Abstract
Background
The outbreak of highly contagious coronavirus disease 2019 (COVID-19) has posed a serious threat to human life and health, especially for those with underlying diseases. However, the impact of COVID-19 epidemic on hemodialysis (HD) centers and HD patients has not been reported.
Methods
We reviewed the whole course of the COVID-19 in the HD center of Renmin Hospital, Wuhan University (from January 14, 2020, to March 12, 2020). We compared the clinical manifestation and immune profiles among different patient groups with healthy individuals.
Results
Forty-two of 230 HD patients (18.26%) and 4 of 33 medical staff (12.12%) were diagnosed with COVID-19 during the study period. Fifteen HD patients (6.52%), including 10 COVID-19 diagnosed, died. Only 2 deaths of the COVID-19 HD patients were associated with pneumonia/lung failure, others were ascribed to cardiovascular/cerebrovascular diseases or hyperkalemia. Except for 3 patients who were admitted to the intensive care unit for a severe condition (8.11%), including 2 who died, most COVID-19 diagnosed patients presented mild or nonrespiratory symptoms. The flow cytometric analysis of peripheral blood showed that multiple lymphocyte populations in HD patients were significantly decreased. HD patients with COVID-19 even displayed more remarkable reduction of serum inflammatory cytokines than other patients with COVID-19.
Conclusions
Compared with the general population, HD patients and health care professionals are the highly susceptible population and HD centers are high-risk areas during the outbreak. Most HD patients with COVID-19 exhibited mild clinical symptoms and did not progress to severe pneumonia, likely due to the impaired cellular immune function and incapability of mounting cytokine storm. More attention should be paid to prevent cardiovascular events, which may be the collateral impacts of the COVID-19 epidemic on HD patients.
Keywords: COVID-19, cytokine storm, epidemic, hemodialysis, immune
Since December 2019, the initial outbreak of COVID-19 in Wuhan city has spread rapidly to all over China and even the world, becoming a serious pandemic.1, 2, 3, 4 As of March 12, 2020, the total laboratory-confirmed COVID-19 cases in Wuhan city, nationwide in China, and globally reached 49,986, 80,981, and 125,260, respectively.5,6 Epidemiological data suggest that patients with COVID-19 with underlying conditions, such as diabetes, hypertension, or cardiovascular disease, or the elderly are at a higher risk of mortality.7,8 Considering the large population size of HD patients (there are 7184 registered patients receiving HD treatment in 61 centers in Wuhan city), high concentration of patients in HD centers, and the compromised immune function of uremic patients,9 necessitates a critical assessment of the impact of COVID-19 among HD patients.
In this study, we reviewed an outbreak of COVID-19 in the HD center in Renmin Hospital of Wuhan University, one of the largest hospitals in Wuhan city. A cluster of HD patients who contracted COVID-19 were surveyed since January 14, 2020, the day when the first case was confirmed, and followed until March 12, 2020. The epidemiological, clinical, laboratory, and radiological characteristics, and outcomes of some of these patients were reviewed. We expect our findings will shed light on the appropriate management of the HD center and HD patients in face of COVID-19 or another similar epidemic that may emerge.
Methods
Study Design and Participants
We reviewed the epidemic course from the first laboratory-confirmed case of COVID-19 infection on January 14 to the control of the epidemic on March 12 in the HD center of Renmin Hospital of Wuhan University. A total 230 HD patients and 33 medical staff were included in this study. Diagnosis of COVID-19 pneumonia was based on the New Coronavirus Pneumonia Prevention and Control Program (fifth edition valid from February 4, 2020, to February 17, 2020, sixth edition valid from February 18, 2020, to March 4, 2020, seventh edition valid from March 4, 2020, until now) published by the National Health Commission of China.10, 11, 12 In the fifth edition, suspected cases of COVID-19 are defined as those with epidemiological history or clinical presentations of fever, respiratory symptoms, or decreased white blood cells or lymphocyte count. The clinically diagnosed case is recognized when the suspected case displays the features of pneumonia. The confirmed case is identified if the suspected cases or clinically diagnosed case is positive in the pathogenic test. In the sixth and seventh editions, the definition of clinically diagnosed case has been removed. The suspect case should meet the criteria of epidemiological history and clinical presentations. If pathogen evidence is present, the suspect case should be confirmed as COVID-19. According to the guidance of the government, all infected patients were transferred to the corresponding designated hospitals for further treatment after disease assessment and classification. The study protocol was approved by the Ethics Committee of Renmin Hospital of Wuhan University (WDRY2020-K064). Written informed consent was waived by the Ethics Commission of the hospital for emerging infectious diseases.
