Abstract
Context
The management of youth with delayed puberty is hampered by difficulty in predicting who will eventually progress through puberty and who will fail to attain adult reproductive endocrine function. The neuropeptide kisspeptin, which stimulates gonadotropin-releasing hormone (GnRH) release, can be used to probe the integrity of the reproductive endocrine axis.
Objective
We sought to determine whether responses to kisspeptin can predict outcomes for individuals with pubertal delay.
Design, Setting, and Participants
We conducted a longitudinal cohort study in an academic medical center of 16 children (3 girls and 13 boys) with delayed or stalled puberty.
Intervention and Outcome Measures
Children who had undergone kisspeptin- and GnRH-stimulation tests were followed every 6 months for clinical evidence of progression through puberty. Inhibin B was measured in boys. A subset of participants underwent exome sequencing.
Results
All participants who had responded to kisspeptin with a rise in luteinizing hormone (LH) of 0.8 mIU/mL or greater subsequently progressed through puberty (n = 8). In contrast, all participants who had exhibited LH responses to kisspeptin ≤ 0.4 mIU/mL reached age 18 years without developing physical signs of puberty (n = 8). Thus, responses to kisspeptin accurately predicted later pubertal outcomes (P = .0002). Moreover, the kisspeptin-stimulation test outperformed GnRH-stimulated LH, inhibin B, and genetic testing in predicting pubertal outcomes.
Conclusion
The kisspeptin-stimulation can assess future reproductive endocrine potential in prepubertal children and is a promising novel tool for predicting pubertal outcomes for children with delayed puberty.
Keywords: Delayed puberty, kisspeptin, constitutional delay, hypogonadotropic hypogonadism
Children presenting with delayed puberty pose a vexing challenge for clinicians because of difficulty in predicting which children will eventually progress through puberty and which children will not (1, 2). Some causes of delayed puberty are readily recognizable, such as primary gonadal insufficiency, anatomic lesions of the hypothalamic/pituitary region, and functional hypogonadotropic hypogonadism (ie, physiologic suppression of the reproductive endocrine axis by chronic illness, stress, or negative energy balance). However, these conditions account for delayed puberty in only 36% to 47% of girls and 11% to 29% of boys presenting for pediatric endocrinology care (3-5). For the majority of girls and boys with delayed puberty, the provider is left with 2 potential diagnoses with markedly different outcomes: constitutional delay and idiopathic hypogonadotropic hypogonadism (IHH, also called congenital hypogonadotropic hypogonadism and isolated gonadotropin-releasing hormone [GnRH] deficiency) (1). Constitutional delay is a self-limited condition in which puberty starts late (or starts then stalls temporarily) but eventually begins and progresses to attainment of full adult reproductive endocrine function. Studies have suggested that, despite being a self-limited condition, constitutional delay may have lasting effects on height, bone mineral density, and psychosocial well-being (6). In contrast, IHH arises from pathologic dysfunction in the secretion or action of GnRH and results in failure to achieve normal adult reproductive endocrine function by a designated age (with a cutoff of 18 years frequently used) (7). Patients with IHH may present with absence of pubertal development or with pubertal development that proceeds partially but then stalls indefinitely.
Children with delayed puberty who later progress through puberty are often indistinguishable at first presentation from those who have abiding hypogonadotropism, and years of waiting may be needed before a given child’s outcome is known. As a result, patients, families, and providers can be faced with a conundrum when deciding between 2 common management approaches for delayed puberty: treatment with sex steroids or watchful waiting without intervention. A prospective method to predict pubertal outcomes would critically inform decision making about the management of delayed puberty.
For decades, clinicians and researchers have tried to develop tests to predict whether a child with pubertal delay will eventually progress through puberty (8). These tests include measurement of baseline (unstimulated) serum gonadotropins (luteinizing hormone, LH, and follicle-stimulating hormone, FSH), gonadotropins after stimulation with GnRH or GnRH analogs, and inhibin B at baseline. Unfortunately, all of these tests have significant deficits in sensitivity, specificity, or both (8). For example, studies have demonstrated significant overlap in serum inhibin B concentrations between boys with constitutional delay and those with IHH, with 10% to 29% of those with constitutional delay and 22% to 45% of those with IHH having an inhibin B that falls in the range of overlap (9-11). Better tests for predicting pubertal outcomes for youth with pubertal delay are needed.
Kisspeptin is a neuropeptide secreted in the hypothalamus that potently stimulates GnRH secretion in all mammalian species studied to date, including humans (12). In studies by our group and others, reproductively intact adults responded to a single bolus of kisspeptin with a robust increase in LH, whereas adults with IHH largely did not (13-19). Thus, provocative testing using kisspeptin can be used to assess an individual’s capacity for GnRH secretion.
