Highlights
-
•
COVID19 patients can develop severe acute respiratory distress syndrome (ARDS).
-
•
ARDS is a fatal complication and the most common cause of death in COVID19 patients.
-
•
Early detection of acute respiratory failure is mandatory to promplty identify critically ill COVID19 patients.
-
•
Elevated LDH and CRP serum concentrations are associated to respiratory failure in CoVID-19 patients.
Keywords: CoVID-19, LDH, CRP, Italian epidemic, CoVID-19 pneumonia, Acute respiratory failure
Abstract
Objective
The dramatic worldwide CoVID-19 infection requires the identification of a reliable and inexpensive tool to quickly discriminate patients with a more unfavorable outcome.
Methods
We performed routine laboratory tests suitable to identify tissue damage and inflammatory status in 123 consecutive CoVID-19 patients admitted to the Emergency Department of the hospital of Piacenza (Emilia-Romagna, Northern Italy). The results were correlated with patients’ respiratory function evaluated by the partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2/FiO2).
Results
The most common laboratory abnormalities were lymphocytopenia and elevated values of C-reactive protein (CRP) and lactate dehydrogenase (LDH). Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatine kinase (CK) were also increased. The respiratory performance (PaO2/FiO2) showed a strong inverse correlation with LDH (r = 0.62, r2 0.38, p value < 0.0001) and CRP (r = 0.55, r2 0.31, p value < 0.0001). PaO2/FiO2 values also showed a significant inverse correlation with age (r = −0.37, p < 0.0001), AST (r = −0.31, p < 0.01), WBC (r = −0.49, p < 0.0001), neutrophils count (r = −0.5, p < 0.001). ROC curves showed a sensitivity of 75% and specificity of 70% for the LDH cut-off value of 450 U/L and a sensitivity of 72% and specificity of 71% for the CRP cut-off value of 11 mg/dl in identifying CoVID-19 with moderate-severe ARDS.
Conclusions
LDH and CRP may be related to respiratory function (PaO2/FiO2) and be a predictor of respiratory failure in CoVID-19 patients. LDH and CRP should be considered a useful test for the early identification of patients who require closer respiratory monitoring and more aggressive supportive therapies to avoid poor prognosis.
1. Introduction
Since the beginning of March 2020, the pandemic outbreak of coronavirus disease (CoVID-19) has heavily tested the Italian healthcare system. At time of writing, Italy has the third largest number of CoVID-19 cases in the world [1]. Piacenza is a small city in Emilia-Romagna (Northern Italy), very close to Codogno (Lombardy), where on February 21st 2020 the first case of CoVID-19 has been reported. In just a few days our hospital has become one of the Italian epicenters of CoVID19 pandemic. While writing, we have dealt with 2516 CoVID-19 cases, among that 1414 needed hospitalization for acute respiratory failure and 210 were admitted to Intensive Care Unit.
The most common symptoms of CoVID-19 infection are dry cough and fever, which can turn into interstitial pneumonia with progression to acute respiratory distress syndrome (ARDS) and end-organ failure [2].
Emergency Departments (ED) are facing an elevated number of critically ill patients, and it is common occurrence that the clinical scenario may not always correspond to the severity of lung damage. In our experience, some patients do not complain dyspnea even in presence of reduced levels of peripheral oxygen saturation and severe lung involvement [3], as documented by point-of-care lung ultrasonography (US) and chest CT scan [4].
The real challenge for the clinicians is to quickly identify CoVID-19 patients at high risk for ARDS. Old age, comorbidities (hypertension, diabetes), lymphocytopenia, elevated inflammatory indices (C-reactive protein, serum ferritin, erythrocyte sedimentation), and organ dysfunction (aspartate aminotransferase, creatinine, lactate dehydrogenase) are risk factors for ARDS in CoVID-19 patients [2], [5], [6]. Unfortunately, the pathogenesis of CoVID-19 has not been completely understood. Certainly, inflammatory cytokine storm and viral evasion of cellular immune responses play a central role in disease progression and severity [7]. Many laboratory abnormalities have been described to be associated to an adverse outcome in COVID-19 patients [8]. In a meta-analysis by Henry et al., biomarkers of inflammation, cardiac and muscle injury, liver and kidney function and coagulation measures were significantly elevated in patients with both severe and fatal COVID-19, in particular Interleukin (IL) -6, IL-10 and serum ferritin were strong discriminators for severe disease [9].