Data Collection
The medical records of all participants were analyzed by the research team. Epidemiological, clinical, laboratory, and radiological characteristics and outcomes data were obtained with data collection forms from electronic medical records or specific data questionnaire. Some data were retrieved from the Hubei Province kidney disease quality control information platform. Information collected included demographic data, medical history, underlying comorbidities, symptoms, signs, blood tests, and chest computed tomographic (CT) scans. During the period of follow-up, the odd episodes of death that happened among these patients were recorded and the cause of death was carefully evaluated by the research team, based on the time, place, and clinical manifestation of the death.
Virologic Studies
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection was done by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) as described previously.13 Briefly, nasopharyngeal swab samples of participants were collected for SARS-CoV-2 test with the detection kit (Bioperfectus, Taizhou, China). The ORF1ab gene (nCovORF1ab) and the N gene (nCoV-NP) were used for real-time RT-PCR according to the manufacturer’s instructions. Reaction mixture were prepared and RT-PCR assay was then performed under the following conditions: incubation at 50 °C for 15 minutes and 95 °C for 5 minutes, 40 cycles of denaturation at 94 °C for 15 seconds, and extending and collecting fluorescence signal at 55 °C for 45 seconds.
Cellular Immune Profiling and Cytokine Measurement
To explore the effect of SARS-CoV-2 infection on host immune responses, we recruited 19 HD patients with COVID-19 and 19 non-COVID-19 HD patients for further blood sample collection with informed consent; 30 healthy volunteers were simultaneously enrolled. Peripheral blood mononuclear cells (PBMCs) and serum were isolated from the blood samples of the participants. Isolated PBMCs were stained with a BD Multitest IMK Kit (Cat340503; BD Biosciences, San Jose, CA) for analyzing the frequency and cell number of total T cells, CD4+ T cells,CD8+ T cells, B cells, and natural killer cells in healthy controls and patients. The stained cells were acquired on an LSR Fortessa Cell Analyzer (BD Biosciences) and data analyzed using the FlowJo software (TreeStar, Ashland, OR). Serum levels of a panel of cytokines (interleukin [IL]-4, IL-6, IL-10, IL-17, tumor necrosis factor-α, and interferon-γ) were assayed using Human Cytokine Standard 27-Plex Assays panel and the Bio-Plex 200 system (Bio-Rad, Hercules, CA) according to the manufacturer’s suggested protocol. All experimental procedures were completed under biosafety level II plus condition.
Statistical Analysis
The measured data were median and interquartile range values and compared using independent group t test. Enumeration data were described as number (%). All statistical analyses were performed using SPSS (IBM Corp., Armonk, NY), and a P value of less than 0.05 was considered as significant difference.
Results
Patient Characteristics and Study Design
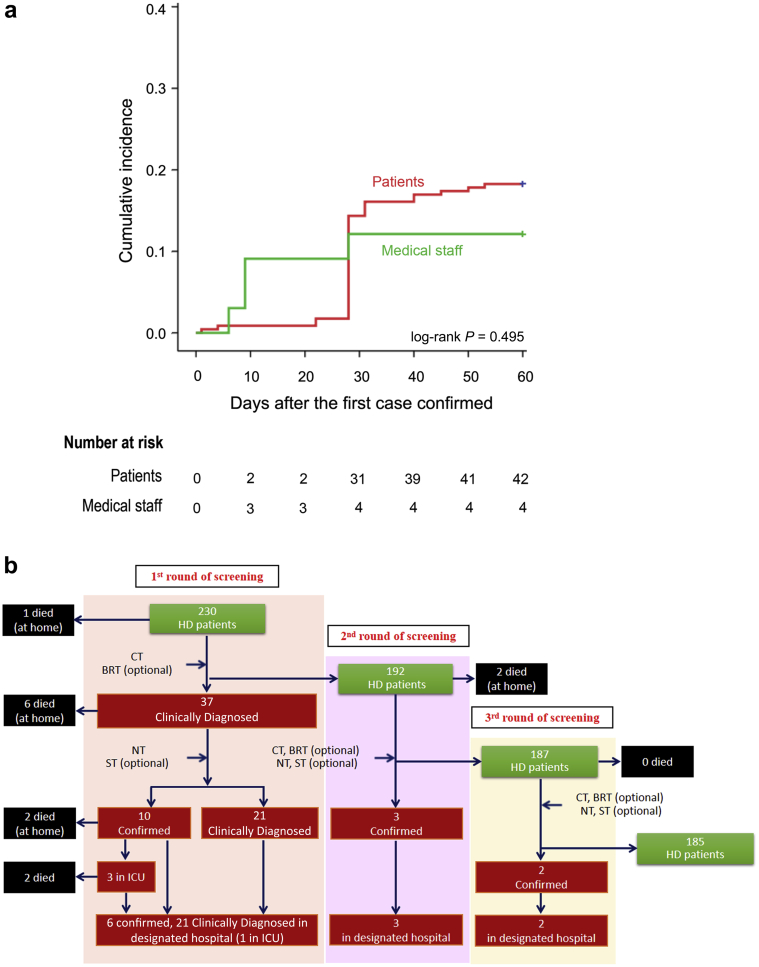
A total of 230 patients and 33 staff in our HD center were included in this study. The cumulative incidence of COVID-19 epidemic in our HD center is presented in Figure 1a. The first COVID-19 patient was diagnosed on January 14, and the second patient was diagnosed on January 17. On January 19, a nurse was confirmed as the first infected medical staff in our HD center. Since January 21, patients with COVID-19 have been quarantined and all medical staff have been asked to upgrade their personal prevention and protection, which includes wearing full protective gear such as waterproof disposable gown, cap, gloves, face shield, and N95 face mask, and more rigorous cleaning and disinfection. Two days later, 2 medical staff were diagnosed. On February 4, 2 new patients were further confirmed with COVID-19. Therefore, the HD center decided to screen all patients and