We have conducted a prospective study to test the hypothesis that a child’s ability to respond to kisspeptin can predict whether that child will subsequently progress through puberty. We had previously shown that children with delayed or stalled puberty have a range of responses to kisspeptin, from no response to robust responses (20). We now report results from follow-up visits conducted every 6 months to assess pubertal outcomes and to determine whether the kisspeptin-stimulation test accurately predicted these outcomes.
Materials and methods
Study approval
The physiologic study on the kisspeptin-stimulation test is approved by the Massachusetts General Hospital (MGH)/Partners Institutional Review Board (protocol 2011P002885) and is registered on ClinicalTrials.gov (NCT01438034). Kisspeptin was used under investigational new drug 113 591, and GnRH was used under investigational new drug 93 353. Studies on the genetics of delayed puberty and IHH were approved by Boston Children’s Hospital (protocol P00017406) and MGH (protocol 1999P006955), respectively. All participants gave written informed assent, and at least one parent gave written informed consent.
Study protocol
Details of the study protocol have been described previously (20). Briefly, inclusion criteria were age 12 years or older for girls and 13.5 years or older for boys, with delayed or stalled puberty defined based on breast development for girls and testicular volume for boys. Children with an identifiable cause of delayed puberty were excluded. Participants had 2 admissions to the MGH Translational and Clinical Research Center (TCRC) for blood sampling every 10 minutes to measure baseline LH secretion overnight, LH secretion in response to kisspeptin-10 0.313 mcg/kg given intravenously (ΔLHkisspeptin), and LH secretion in response to intravenous GnRH 75 ng/kg (ΔLHGnRH). ΔLHkisspeptin and ΔLHGnRH were assessed both before and after pituitary “priming” with pulsatile subcutaneous GnRH 75 ng/kg every 2 hours for 6 days to ensure robust pituitary responsiveness to GnRH (8).
Participants subsequently returned every 6 months for follow-up visits to undergo physical examinations and measurement of FSH, LH, and either estradiol or testosterone to assess reproductive endocrine activity. On reaching age 18 years, participants underwent a final evaluation for physical and laboratory signs of reproductive endocrine activity. For participants receiving sex-steroid treatment, treatment was held prior to laboratory studies to ensure that sex-steroid measurements reflected endogenous production rather than exogenous administration (for 4 weeks for estradiol, for 6 weeks for injected testosterone, and for 2 weeks for transdermal testosterone).
Laboratory assays
For the TCRC visits, LH, FSH, testosterone, and estradiol were measured by the MGH Clinical Laboratory Research Core, the Brigham and Women’s Hospital Research Assay & Analysis Core, and LabCorp as previously described (20). Precision of the LH assay was 4.3% to 6.4%. Inhibin B was measured in a single batch by immunoassay by the University of Virginia Ligand Assay Core, with an intra-assay coefficient of variation of 2.5%. For follow-up visits, laboratory studies were performed by LabCorp and Quest Diagnostics. For participants outside the Boston area, follow-up data were obtained through their routine clinical care from their local endocrinologists and clinical laboratories.
Genetic testing
Whole-exome sequencing was performed at the Broad Institute in Cambridge, Massachusetts. Exome-sequencing data were screened for variants in 30 genes associated with IHH/Kallmann syndrome, delayed puberty, or both (listed in Supplemental Table 1 [21]). Variants were classified according to criteria of the American College of Medical Genetics and Genomics (22).
Statistics
The Fisher exact test was used to assess the association between results of the kisspeptin-stimulation test and pubertal outcomes. A P value of less than .05 was considered significant. The Jeffreys interval was used to calculate confidence intervals for sensitivity and specificity.