In the present study, we tested the hypothesis that some routine laboratory markers, used to detect tissue damage and inflammatory status, can be useful to predict the severity of respiratory failure in CoVID-19 patients, especially in small emergency departments worldwide, where CT scan is not available.
2. Patients and Methods
This is a retrospective observational study, including 123 patients (91 males; 32 females) consecutively admitted to the ED of “Guglielmo da Saliceto” Hospital in Piacenza, Emilia-Romagna, Italy, from February 27th to March 19th 2020, and diagnosed with CoVID-19 pneumonia. The study was approved by the local ethics committee.
The mean age was 63.1 years (ranging from 22 to 94). All the patients resulted positive for 2019-nCoV by Real Time-Polymerase Chain Reaction from the nasopharyngeal swab [10] and were investigated at admission with lung US and chest CT scan that documented interstitial pneumonia at different stages.
Moreover at admission, we sampled arterial blood gas for all the patients to evaluate respiratory function based on partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio (PaO2/FiO2). ARDS was diagnosed according to the Berlin definition [11]: acute onset, bilateral opacities on chest CT scan, respiratory failure not fully explained by heart failure or fluid overload. Severity of ARDS was established according to PaO2/FiO2 value: mild (PaO2/FiO2 200–300 mmHg), moderate (PaO2/FiO2 100–200 mmHg), severe (PaO2/FiO2 < 100 mmHg). Arterial blood samples were analyzed on a ABL90 FLEX Plus blood gas analyzer (Radiometer Medical ApS, Brønshøj, Denmark).
Blood count and serum values of creatinine, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine kinase (CK) and C-reactive protein (CRP) were quantified in all patients enrolled in the study. A Sysmex automated hematology analyzer was used to perform blood count according to the manufacturer's protocol (Sysmex Partec, Milan, Italy). Serum samples were analyzed on a fully automated clinical chemistry Instrument (Beckman Olympus, Beckman, Germany). In particular CRP was detected by a turbidimetric reaction and LDH by an enzymatic reaction (oxidation of lactate to pyruvate), based on recommendations of IFCC.
The normal distribution of variables was analyzed using the Kolgomorov-Smirnov Test; means, medians, standard deviation (SD), interquartile range (IQR) have been calculated when appropriate. A log-transformation was applied to the data in the case of further analysis requiring the assumption of a normal distribution. Correlation analysis was performed using the Pearson product moment correlation test; p values lower than 0.05 were considered significant. Multivariate analysis using a logistic regression model with PaO2/FiO2 < 200 mmHg as dichotomic dependent variable and sex, age, neutrophils, lymphocytes, platelets count, creatinine, LDH, AST, CK and CRP serum concentrations, as covariates.
Receiver Operating Characteristic (ROC) curve was made for LDH and CRP serum values of patients with PaO2/FiO2 less than 200 mmHg. ROC curve plots the true-positive rate (sensitivity) against the false-positive rate (1-specificity) for all possible cut off values. The area under ROC curve was also calculated.
Statistical analysis was performed using SPSS 26.0.0.1 (IBM Corp. IBM SPSS Statistics for Mac, Armonk, NY: IBM Corp.).
3. Results
One hundred twenty-three consecutive patients with diagnosis of CoVID-19 pneumonia were included in the study: 91/123 (74%) were male (mean age 61.9 ± 14.9 years), and 32/123 (26%) female (mean age 66.5 ± 15.0 years).
At admission, 45% of patients had a PaO2/FiO2 value ranging between 200 and 300 mmHg (mild ARDS), 18% had a PaO2/FiO2 value between 100 and 200 mmHg (moderate ARDS), 6% a PaO2/FiO2 lower than 100 mmHg (severe ARDS) (Table 1 ).
Table 1.