staff with chest CT and selective blood test. On February 10, there were 30 newly diagnosed cases with COVID-19, including 29 HD patients and 1 medical staff. On February 13, 4 more new COVID-19 cases were confirmed in HD patients. Since then, until the first screening was fully completed on February 17, 2020, no new COVID-19 case occurred. To determine potentially infected but asymptomatic cases in their incubation period, we launched the second round of screening from February 22, 2020, to March 3, 2020, and the third round of screening from March 3, 2020, to March 12, 2020. There were 3 cases in the second screening, and 2 cases in the third screening that were confirmed with the diagnosis of COVID-19.
Figure 1.
Retrospective survey of the course of COVID-19 emerging in one hemodialysis (HD) facility. (a) The cumulative incidence of COVID-19 epidemic in our HD center. The first COVID-19 patient was diagnosed on January 14. The second patient was diagnosed on January 17. The first infected staff member was reported on January 19. The personal prevention and protection of medical staff was upgraded on January 21. Two days later, 2 medical staff were diagnosed. On February 4, 2 new patients and were further confirmed with COVID-19. Twenty-nine HD patients and 1 medical staff were diagnosed on February 10. Four new HD patients were diagnosed with COVID-19 on February 13. (b) The management and the outcomes of the cluster during the epidemic. Thirty-seven patients and 4 medical staff were diagnosed with COVID-19 in our center. Six patients confirmed with COVID-19 had died and the other 31 patients were distributed to the designated hospital for treatment. The presumed causes of death were heart failure, hyperkalemia, and cerebrovascular disease. BRT, blood routine test; CT, computed tomography; ICU, intensive care unit; NT, nucleic acid test; ST, serological test.
Clinical Manifestation, Management, and Patient Outcome
During the period of screening, all infected patients and staff were classified, quarantined, or transferred to the designated hospital according to the government instruction. Figure 1b summarizes the management flow and the outcomes of the followed cluster in the epidemic. Of the total 42 (18.26%) patients who were diagnosed with COVID-19, 13 have died since the epidemic outbreak, including 10 patients who contracted COVID-19. Among the infected patients, 3 were admitted to the intensive care unit and 2 died later; 1 patient is still stable. We launched 3 rounds of screening to find the infected patients in the HD center. The first round started on February 4, 2020, and closed on February 17, 2020, with 37 patients being clinically diagnosed with COVID-19. Ten patients were confirmed with the diagnosis of COVID-19 based on the positive test of SARS-CoV-2 nucleic acid on nasopharyngeal swab. All these 31 patients were transferred to the designated hospitals, and those left remained in our hospital under indicated quarantine or isolation, followed by the second screening, beginning on February 22, 2020, for the purpose of determining potential infected cases who were in the incubation period during previous testing. Three patients were confirmed with the diagnosis and another 2 patients died in this phase. The third round of screening was carried out on 187 patients and it was determined that 2 patients contracted COVID-19. As of February 14, 2020, the day this manuscript was finished, there were 185 HD patients remaining in our hospital and 32 in the designated hospital and their demographic data are summarized in Table 1. All of the deaths were followed and reviewed by our research team. The 2 patients who died in the intensive care unit (Table 1) and the other 11 cases who died at home showed no obvious symptoms of pneumonia; the inferred cause of death was heart failure, hyperkalemia, and cerebrovascular disease, and none of them succumbed to severe pneumonia based on the clinical manifestations (Figure 1).
Table 1.