Results
Responses to kisspeptin
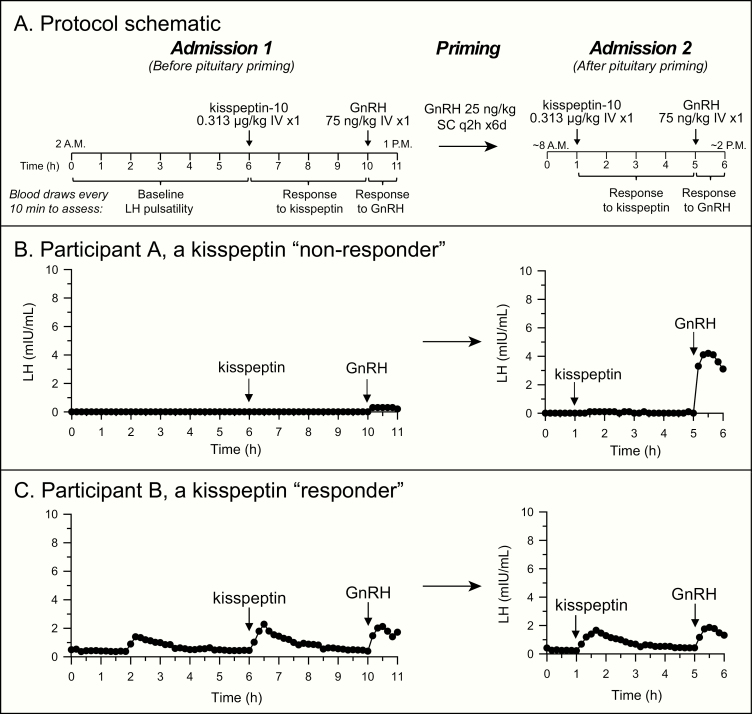
Seventeen participants with delayed or stalled puberty participated in this study (5 girls and 12 boys, Fig. 1). Results of kisspeptin- and GnRH-stimulation testing for 15 of these participants (participants 1-15) were previously reported in Chan et al (20). Participant A, new to this study, demonstrated a “kisspeptin nonresponder” pattern, whereas participant B, also new to this study, demonstrated a “kisspeptin responder” pattern (Fig. 2).Characteristics of the participants and their neuroendocrine phenotypes are provided in Table 1 and Supplemental Table 2 (21).
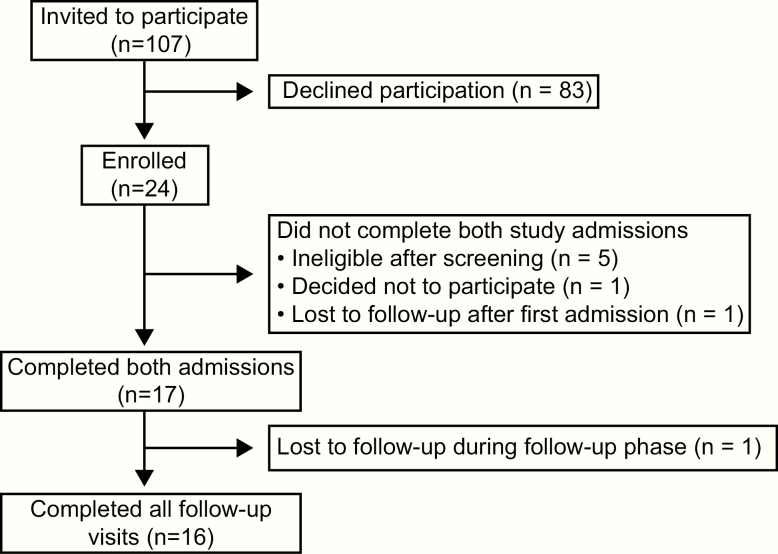
Figure 1.
Summary of recruitment and participation.
Figure 2.
Neuroendocrine characteristics of children presenting with delayed or stalled puberty. A, A schematic of the protocol. Details of the protocol are given in Chan et al (20). At the first visit, participants had serum luteinizing hormone (LH) measured to assess spontaneous pulsatility overnight and to chart responses to kisspeptin and gonadotropin-releasing hormone (GnRH). Participants then received exogenous pulsatile GnRH to enhance pituitary responsiveness to GnRH. They then returned for a second visit to measure LH secretion in response to kisspeptin and GnRH after this pituitary “priming.” B, Results for participant A, a “kisspeptin nonresponder.” C, Results for participant B, a “kisspeptin responder.”
Table 1.
Participant Characteristics and Reproductive Endocrine Evaluation
| IDa | Sex | At enrollment | Before pituitary priming | After pituitary priming | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Pubertal development | LH, mIU/mL | FSH, mIU/mL | Overnight LH pulses | Inhibin B, pg/mL | ΔLHGnRH, mIU/mL | ΔLHkisspeptin,mIU/mL | Pubertal progression? | ||
| 1 | M | 17.0 | Absent | 0.1 | 0.2 | Absent | < 17 | 0.2 | 0 | N |
| 2 | M | 15.7 | Absent | 0.2 | 0.2 | Absent | < 17 | 0.2 | 0 | N |
| 3 | F | 16.7 | Absent | < 0.2 | 0.2 | Absent | ND | 0.5 | 0 | N |
| 4 | M | 15.6 | Absent | 0.1 | 0.7 | Absent | < 17 | 0.6 | 0 | N |
| A | M | 16.4 | Absent | < 0.1 | 0.3 | Absent | 23 | 0.7 | 0 | N |
| 5 | F | 15.2 | Absent | 0.1 | 0.5 | Absent | ND | 0.8 | 0 | Lost to follow-up |
| 6 | F | 16.5 | Absent | < 0.2 | 0.6 | Absent | ND | 1.3 | 0 | N |