Patients’ demographic and laboratory findings at admission. M: male, F: female. Laboratory tests are expressed as median values and Interquartile range (IQR): LDH: lactate Dehydrogenase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, CK: creatine kinase, CRP: c-reactive protein. PaO2/FiO2: partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio (number and percentage of patients for the different subclasses of ARDS).
| Demographics | ||
| Subjects [n] | 123 | |
| Sex [M/F] | 91/32 | |
| Age [mean] (min–max) | 63.1 (22–94) years | |
| Laboratory findings | ||
| Median value (IQR) | Normal range | |
| White blood cells [× 103 per µL] | 7.54 (5.17–9.66) | 4.0–10.0 |
| Neutrophils [× 103 per µL] | 5.75 (3.40–7.95) | 2.0–8.0 |
| Lymphocytes [× 103 per µL] | 0.98 (0.74–1.22) | 1.5–4.0 |
| Platelets [× 103 per µL] | 187 (145–242) | 150–450 |
| Eosinophils [× 103 per µL] | 0.0000 (0.0000–0.01) | 0.10–0.50 |
| Monocytes [× 103 per µL] | 0.39 (0.30–0.56) | 0.10–1.00 |
| Basophils [× 103 per µL] | 0.01 (0.0000–0.01) | 0.00–0.20 |
| Creatinine [mg/dL] | 1.0 (0.85–1.19) | 0.6–1.0 |
| LDH [U/L] | 410 (303–525) | 0–247 |
| AST [U/L] | 47 (33–68) | 10–37 |
| ALT [U/L] | 33 (22–56) | 10–37 |
| CK [U/L] | 164 (89–255) | 0–149 (F) 0–172 (M) |
| CRP [mg/dL] | 9.02 (4.47–14.46) | 0–0.5 |
| Respiratory function | ||
| PaO2/FiO2 > 300 mmHg [n (%)] | 38 (31) | |
| PaO2/FiO2 > 200 < 300 mmHg [n (%)] | 56 (45) | |
| PaO2/FiO2 > 100 < 200 mmHg [n (%)] | 22 (18) | |
| PaO2/FiO2 < 100 mmHg [n (%)] | 7 (6) | |
LDH was increased in 89% of patients, AST in 66%, ALT in 43%, CK in 46%, creatinine in 48%.
CRP resulted high in 98% of patients, only 21% of patients presented pathological values of white blood cell (WBC), but 18% had a neutrophils count above the upper normal range value, while 89% of patients had lymphocytes count below the lower normal range value, as previously reported [2], [8].
Our CoVID-19 patients showed elevated concentrations of: LDH (median 410 U/L (IQR 303–525); normal range 0–247), CRP (median 9.02 mg/dL (IQR 4.47–14.46); normal range 0–0.05), AST (median 47 U/L (IQR 10–37); normal range 10–31), ALT (median 33 U/L (IQR 22–56); normal range 10–31) and CK (median 164 U/L (IQR 89–255); normal range 0–149 for females and 0–172 for males). Creatinine (median 1.0 mg/dL (IQR 0.85–1.19); normal range 0.6–1) was slightly affected (Table 1).
Correlation analysis showed a significant direct relation between LDH and AST (r = 0.62, p < 0.0001), ALT (r = 0.371, p < 0.0001), CK (r = 0.452, p < 0.0001), creatinine (r = 0.36, p < 0.001), WBC count (r = 0.44, p < 0.0001), neutrophils count (r = 0.52, p < 0.0001), CRP (r = 0.57, p < 0.0001). CRP values correlated with WBC count (r = 0.51, p < 0.0001), neutrophils count (r = 0.36, p < 0.0001), and showed a slight inverse correlation with lymphocyte count (r = -0.21, p < 0.05).
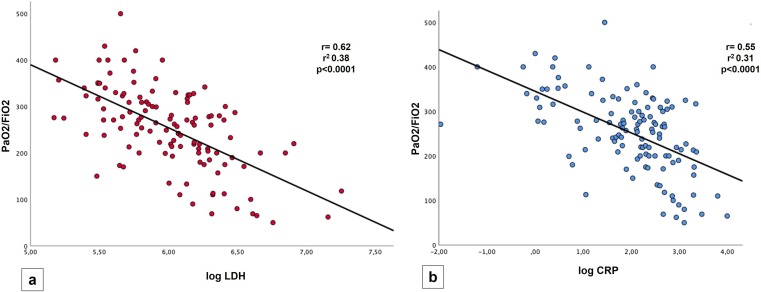
The most interesting findings were the strong inverse correlation between LDH and PaO2/FiO2 values (r = 0.62, r2 0.38, p value < 0.0001) (Fig. 1 a) and between CRP and PaO2/FiO2 values (r = 0.55, r2 0.31, p value < 0.0001) (Fig. 1b). PaO2/FiO2 values also showed a significant inverse correlation with: age (r = -0.37, p < 0.0001), AST (r = -0.31, p < 0.01), WBC (r = -0.49, p < 0.0001), neutrophils count (r = -0.5, p < 0.001); a trend of a slight direct correlation with lymphocytes count were found (r = 0.18) without reaching significance (0.051).