Demographic and clinical characteristics of the hospitalized patients and patients who died with COVID-19
| Characteristics | Hospitalized | Died |
|---|---|---|
| No. of patients | 32 | 2 |
| Sex, n (%) | ||
| Male | 21 (66) | 2 (100) |
| Female | 11 (34) | 0 |
| Age, y | ||
| Median (IQR) | 66 (44–73) | 79 (77–81) |
| HD age, mo | ||
| Median (IQR) | 36 (14–48) | 67.5 (41–94) |
| Albumin, g/l | ||
| Median (IQR) | 38.5 (36–43) | 37.5 (37–38) |
| Hemoglobin, g/l | ||
| Median (IQR) | 127 (100–152) | 116.5 (105–128) |
| Kt/V | ||
| Median (IQR) | 1.21 (1.17–1.41) | 1.22 (1.18–1.26) |
| iPTH, pg/ml | ||
| Median (IQR) | 387 (160–518) | 405 (244–566) |
| Primary cause of end-stage renal disease, n (%) | ||
| Glomerulonephritis | 19 (59) | 0 |
| Hypertensive nephropathy | 8 (25) | 2 (100) |
| Diabetic nephropathy | 4 (13) | 0 |
| Polycystic kidney disease | 1 (3) | 0 |
| Access, n (%) | ||
| CVC | 11 (34) | 1 (50) |
| AVF | 21 (66) | 1 (50) |
| Artificial blood vessel | 0 | 0 |
AVF, arteriovenous fistula; CVC, central venous catheter; iPTH, intact parathyroid hormone; IQR, interquartile range.
Demographic data of 42 COVID-19 HD patients are summarized in Table 2. Among 15 patients with confirmed diagnosis of COVID-19, 10 were men and 5 were women, with median age of 71 years (range, 54–76 years). Of the 27 clinically diagnosed patients with COVID-19, 15 were men and 12 were women, with median age of 61 years (range, 47–68 years). The primary causes of end-stage renal disease in these patients were glomerulonephritis (8 of 15 with confirmed diagnosis, 15 of 27 clinically diagnosed), hypertension (5 of 15 with confirmed diagnosis, 8 of 27 clinically diagnosed), diabetic nephropathy (1 of 15 with confirmed diagnosis, 4 of 27 clinically diagnosed). The HD duration of these patients was approximately 30 months.
Table 2.
Clinical characteristics of the infected and noninfected patients
| Characteristics | Confirmed diagnosis | Clinically diagnosed | Noninfected |
|---|---|---|---|
| No. of patients | 15 | 27 | 188 |
| Sex, n (%) | |||
| Male | 10 (67) | 15 (56) | 107 (57) |
| Female | 5 (33) | 12 (44) | 81 (42) |
| Age, years | |||
| Median (IQR) | 71 (54–76) | 61 (47–68) | 58 (22–91) |
| HD age, mo | |||
| Median (IQR) | 32 (14–43) | 35 (16–61) | 47 (2–261) |
| Albumin, g/l | |||
| Median (IQR) | 39 (36–44) | 40 (35–46) | 40 (15–56) |
| Hemoglobin, g/l | |||
| Median (IQR) | 124 (100–152) | 112 (103–141) | 110 (93–118) |
| Kt/V | |||
| Median (IQR) | 1.19 (1.15–1.45) | 1.33 (1.17–1.56) | 1.36 (1.05–1.42) |
| iPTH, pg/ml | |||
| Median (IQR) | 400 (196–555) | 248 (86–329) | 356 (169–684) |
| Primary cause of end-stage renal disease, n (%) | |||
| Glomerulonephritis | 8 (53) | 15 (55) | 52 (31) |
| Hypertensive nephropathy | 5 (33) | 8 (30) | 46 (27) |
| Diabetic nephropathy | 1 (7) | 4 (15) | 11(7) |
| Polycystic kidney disease | 1 (7) | 0 | 3 (2) |
| Access, n (%) | |||
| CVC | 6 (40) | 8 (28) | 39 (21) |
| AVF | 9 (60) | 19 (72) | 147 (78) |
| Artificial blood vessel | 0 | 0 | 2 (1) |
AVF, arteriovenous fistula; CVC, central venous catheter; iPTH, intact parathyroid hormone; IQR, interquartile range.