| 7 | M | 14.9 | Absent | 0.1 | 0.3 | Absent | < 17 | 1.6 | 0.1 | N |
| 8 | M | 14.6 | Absent | 0.8 | 1.4 | Absent | 47 | 7.5 | 0.4 | N |
| 9 | M | 16.0 | Stalled | 0.1 | 0.5 | Present | 209 | 3.7 | 0.8 | Y |
| 10 | M | 14.9 | Absent | < 0.3 | 1.1 | Present | 96 | 3.0 | 0.8 | Y |
| B | F | 15.0 | Absent | 0.1 | 0.2 | Absentb | ND | 1.2 | 0.9 | Y |
| 11 | M | 15.1 | Stalled | 0.1 | 0.3 | Absentb | 156 | 6.3 | 1.0 | Y |
| 12 | M | 14.8 | Absent | 0.1 | 1.7 | Present | 44 | 3.1 | 1.4 | Y |
| 13 | F | 13.9 | Absent | 0.1 | 1.0 | Present | ND | 1.7 | 1.4 | Y |
| 14 | M | 17.5 | Stalled | 0.2 | 0.7 | Present | 66 | 15.4 | 1.5 | Y |
| 15 | M | 14.2 | Absent | 0.6 | 3.4 | Present | 39 | 2.0 | 1.7 | Y |
Abbreviations: ΔLHkisspeptin, increase in luteinizing hormone (LH) after kisspeptin; ΔLHGnRH, increase in LH after gonadotropin-releasing hormone (GnRH), F, female; FSH, follicle-stimulating hormone; ID, identification; M, male; N, no; ND, not done; Y, yes.
aParticipants with numerical IDs are numbered as in Chan et al (20); participants with alphabetical IDs enrolled in the study after that report.
bParticipants B and 11 were receiving exogenous sex steroids at the time of their prepriming visits. Some data in this table were previously reported in Chan et al (20).
Long-term follow-up
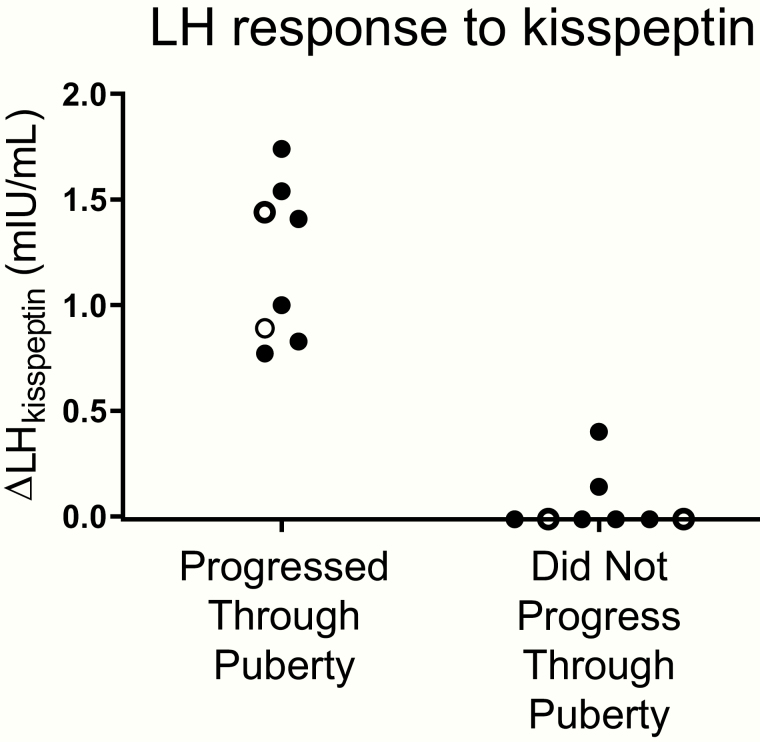
After this initial neuroendocrine characterization, participants returned for follow-up visits every 6 months. Eight participants (2 girls and 6 boys) progressed through puberty during the follow-up period (Table 1, Supplemental Table 3 [21]). The boys demonstrated progressive increases in testicular volume, and the girls exhibited progressive breast development in the absence of exogenous treatment. All of these participants were “kisspeptin responders” who had responded to exogenous kisspeptin with a rise in LH of 0.8 mIU/mL or greater (Fig. 3, Table 1).
Figure 3.
Distinct responses to kisspeptin in children who progressed through puberty and those who did not. Girls (open circles) and boys (filled circles) presenting with delayed or stalled puberty underwent kisspeptin-stimulation testing to assess the change in luteinizing hormone in response to exogenous kisspeptin (∆LHkisspeptin). Participants were then followed until age 18 years to determine whether they progressed through puberty spontaneously.
