachi I. US state- and county-level social capital in relation to obesity and physical inactivity: a multilevel, multivariable analysis. Soc Sci Med 2006;63:1045–59. [DOI] [PubMed] [Google Scholar]
- 19.Avlund K, Lund R, Holstein BE, Due P. Social relations as determinant of onset of disability in aging. Arch Gerontol Geriatr 2004;38:85–99. [DOI] [PubMed] [Google Scholar]
- 20.Kelly ME, Duff H, Kelly S, et al. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: a systematic review. Syst Rev 2017;6:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krueger KR, Wilson RS, Kamenetsky JM, Barnes LL, Bienias JL, Bennett DA. Social engagement and cognitive function in old age. Exp Aging Res 2009;35:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn Sci 2009;13:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanamori S, Kai Y, Aida J, et al. Social participation and the prevention of functional disability in older Japanese: the JAGES cohort study. PLoS One 2014;9:e99638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levasseur M, Desrosiers J, Whiteneck G. Accomplishment level and satisfaction with social participation of older adults: association with quality of life and best correlates. Qual Life Res 2010;19:665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. How to use the ICF: a practical manual for using the International Classification of Functioning, Disability and Health (ICF). Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 26.Holt NE, Percac-Lima S, Kurlinski LA, et al. The Boston Rehabilitative Impairment Study of the Elderly: a description of methods. Arch Phys Med Rehabil 2013;94:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE). Psychopharmacol Bull 1988;24:689–92. [PubMed] [Google Scholar]
- 28.Ganz DA, Higashi T, Rubenstein LZ. Monitoring falls in cohort studies of community-dwelling older people: effect of the recall interval. J Am Geriatr Soc 2005;53:2190–4. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D Wechsler Adult Intelligence Scale Administration and scoring manual. 3rd ed. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 30.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol 1999;13:348–58. [DOI] [PubMed] [Google Scholar]
- 31.Benedict R, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test - Revised: normative data and analysis of inter-form and test-retest reliability. Neuropsychologist 1998;12:43–55. [Google Scholar]
- 32.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–8. [DOI] [PubMed] [Google Scholar]
- 33.Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 2009;17:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jette AM, Haley SM, Coster WJ, et al. Late life function and disability instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci 2002;57:M209–16. [DOI] [PubMed] [Google Scholar]
- 35.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- 36.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–31. [DOI] [PubMed] [Google Scholar]
- 37.Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA 2009;302: 2214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain 2004;5:133–7. [DOI] [PubMed] [Google Scholar]
- 39.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a selfreport version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–44. [DOI] [PubMed] [Google Scholar]
- 40.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49:156–63. [DOI] [PubMed] [Google Scholar]
- 41.Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age Ageing 2012;41:299–308. [DOI] [PubMed] [Google Scholar]
- 42.Luukinen H, Koski K, Laippala P, Kivela SL. Factors predicting fractures during falling impacts among home-dwelling older adults. J Am Geriatr Soc 1997;45:1302–9. [DOI] [PubMed] [Google Scholar]
- 43.Herrmann E, Call J, Hernandez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 2007;317:1360–6. [DOI] [PubMed] [Google Scholar]
- 44.Mendes de Leon CF, Glass TA, Berkman LF. Social engagement and disability in a community population of older adults: the New Haven EPESE. Am J Epidemiol 2003;157:633–42. [DOI] [PubMed] [Google Scholar]
- 45.Avin KG, Hanke TA, Kirk-Sanchez N, et al. Management of falls in community-dwelling older adults: clinical guidance statement from the Academy of Geriatric Physical Therapy of the American Physical Therapy Association. Phys Ther 2015;95:815–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tinetti ME, Kumar C. The patient who falls: “It’s always a trade-off”. JAMA 2010;303:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med 2003;348:42–9. [DOI] [PubMed] [Google Scholar]