Hematology abnormalities such as lymphocytopenia and thrombocytopenia are common in patients with COVID-19 as previously reported.7,8,14,15 These features were also present in the COVID-19 contracted HD patients (Table 3). Anemia was also commonly seen in these patients, but primarily ascribed to end-stage renal disease complications. With respect to the clinical symptoms in HD patients with COVID-19, we found that only 2 (13%) with confirmed diagnosis, and 2 (7%) in clinically diagnosed patients had fever, or fatigue. Three patients had the symptom of dry cough and only 1 clinically diagnosed patient had chest pain. One with confirmed diagnosis and 1 clinically diagnosed patient had nausea. Most of the patients present no obvious symptoms (Table 3). Radiological examination by chest CT scan showed that 2 (13%) patients with confirmed diagnosis and 8 (30%) clinically diagnosed patients had unilateral involvement, 12 (80%) with confirmed diagnosis and 19 (70%) clinically diagnosed patients had bilateral involvement. Twelve (80%) patients with confirmed diagnosis patients and 15 (65%) clinically diagnosed patients had multiple “ground-glass opacity” lesions in the lung, which were regarded as the key characteristics of COVID-19 pneumonia (Table 4). Table 5 concluded the epidemiological and outcomes of all patients and staff in our HD unit. These clinical manifestations summarized previously indicated that most cases of COVID-19 infected HD patients are mild conditions, which is quite different from previous findings on other COVID-19 contracted patients with comorbidities such as diabetes, hypertension, cardiovascular disease, or the elderly.7,8,14
Table 3.
Blood routine findings of infected and noninfected patients
| Parameters | Confirmed diagnosis (n = 15) | Clinically diagnosed (n = 27) | Noninfected (n = 188) |
|---|---|---|---|
| Leucocytes (×109/l; NR, 3.5–9.5) | |||
| Median (IQR) | 7.18 (6.15–10.10) | 6.38 (4.79–7.66) | 6.46 (2.10–17.42) |
| Increased, n (%) | 1 (7) | 0 | 11 (6) |
| Decreased, n (%) | 0 | 2 (7) | 88 (47) |
| Neutrophils (×103/l; NR, 1.8–6.3) | |||
| Median (IQR) | 4.92 (4.23–7.06) | 4.08 (3.31–6.01) | 4.98 (3.00–17.58) |
| Increased, n (%) | 4 (27) | 3 (11) | 32 (17) |
| Decreased, n (%) | 0 | 3 (11) | 1(1) |
| Lymphocytes (×109/l; NR, 1.1–3.2) | |||
| Median (IQR) | 1.42 (0.85–1.56) | 0.73(0.45–1.33) | 1.01 (0.55–1.61) |
| Increased, n (%) | 1 (7) | 0 | 0 |
| Decreased, n (%) | 7 (47) | 18 (67) | 98 (52) |
| Monocytes (×109/l; NR, 0.1–0.6) | |||
| Median (IQR) | 0.58 (0.39–0.76) | 0.46 (0.31–0.69) | 0.49 (0.27–0.77) |
| Increased, n (%) | 4 (27) | 9 (33) | 39 (21) |
| Decreased, n (%) | 0 | 0 | 1 (1) |
| Red blood cells (×1012/l; NR, 4.3–5.8) | |||
| Median (IQR) | 3.94 (3.54–4.42) | 3.58 (3.14–3.99) | 3.43 (2.96–4.45) |
| Increased, n (%) | 0 | 0 | 0 |
| Decreased, n (%) | 8 (53) | 15 (56) | 122 (65) |
| Blood platelets (×109/l; NR, 125–350) | |||
| Median (IQR) | 154 (140–200) | 159 (122–189) | 171 (36–489) |
| Increased, n (%) | 0 | 0 | 3 (2) |
| Decreased, n (%) | 7 (47) | 16 (59) | 41 (22) |
| C-reactive protein (mg/l, NR <10) | |||
| Increased, n (%) | 7 (47) | 6 (22) | 6 (3) |
| Serum amyloid A (mg/l, NR <10) | |||
| Increased, n (%) | 6 (40) | 9 (33) | 56 (30) |
IQR, interquartile range; NR, normal range.
Table 4.
Clinical symptoms and chest CT results of confirmed and clinically diagnosed patients
| Characteristics | Confirmed patients (n = 15) | Clinical diagnosed patients (n = 27) |
|---|---|---|
| Symptoms | ||
| Fever | 2 (13) | 2 (7) |
| Fatigue | 2 (13) | 1 (4) |
| Dry cough | 3 (20) | 0 |
| Chest pain | 0 | 1 (4) |
| Nausea | 1 (7) | 1 (4) |
| Chest CT | ||
| Unilateral pneumonia | 2 (13) | 8 (30) |
| Left | 1 (7) | 2 (7) |
| Right | 1 (7) | 6 (22) |
| Bilateral pneumonia | 12 (80) | 19 (70) |
| No pneumonia | 1 (7) | 0 |
| Multiple “ground-glass opacity” lesions | 12 (80) | 15 (65) |
CT, computed tomography.
Data are n (%).
Table 5.
Epidemiological and clinical characteristics in HD unit
| Characteristics | HD patients (n = 230) | Medical staff (n = 33) |
|---|---|---|
| No. of infections | 42 (18.3) | 4 (12.1) |
| No. of mild cases | 37 (88.1) | 4 (100) |
| No. of intensive care unit cases | 3 (7.1) | 0 |
| No. of deaths | 2 (4.7) | 0 |
HD, hemodialysis.