In contrast, 8 participants (2 girls and 6 boys) failed to enter puberty by age 18 years (Table 1, Supplemental Table 3 [21]). These participants exhibited persistent sexual immaturity on physical examination; boys had testicular volumes of less than 4 mL, and the girls did not have breast development until starting treatment with exogenous estradiol. On assessment of endogenous reproductive endocrine activity after discontinuation of sex-steroid treatment, all of these participants had serum concentrations of sex steroids (estradiol for girls, testosterone for boys) that were below the adult reference range and serum gonadotropin concentrations that were either low or inappropriately normal in the context of low sex steroids. These individuals who failed to enter puberty were 7 “kisspeptin nonresponders” (2 girls and 5 boys) who had shown little to no response to kisspeptin (an eighth “nonresponder” was lost to follow-up) and 1 male “intermediate responder” who had shown an LH response to kisspeptin of 0.4 mIU/mL (Fig. 3, Table 1).
Thus, the participants’ responses to kisspeptin clearly distinguished those who later progressed through puberty from those who did not (P = .0002). Sensitivity and specificity for the kisspeptin-stimulation test were both 100% (95% CI 74%-100%).
Luteinizing hormone measurements under 3 conditions: daytime, overnight, and after gonadotropin-releasing hormone stimulation
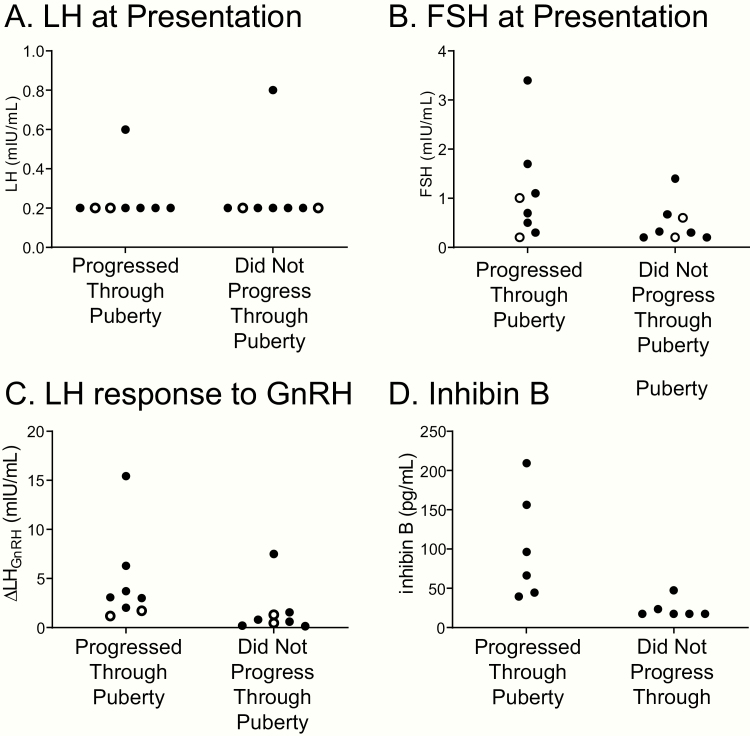
Unstimulated LH concentrations (measured during the day or overnight) and LH measured after stimulation by exogenous GnRH or GnRH analogs have been studied as methods to predict whether a child will eventually enter puberty (8). In this study, 2 boys had unstimulated serum LH concentrations in the pubertal range at the time of enrollment, and 1 was later found to progress through puberty. Of the remaining 14 participants with LH in the prepubertal range at enrollment, 7 later progressed through puberty and 7 did not (Fig. 4, Table 1). Thus, unstimulated LH did not accurately predict pubertal outcomes. Unstimulated FSH at the time of enrollment similarly did not predict pubertal outcomes (Fig. 4, Table 1).
Figure 4.
Additional hormonal evaluation of children who progressed or did not progress through puberty. Girls (open circles) and boys (filled circles) presenting with delayed and stalled puberty were evaluated for A, serum luteinizing hormone (LH) and B, follicle-stimulating hormone (FSH) at the time of presentation and C, the change in LH in response to exogenous gonadotropin-releasing hormone (GnRH). D, Boys were additionally evaluated for serum inhibin B.
In early puberty, daytime gonadotropin measurements may not accurately reflect activity of the reproductive endocrine axis because pulses of GnRH and LH secretion occur only at night during the deep stages of sleep (23). We had therefore conducted overnight blood sampling to detect sleep-associated LH secretion. All 6 children who had at least one pulse of LH secretion overnight were later found to progress through puberty (Table 1). In contrast, variable outcomes were observed for the 10 children who had no LH pulses overnight. Eight failed to progress through puberty, and 2 exhibited pubertal progression: one girl and one boy (participants B and 11) who at the time of neuroendocrine evaluation were being treated with exogenous sex steroids that may have suppressed endogenous LH secretion.