Fig. 1.
Linear regression graphs showing in (a) an inverse correlation between LDH and PaO2/FiO2, in (b) an inverse correlation between CRP and PaO2/FiO2.
Multivariate logistic correlation confirmed that PaO2/FiO2 values less than 200 mmHg (moderate or severe ARDS) were significantly associated with higher LDH and CRP values (p < 0.05).
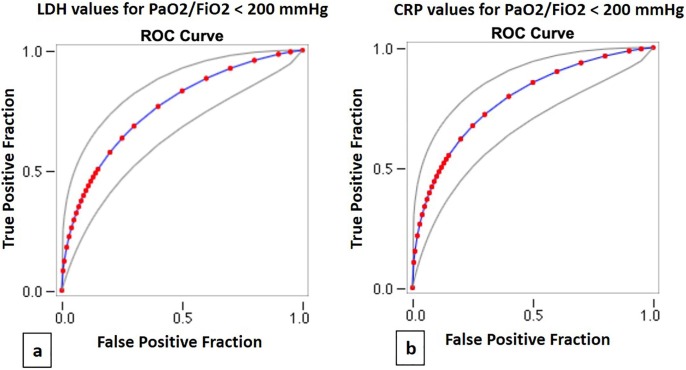
ROC curves for LDH showed that a cut-off value of 450 U/l had a sensitivity of 75% and a specificity of 70% in recognizing moderate and severe ARDS (Area under the curve (AUC) 0.76, p < 0.0001) (Fig. 2 a); for CRP a cut-off value of 11 mg/dl had a sensitivity of 72% and a specificity of 71% (AUC 0.78, p < 0.0001) (Fig. 2b).
Fig. 2.
Receiver operating characteristic (ROC) curves for LDH (a) and CRP values (b) in patients with PaO2/FiO2 < 200 mmHg.
4. Discussion
The clinical spectrum of CoVID-19 infection can vary from asymptomatic forms to interstitial pneumonia with different lung damage and the development of ARDS.
All enrolled patients complained at admission flu-like symptoms, and some of them dyspnea with a wide spectrum of respiratory failure based on PaO2/FiO2 ratio as reported in Table 1. Lung US and CT scan findings documented interstitial pneumonia at different stages in all the patients. ARDS is the most frequent fatal complication of CoVID-19 pneumonia [12].
The strongest and most interesting findings of our study were the correlations between LDH and CRP serum concentrations with PaO2/FiO2 values. According to the multivariate analysis, these relations were not influenced by sex, age, neutrophils, lymphocytes, platelets count, creatinine, AST, ALT and CK serum values. Moreover, LDH correlate with CRP and other inflammation markers suggesting a possible relationship between tissue damage and the infective status.
LDH is an enzyme involved in energy production by conversion of lactate to pyruvate and it is present in almost all body cells with highest levels in heart, liver, lungs, muscles, kidneys and blood cells. LDH is a general indicator of an acute or chronic tissue damage and is considered an inflammatory marker [13]. LDH has been described to be increased during acute and severe lung damage, and elevated LDH values has been found in other interstitial lung infections [14]. CRP is a reliable marker of acute inflammation. CRP is a hepatic protein regulated at the transcriptional level by the cytokine IL-6 and IL-1 [15].
In emerging literature, ARDS in CoVID-19 patients has been related to a systemic hyper-inflammation or cytokine storm, sustained by IL-6 and IL-1 increase [16], and multiple trials are ongoing on anti-cytokine therapy [17].
In CoVID-19 patients, LDH and CRP might represent an expression of lung damage and might reflect the respiratory distress consequent to the abnormal inflammation status. In a small cohort of 27 patients, CRP correlated with CT findings and resulted significantly increased at the early stage of severe COVID-19 before changes in the CT score [18].