Data are n (%).
HD Patients Exhibit Blunted Immune Responses
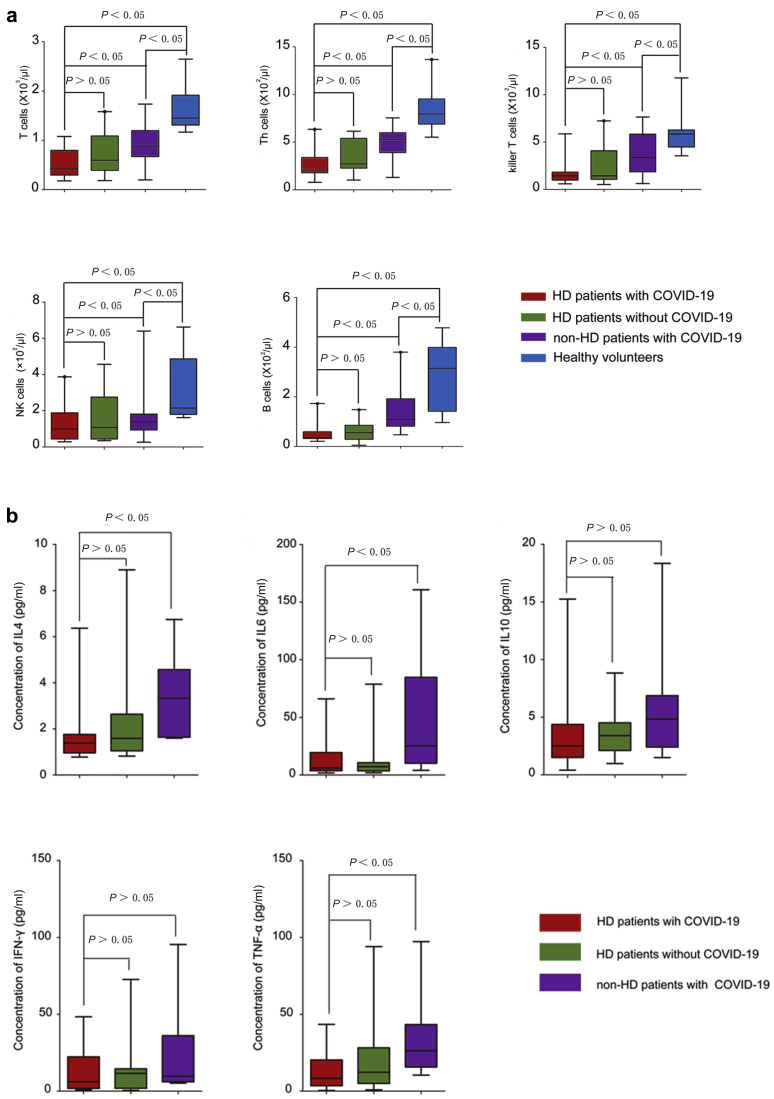
The immune system plays an essential role in protecting the host from various pathogens, including viruses. In response to viral infection, both cellular immunity and humoral immunity are activated and work together to fight the infection by directly lysing infected cells or releasing neutralizing antibodies, respectively. Once SARS-CoV-2 infects the host’s respiratory tract, it multiplies in cells of the airway, triggering extensive immune activation and releasing massive proinflammatory cytokines. This “cytokine storm” effect is responsible for severe conditions or even eventually leads to death of patients with COVID-19.14 As we mentioned earlier, most of the HD patients with SARS-CoV-2 infection showed relatively mild symptoms. We speculated that this might be related to the compromised immunity in HD patients. To test this hypothesis, we enumerated the absolute numbers of T cells and natural killer cells, as well as B cells in PBMCs of HD patients in the presence of absence of SARS-CoV-2 infection. We found that the numbers of T cells, CD4+ T cells, CD8+ T cells, natural killer cells, and B lymphocytes in PBMCs of HD patients were significantly lower compared with those of non-HD patients. These numbers in HD patients with SARS-CoV-2 infection were further decreased (Table 6 and Figure 2a). Similar to the numbers of lymphocytes, we also observed that the serum levels of IL-4, IL-6, and tumor necrosis factor-α in non-HD patients with SARS-CoV-2 infection were significantly higher than the normal level, whereas the serum levels of these cytokines in HD patients with or without SARS-CoV-2 infection are significantly lower than those in non-HD patients with SARS-CoV-2 infection (Table 7 and Figure 2b). These results suggest that HD patients have a compromised immune system, which may be detrimental for mounting effective antiviral responses, but beneficial for limiting tissue damage by dampening the cytokine release.