GnRH-stimulated LH secretion has also been studied as a test to predict pubertal outcomes (8). In our cohort, LH responses to GnRH were overlapping, ranging from 1.2 to 15.4 mIU/mL in those who later progressed through puberty and from 0.2 to 7.5 mIU/mL in those who did not (Fig. 4, Table 1).
Thus, none of these tests—unstimulated LH, whether measured during the day or at night, or GnRH-stimulated LH—was as accurate as the kisspeptin-stimulation test for predicting pubertal outcomes in this study cohort.
Inhibin B
Measurement of serum inhibin B has been proposed as a method to predict pubertal outcomes for children with delayed puberty (8-11). Serum inhibin B ranged from 39 to 209 pg/mL in boys who later progressed through puberty and from less than 17 pg/mL to 48 pg/mL in boys who did not (Fig. 4, Table 1). Thus, inhibin B did not accurately distinguish those who would later progress through puberty from those who would not.
Genetic testing
A subset of participants consented to exome sequencing. Pathogenic and likely pathogenic variants in 30 IHH genes (Supplemental Table 1 [21]) were identified in 2 of 5 participants who later progressed through puberty and 2 of 6 participants who did not enter puberty (Table 2). No pathogenic or likely pathogenic variants were identified in IGSF10, a gene potentially associated with constitutional delay. Thus, genetic testing could not predict pubertal outcomes for children with pubertal delay.
Table 2.
Pathogenic and Likely Pathogenic Variants in Genes Associated With Idiopathic Hypogonadotropic Hypogonadism
| ID | Sex | Pubertal progression? | Variant identified |
|---|---|---|---|
| 1 | M | N | ND |
| 2 | M | N | No qualifying variants found |
| 3 | F | N | FGFR1 p.R609X |
| 4 | M | N | ANOS1 a p.T193Kfs*24 |
| A | M | N | No qualifying variants found |
| 6 | F | N | No qualifying variants found |
| 7 | M | N | ND |
| 8 | M | N | No qualifying variants found |
| 9 | M | Y | ND |
| 10 | M | Y | No qualifying variants found |
| B | F | Y | ND |
| 11 | M | Y | No qualifying variants found |
| 12 | M | Y | FGFR1 p.I676Dfs*7 |
| 13 | F | Y | No qualifying variants found |
| 14 | M | Y | ND |
| 15 | M | Y | TACR3 p.W208X |
Abbreviations: F, female; ID, identification; M, male; N, no; ND, not done; Y, yes.
aFormerly named KAL1.
Discussion
We found that responses to the kisspeptin-stimulation test accurately predicted pubertal outcomes in a cohort of 16 girls and boys presenting with delayed or stalled puberty who were followed longitudinally. Furthermore, the kisspeptin-stimulation test predicted outcomes more accurately than other tests for evaluating delayed puberty, including inhibin B, LH after GnRH stimulation, overnight LH at baseline, and genetic testing.
The kisspeptin-stimulation test overcomes 2 fundamental challenges in predicting pubertal outcomes for children with delayed puberty. The first challenge is that there has not been a method to distinguish the physiologic hypogonadotropic hypogonadism of a normal prepubertal child from the pathologic hypogonadotropic hypogonadotropism of a child who has IHH. Being a provocative test, the kisspeptin-stimulation test provides the first available method to measure a child’s future potential for GnRH secretion. Indeed, we found that kisspeptin could elicit LH responses in children who eventually progressed through puberty at a time when they appeared prepubertal on physical examination and daytime laboratory evaluation.
The second challenge is distinguishing a child with a temporary pause in pubertal development from a child with IHH and partial pubertal development that has permanently stalled. We had previously shown that adults with IHH and partial reproductive endocrine activity fail to respond to kisspeptin (19). Similarly, in the current study, one participant who exhibited partial reproductive endocrine activity (participant 8) had diminished responses to kisspeptin that correctly predicted his lack of pubertal development by age 18 years, whereas inhibin B and GnRH-induced LH suggested that he would later progress through puberty.
In 2 participants (participants B and 11), the lack of LH pulses on overnight measurements did not correctly anticipate the participants’ eventual progress through puberty, whereas the kisspeptin-stimulation test accurately predicted the participants’ outcomes. These participants had been receiving sex-steroid treatment at the time of their study visits, and this may have suppressed endogenous LH secretion. In contrast, their responses to kisspeptin were robust, and thus the kisspeptin-stimulation test reliably predicted their eventual pubertal progression even when performed in the context of exogenous sex-steroid treatment.