Early identification and adequate treatment of CoVID-19 patients at high risk for acute respiratory failure are paramount to avoid ARDS and end-organ damage. As reported by Pan et al, chest CT has a pivotal role for the diagnosis and assessment of the severity of lung involvement in COVID-19 pneumonia [19]. Nowadays CT protocols are used to estimate the pulmonary damage [20], and CT findings can be useful to predict adverse outcome [21], but unfortunately CT scan is not available in all the Emergency Departments. Based on our results, we believe that dosing LDH and CRP could be useful to the early identification of patients at high risk for acute respiratory failure, even in patients who do not complain dyspnea or affected by slight respiratory failure. These patients could be benefit from a prompt hospitalization, a closer observation and correct treatments.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The Authors are grateful to all the medical staff of their hospital for the strength, courage and energy to manage such a hard disaster.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cca.2020.06.012.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wordometers, https://www.worldometers.info/coronavirus/#countries, 2020, Accessed April 20, 2020.
- 2.C. Huang, Y. Wang, X. Li, et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30], Lancet. 395(10223) (2020) 497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed]
- 3.E. Poggiali, P.M. Ramos, D. Bastoni, A. Vercelli, A. Magnacavallo, Abdominal pain: a real challenge in novel COVID-19 infection, Eur. J. Case Rep. Int. Med. 7(4) (2020) 001632. Published 2020 Mar 26. doi:10.12890/2020_001632. [DOI] [PMC free article] [PubMed]
- 4.Poggiali E., Dacrema A., Bastoni D. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020;295(3):E6. doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.J. Harenberg, E. Favaloro, COVID-19: progression of disease and intravascular coagulation - present status and future perspectives [published online ahead of print, 2020 May 14], Clin. Chem. Lab Med. 2020;/j/cclm.ahead-of-print/cclm-2020-0502/cclm-2020-0502.xml. doi:10.1515/cclm-2020-0502. [DOI] [PubMed]
- 6.I. Lapić, D. Rogić, M. Plebani, Erythrocyte sedimentation rate is associated with severe coronavirus disease 2019 (COVID-19): a pooled analysis [published online ahead of print, 2020 May 9], Clin Chem Lab Med. 2020;/j/cclm.ahead-of-print/cclm-2020-0620/cclm-2020-0620.xml. doi:10.1515/cclm-2020-0620. [DOI] [PubMed]
- 7.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.G. Lippi, M. Plebani, Laboratory abnormalities in patients with COVID-2019 infection [published online ahead of print, 2020 Mar 3], Clin. Chem. Lab. Med. 2020;/j/cclm.ahead-of-print/cclm-2020-0198/cclm-2020-0198.xml. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed]
- 9.B.M. Henry, M.H.S. de Oliveira, S. Benoit, M. Plebani, G. Lippi, Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis [published online ahead of print, 2020 Apr 10]. Clin. Chem. Lab. Med. 2020;/j/cclm.ahead-of-print/cclm-2020-0369/cclm-2020-0369.xml. doi:10.1515/cclm-2020-0369. [DOI] [PubMed]
- 10.World Health Organization. Novel Coronavirus (2019-nCoV) technical guidance: Laboratory testing for 2019-nCoV in humans. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance, 2020. Accessed May, 2020.
- 11.Definition Task Force A.R.D.S., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 12.J.J. Marini, L. Gattinoni, Management of COVID-19 Respiratory Distress [published online ahead of print, 2020 Apr 24], JAMA. 2020;10.1001/jama.2020.6825. doi:10.1001/jama.2020.6825.
- 13.J. Sepulveda, Challenges in Routine Clinical Chemistry Analysis: Proteins and Enzymes. Editor(s): A. Dasgupta, J. L. Sepulveda, Chapter 9, Accurate Results in the Clinical Laboratory, Elsevier,2013:131-148.
- 14.McFadden R.G., Oliphant L.D. Serum lactate dehydrogenase in interstitial lung disease. Chest. 1991;100(4):1182. doi: 10.1378/chest.100.4.1182-b. [DOI] [PubMed] [Google Scholar]
- 15.Black S., Kushner I., Samols D. C-reactive protein. J. Biol. Chem. 2004;279(47):48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 16.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19(6) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan C., Huang Y., Shi F., Tan K., Ma Q., Chen Y., Jiang X., Li X. C-reactive protein correlates with CT findings and predicts severe COVID-19 early. J. Med. Virol. 2020 doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan F., Ye T., Sun P. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung M., Bernheim A., Mei X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D. Colombi, F.C. Bodini, M. Petrini, et al., Well-aerated Lung on Admitting Chest CT to Predict Adverse Outcome in COVID-19 Pneumonia. Radiology, 2020;201433. doi:10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.