Table 6.
The frequency of immunocytes in the PBMCs of patients
| Parameters | HD patients with COVID-19 | HD patients without COVID-19 | Non-HD patients with COVID-19 | Heathy volunteers | |
|---|---|---|---|---|---|
| T cells, /μl | 422 (309–819) | 594 (397–1045) | 884 (684–1166) | 1452 (1327–1839) | |
| Th cells, /μl | 207 (187–337) | 272 (231–531) | 527 (395–579) | 794 (696–926) | |
| Killer T cells, /μl | 143 (110–171) | 143 (114–394) | 335(200–592) | 585 (457–626) | |
| Natural killer cells, /μl | 99 (48–196) | 108 (55–259) | 138 (99–178) | 215 (181–466) | |
| B cells, /μl | 35 (32–56) | 56 (29–83) | 109 (83–175) | 315 (150–388) | |
HD, hemodialysis; IQR, interquartile range; PBMC, peripheral blood mononuclear cell.
Data are median (IQR). n = 19.
Figure 2.
Immunological profile of the hemodialysis (HD) patients under COVID-19 infection. (a) The frequency of lymphocytes in the PBMCs of patients with or without COVID-19. The proportion of T cells, CD4+ T cells, CD8+ T cells, and B cells of HD patients with or without COVID-19, non-HD COVID-19 patients, or healthy volunteers. (b) The serum levels of cytokines in indicated patients. IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Table 7.
The serum level of cytokines in indicated patients
| Parameters | HD patients with COVID-19 | HD patients without COVID-19 | Non-HD patients with COVID-19 |
|---|---|---|---|
| IL-4, pg/ml | 1.39 (0.977–1.76) | 1.59 (1.10–2.49) | 3.43 (1.89–4.57) |
| IL-6, pg/ml | 6.04 (3.98–19.45) | 7.24 (3.96–9.90) | 25.41 (11.38–81.39) |
| IL-10, pg/ml | 2.51 (1.66–4.37) | 3.40 (2.30–4.40) | 4.84 (2.60–6.90) |
| Interferon- γ, pg/ml | 6.07 (1.83–22.27) | 11.57 (1.95–14.33) | 10.74 (6.05–36.06) |
| Tumor necrosis factor- α, pg/ml | 8.26 (4.74–20.27) | 12.27 (5.26–26.22) | 26.68 (18.23–42.21) |
HD, hemodialysis; IL, interleukin; IQR, interquartile range.
Data are median (IQR). n = 19.
Discussion
As the epicenter of the COVID-19 in China, Wuhan city has reported 49,991 confirmed cases, 8329 of which are in severe condition and 2436 have died as of March 12.5 Previous epidemiological survey showed that the elderly or patients with comorbidities were more susceptible to COVID-19, and the incidence of severe cases and the risk of death were high.14,16, 17, 18 However, there is no report about the impacts of the COVID-19 epidemic on HD patients. HD patients are a distinctly different cluster from other populations because of the following: (i) They make up a large-scale cluster. For instance, there are more than 7000 HD patients in Wuhan city. (ii) They are scattered in different centers across the city. (iii) They are highly mobile. Patients must travel regularly between their residences and hospitals. (iv) They usually receive concentrated dialysis treatment. (v) Their immune functions are compromised, so they may become potential “super-spreaders.” (vi) The epidemic is very likely to spread in clusters with those who are receiving dialysis in the same shift with 1 patient with undiagnosed COVID-19, the adjacent patients are at particularly high risk of infection. Considering these factors, HD patients and HD centers should be given priority in epidemic prevention and control. According to our investigation on single center, the infection rate of HD patients in the COVID-19 epidemic is indeed much higher than that of other populations, and the staffs in the HD center are also at high risk of infection.
Reviewing the epidemic situation in our center can bring some important experiences and lessons that have been described elsewhere.19,20 The COVID-19 epidemic initially emerged in our center on January 14, 2020, and the first infected medical staff appeared on January 19, 2020, but until January 21, actions were taken by our center to stand up to, we must admit that the best time had been missed. Nevertheless, a series of measures including upgrading prevention and protection, quarantine and isolation, seem to be effective to contain the epidemic, but the most critical means we think is thoroughly determining the infected cases by repeated screening, which is mainly based on the results of chest CT scan. The suspected infections with abnormal radiological images were further subjected to SARS-CoV-2 nuclear acid test. Once the diagnosis of COVID-19 was made, the patient was transferred to the designated hospital within 4 hours according to the contingent plan. Another issue that needs to be noted is that the outbreak of COVID-19 in our center left 13 deaths, with a mortality of 5.65%, which is far higher than that of the same period in history. The major causes of deaths were cardiovascular and cerebrovascular complications or hyperkalemia rather than pneumonia, the real reason we speculated was inadequate dialysis. It is not worth it to avoid infection by reducing the frequency of dialysis, because COVID-19 infection in HD patients seemed to be less severe or fatal.