Despite an ever-growing understanding of the genetics of IHH and constitutional delay, genetic testing is currently inadequate for predicting pubertal outcomes. Currently, fewer than half of IHH patients have an identifiable mutation in an IHH gene (7). Furthermore, even if a mutation is found, the predictive power is limited by variable penetrance and expressivity; within the same family, some carriers of an IHH gene mutation may have IHH and others may have constitutional delay (24). In our cohort, patients with pathogenic variants in TACR3 and FGFR1 were found to progress through puberty, highlighting the phenotypic variability associated with IHH gene mutations and demonstrating the limited prognostic value of genetic testing at the current time. Future work will undoubtedly enhance our understanding of the contribution of both rare and common genetic variation to IHH and constitutional delay, and clinical genetic testing may eventually come to have a valuable role in the evaluation of delayed puberty.
Strengths of this study include the inclusion of girls and boys, the detailed neuroendocrine characterization (which, informed by data from this study, can be simplified for future studies and eventual clinical use), and the prospective, longitudinal follow-up of up to 4 years to establish pubertal outcomes. One limitation of this study is the limited sample size; large-scale evaluation of the kisspeptin-stimulation test will be needed for a more accurate assessment of the sensitivity and specificity of the test for predicting pubertal outcomes. Nevertheless, our study enrolled a sufficient number of participants to demonstrate clear differences in kisspeptin responsiveness between those who progressed and did not progress through puberty, as well as to provide an example in which the kisspeptin-stimulation test outperformed currently proposed diagnostic tests. Another limitation is that some participants had clinical features, such as anosmia, that suggested a diagnosis of IHH/Kallmann syndrome. The purpose of this initial study was to assess the ability of the kisspeptin-stimulation test to predict pubertal outcomes, not to “solve” individual cases, and inclusion of participants with a range of characteristics was useful and necessary for this purpose. A third limitation is that, though not observed in this study cohort, some patients with IHH would be predicted to have intact responses to kisspeptin (eg, those with mutations in TAC3 and TACR3, the genes for neurokinin B and its receptor, which appear to function upstream of kisspeptin, or in KISS1, which encodes kisspeptin itself) (25, 26). However, this is likely to be only a small subset of IHH patients because mutations in TACR3 are present in only about 5% of patients with normosmic IHH, and mutations in TAC3 and KISS1 are rare causes of IHH (27-30). Finally, although the kisspeptin-stimulation test can assess the potential for future reproductive endocrine function, it may not be able to predict when that function will emerge. In this study cohort, all participants who progressed through puberty did so before age 18 years.
Despite these limitations, our results suggest that the kisspeptin-stimulation test has the potential to be a significant advance in evaluating delayed puberty. Coupling the kisspeptin-stimulation test with other tests may provide the greatest accuracy in predicting outcomes for patients presenting with delayed puberty and, in turn, guiding appropriate management of these patients to optimize health outcomes in adolescence and adulthood.
Acknowledgments
We thank our study participants and their families, the MGH TCRC, research staff (Cindy Li, Tairmae Kangarloo, Joanna Clair, Casey Cokkinias, Voula Christopoulos, Dianali Rivera Morales, Ryan Ciarlo, Temitope Kusa, Isabella McDonald, Margaret E. Chen, Elfa Jonsdottir-Lewis, Amalia Feld, and Katerina Hoskova), and colleagues who referred patients (Michael Agus, Paul Boepple, Rosalind Brown, Gregory Goodwin, Nelly Mauras, and Eray Savgan-Gurol).
Financial Support: This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grants R01 HD043341, K12 HD052896, R01 HD090071, and P50 HD028138), the National Institutes of Health National Center for Advancing Translational Sciences (Grant UL1 TR001102), the Harvard Catalyst, the Doris Duke Charitable Foundation (Award 2013110), the Charles H. Hood Foundation, the Massachusetts General Hospital Executive Committee on Research Fund for Medical Discovery, and the Robert and Laura Reynolds MGH Research Scholar Program.
Clinical Trial Information: This trial has been registered is registered on ClinicalTrials.gov (NCT01438034).
Glossary
Abbreviations
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- IHH
idiopathic hypogonadotropic hypogonadism
- LH
luteinizing hormone
- MGH
Massachusetts General Hospital
- TCRC
Translational and Clinical Research Center
Additional Information
Disclosure Summary: Y.-M.C. reports personal fees from Abbvie, Endo Pharmaceuticals, and Becker Pharmaceuticals. Y.-M.C. and S.B.S. have a provisional patent for kisspeptin-10. The remaining authors have nothing to disclose.
Data Availability: The data sets generated and analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Palmert MR, Dunkel L. Clinical practice. Delayed puberty. N Engl J Med. 2012;366(5):443-453. [DOI] [PubMed] [Google Scholar]
- 2. Wei C, Crowne EC. Recent advances in the understanding and management of delayed puberty. Arch Dis Child. 2016;101(5):481-488. [DOI] [PubMed] [Google Scholar]
- 3. Sedlmeyer IL, Palmert MR. Delayed puberty: analysis of a large case series from an academic center. J Clin Endocrinol Metab. 2002;87(4):1613-1620. [DOI] [PubMed] [Google Scholar]
- 4. Lawaetz JG, Hagen CP, Mieritz MG, Blomberg Jensen M, Petersen JH, Juul A. Evaluation of 451 Danish boys with delayed puberty: diagnostic use of a new puberty nomogram and effects of oral testosterone therapy. J Clin Endocrinol Metab. 2015;100(4):1376-1385. [DOI] [PubMed] [Google Scholar]
- 5. Varimo T, Miettinen PJ, Känsäkoski J, Raivio T, Hero M. Congenital hypogonadotropic hypogonadism, functional hypogonadotropism or constitutional delay of growth and puberty? An analysis of a large patient series from a single tertiary center. Hum Reprod. 2017;32(1):147-153. [DOI] [PubMed] [Google Scholar]
- 6. Zhu J, Chan YM. Adult consequences of self-limited delayed puberty. Pediatrics. 2017;139(6):e20163177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balasubramanian R, Crowley WF Jr. Isolated gonadotropin-releasing hormone (GnRH) deficiency. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews. Seattle,WA: University of Washington, Seattle;1993. [PubMed] [Google Scholar]
- 8. Harrington J, Palmert MR. Clinical review: distinguishing constitutional delay of growth and puberty from isolated hypogonadotropic hypogonadism: critical appraisal of available diagnostic tests. J Clin Endocrinol Metab. 2012;97(9):3056-3067. [DOI] [PubMed] [Google Scholar]
- 9. Adan L, Lechevalier P, Couto-Silva AC, et al. Plasma inhibin B and antimüllerian hormone concentrations in boys: discriminating between congenital hypogonadotropic hypogonadism and constitutional pubertal delay. Med Sci Monit. 2010;16(11):CR511-CR517. [PubMed] [Google Scholar]
- 10. Binder G, Schweizer R, Blumenstock G, Braun R. Inhibin B plus LH vs GnRH agonist test for distinguishing constitutional delay of growth and puberty from isolated hypogonadotropic hypogonadism in boys. Clin Endocrinol (Oxf). 2015;82(1):100-105. [DOI] [PubMed] [Google Scholar]
- 11. Rohayem J, Nieschlag E, Kliesch S, Zitzmann M. Inhibin B, AMH, but not INSL3, IGF1 or DHEAS support differentiation between constitutional delay of growth and puberty and hypogonadotropic hypogonadism. Andrology. 2015;3(5):882-887. [DOI] [PubMed] [Google Scholar]
- 12. Harter CJL, Kavanagh GS, Smith JT. The role of kisspeptin neurons in reproduction and metabolism. J Endocrinol. 2018;238(3):R173-R183. [DOI] [PubMed] [Google Scholar]
- 13. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609-6615. [DOI] [PubMed] [Google Scholar]
- 14. Dhillo WS, Chaudhri OB, Thompson EL, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92(10):3958-3966. [DOI] [PubMed] [Google Scholar]
- 15. Chan YM, Butler JP, Pinnell NE, et al. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab. 2011;96(6):E908-E915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. George JT, Veldhuis JD, Roseweir AK, et al. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab. 2011;96(8):E1228-E1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jayasena CN, Nijher GM, Comninos AN, et al. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96(12):E1963-E1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan YM, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J Clin Endocrinol Metab. 2012;97(8):E1458-E1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan YM, Lippincott MF, Butler JP, et al. Exogenous kisspeptin administration as a probe of GnRH neuronal function in patients with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2014;99(12):E2762-E2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan YM, Lippincott MF, Kusa TO, Seminara SB. Divergent responses to kisspeptin in children with delayed puberty. JCI Insight. 2018;3(8):e99109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan YM.Supplementary tables for “Using kisspeptin to predict pubertal outcomes for youth with pubertal delay.” Posted January 26, 2020. figshare https://figshare.com/articles/Supplementary_Tables_1-3/11587935. [DOI] [PMC free article] [PubMed]
- 22. Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med. 1972;287(12):582-586. [DOI] [PubMed] [Google Scholar]
- 24. Zhu J, Choa RE, Guo MH, et al. A shared genetic basis for self-limited delayed puberty and idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2015;100(4):E646-E654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Young J, George JT, Tello JA, et al. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology. 2013;97(2):193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lippincott MF, León S, Chan YM, et al. Hypothalamic reproductive endocrine pulse generator activity independent of neurokinin B and dynorphin signaling. J Clin Endocrinol Metab. 2019;104(10):4304-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tusset C, Noel SD, Trarbach EB, et al. Mutational analysis of TAC3 and TACR3 genes in patients with idiopathic central pubertal disorders. Arq Bras Endocrinol Metabol. 2012;56(9):646-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629-635. [DOI] [PubMed] [Google Scholar]