Previous studies have shown that SARS-CoV-2 infection would trigger release of inflammatory cytokines. Cytokine storm may be the key cause of the worsened condition and even death of patients.14 However, our study demonstrated that HD patients were unable to mount effective cellular immune response on the invasion of SARS-CoV-2, thus results in no cytokine storm and no severe organ damage, which may be beneficial for patient survival but also means longer time to clear the virus, and persistent virus shedding duration, which has been observed in SARS-CoV infected HD patients in the SARS outbreak.21 In the current clinical guidelines and practice for COVID-19 therapy, glucocorticoids are recommended and usually prescribed.7,12 However, with regard to the management of COVID-19 in HD patients, we suggest the administration of glucocorticoids should be prudent because the immune system in HD patients has already been suppressed.22
Disclosure
All the authors declared no competing interests.
Acknowledgments
Dr. Gowthaman Uthaman, from the Department of Laboratory Medicine of Yale University School of Medicine, offered advice and assistance in the manuscript writing.
This work was supported by grants from the National Natural Science Foundation of China (#81370800 to HW, #81800615 to YM) and the Key Project on Science and Technology Innovation of Hubei Province (#2019ACA137 to HW).
References
- 1.Zhu N., Zhang D.Y., Wang W.L. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahase E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 3.Eurosurveillance Editorial Team Latest updates on COVID-19 from the European Centre for Disease Prevention and Control. Euro Surveill. 2020;25:2002131. doi: 10.2807/1560-7917.ES.2020.25.6.2002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 2020. World Health Organization. WHO characterizes COVID-19 as a pandemic. Geneva, Switzerland. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed July 9, 2020. [Google Scholar]
- 5.National Health Commission of the People’s . 2020. Republic of China. COVID-19 epidemic situation report. Beijing, China. Available at: http://www.nhc.gov.cn/xcs/yqtb/202003/37c1536b6655473f8c2120ebdc475731.shtml. Accessed July 9, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . 2020. Coronavirus disease 2019 (COVID-19) Situation Report – 52. Geneva, Switzerland. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200312-sitrep-52-covid-19.pdf?sfvrsn=e2bfc9c0_4. Accessed July 9, 2020. [Google Scholar]
- 7.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaier M., Leick A., Uhlmann L. End-stage renal disease, dialysis, kidney transplantation and their impact on CD4(+) T-cell differentiation. Immunology. 2018;155:211–224. doi: 10.1111/imm.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Health Commission of China . 2020. New coronavirus pneumonia prevention and control program (5th edition) Beijing, China. Available at: http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440/files/7260301a393845fc87fcf6dd52965ecb.pdf. Accessed July 9, 2020. [Google Scholar]
- 11.National Health Commission . 2020. New coronavirus pneumonia prevention and control program (6th edition) Beijing, China. Available at: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf. Accessed July 9, 2020. [Google Scholar]
- 12.National Health Commission of China . 2020. New coronavirus pneumonia prevention and control program (7th edition) Beijing, China. Available at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf. Accessed July 9, 2020. [Google Scholar]
- 13.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan F., Ye T., Sun P. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwan B.C., Leung C.B., Szeto C.C. Severe acute respiratory syndrome in dialysis patients. J Am Soc Nephrol. 2004;15:1883–1888. doi: 10.1097/01.asn.0000131522.16404.1f. [DOI] [PubMed] [Google Scholar]
- 18.Girndt M., Sester M., Sester U. Molecular aspects of T- and B-cell function in uremia. Kidney Int Suppl. 2001;78:S206–S211. doi: 10.1046/j.1523-1755.2001.59780206.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang H. Maintenance hemodialysis and Coronavirus Disease 2019 (COVID-19): saving lives with caution, care, and courage. Kidney Med. 2020;2:365–366. doi: 10.1016/j.xkme.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su K, Ma Y, Wang Y, et al. How we mitigate and contain COVID-19 outbreak in hemodialysis center (HD): lessons and experiences [e-pub ahead of print]. Infect Control Hosp Epidemiol. https://doi.org/10.1017/ice.2020.161. Accessed July 9, 2020. [DOI] [PMC free article] [PubMed]
- 21.Girndt M., Sester U., Sester M. Impaired cellular immune function in patients with end-stage renal failure. Nephrol Dial Transplant. 1999;14:2807–2810. doi: 10.1093/ndt/14.12.2807. [DOI] [PubMed] [Google Scholar]
- 22.Vaziri N.D., Pahl M.V., Crum A., Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22:149–156. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]